Abstract
Purpose
B-cell chronic lymphocytic leukemia (CLL) is an incurable disease despite aggressive therapeutic approaches. We previously found that Axl receptor tyrosine kinase (RTK) plays a critical role in CLL B-cell survival. Here, we explored the possibility of using a high-affinity Axl inhibitor as a single agent or in combination with Bruton’s tyrosine kinase (BTK) inhibitors for future clinical trial to treat CLL patients.
Experimental Design
Expression/activation status of other members of the TAM (Tyro3, Axl, MER) family of RTKs in CLL B-cells was evaluated. Cells were treated with a high-affinity orally bioavailable Axl inhibitor TP-0903 with or without presence of CLL bone marrow stromal cells (BMSCs). Inhibitory effects of TP-0903 on Axl signaling pathway was also evaluated in CLL B-cells. Finally, cells were exposed to TP-0903 in combination with BTK inhibitors to determine any synergistic/additive effects of the combination.
Results
CLL B-cells overexpress Tyro3, but not MER. Of interest, Tyro3 remains as constitutively phosphorylated and form a complex with Axl in CLL B-cells. TP-0903 induces massive apoptosis in CLL B-cells with LD50 values of nanomolar ranges. Importantly, CLL BMSCs could not protect the leukemic B-cells from TP-0903 induced apoptosis. A marked reduction of the anti-apoptotic proteins Mcl-1, Bcl-2, XIAP and upregulation of the pro-apoptotic protein BIM in CLL B-cells were detected as a result of Axl inhibition. Finally, combination of TP-0903 with BTK inhibitors augments CLL B-cell apoptosis.
Conclusion
Administration of TP-0903 either as a single agent or in combination with BTK inhibitors may be effective in treating CLL patients.
Keywords: CLL, Axl, Tyro3, BTK, TP-0903
Introduction
CLL is the most common form of leukemia in the Western hemisphere and accounts for ~11% of all newly diagnosed hematologic neoplasms, and given its chronic nature, there are estimated to be over 100,000 individuals in the United States living with CLL(1). Initial therapy of progressive CLL is effective, but when a patient relapses the treatment options are limited. Since we do not routinely cure CLL patients, we are therefore frequently faced with second-line therapies that have very inferior levels of deep clinical responses and durability. In addition, the current therapies for upfront treatments utilize agents that are toxic to the marrow/immune system and are difficult to use in older patients (≥65). Thus, any insights into drugs with unique mechanism(s) of action with less severe toxic profiles are needed. Given this we have been focused on developing insights into CLL B-cell biology that relate to disease progression events and provide unique therapeutic targets.
In our recent studies we reported a previously undefined RTK Axl, a member of the TAM family of RTKs (2, 3), expressed in CLL B-cells and is likely playing a critical role in regulating CLL B-cell survival(4, 5). We found that CLL B-cells from majority of CLL patients express constitutively active (phosphorylated) Axl and that it is physically associated with multiple non-receptor cellular kinases including PI3K, Syk/ZAP70 and Lyn(4). Most recently, we have detected that p53 functionality is linked to Axl expression in CLL B-cells(5). Importantly, we have also found that inhibition of Axl induces apoptotic cell death in CLL B-cells(4, 5).
B-cell receptor (BCR) signaling plays a critical pathogenic role in CLL. In normal B-cells, ligation of the BCR results in a signaling cascade that can lead to proliferation, apoptosis, or anergy depending on the stage of development and antigen ligated(6). In CLL B-cells, however, the BCR is dysregulated, and activation through antigen ligation or auto-stimulation results in the progression of proliferative and prosurvival signals(7, 8). Although multiple agents are in clinical development that target BCR, one of the most exciting is the BTK inhibitor, ibrutinib which has emerged as an attractive agent in CLL therapy(9). Early phase 2 or 3 trials show that oral therapy with BTK inhibitors is very potent in relapsed/refractory CLL. With the single agent BTK inhibitor, the majority of the treated cohort only experienced a partial response(9, 10). However, this agent is not curative and resistance develops. Thus it is likely that combination of other drugs with BTK inhibitors will be needed to improve the clinical responses seen with this single agent.
Given this, we further defined the role of the TAM family RTKs in CLL B-cell survival and apoptosis resistance. Our studies show that CLL B-cells express higher levels of Tyro3, but not MER, as compared to normal B-cells and that it is constitutively phosphorylated. We also found that combined treatment of CLL B-cells with Axl and certain BTK inhibitors augmented apoptosis levels.
Materials and Methods
B-cell isolation and cell culture
All patients provided written informed consent according to the Declaration of Helsinki to the Mayo Clinic Institutional Review Board, which approved these studies. Primary CLL B-cells were isolated and purified from blood of the CLL patients with the use of RosetteSep B-cell enrichment kit (Stem Cell Technologies). The typical purification range of CD5+/CD19+ CLL B-cells for this work was >95–99%. Peripheral blood mononuclear cells (PBMC) from healthy individuals were included as normal controls wherever appropriate. Normal B-cells were also purified from healthy control individuals(4). Cells were cultured in serum-free AIM-V (GIBCO) medium as needed. Primary bone marrow stromal cells (BMSCs) were isolated from the bone biopsy materials and were maintained in vitro as previously described(11, 12). MDA-MB-231 breast epithelial carcinoma cells (American Type Culture Collection, Rockville, MD) were maintained in DMEM/F12 medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS).
Reagents
A high-affinity orally bioavailable Axl inhibitor TP-0903 and a reversible BTK inhibitor TP-4216 were obtained from Tolero Pharmaceuticals Inc., PCI-32675 (ibrutinib) was purchased from Selleck Chemical LLC. Bcl-2 antibody was purchased from BD Pharmingen and antibodies to Actin, Axl, and BIM were purchased from Santa Cruz Biotechnologies. Antibody to poly (ADP-ribose) polymerase (PARP) and phosphotyrosine mouse monoclonal antibody (4G10) were purchased from BIOMOL and Millipore, respectively. All other antibodies were obtained from Cell Signaling Technology.
