Abstract
Parkinson’s Disease (PD) is the second most common neurodegenerative disease, yet its etiology and pathogenesis are poorly understood. PD is characterized by selective dopaminergic (DAergic) degeneration and progressive hypokinetic motor impairment. Mutations in dj-1 cause autosomal recessive early-onset PD. DJ-1 is thought to protect DAergic neurons via an antioxidant mechanism, but the precise basis of this protection has not yet been resolved. Aging and manganese (Mn) exposure are significant non-genetic risk factors for PD. Caenorhabditis elegans (C. elegans) is an optimal model for PD and aging studies because of its simple nervous system, conserved DAergic machinery, and short 20-day lifespan. Here we tested the hypothesis that C. elegans DJ-1 homologues were protective against Mn-induced DAergic toxicity in an age-dependent manner. We showed that the deletion of C. elegans DJ-1 related (djr) genes, djr-1.2, decreased survival after Mn exposure. djr-1.2, the DJ-1 homologue was expressed in DAergic neurons and its deletion decreased lifespan and dopamine (DA)-dependent dauer movement behavior after Mn exposure. We also tested the role of DAF-16 as a regulator of dj-1.2 interaction with Mn toxicity. Lifespan defects resulting from djr-1.2 deletion could be restored to normal by overexpression of either DJR-1.2 or DAF-16. Furthermore, dauer movement alterations after djr-1.2 deletion were abolished by constitutive activation of DAF-16 through mutation of its inhibitor, DAF-2 insulin receptor. Taken together, our results reveal PD-relevant interactions between aging, the PD environmental risk factor manganese, and homologues of the established PD genetic risk factor DJ-1. Our data demonstrate a novel role for the DJ-1 homologue, djr-1.2, in mitigating Mn-dependent lifespan reduction and DA signaling alterations, involving DAF-2/DAF-16 signaling.
INTRODUCTION
For nearly a century, we have understood the progressive motor impairment in Parkinson’s Disease (PD), including rigidity and hyperkinesia, results from the loss of dopaminergic (DAergic) cells in the midbrain substantia nigra (SN) 1; yet the molecular mechanisms initiating DAergic cell loss remain unclear. PD affects over 2% of the world population over 60-years-of-age, yet the majority of PD cases have an unknown genetic or environmental cause, and are defined as idiopathic PD. Modeling the complex interaction of known genetics, environmental exposure, and aging risk factors for PD in the simple model organism Caenorhabditis elegans (C. elegans) represents a promising path to understanding the etiology and pathogenesis of PD and parkinsonism.
Studies of familial PD have identified 11 genes associated with heritable PD, including dj-12, 3. Loss of function mutations in dj-1 (PARK7) represent the second most common cause of autosomal recessive PD 4, 5 and are associated with early-onset (less than 40 years of age) PD-symptoms 6, 7. Examination of DJ-1 in PD patients and animal models has highlighted a diverse genetic network required for DAergic cell survival in aging and after exposure to toxic insults. Originally identified as an oncogene 8, 9, DJ-1 has been implicated in as a cytoprotective antioxidant protein under a variety of toxic insults, including other toxicants used in modeling PD, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) 10, 6-hydroxydopamine(6-OHDA) 11, rotenone 12 and paraquat 13. DJ-1 has reactive oxygen species (ROS) quenching abilities due to self-oxidation at the cysteine (Cys)-106 residue 14. DJ-1 has also been linked to oxidative stress pathways, including glutathione (GSH)15 and nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) 16, 17. A study from our laboratory was the first to address the role of DJ-1 in Mn-toxicity, indicating DJ-1 expression was decreased upon Mn exposure in astrocytes 18. This is consistent with the facts that mitochondrial morphology is altered in DJ-1 deficient neurons with increased autophagic activity 19, and Mn is known to target mitochondria. These data argue for a hypothesis in which DJ-1 may operate at the intersection of environmental stressors and aging.
Genetic risk factors explain only 10-12% of all PD occurrences 3, suggesting that environmental factors plays a strong role in idiopathic PD etiology. Overexposure to excessively high Mn levels causes a parkinsonian disorder, manganism, associated with basal ganglia dysfunction, including DAergic dysfunction and degeneration 20-24. Occupational exposure is the most common path to Mn intoxication, with metal welders and miners representing the largest cohort in manganism, although environmental exposure to Mn through drinking water has also been reported to cause Mn intoxication 21. Mn is an established environmental risk factor for parkinsonism and/or PD 25-28. In 2012, mutations in SCL30A10 have been shown to cause Parkinsonism and dystonia concomitant with hypermanganesemia and polycythemia 29, More recently, Leyva-Illades et al., identified SLC30A10 as a Mn exporter of intracellular Mn 30. Mn neurotoxicity reflects known pathogenic mechanisms in PD including increased ROS and induction of antioxidant response pathways such as Nrf-2 and GSH 31, 32,33, 34.
C. elegans is a model organism for lifespan and PD interaction studies because its genetically malleable, has simple husbandry and amendable living conditions, shows predictable and well characterized life stages with short lifespan, and has a simple and easily visualized nervous system 34, 35. In addition, our lab has recently established C. elegans as a model of Mn neurotoxicity 21, 31, 34, 36-38. The C. elegans nervous system is comprised of 302 cells sharing remarkably high gene conservation with the human nervous system. In particular, the machinery of the DAergic system is conserved in worms, making them an ideal model for PD research 35, 39, 40. The C. elegans lifecycle includes an egg stage, four larval stages (L1-L4), a reproductively active adult stage, as well as an alternative life-stage referred to as dauer. Worms enter dauer stage after L2 as an alternative to L3 when conditions for reproduction are poor, either because of lack of food or presence of a chemical or physical stressor (i.e. high or low temperature) 41, 42.
The average lifespan for C. elegans is less than a month, but there are a number of known genes that alter lifespan by as much as tripling or halving it. One of the important regulators of lifespan, abnormal dauer formation 16 (DAF-16)/forkhead box O (FOXO), is also a transcriptional regulator of antioxidant response, suggesting aging coincides with oxidant management. Dauer stage entrance is also regulated by daf-16 and its upstream regulator daf-2/InR (insulin receptor) 42-45. Insulin signaling via daf-2/daf-16/FOXO pathway has been implicated in the control of a variety of downstream antioxidant genes responsible for antioxidant response including positive self regulation ofdaf-1644, 46, 47, and combined or individual regulation of hsp-16.2, sod-3, sod-1 and gst-4 43, although the contribution of DAF-16 to lifespan and DAergic associated response to Mn has not been previously examined.