Treatment of CLL B-cells with inhibitors and flow cytometric analysis
CLL B-cells (2 × 106 cells/ml) from CLL patients with low-risk FISH (13q14- deletion, trisomy 12 or no chromosomal abnormalities; n=20) or with high-risk FISH (17p13.1-deletion; n=8, and 11q22.3-deletion; n=10) were treated with increasing doses of TP-0903 (0.01–0.25μM) for 24 hours. Normal PBMC cultured in serum-free AIM-V media were also treated with TP-0903 (0.01–0.5μM) for 24 hours. Cells were harvested, and induction of apoptosis was determined by flow cytometry (FACScan, Becton Dickinson) after staining with annexin/propidium iodide (PI). Of note, we did not supplement FBS to CLL B-cell culture as prior study found that FBS induces spontaneous apoptosis in CLL B-cells(13), instead, we used serum-free AIM-V basal media which contains human serum albumin to support primary CLL B-cell growth. Therefore, for comparison, we cultured PBMC isolated from healthy, normal individuals in serum-free AIM-V media, instead of RPMI+10% FBS.
In separate experiments, CLL B-cells (2 × 106 cells/ml) were treated with increasing doses (0.05–0.15 μM) of TP-0903 as a single agent or in combination with increasing doses (0.25–0.75μM) of ibrutinib or a reversible BTK inhibitor TP-4216 at a constant dose ratio (1:5) for 24 hours. Cells were harvested and apoptosis induction was determined as described above. Combination effects of the two drugs were analyzed using the CalcuSyn software program, which uses the method of Chou and Talalay(14). A combination index (CI) value of 1 indicates an additive effect; values >1 indicate an antagonistic effect and values <1 indicate a synergistic effect of combined treatment.
Axl expression on CLL B-cells or normal immune cells (B-/T-/NK-cells) was determined by flow cytometry using a specific antibody to Axl (Cell Signaling) as described previously(4, 5). For the detection of B-cells and T-cells, chromogen-conjugated antibody to CD19 or CD3 was used respectively to stain the cells prior analysis on flow cytometer.
Treatment of CLL B-cells with TP-0903 in co-culture with stromal cells
CLL BMSCs were plated in 24-well tissue-culture plates (5.0 × 104 cells/well) and cultured until the cells were ~80% confluent. After washing, CLL BMSCs were co-cultured with CLL B-cells at a cell density of 2.0 × 106 cells/well in serum-free AIM-V medium. Cells were subsequently treated with TP-0903 (0.1 and 0.175μM) or DMSO. For comparison, CLL B-cells cultured alone were treated similarly with TP0903 or DMSO. After 24 hours, CLL B-cells and CLL BMSCs were harvested and apoptosis induction in both the cell types was determined by flow cytometry as described above.
Transfection, immunoprecipitation and Western blot analysis
Purified CLL B-cells (4.0 × 106/ml) treated with DMSO or TP-0903 (0.1μM) or ibrutinib/TP-4216 (0.75 μM) for 20–24 hours were lysed in NP40-lysis buffer, and whole cell extract was prepared as described previously(4, 5). Normal B-cell lysates were also prepared in NP40-lysis buffer as control. For immunoprecipitation (IP) experiments, 0.2–0.3mg of total protein from CLL B-cell lysates was used to IP specific RTKs using appropriate antibodies as described elsewhere(4). The precipitated immune complex was electrophoresed on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, followed by detection of proteins of interest using specific antibodies. MDA-MB-231 cells were transfected with a specific siRNA to Axl (Santa Cruz) or scrambled (sc) siRNA (Santa Cruz) using Lipofectamine2000 (Invitrogen) according to manufacturer’s instructions. Cells were harvested after 48 hours, lysed and analyzed for the expression of Axl, XIAP, Bcl-2, Mcl-1 and phosphorylation status of AKT using specific antibodies by Western blots as described above.
Results
CLL B-cells overexpress constitutively phosphorylated Tyro3
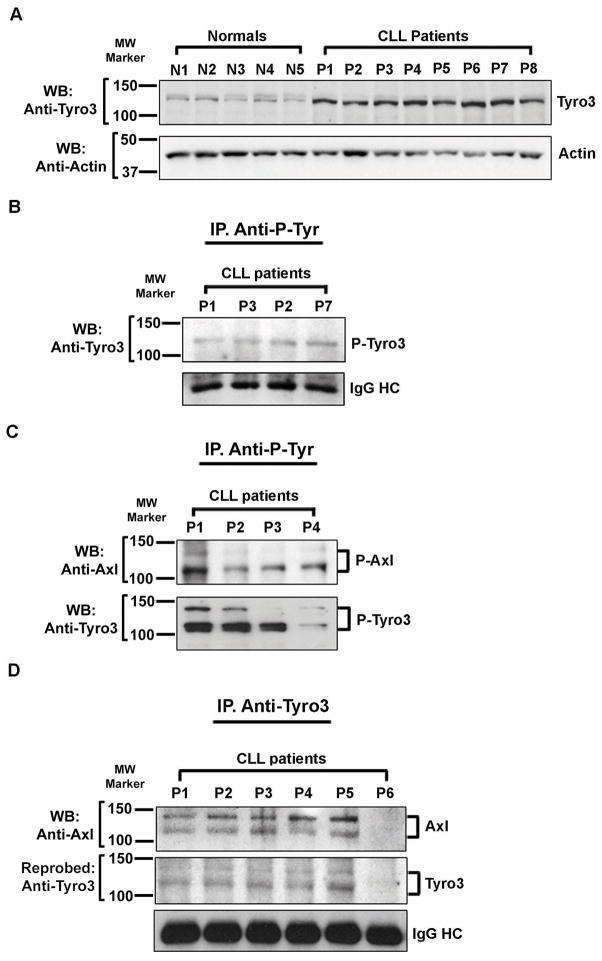
We have reported earlier that CLL B-cells express higher levels of constitutively phosphorylated Axl as compared to normal B-cells(4) however; expression status of other members of the TAM RTK family including Tyro3 and MER in CLL B-cells is not defined. Therefore, to more completely define the expression/activation status of the TAM family members, CLL B-cell lysates were analyzed for the expression of Tyro3 and MER by Western blots using specific antibodies. While MER RTK expression was undetectable (data not shown), Tyro3 expression was substantially elevated in CLL B-cells as compared to normal B-cells (Fig. 1A).