The present study tests the hypothesis that C. elegans dj-1 homologues are protective against Mn-induced DAergic toxicity in an age-dependent manner. The three-way intersection of aging, genetic and environmental risk factors represents a key gap in our understanding of PD etiology. Given the evidence described above linking DJ-1 with neuroprotection against oxidative injury, the known connections between Mn exposure and oxidative injury, and the established links between oxidative stress, aging and the daf-2/daf-16/FOXO pathways makes for a compelling argument to test this hypothesis in the C. elegans model system. We report that DJ-1 is required for normal survival, lifespan, and dauer movement upon Mn exposure and that the daf-16 signaling modulates DJ-1 dependent DAergic associated behaviors in lifespan and dauer movement. Our results establish that daf-16 signaling regulates effects of DJ-1 deficient DAergic cellular pathologies. Further, we establish a clear interaction between three distinct, but established risk factors for PD/parkinsonism (aging, Mn-exposure and loss-of-function alleles of DJ-1).
MATERIALS AND METHODS
C. elegans Strains and Maintenance
C. elegans strains were maintained at 20°C, as previously described unless otherwise noted 31. The following strains were used: N2 (wildtype), MAB147 (mjaEx109[DJR-1.1::GFP; rol-6(su1006)]), MAB821 (mjaEx050[DJR-1.2::GFP; rol-6(su1006)]; otls181), MAB810 (djr-1.1(tm918)), MAB811 (djr-1.2(tm1346)), MAB809 (djr-1.1(tm918); djr-1.2(tm1346)), MAB822 (mjaEx050[DJR-1.2::GFP; rol-6(su1006)]; otls181; djr-1.1(tm918); djr-1.2(tm1346)), MAB823 (mjaEx109[DJR-1.1::GFP; rol-6(su1006)]; djr-1.1(tm918); djr-1.2(tm1346)), MAB799 (djr-1.2(ok1413)), CB1112 (cat-2(e1112)), CF1041 (daf-2(e1370)), MAB808 (cat-2(e1112); djr-1.2(1346)), MAB812 (daf-2(e1370); djr-1.2(tm1346)), MAB8128 (daf-2(e1370); djr-1.2(tm1346); cat-2(e1112)), TJ356 (zIs356[DAF-16::GFP; rol-6(su1006)]), MAB813 (zIs356[DAF-16::GFP; rol-6(su1006)], djr-1.2(tm1346)).
DJR::GFP and DAF-16::GFP expression
Wildtype (WT) worms (N2) were microinjected with plasmids carrying either djr-1.1 or djr-1.2 full-length promoter and genes in frame with green fluorescent protein (GFP), along with a selectable marker (rol-6(su1006)). Progeny with the roller phenotype were individually plated and screened for GFP expression. Worms carrying DJR-1.2::GFP were crossed using conventional genetics with OH7193 (otIs181 [Pdat-1::mCherry + Pttx-3::mCherry]; him-8(e1489) IV) worms carrying mCherry labeled DAergic neurons and AIY interneurons. Worms carrying DJR-1.1::GFP, DJR-1.2::GFP, or DAF-16::GFP were observed at the noted time points by confocal microscopy.
Acute Mn Exposure
Acute Mn exposure was carried out as previously described 31. Briefly, approximately 2,500 synchronous larval stage 1 (L1) worms per strain were exposed to doses of MnCl2 (0-1M) for 30 minutes. Synchronous populations were obtained by bleach treatment of gravid adults and collection of egg populations. After Mn exposure, worms were plated on nematode growth media (NGM) plates and exposure concentration were blinded for observation, survival, lifespan, and dauer movement assays.
Survival
Survival after acute Mn exposure was assessed as previously described 37. Briefly, approximately 2,500 synchronous larval stage 1 (L1) worms per strain were exposed to doses of MnCl2 (0-1M) for 30 minutes, then plated upon NGM plates containing OP50 bacterial food source and stored at 20°C. Worm survival (live/live+dead) was determined for ~200 worms in triplicate for each treatment dose and each genotype. Each curve represents N>3.
Lifespan
Lifespan experiments were completed as previously described 37. Briefly, after acute Mn exposure to 0mM or 50mM MnCl2 for 30 minutes, worms were plated on NGM plates with OP50 and stored at 20°C. Worms were grown to L4 stage after treatment. Next 30 worms per dose-genotype combination were placed on fresh NGM plates containing OP50 bacteria (day 0). Worms were moved every two days during the first week to avoid confusion of treated worms with developing progeny. After reproduction declined, worms were moved ever 4 days. Lifespan survival was assessed as living when worm was responsive to plate shaking or platinum wire stimulation. Worms were considered dead only when they no longer responded to gentle prodding with a platinum wire.
Dauer Movement
After acute Mn exposure to 0 or 50mM MnCl2 for 30 minutes, worms were plated on unseeded NGM plates and stored at 20°C for 72 hours to induce dauer formation. Dauer movement was scored as previously described 48. For each strain, 30 dauer worms per strain were scored for dauer movement per experiment (N=6). Dauers were considered moving if they moved greater than one body bend forward or backwards under a one-minute observation with a dissecting microscope. Care was taken to avoid disturbing the plates within 10 minutes of observation to avoid scoring stimulus-induced movement. For strains carrying daf-2(e1370) temperature sensitive mutation, worms were maintained at 25°C for 72 hours before observation. daf-2(e1370) strains were also maintained on unseeded plates for dauer movement scoring to maintain continuity across dauer movement experiments.
Statistics
Survival
Dose-response curves were compared using sigmoidal dose-response model with a top constraint at 100% to draw the curves and determine the lethal dose 50 (LD50). Statistical analysis of significance is carried out by one-way ANOVA for the dose-response curves with Graphpad Prism 7.
Lifespan
Lifespan curves were generated with Graphpad Prism 7 and compared by the log-rank test with 95% confidence intervals.
Dauer Movement
Dauer movement bar graphs were generated using Graphpad Prism 7. Movement was analyzed with one-way analysis of variance (ANOVA) to compare differences between two individual strains or treatment conditions.
RESULTS
Acute Mn exposure alters DJR expression
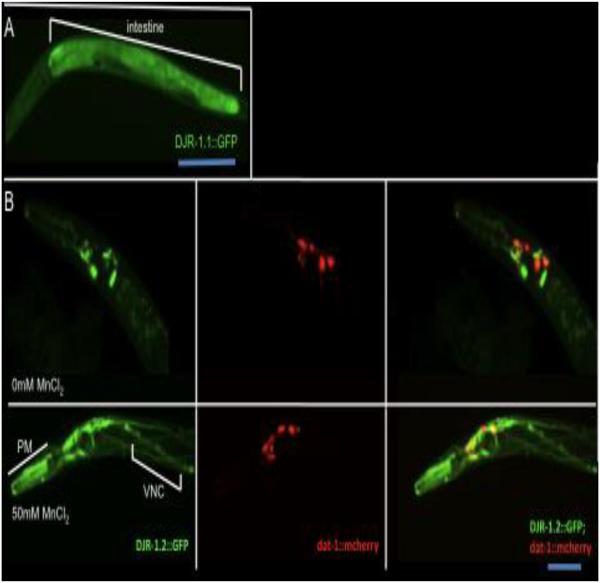
C. elegans has two DJ-1 homologues: DJR-1.1 and DJR-1.2. The homology between these two proteins is about 62.5% (Fig. S1). We generated worms expressing full-length DJ-1 homologues DJR-1.1 and DJR-1.2 tagged with GFP, in order to examine spatial and temporal expression of DJR, as well as expression in response to Mn exposure. DJR-1.1::GFP is expressed in the intestine (Fig. 1A). Under normal conditions DJR-1.2::GFP is expressed in the head neurons, pharyngeal muscles, as previously reported 49 (Fig. 1B). To check whether DJR-1.2 expressed in DAergic neurons, Worms carrying DJR-1.2::GFP were crossed with OH7193 worms carrying mCherry labeled DAergic neurons. Head neurons expressing DJR-1.2::GFP include the DAergic neurons expressing dat-1::mCherry (Fig. 1B).
Figure 1.
DJR-1.1::GFP and DJR-1.2::GFP expression patterns..(A) DJR-1.1::GFP is expressed in the intestine of L3 worm. (B) DJR-1.2::GFP is expressed in the head neurons of young adult worms, including the DAergic neurons. Twenty-four hours after acute Mn exposure (50mM Mn), DJR-1.2 expression is increased in pharyngeal muscles (PM) and head neurons, including DAergic neurons, as well as the ventral nerve chord (VNC). (Note: The DJR-1.2::GFP strain is a stable line with mosaic expression, therefore, DJR-1.2::GFP is not seen in all DAergic neurons).
To investigate the effect of Mn exposure on DJR-1.2 expression, worms were treated for 30 min with Mn at the L1 stage (acute Mn exposure). This combination of stage and timing allowed us to assess the largest Mn effect in the dose–response survival curves. In all experiments except survival dose-response, only animals that survived 24 hours post treatment were observed. Twenty-four-hours after acute Mn exposure (50mM MnCl2), expression of DJR-1.2::GFP was also observed in the ventral nerve chord (Fig. 1B, lower panels). These results confirm DJR-1.2 under its native promoter is expressed in DAergic cells, and neuronal expression of DJR-1.2 in the head and ventral nerve chord (VNC) neurons is enhanced upon acute Mn exposure. Thus, DJR-1.2 appears to be a neuronal expressed, Mn responsive DJ-1 homologue in C. elegans.
djr deletion decreases survival after acute Mn exposure
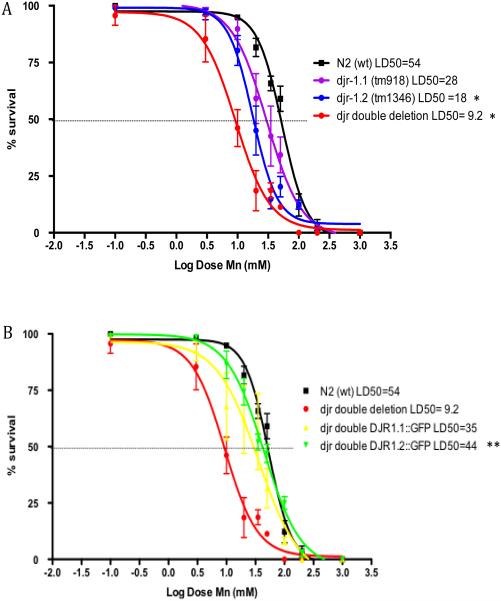
In order to test the effects of djr deletion on Mn sensitivity, we utilized worms with djr deletions, djr-1.1(tm918), djr-1.2(tm1346) and djr double deletion (djr-1.1(tm918); djr-1.2(tm1346)). Deletion of djr-1.2 alone or djr-1.1; djr-1.2 together led to significantly increased sensitivity to acute Mn exposure, as represented by the leftward shift in the dose-response curve for the deletion strains when compared to WT N2 strain (Fig. 2A) (p<0.05 and P<0.001, respectively, N=6). Notable, although it is not significant (p=0.3206, N=6), deletion of djr-1.1 alone appeared more sensitive to Mn exposure than N2 worms, which is consistent with previous finding that djr-1.1 deletion worms accumulated higher levels of Mn after Mn exposure 50. In order to test the roles of the DJR homologues in the survival response to acute Mn exposure, we crossed djr double deletion mutants with either DJR-1.1::GFP or DJR-1.2::GFP. djr double deletion worms carrying DJR-1.2::GFP, but not DJR-1.1::GFP, showed a significant rightward shift in the dose-response curves (Fig. 2B) (p<0.01, N=6), suggesting DJR-1.2::GFP is sufficient to overcome the increased sensitivity of djr double deletions to the acute effect of Mn.
Figure 2.
Survival upon acute Mn exposure is influenced by djr-1.2. (A) djr deletion strains are hypersensitive to Mn-induced lethality (djr-1.1(tm918): p=0.02*, djr-1.2(tm1346): p<0.01**; djr double deletion: p<0.01**, n=6). (B) DJR-1.2::GFP, but not DJR-1.1::GFP, increases survival of djr double deletion strain after acute Mn exposure (p<0.01**, n=6). Statistical analysis was carried out by mean of one-way ANOVA with Dunnett’s multiple comparisons test.