Figure 1. CLL B-cells overexpress Tyro3 and associates with Axl.
A. Tyro3 is overexpressed in CLL B-cells. Lysates from purified CLL B-cells (P1–P8) and normal B-cells obtained from healthy individuals (N1–N5) were examined for the expression of Tyro3 by Western blot analysis using a specific antibody. Actin was used as loading control. CLL patients and normal individuals are indicated by assigning arbitrary numbers. B. Tyro3 is constitutively phosphorylated in CLL B-cells. Total tyrosine-phosphorylated proteins were immunoprecipitated from purified CLL B-cell lysates used above as indicated using a phospho-tyrosine specific antibody (4G10) followed by Western blot analysis using a specific antibody to Tyro3. Presence of immunoglobulin G (IgG) heavy chain was used as loading control C. CLL B-cells co-express constitutively phosphorylated Axl and Tyro3. Tyrosine-phosphorylated proteins were immunoprecipitated from CLL B-cell lysates using 4G10 antibody and phospho-Axl or phospho-Tyro3 was detected in Western blot analysis using a specific antibody to Axl or Tyro3, respectively. CLL patients are indicated by assigning arbitrary numbers. D. Axl and Tyro3 form a complex. Tyro3 was immunoprecipitated from lysates of CLL B-cells using an antibody to Tyro3, followed by Western blot analysis to detect Axl. The blot was stripped and reprobed with an antibody to Tyro3. IgG heavy chain was used as loading control. CLL patients are indicated by assigning arbitrary numbers. Molecular sizes are indicated using standard molecular weight protein markers (Bio-Rad).
Next, we determined the phosphorylation status of Tyro3 in CLL B-cells as we found earlier for Axl(4). For this, we immunoprecipitated tyrosine-phosphorylated proteins from CLL B-cell lysates, followed by Western blot analysis to detect Tyro3 or Axl. As shown in Fig. 1B we detected phosphorylation on Tyro3 in CLL B-cells. Further analysis demonstrates that both Tyro3 and Axl remain as constitutively phosphorylated RTKs in these leukemic B-cells (Fig. 1C).
Do Axl and Tyro3 exist in the same complex?
To further explore the role of Tyro3 in the TAM RTK signaling, we interrogated whether Axl and Tyro3 heterodimerize or coexist in the same molecular complex in CLL B-cells. To address this, Tyro3 was immunoprecipitated from CLL B-cell lysates and examined the immune complex to detect Axl in Western blot analysis. Indeed, our findings demonstrate that Axl and Tyro3 are physically associated or exist in the same complex (Fig. 1D).
CLL B-cells are sensitive to Axl inhibition
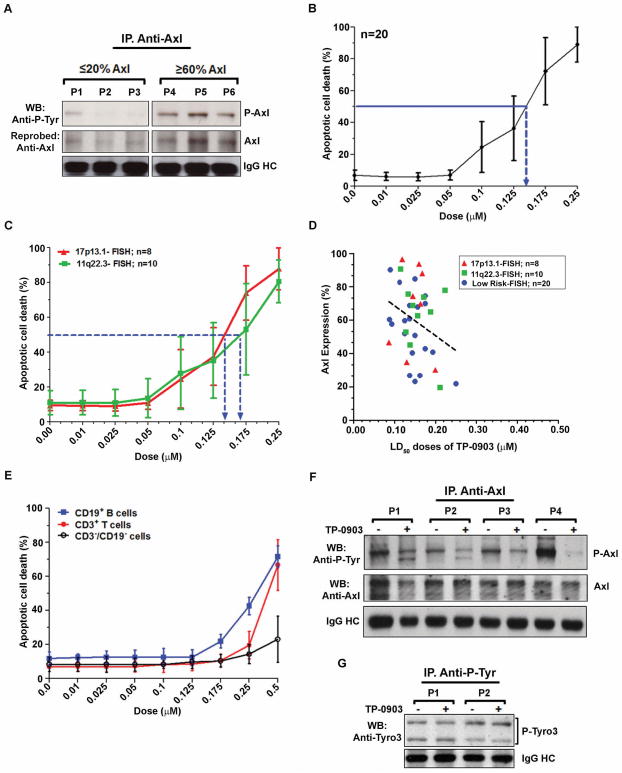
To address whether Axl expression is positively correlated with its phosphorylation levels, Axl was immunoprecipitated from lysates of CLL B-cells expressing low levels (≤20%) or high levels (≥60%) of Axl on cell surface as determined by flow cytometry prior cell lysis. Immunoprecipitated Axl was analyzed for phosphorylation levels by Western blots using 4G10 antibody. Indeed for CLL B-cells with higher Axl expression (≥60%) there was much more evident phosphorylation as compared to those expressing low levels of Axl (Fig. 2A). We hypothesized that CLL B-cells expressing constitutively phosphorylated Axl even at low levels can be impacted when exposed to a very high-affinity Axl inhibitor TP-0903(15). To address this, CLL B-cells from previously untreated CLL patients with low-risk FISH (n=20) were treated with increasing doses of TP-0903 for 24 hours and induction of apoptosis was determined. Indeed, TP-0903 induced robust levels of apoptosis in CLL B-cells from all the CLL patients (Fig. 2B) irrespective of their disease stages or prognostic factors (Supplementary Table 1) with a mean LD50 dose of 0.14μM. Most recently, we detected that CLL B-cells with 17p13.1 defects expressed increased levels of Axl(5). Therefore, to test whether TP-0903 exerts cytotoxic effects on these high-risk leukemic B-cells, CLL B-cells (Supplementary Table 1) with 17p13.1-deletion or 11q22.3-defects (confer heterologous deletion of the ATM gene) were treated with increasing doses of TP-0903 and apoptosis induction was determined after 24 hours as described above. A dose-dependent induction of massive apoptosis was noted in these high-risk CLL B-cells following TP-0903 treatment (Fig. 2C). The mean LD50 doses of both the CLL cohorts (17p13.1 and 11q22.3) were comparable and very close to that obtained for the low-risk CLL B-cells (Fig. 2B). Together, these results suggest that TP-0903 could be an effective therapeutic option across all the CLL risk groups whose leukemic B-cells express constitutively phosphorylated Axl RTK.
Figure 2. Impact of Axl inhibition on CLL B-cell survival.
A. Axl expression level is positively associated with its phosphorylation status. Axl was immunoprecipitated from equal amount of lysates of CLL B-cells expressing low levels of Axl (≤20%) or high levels (≥60%) as determined by flow cytometric analysis. The immune complex was then analyzed for the presence of phosphorylated Axl in Western blot using 4G10 antibody. The blot was stripped and reprobed with an anti-Axl antibody. IgG heavy chain was used as loading control. B. Axl inhibition induces robust apoptotic cell death. CLL B-cells conferring low-risk FISH detectable chromosomal abnormalities or not from previously untreated CLL patients (n=20) were treated with increasing doses of an orally bioavailable high affinity Axl inhibitor TP-0903 for 24 hours. Cells were harvested, stained with annexin V-FITC/PI and analyzed on flow cytometer to determine total apoptotic cell death. Results are presented as mean values with standard deviations (SD) at each dose of TP-0903. The mean LD50 (~0.14 μM) value is indicated by the arrow. C. Impact of Axl inhibition on high-risk CLL B-cell survival. CLL B-cells of high-risk as determined by FISH detectable chromosomal abnormalities at chromosome 17p13.1 (n=8) or 11q22.3 (n=10) were treated with increasing doses of TP-0903 for 24 hours. Apoptosis induction was determined as described above. Results are presented as mean values with SD. Arrows indicate mean LD50 values of TP-0903 for the CLL cohorts. D. Relation of Axl expression levels and sensitivity of CLL B-cells to TP-0903. Axl expression on CLL B-cells was determined by flow cytometric analysis using a specific antibody to Axl prior treatment with TP-0903 as described above (panels B&C). LD50 doses for individual CLL samples were calculated from the dose-response curve and plotted against % Axl expression. E. Impact of TP-0903 on normal immune cells. PBMC isolated from normal, healthy individuals (n=5) were treated with increasing doses of TP-0903 (0.01–0.5μM) and cultured in serum-free AIM-V medium for 24 hours. Apoptosis induction was determined as described above. Results are presented as mean values with SD. F. TP-0903 targets phosphorylated Axl in CLL B-cells. Axl was immunoprecipitated from lysates of CLL B-cells treated with a sub-lethal dose of TP-0903 (0.1μM for 20–24 hours), followed by Western blot analysis to detect the levels of phosphorylation on Axl using 4G10 antibody. The blot was stripped and probed with an antibody to Axl. IgG heavy chain was used as loading control. G. TP-0903 does not target P-Tyro3 in CLL B-cells. Tyrosine-phosphorylated proteins were immunoprecipitated from the above TP-0903 treated CLL B-cell lysates, followed by Western blot analysis to detect Tyro3 using a specific antibody. IgG heavy chain was used as loading control. CLL patients are indicated by assigning arbitrary numbers.
To further explore whether sensitivity of CLL B-cells to TP-0903 depends on the levels of Axl expression, we determined levels of Axl expression on CLL B-cells obtained from all the CLL patients (n=38) prior treatment of the cells with TP-0903 (as shown in Figs. 2B&C) by flow cytometry using a specific antibody to Axl. LD50 doses were evaluated from the TP-0903 dose-response curve for each sample and plotted against Axl expression on the leukemic B-cells. Although we did not find a perfect correlation between the Axl expression levels and sensitivity of the leukemic B-cells to TP-0903 (Fig. 2D), it appears that CLL B-cells from majority of CLL patients expressing >20% Axl are sensitive to TP-0903 treatment over a narrow range of LD50 doses (0.09–0.2μM).
Given these findings we concluded that TP-0903 is highly active in inducing apoptosis in CLL B-cells expressing Axl. To this end, it is also critical to find out if TP-0903 targets normal immune cells. Previously, we have detected that CLL B-cells express significantly higher levels of Axl compared to normal B-cells(4). To further detail Axl expression patterns in nonmalignant immune cells, we examined Axl expression on PBMC from healthy, age-matched individuals (n=11) by flow cytometry and sensitivity of the latter cell types to TP-0903. Granulocytes and T-cells do not express Axl (data not shown) as we reported earlier(4). However, we did find that non-B-/non-T-cells (CD19−/CD3−) i.e. NK-cells express low levels of Axl [mean: 7.23 ± 3.8 (SD)]. To test the impact of TP-0903 on normal immune cells in vitro and compare with CLL B-cells, we cultured normal PBMC in serum-free AIM-V media with increasing doses of TP-0903 for 24 hours. Cells were stained with annexin/PI and antibody to CD19 (B-cells), or CD3 (T-cells) to detect induction of apoptosis by flow cytometry. We found the LD50 doses of TP-0903 for normal B-cells and T-cells were ~0.3μM and ~0.4μM, respectively (Fig. 2E), which were higher than that found for the malignant B-cells under similar experimental conditions. However, CD3−/CD19− cells (NK cells) did not show any significant level of sensitivity to TP-0903 (Fig. 2E). Although the observed LD50 doses of TP-0903 for the normal B-/T-cells were only 2–3 fold higher than that of CLL B-cells, it is important to note that while serum-free AIM-V medium supports well primary CLL B-cell growth and survival (>90% viability) it is not the optimal culture medium for the PBMC in vitro. In total, these in vitro data indicate that cytotoxic effects of TP-0903 on normal immune cells are moderate at the doses (LD50 dose range 0.09–0.2μM) which effectively induce apoptosis in leukemic B-cells under similar in vitro culture conditions.