Lifespan decreases after acute Mn exposure in a djr-1.2 dependent manner
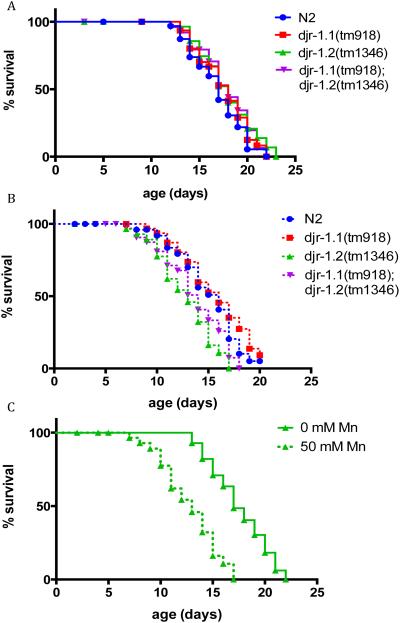
Identical strains were used in the lifespan assessments as in the survival experiments: djr deletions, djr-1.1(tm918), djr-1.2(tm1346), and djr double deletion (djr-1.1(tm918); djr-1.2(tm1346)). Forty worms per genotype were followed for the duration of lifespan. None of the djr deletion strains showed lifespan alterations under control treatment conditions (0mM Mn,, Fig. 3A). Deletion of djr-1.1 (djr-1.1(tm918)) failed to alter lifespan compared to WT worms both in the presence or absence of acute Mn (50mM) treatment (Fig. 3A&B, Table 1). Interestingly, deletion of djr-1.2 (djr-1.2(tm1346)) did not alter lifespan in the absence of Mn when compared with WT worms (Fig. 3A). However, it led to reduced lifespan after acute Mn (50mM) treatment (Fig. 3B, Table 1). Deletion of djr-1.2 sensitized the worms to acute Mn (50mM) treatment (Fig. 3C, Table 1), which was not seen in djr-1.1(tm918) worms. djr double deletion mutants showed the same trend as djr-1.2(tm1346) mutant, with increased sensitivity to acute Mn (50mM) treatment (p<0.001, Table 1). Meanwhile, djr double deletion mutants treated with Mn (50mM) showed a trend towards increased sensitivity to Mn when compared to similarly treated N2 animals (p=0.066, table 1). These results suggest that djr-1.2 is responsible for the life-long effects of acute Mn treatment.
Figure 3.
djr deletion-dependent lifespan alteration in response to Mn treatment. A) djr deletion does not affect lifespan under control conditions; (B) djr-1.2 deletion mutants show increased sensitivity to 50mM Mn when compared to N2 (wt) animals (p<0.01) (C) djr-1.2 deletion mutants show reduced lifespan after acute Mn exposure (p<0.01). Lifespans were compared with the log-rank test (n=30).
Table 1.
Significant comparison (P-values) from lifespan experiments (Figure 3).
| Comparison | p-value (log-rank test) |
|---|---|
| N2 0mM MnCl2 vs. N2 50mM MnCl2 | p=0.15 |
| djr-1.1(tm918) 0mM MnCl2 vs. djr-1.1(tm918) 50mM MnCl2 | p=0.54 |
| djr-1.2(tm1346) 0mM MnCl2 vs. djr-1.2(tm1346) 50mM MnCl2 | p<0.001*** |
|
djr double deletion 0mM MnCl2 vs. djr double deletion 50mM MnCl2 |
p<0.001*** |
| N2 50mM MnCl2 vs. djr-1.1(tm918) 50mM MnCl2 | p=0.34 |
| N2 50mM MnCl2 vs. djr-1.2(tm1346) 50mM MnCl2 | p<0.01** |
| N2 50mM MnCl2 vs. djr double deletion 50mM MnCl2 | p=0.066 |
Dauer djr-1.2 mutants show increased movement indicative of reduced DA signaling
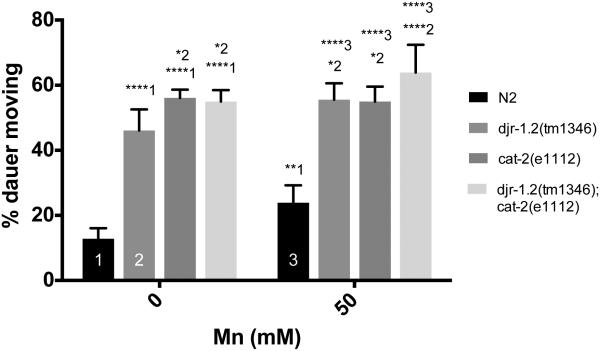
To investigate the role of djr-1.2 in DA signaling in response to Mn treatment, we employed a dauer spontaneous movement assay that is regulated by DAergic signaling 48. Dauer induction is independent of DAergic signaling, but once worms enter the dauer stage, animals are sensitive to changes in DAergic signaling and respond to decreased DA levels with increased movement 48. Dauer animals with djr-1.2 deletion showed increased dauer movement compared to control animals, indicating reduced DAergic signaling (Percent moving ± SEM: djr-1.2 46 ± 2.6 vs N2 13 ± 1.3, p<0.0001, n=6)(Fig. 4). CAT-2 is the C. elegans homologue of tyrosine hydroxylase (TH). cat-2 deletion mutants also showed increased dauer movement compared to control animals, corroborating previous findings that reduced DAergic signaling increased dauer movement 48 (percent moving ± SEM: cat-2 56 ± 1.0; p<0.0001, n=6) (Fig. 4). With 50mM Mn exposure, N2 (wt) animals showed increased dauer movement when compared with untreated animals (percent moving ± SEM: N2 50mM 23 ± 2.2, p=0.002, n=6)(Fig. 4). djr-1.2 (tm1346) deletion mutant dauers also showed increased movement after Mn exposure suggesting DAergic signaling is further impaired upon Mn exposure (percent moving ± SEM: djr-1.2 50mM 56 ± 2.0, p=0.02, n=6). In contrast, cat-2(e1112) and djr-1.2;cat-2 double deletion mutants did not show altered movement in the absence and presence of Mn exposure (Fig. 4).
Figure 4.
djr-1.2 deletion alters DA-dependent dauer movement. 1 represents comparisons to N2 animals, 2 represents comparisons to djr-1.2(tm1346) animals, 3 represents comparisons to N2 50mM Mn treatments. Significance indicated: *, p<0.05; **, p<0.01; ****, p<0.0001. N=6 Statistical analysis was carried out by mean of one-way ANOVA with Dunnett’s multiple comparisons test.