TP-0903 targets phosphorylated Axl in CLL B-cells
To interrogate whether TP-0903 is able to “hit” its target P-Axl, purified CLL B-cells were treated with a sub-lethal dose of TP-0903 (0.1μM) or left untreated for 20–24 hours. Axl was then immunoprecipitated from the cell lysates, followed by Western blot analysis using a phospho-tyrosine specific antibody (4G10) to detect P-Axl level. Results demonstrate substantial reduction of the P-Axl level upon treatment of CLL B-cells with TP-0903 (Fig. 2F).
To further examine whether TP-0903 could also target P-Tyro3, tyrosine-phosphorylated proteins were pulled down from TP-0903-treated CLL B-cell lysates by IP using 4G10-antibody, followed by Western blot analysis to detect Tyro3. We found that TP-0903 was unable to target P-Tyro3 in CLL B-cells at this dose (Fig. 2G). This is consistent with an earlier study indicating that the inhibitor possesses lower affinity for this RTK(15).
Inhibition of P-Axl alters activation status of Src/AKT and expression of anti-apoptotic proteins in CLL B-cells
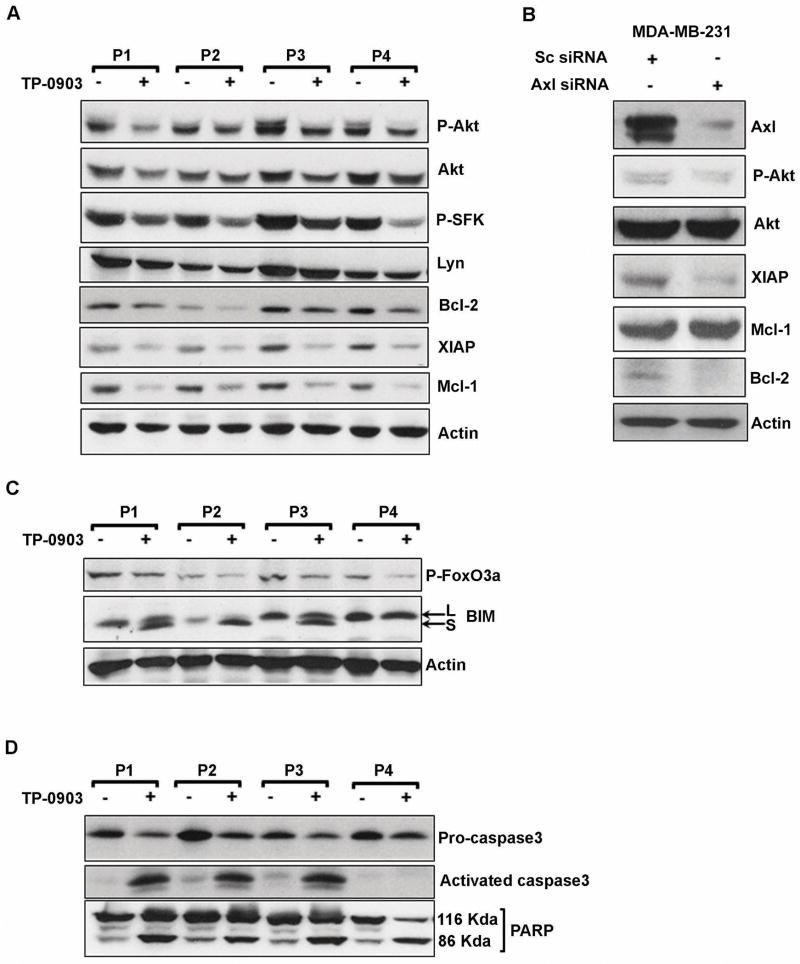
To further examine the impact of Axl inhibition on its downstream signaling components (i.e., P-AKT and P-Src family kinases [SFK]) and anti-apoptotic proteins, Mcl-1, Bcl-2 and XIAP, TP-0903-treated CLL B-cell lysates used above in Fig. 2F were further analyzed in Western blots using specific antibodies. TP-0903 treatment inhibited constitutively active SFK/AKT and reduced Mcl-1, Bcl-2 and XIAP levels in CLL B-cells (Fig. 3A). These results indicate that targeting the Axl signaling pathway by TP-0903 robust apoptosis can be induced which is linked to reduction of Mcl-1, Bcl-2 and XIAP expression levels, known to play a critical role in CLL B-cell resistance to apoptosis.
Figure 3. Impact of TP-0903 treatment on Axl downstream targets, caspase 3 activation and PARP cleavage.
A. TP-0903 reduces phosphorylation on AKT/Src kinase and expression of anti-apoptotic proteins. DMSO or TP-0903 treated CLL B-cell lysates used in Fig. 2F were analyzed for the status of AKT and Src phosphorylation as downstream signaling mediators of Axl by Western blots using specific antibodies to P-AKT or P-Src. Respective blots were stripped and reprobed with AKT or Src antibody. Status of anti-apoptotic proteins Bcl-2, XIAP and Mcl-1 were also analyzed in these TP-0903 treated CLL B-cell lysates in Western blots using specific antibodies. Actin was used as loading control. B. Depletion of Axl in MDA-MB-231 cells reduces expression of XIAP and Bcl-2, but not Mcl-1. MDA-MB-231 cells were transfected with a siRNA targeted to Axl or sc-siRNA as control. After 48 hours of transfection, cell lysates were prepared and expression of Axl, XIAP, Mcl-1 and Bcl-2 was analyzed in Western blots using specific antibodies. Phosphorylation status of AKT (Ser-473) was also examined by Western blot analysis. The blot was stripped and reprobed to detect total AKT. Actin was used as loading control. C. TP-0903 reduces phosphorylation on FOXO3a and upregulates BIM expression. DMSO or TP-0903 treated CLL B-cell lysates used above (panel A) were further analyzed for the phosphorylation status of FOXO3a, a downstream target of AKT and an upstream transcriptional activator of the BIM gene, in Western blots using a specific antibody. Expression of the pro-apoptotic protein BIM was also examined by Western blot using a specific antibody which recognizes both the large (BIML) and short (BIMS) forms of BIM. Actin was used as a loading control. D. TP-0903-induced apoptosis involves caspase 3 activation and PARP-cleavage. DMSO or TP-0903 treated CLL B-cells used above were further analyzed for the activation of caspase 3 and PARP-cleavage by Western blots using specific antibodies. CLL patients are indicated by assigning arbitrary numbers.
To further validate that the observed inhibitory effect of TP-0903 on Mcl-1, Bcl-2 and XIAP levels in CLL B-cells was as a result of P-Axl inhibition, not off-target effects, we depleted Axl by RNA interference in MDA-MB-231 breast cancer cells. We chose MDA-MB-231 cells as (i) these cells express high level of Axl(16), (ii) easy to transfect and (iii), Axl does not appear to be a primary survival factor(16). Indeed, siRNA-mediated depletion of Axl in MDA-MB-231 cells did not induce cell death (data not shown) as was observed in an earlier study(16). However, reduction of Axl expression in MDA-MB-231 cells was accompanied by significant downregulation of the anti-apoptotic proteins, XIAP and Bcl-2, but not Mcl-1 (Fig. 3B). It is likely that Axl signaling depends on cellular context. In total, these results suggest that TP-0903 primarily targets Axl in CLL B-cells to induce apoptosis.
Inhibition of the Axl/AKT axis induces BIM expression
Amongst BH3-only proteins, BIM is critical for hematopoietic development and homeostasis of both lymphoid and myeloid cell series(17). BIM has at least three major isoforms (BIMEL, BIML and BIMS) generated by alternative splicing(18). The pro-apoptotic activity of BIM is regulated transcriptionally, post-transcriptionally and post-translationally. FOXO3a is one of the major transcription factors for BIM, and is phosphorylated by AKT which inhibits its nuclear translocation from cytoplasm in healthy cells(19, 20). As TP-0903 inhibited the Axl/AKT axis in CLL B-cells, we wished to examine whether BIM was upregulated as a result of AKT inhibition. Western blot analysis of the TP-0903 treated CLL B-cell lysates revealed an increased expression of BIM compared to the cells left unexposed to TP-0903 (Fig. 3C). Further analysis suggests that increase of BIM levels was due to reduction of inhibitory phosphorylation levels of its upstream transcriptional regulator FOXO3a in TP-0903-treated CLL B-cells (Fig. 3C). Finally, we also observed that TP-0903 mediated apoptosis of CLL B-cells involves PARP cleavage and caspase 3 activation in 3 of 4 CLL patients (except P4) (Fig. 3D). It is likely that either there was activation of other executioner caspase (e.g., caspase 7) or accumulation of AIF from mitochondria to nucleus(21) in the cells from the CLL patient P4.
TP-0903 overcomes CLL BMSC mediated protection of the leukemic B-cells
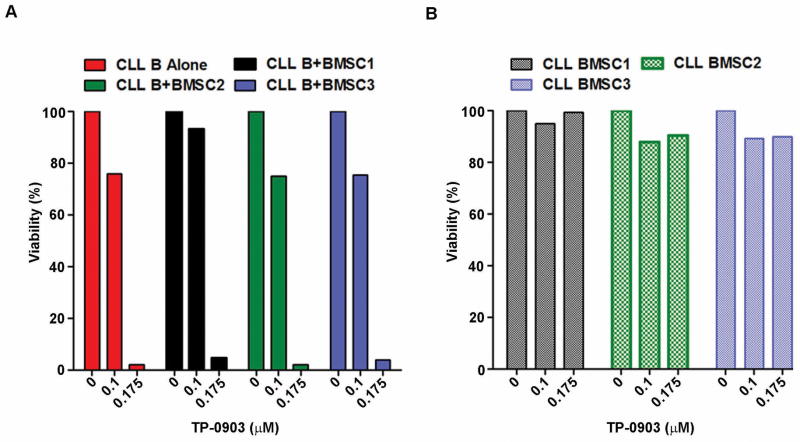
The stromal protection of leukemic cells is an important factor contributing to drug resistance in vivo(22). Previously, we and others have shown that BMSCs protect CLL B-cells from spontaneous and drug-induced apoptosis through soluble- or contact-mediated interactions(12, 13, 23). Here we tested whether TP-0903 could overcome CLL BMSC mediated protection of CLL B-cells from apoptosis induction. For this, purified CLL B-cells sensitive to TP-0903 were co-cultured with CLL BMSCs obtained from CLL patients (n=3), followed by treatment with TP-0903 at two doses: 0.1μM (lower than the mean LD50 dose 0.14μM) and 0.175μM (higher than the mean LD50) for 24 hours. Cells were harvested and induction of apoptosis was determined by flow cytometric analysis after annexin/PI staining. Results indicate that CLL BMSCs could not protect the leukemic B-cells from TP-0903 induced apoptosis (Fig. 4A).
Figure 4. TP-0903 overcomes CLL BMSC mediated protection of CLL B-cells from apoptosis.