Increased DAF-16 expression abolishes Mn-dependent lifespan and DA-dependent dauer movement alterations in djr-1.2 mutant animals
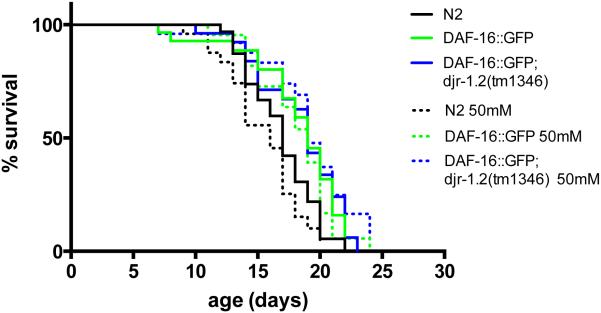
Next, we addressed the role of the C. elegans DAF-16/FOXO pathway in djr mutants and Mn exposure. We used a DAF-16::GFP strain, which overexpresses DAF-16, to study the effect of insulin signaling on the lifespan of djr-1.2 mutants exposed to Mn. DAF-16::GFP animals showed significantly extended lifespan when compared to WT N2 worms in the absence of an acute Mn exposure (Fig 5, Table 2), which is consistent with previous findings 45, 51. The extended lifespan remained the same in the presence of 50mM Mn exposure (Fig. 5, Table 2). Previously, we showed that djr-1.2 deletion mutants had reduced lifespan after exposure to 50mM Mn (Fig. 3C). This Mn-dependent lifespan reduction in djr-1.2 mutant was attenuated by DAF-16 overexpression, as the lifespan of djr-1.2(tm1346);DAF-16::GFP worms remained the same in the absence or presence of 50 mM Mn exposure (Fig. 5, Table 2). Previous studies from our lab have shown that alterations in DAergic signaling alter lifespan 31, as increased extracellular dopamine is associated with decreased lifespan. These lifespan alterations were abolished in cat-2(e1112) mutants 48. Furthermore, djr-1.2 deletion is associated with altered DAergic signaling (Fig. 4). DAF-16 overexpression reversed the lifespan reduction seen in djr-1.2 deletion mutant to levels indistinguishable from DAF-16::GFP animals, as DAF-16::GFP; djr-1.2(tm1346) worms had similar lifespan to worms with DAF-16::GFP alone, but significantly longer than N2 worms (Fig. 5, Table 2).
Figure 5.
DAF-16 overexpression recovers lifespan reduction resulting from djr-1.2 deletion upon Mn exposure (50mM MnCl2, 30min). Overexpression of DAF-16::GFP restored lifespan in djr-1.2(tm1346) deletion animals. DAF-16::GFP alone and DAF-16::GFP; djr-1.2(tm1346) worms show increased lifespan compared with N2 worms both in the absence of Mn exposure (p<0.05 and p<0.05, respectively) and in the presence of Mn exposure (p<0.05 and p<0.01, respectively). No statistically significant differences were noted between same strains with or without Mn exposure (See Table 2 for all p values). Lifespans were compared with the log-rank test (n=30).
Table 2.
Significant comparison (P-values) from lifespan experiments (Figure 6).
| Comparison | p-values (log-rank test) |
|---|---|
| N2 0mM MnCl2 vs. DAF-16::GFP 0mM MnCl2 | p<0.05* |
| N2 0mM MnCl2 vs. DAF-16::GFP; pdr-1.2(tm1346) 0mM MnCl2 | p<0.05* |
| DAF-16::GFP 0mM MnCl2 vs. DAF-16::GFP; pdr-1.2(tm1346) 0mM MnCl2 |
p=0.7812 |
| N2 0mM MnCl2 vs. N2 50mM MnCl2 | p=0.1799 |
| DAF-16::GFP 0mM MnCl2 vs. DAF-16::GFP 50mM MnCl2 | p=0.4896 |
| DAF-16::GFP; pdr-1.2(tm1346) 0mM MnCl2 vs. DAF-16::GFP; pdr- 1.2(tm1346) 50mM MnCl2 |
p=0.4671 |
| N2 50mM MnCl2 vs. DAF-16::GFP 50mM MnCl2 | P<0.05* |
| N2 50mM MnCl2 vs. DAF-16::GFP; pdr-1.2(tm1346) 50mM MnCl2 | p<0.01** |
| DAF-16::GFP 50mM MnCl2 vs. DAF-16::GFP; pdr-1.2(tm1346) 50mM MnCl2 |
p=0.1903 |
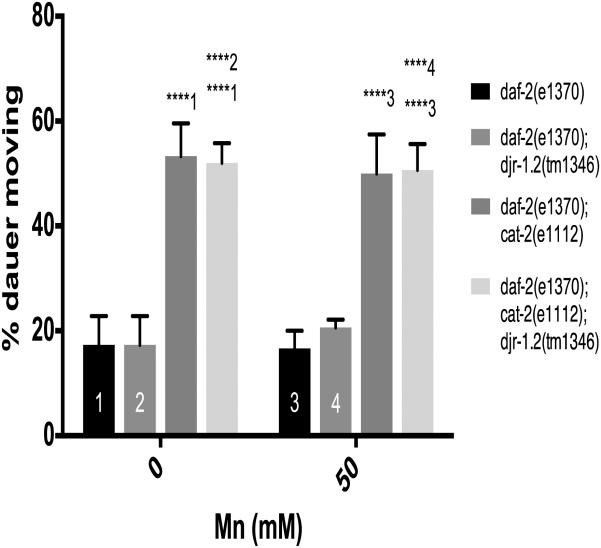
To investigate the role of the daf-16 signaling pathway in djr-1.2 and DA dependent dauer movement, we crossed djr-1.2 (tm1346) with daf-2(e1370) animals. DAF-2 is the C. elegans insulin receptor (InR) homologue and it functions as a suppressor of daf-16 36, 52. daf-2(e1370) mutation led to constitutively active daf-16 signaling 53. Unlike WT animals (Fig. 4), djr-1.2 (tm1346); daf-2(e1370) double mutant dauers did not exhibit altered movement when compared to dauers carrying daf-2(e1370) alone (percent moving ± SEM: djr-1.2 daf-2 18 ± 2.5 vs daf-2 19 ± 1.7, n.s, n=5)(Fig. 6). Furthermore, there was no statistically significant difference in dauer movement between control and Mn-exposed djr-1.2 (tm1346); daf-2(e1370) double-mutant dauers. This suggests that activating daf-16 through a daf-2(e1370) mutation removed the DAergic effects seen in djr-1.2 mutant dauer movement, which indicates that activating daf-2/daf-16 signaling pathway protected djr-1.2 (tm1346) worms from Mn induced DAergic neurotoxicity. In contrast, cat-2(1112); daf-2(e1370) double mutant dauers continued to show increased movement (percent moving ± SEM: daf-2 cat-2 53 ± 2.8, p<0.001, n=5) irrespective of Mn exposure (Fig. 6), suggesting that cat-2 mutation was epistatic to djr-1.2/daf-2 dauer movement effects.