A. Impact of TP-0903 on CLL B-cells in co-culture with CLL BMSCs. Purified CLL B-cells sensitive to TP-0903 were cultured alone or co-cultured with CLL BMSCs from 3 different CLL patients and treated with a dose (0.1μM) of TP-0903 which is lower than the mean LD50 dose (0.14μM) or a dose (0.175μM) of TP-0903 which is higher than the LD50 dose for 24 hours. CLL B-cells cultured alone were also treated with the above doses of TP-0903. Induction of apoptosis in CLL B-cells was determined by flow cytometric analysis using annexin V/PI staining and presented as percent viability. B. TP-0903 does not exert cytotoxic effect on BMSCs. CLL BMSCs co-cultured with CLL B-cells above were harvested following treatment with TP-0903 and analyzed for apoptosis induction as described above. Results are presented as percent viability of the cells.
To rule out any impact of TP-0903 on stromal cell viability, CLL BMSCs from co-culture were harvested and induction of apoptosis was determined. Our findings demonstrate that TP-0903 overcomes stromal cell mediated protection of CLL B-cells without exerting any significant cytotoxic effect on CLL BMSCs at both doses (Fig. 4B). In total, TP-0903 is able to target P-Axl in CLL B-cells and induces apoptosis even in presence of CLL BMSCs without impacting on the CLL bone marrow stroma.
Combined treatment of CLL B-cells with TP-0903 with BTK inhibitors augments apoptosis levels
Most recently, ibrutinib which irreversibly targets BTK of the BCR-signaling pathway has emerged as a very effective therapeutic intervention in CLL therapy(9). Despite the fact that the CLL patients achieve a longer period of progression-free survival (PFS), ibrutinib is not curative and resistance develops(9, 10, 24). Thus, we tested whether combined treatment of CLL B-cells with TP-0903 and ibrutinib augments leukemic B-cell apoptosis levels. CLL B-cells were treated with increasing doses of TP-0903 in combination with ibrutinib or as a single agent for 24 hours. For comparison, CLL B-cells were also treated with a reversible BTK inhibitor, TP-4216 in combination with TP-0903 using a similar in vitro treatment strategy. Cells were harvested and induction of apoptosis was determined by flow cytometry after staining with annexin/PI. Here we found that the single agent ibrutinib or TP-4216 did not induce significant level of apoptosis in CLL B-cells in the dose range used in this study but, combination with TP-0903 augmented apoptosis levels in CLL B-cells (Fig. 5A). Interestingly, combined effects of TP-0903 and TP-4216 on CLL B-cell survival were more pronounced than that of TP-0903 and ibrutinib.
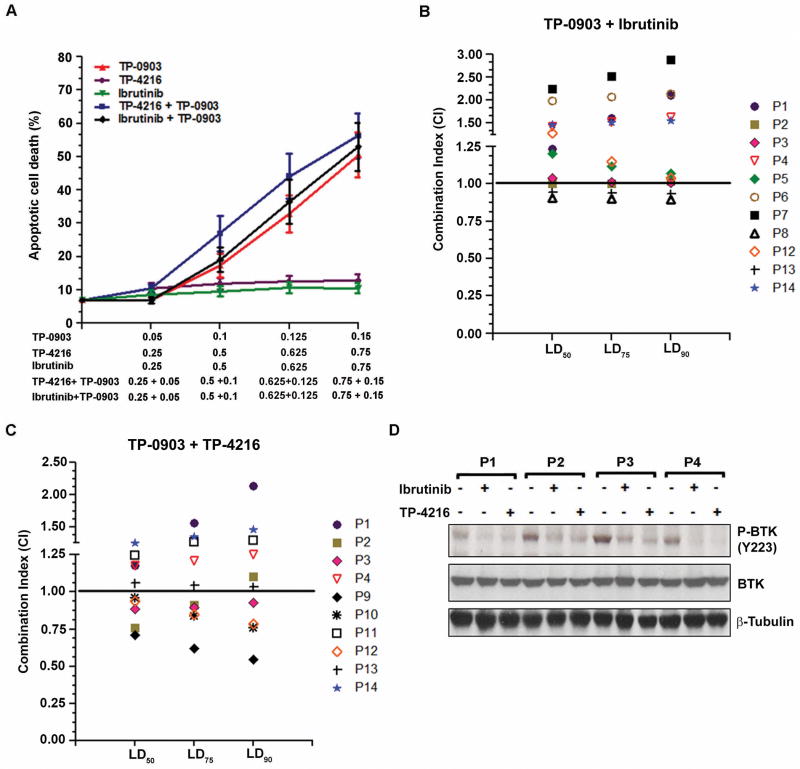
Figure 5. Combined effect of Axl and BTK inhibition on CLL B-cell survival.
A. Combined treatment of CLL B-cells with TP-0903 and BTK inhibitors augments apoptosis levels. CLL B-cells from different CLL patients were treated with increasing doses of TP-0903, ibrutinib or TP-4216, a reversible BTK inhibitor, as a single agent or in combination of TP-0903 with ibrutinib or with TP-4216 as indicated. After 24 hours, cells were harvested and induction of apoptosis was determined as described elsewhere. Results are presented as mean values with standard deviations. B. Combined treatment of CLL B-cells with TP-0903 and ibrutinib shows moderate cytotoxic effects. Combination index (CI) of the results obtained from individual CLL samples following treatment with TP-0903 and ibrutinib as presented in panel A was calculated following the method of Chou and Talalay. CI values <1 indicate a synergistic effect, CI value of 1 indicates additive effects and values >1 indicate antagonistic effects of combined treatment. C. Administration of TP-0903 with a reversible BTK inhibitor TP-4216 has more effective combination effects. CI values of the results obtained from combined administration of TP-0903 and TP-4216 on CLL B-cells from individual CLL patients were calculated similarly as described above. CLL B-cells from majority of CLL patients show additive/synergistic effects (6 of 10) to the combined treatment with TP-0903 and TP-4216. D. BTK inhibitors target phosphorylated BTK in CLL B-cells. Purified CLL B-cells were treated with DMSO, ibrutinib (0.75μM) or TP-4216 (0.75μM) for 24 hours. Cells were harvested, lysed and examined to detect expression of P-BTK (Y223) in Western blot using a specific antibody. The blot was stripped and reprobed with an antibody to BTK. β-tubulin was used as loading control. CLL patients are indicated by assigning arbitrary numbers.