Figure 6.
Constitutively overexpressing DAF-16 through daf-2(e1370) mutation abolishes djr-1.2 deletion- and Mn-dependent increases in dauer movements. 1 represents comparisons to daf-2(e1370) dauers, 2 represents comparisons to daf-2(e1370); djr-1.2(tm1346) dauers, 3 represents comparisons to daf-2(e1370) under 50mM MnCl2 treatment conditions, 4 represents daf-2(e1370); djr-1.2(tm1346) under 50mM MnCl2 treatment conditions. Significance indicated: **, p<0.01; ****, p<0.0001. N=5. Statistical analysis was carried out by mean of one-way ANOVA with Dunnett’s multiple comparisons test.
Discussion
We systematically examined the role of DJ-1 homologues (djr-1.1 and djr-1.2) in the context of Mn exposure in C. elegans. We found that DJR-1.2, but not DJR-1.1 is responsible for the alternations observed in worm survival, lifespan and dauer movement upon Mn exposure, suggesting DAF-16 is a possible regulator of DJR-1.2 x Mn interaction in C. elegans.
In C. elegans, expression of DJR-1.1 is restricted to the intestine, while DJR-1.2 is expressed in a variety of tissues, including the pharyngeal muscles and various neuronal populations in the head. We confirmed that DJR-1.2 is expressed in the six DAergic neurons in the worm’s head, indicating it might play a role in DA signaling. DJR-1.2 is also expressed in other neurons, but the levels are lower than in DAergic neurons. Acute Mn exposure led to increased number of DJR-1.2::GFP positive neurons (Fig. 1). Twenty-four hours after Mn exposure, DJR-1.2::GFP was observed in all ventral nerve chord (VNC) neurons, which consist of cholinergic and gamma-aminobutyric acid-ergic (GABAergic) motor neuron populations. The VNC neurons express the D2-like dopamine (DA) receptor DOP-3 and are known to function in other DA-associated paralysis behaviors 54. These results established DJR-1.2 expression in DAergic neurons, and upon Mn treatment, in cells expressing DA receptors.
The DJR-1.2 human homologue DJ-1 has been proposed as an antioxidant gene 12, 55-57, with contradictory evidence that it may act as a positive regulator of Nrf-2 16, 58. Given that Mn exposure had long lasting effects on DJR-1.2::GFP expression in neurons involved in DAergic signaling, we were interested in the long-term combined effects of DJR-1.2 and Mn exposure on lethality, DA signaling and lifespan.
djr deletion alters survival in response to Mn exposure, analogous to the effect of DJ-1 deletion in other PD models 11, 13, 59, 60. Studies with DJ-1 deletion alone showed no increase in DAergic cell loss in experimental models of normal aging, but when a toxicant was introduced, DJ-1 null animals showed reduced survival and increased DAergic cell loss 11, 13, 59, 60. Here we showed that Mn exposure reduced survival in the context of DJ-1 homologue djr-1.2 deletion. Furthermore, DJR-1.2 overexpression was sufficient to restore the protection against Mn-induced lethality in djr double deletion mutant, indicating DJR-1.2 is necessary and sufficient for maintaining survival in the context of Mn exposure (Fig. 2).
An established characteristic of human PD patients with DJ-1 mutations is reduced lifespan, along with early onset of PD symptoms 61. Lifespan reductions have also been shown in a Drosophila model of DJ-1 mutation and paraquat exposure 59. In order to examine the life-long effects of djr deletion and Mn exposure we examined the lifespans of C. elegans djr deletion mutants in the context of acute Mn exposure. djr-1.2 deletion alone showed no significant reduction in lifespan, but upon Mn exposure, lifespan of djr-1.2 deletion mutants was significantly decreased compared with N2 control worms. djr double deletion showed the same trend as with deletion of djr-1.2 alone, suggesting that djr-1.2 is the major regulator (compared with djr-1.1) of the lifespan effects seen in response to Mn exposure.
We examined if the daf-2/daf-16 signaling pathway, the master regulatory pathway for lifespan, might be involved in the long-lasting effects of djr-1.2 upon Mn exposure. DAF-16 is the C. elegans orthologue of human FOXO, and it functions as a transcription factor responsible for antioxidant response. daf-16 deletion has been shown to greatly reduce lifespan and stress tolerance 42, 44, 45, 62. Here we show that overexpression of DAF-16::GFP fully reversed lifespan reduction caused by djr-1.2 deletion and Mn exposure (Fig. 5), suggesting that DAF-16 acts to regulate lifespan mediated by DJR-1.2 and Mn exposure. Previous work from our lab has shown that DAergic signaling is involved in lifespan regulation in the presence of Mn exposure 31. Thus, we suggest that DAergic signaling pathways might also regulate lifespan reductions in djr-1.2 deletion mutants upon Mn exposure.
We further addressed daf-16’s role in mediating the effects of djr-1.2 deletion and Mn exposure, given that both DA and daf-2/daf-16 signaling are involved in regulating dauer movement behavior 48, consistent with our results that DJR-1.2 is expressed in the DAergic cells and signaling pathway. Mn exposure in WT worms increased the dauer movement, indicating impaired DA signaling (Fig. 4). djr-1.2 deletion alone significantly increased dauer movement, indicating djr-1.2 deletion disrupts DA signaling. djr-1.2 deletion combined with Mn exposure led to increased dauer movement when compared to untreated djr-1.2 deletion dauers and WT dauer animals treated with Mn. Taken together, these observations establish that Mn exposure represents a risk factor for DAergic dysfunction and exacerbates losses in DJ-1 function. Interestingly, overexpression of DAF-16 restored the lifespan reduction caused by djr-1.2 deletion and Mn exposure. Moreover, daf-2 mutants, with continuously activated daf-16 signaling, were able to fully rescue the dauer movement defect seen in the Mn exposed djr-1.2 deletion worms. These results indicate that the insulin signaling pathway plays an important role in regulating toxicity induced by loss of DJ-1 function and Mn exposure.
Conclusion
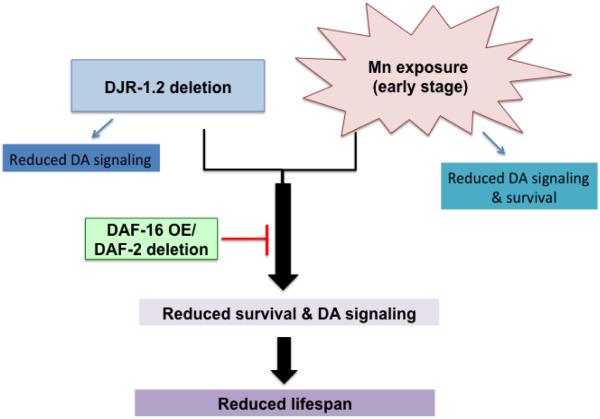
In cells, DJ-1 functions as a senor or chaperone of oxidative stress. In the absence of cellular stress or environmental insults, a cell is able to survive in the absence of DJ-1, although it may not fulfill all of its normal functions. However, when cells are exposed to stress conditions (i.e. Mn exposure), DJ-1 is required to attenuate the stress or to activate stress response pathways. Cells deficient in DJ-1 are more susceptible to environmental insults and prone to die. This explains why DJ-1 deletion alone showed no increase in DAergic cell loss, but when a toxicant was introduced, DJ-1 null animals exhibited reduced survival and increased DAergic cell loss 11, 13, 59, 60. Here, we show a novel interaction between djr-1.2 and Mn exposure, which is regulated by insulin signaling pathway in C. elegans (Fig 7). Loss of DJ-1.2 alone in C. elegans only affects DA signaling (indicated by abnormal DA mediated dauer movement), but does not affect survival or lifespan; however, it significantly reduces survival rate and shortens lifespan when combined with environmental Mn exposure. The reduction in survival and lifespan as well as altered DA signaling are regulated via DAF-2/DAF-16 signaling, since increased DAF-16 expression fully restores the effects caused by DJR-1.2 deletion and Mn exposure. It is noteworthy that insulin insensitivity, as in type-2 diabetes, is comorbid with PD 63, 64. Our results establish that DAF-2/DAF-16 signaling plays a critical role in PD caused by DJ-1 mutations. This is consistent with aberrant insulin signaling pathway as a key regulator of PD in combination with genetic and environmental factors. Our results also establish that insulin signaling regulates Mn toxicity and motor impairment in PD, necessitating additional studies to address the role of DJ-1 in these processes.
Figure 7.
Model for the effect of djr-1.2 deletion with Mn exposure on DA signaling, survival and lifespan. Loss of DJ-1.2 alone in C. elegans only affects DA signaling, but not survival or lifespan. Mn exposure at early stage (L1) only affects DA signaling and survival, but not lifespan. When combining loss of DJR-1.2 and Mn exposure, all three are significantly affected, including lifespan. The reduction in survival and lifespan as well as alteration in DA signaling are regulated through DAF-2/DAF-16 signaling, as DAF-16 overexpression and daf-2 mutant tend to restore survival, lifespan and dauer movement. Increased DAF-16 expression restores the alterations induced by DJR-1.2 deletion and Mn exposure. OE, overexpression.
Supplementary Material
REFERENCES
- 1.Lees AJ, Selikhova M, Andrade LA, Duyckaerts C. Movement disorders : official journal of the Movement Disorder Society. 2008;23:777–783. doi: 10.1002/mds.21855. [DOI] [PubMed] [Google Scholar]
- 2.Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Human mutation. 2010 doi: 10.1002/humu.21277. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifati V. Minerva medica. 2005;96:175–186. [PubMed] [Google Scholar]
- 4.De Marco EV, Annesi G, Tarantino P, Nicoletti G, Civitelli D, Messina D, Annesi F, Arabia G, Salsone M, Condino F, Novellino F, Provenzano G, Rocca FE, Colica C, Morelli M, Scornaienchi V, Greco V, Giofrè L, Quattrone A. Clinical genetics. 2010;77:183–188. doi: 10.1111/j.1399-0004.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 5.Kahle PJ, Waak J, Gasser T. Free radical biology & medicine. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Clark L, Afridi S, Mejia-Santana H, Harris J, Louis E, Cote L, Andrews H, Singleton A, Wavrant De-Vrieze F, Hardy J, Mayeux R, Fahn S, Waters C, Ford B, Frucht S, Ottman R, Marder K. Movement Disorders. 2004;19:796–800. doi: 10.1002/mds.20131. [DOI] [PubMed] [Google Scholar]
- 7.Trinh J, Farrer M. Nat Rev Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H. The Journal of biological chemistry. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- 9.Kim YC, Kitaura H, Iguchi-Ariga SMM, Ariga H. FEBS Letters. 2010;584:3891–3895. doi: 10.1016/j.febslet.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW, Park DS. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, An C, Pu XP. Brain research bulletin. 2012;88:609–616. doi: 10.1016/j.brainresbull.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Nguyen JL, Hulleman JD, Li L, Rochet J. Journal of neurochemistry. 2008;105:2435–2453. doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- 13.Kwon HJ, Heo JY, Shim JH, Park JH, Seo KS, Ryu MJ, Han JS, Shong M, Son JH, Kweon GR. Toxicology letters. 2011;202:85–92. doi: 10.1016/j.toxlet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. EMBO reports. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W. Journal of Biological Chemistry. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 16.Gan L, Johnson DA, Johnson JA. The European journal of neuroscience. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E, Yin Z, Sidoryk-Węgrzynowicz M, Jiang H, Aschner M. Free radical biology & medicine. 2012;52:1067–1074. doi: 10.1016/j.freeradbiomed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MWC, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS. Human Molecular Genetics. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- 20.Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M. Journal of Trace Elements in Medicine and Biology. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aschner M, Erikson KM, Hernández EH, Tjalkens R. Neuromolecular medicine. 2009 doi: 10.1007/s12017-009-8083-0. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erikson KM, Syversen T, Aschner JL, Aschner M. Environmental toxicology and pharmacology. 2005;19:415–421. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 23.Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- 25.Normandin L, Panisset M, Zayed J. Reviews on environmental health. 2002;17:189–217. doi: 10.1515/reveh.2002.17.3.189. [DOI] [PubMed] [Google Scholar]
- 26.Hudnell HK. Neurotoxicology. 1999;20:379–397. [PubMed] [Google Scholar]
- 27.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- 28.Racette BA, Criswell S, Lundin J, Hobson A, Seixas N, Kotzbauer P, Evanoff B, Perlmutter JS, Zhang J, Sheppard L, Checkoway H. Neurotoxicology. 2012;33:1356–1361. doi: 10.1016/j.neuro.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quadri M, Federico A, Zhao T, Breedveld Guido J., Battisti C, Delnooz C, Severijnen L-A, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra Ben A., van de Warrenburg Bart P., Bonifati V. The American Journal of Human Genetics. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, Swaim CD, Morrisett RA, Bowman AB, Aschner M, Mukhopadhyay S. The Journal of Neuroscience. 2014;34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. PLoS genetics. 2010:6. doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Toxicology and applied pharmacology. 2009 doi: 10.1016/j.taap.2009.07.004. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Neurotoxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Benedetto A, Au C, Aschner M. Chemical reviews. 2009 doi: 10.1021/cr800536y. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p44. [DOI] [PubMed] [Google Scholar]
- 35.Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Toxicological sciences : an official journal of the Society of Toxicology. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avila DS, Benedetto A, Au C, Manarin F, Erikson K, Soares FA, Rocha JB, Aschner M. Free radical biology & medicine. 2012;52:1903–1910. doi: 10.1016/j.freeradbiomed.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Exil V, Silva Avila D, Benedetto A, Exil E, Adams M, Au C, Aschner M. PloS one. 2010;5:e14228. doi: 10.1371/journal.pone.0014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, Aschner M. PloS one. 2009;4:e7792. doi: 10.1371/journal.pone.0007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nass R, Merchant KM, Ryan T. Molecular interventions. 2008;8:284–293. doi: 10.1124/mi.8.6.6. [DOI] [PubMed] [Google Scholar]
- 40.Harrington AJ, Hamamichi S, Caldwell G, Caldwell K. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:1282–1295. doi: 10.1002/dvdy.22231. [DOI] [PubMed] [Google Scholar]
- 41.Gaglia M, Jeong D, Ryu E, Lee D, Kenyon C, Lee S. PLoS genetics. 2012;8:e1003133. doi: 10.1371/journal.pgen.1003133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calixto A, Jara J, Court F. PLoS genetics. 2012;8:e1003141. doi: 10.1371/journal.pgen.1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Back P, Matthijssens F, Vlaeminck C, Braeckman BP, Vanfleteren JR. Experimental gerontology. 2010 doi: 10.1016/j.exger.2010.01.014. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p1654. [DOI] [PubMed] [Google Scholar]
- 44.Park SK, Tedesco PM, Johnson TE. Aging cell. 2009;8:258–269. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson ST, Bonafè M, Johnson TE. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- 46.Inoue H. Genes & development. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dingley S, Polyak E, Lightfoot R, Ostrovsky J, Rao M, Greco T, Ischiropoulos H, Falk MJ. Mitochondrion. 2010;10:125–136. doi: 10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaglia M, Kenyon C. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7302–7314. doi: 10.1523/JNEUROSCI.3429-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, Park C. Human molecular genetics. 2012:1–11. doi: 10.1093/hmg/dds155. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p5730. [DOI] [PubMed] [Google Scholar]
- 50.Bornhorst J, Chakraborty S, Meyer S, Lohren H, Gro, Knight AL, Caldwell KA, Caldwell GA, Karst U, Schwerdtle T, Bowman A, Aschner M. Metallomics. 2014;6:476–490. doi: 10.1039/c3mt00325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi W, Huang X, Neumann-Haefelin E, Schulze E, Baumeister R. PLoS genetics. 2012;8:e1002836. doi: 10.1371/journal.pgen.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aitlhadj L, Avila DS, Benedetto A, Aschner M, Stürzenbaum SR. Environmental health perspectives. 2010 doi: 10.1289/ehp.1002522. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amrit F, Boehnisch C, May R. PloS one. 2010;5:e9978. doi: 10.1371/journal.pone.0009978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardaway J, Hardie S, Whitaker S, Baas S, Zhang B, Bermingham D, Lichtenstein A, Blakely R. G3: Genes/Genomes/Genetics. 2012;2:961–975. doi: 10.1534/g3.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inden M, Kitamura Y, Takahashi K, Takata K, Ito N, Niwa R, Funayama R, Nishimura K, Taniguchi T, Honda T, Taira T, Ariga H. Journal of pharmacological sciences. 2011 doi: 10.1254/jphs.11151fp. DOI: papers://1EA7C98D-1F33-42AC-9968-0D386BC21048/Paper/p5568. [DOI] [PubMed] [Google Scholar]
- 56.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. PLoS biology. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. American journal of respiratory and critical care medicine. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Meulener MC, Xu K, Thomson L, Thompson L, Ischiropoulos H, Bonini NM. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Im JY, Lee KW, Junn E, Mouradian MM. Neuroscience research. 2010;67:203–208. doi: 10.1016/j.neures.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Science (New York, NY) 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 62.Johnson TE, Henderson S, Murakami S, de Castro E, de Castro SH, Cypser J, Rikke B, Tedesco P, Link C. Journal of Inherited Metabolic Disease. 2002;25:197–206. doi: 10.1023/a:1015677828407. [DOI] [PubMed] [Google Scholar]
- 63.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Brain : a journal of neurology. 2013;136:374–384. doi: 10.1093/brain/aws009. [DOI] [PubMed] [Google Scholar]
- 64.Cereda E, Barichella M, Pedrolli C, Klersy C, Cassani E, Caccialanza R, Pezzoli G. Movement disorders : official journal of the Movement Disorder Society. 2013;28:257–261. doi: 10.1002/mds.25211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.