To define more clearly the combination effects of these agents, CI values of the data from combined treatment of CLL B-cells from individual CLL patients was determined using the CalcuSyn software(14). Based on the CI values, we found while the in vitro combination of TP-0903 and ibrutinib showed both additive (2 of 11) and synergistic (2 of 11) effects in inducing apoptosis, CLL B-cells from the remaining 7 CLL patients showed antagonistic effects (Fig. 5B). However, we observed synergistic effects of TP-0903 in combination with TP-4216 in induction of apoptosis in CLL B-cells from 5 of 10 CLL patients and additive effect in CLL B-cells from 1 CLL patient (P13) with a CI value of 1.03 (Fig. 5C). Cells from the remaining 4 CLL patients displayed antagonistic effects throughout the dosing range of the drugs with CI values of >1.1 (Fig. 5C). In total, these results suggest that while TP-0903 is quite effective in inducing robust CLL B-cell apoptosis as a single agent, the combination with a BTK inhibitor, particularly the reversible BTK inhibitor TP-4216 could be a more effective strategy to reduce tumor burden in most CLL patients.
Finally, as we did not see any significant level of apoptosis induction in CLL B-cells upon treatment with ibrutinib or TP-4216 at the highest dose used in this study, we examined whether (i) CLL B-cells expressed P-BTK and (ii) these agents were able to target P-BTK under our in vitro culture conditions. Results from Western blot analysis demonstrate that CLL B-cells express constitutively phosphorylated BTK (Y223), albeit at differential levels, which was inhibited by TP-4216 or ibrutinib (Fig. 5D).
Discussion
We previously reported that CLL B-cells express constitutively active Axl, albeit at differential levels(4, 5). Most recently, we have shown that expression of Axl in CLL B-cells is linked to the functional status of p53(5). To further define whether CLL B-cells express other RTKs of the TAM family including Tyro3 and MER, we analyzed CLL B-cells for their expression. Indeed our findings demonstrate that CLL B-cells overexpress Tyro3, but not MER, as compared to normal B-cells. Importantly, Tyro3 remains as a constitutively phosphorylated RTK as the Axl in CLL B-cells. In addition we detected Axl and Tyro3 in the same molecular complex in CLL B-cells, suggesting that these two TAM RTKs may heterodimerize and their dynamic interactions likely play a critical role in transmitting survival signals. However, the exact functional implication of Tyro3 expression in CLL B-cells requires further investigation.
Upregulation of Axl has been reported in a variety of cancers including breast(25), gastric(26), prostate(27), ovarian(28, 29), and lung(30, 31). Overexpression of Axl was shown to correlate with poorer prognosis(32, 33), as well as increased invasiveness(34) indicating that Axl has strong oncogenic potential. Recent studies reported that Axl is overexpressed and activated in several drug-resistant cancer cell lines, suggesting that Axl may play a role in chemotherapy-resistant cancers. Increased Axl levels have been linked with Imatinib-resistant gastrointestinal stromal tumors, Nilotinib-resistant chronic myeloid leukemia cells, BMS-754087-resistant Rhabdomyosarcoma, Lapatinib-resistant HER-2 positive breast tumor cells and resistance to cisplatin in ovarian and esophageal adenocarcinoma(35–41). A most recent study show that overexpression of Axl is sufficient to mediate acquired resistance to cetuximab which targets epidermal growth factor receptor in models of non-small cell lung cancer and head and neck squamous cell carcinoma(42). Although Axl is consistently associated with resistance to chemotherapy in cancer cells, the underlying pathways of Axl upregulation in this context remain unknown(43).
This information on Axl and our earlier in vitro findings indicate that Axl can be an attractive target in CLL therapy(4, 5). Here, we utilized a high-affinity Axl inhibitor TP-0903 to generate preliminary information on its efficiency to specifically target Axl and the level of apoptosis induction in CLL B-cells. We found that TP-0903 as a single agent induced robust apoptosis in CLL B-cells within 24 hours of exposure at nanomolar doses. Importantly, these doses are easily achievable in vivo (courtesy: Tolero Pharmaceuticals). Of note, cytotoxic impact of TP-0903 on CLL B-cells was found to be independent of the disease stage, IgVH mutational status and/or the presence of chromosomal abnormalities including 17p13.1-/11q22.3-deletions of the CLL patients. We also found that TP-0903 is able to specifically target P-Axl, but not P-Tyro3, to induce apoptosis. This latter information suggests that Axl seems to be a highly active and predominant RTK of the TAM family in CLL B-cells despite the expression of significant levels of P-Tyro3. However, the exact role of Tyro3 expression and phosphorylation in CLL B-cell survival and pathogenesis remains undefined. It is likely that Tyro3 plays a secondary role in CLL B-cells as inhibition of P-Axl alone was able to induce a robust level of apoptosis.
Next, we assessed the activation status of the downstream Axl signaling pathway in TP-0903-exposed leukemic B-cells. Our analyses demonstrated that targeting P-Axl by TP-0903 resulted in inhibition of SFK and AKT activation. Of note, these two signaling components are the central and converging points of multiple signaling pathways including BCR signaling in CLL B-cells(44). Importantly, findings from our current study noted a significant reduction of the anti-apoptotic proteins, XIAP, Bcl-2 and Mcl-1 in CLL B-cells upon Axl inhibition. In addition, we also found that expression of the pro-apoptotic protein BIM was upregulated in TP-0903 treated CLL B-cells and reduction of AKT-mediated phosphorylation on FOXO3a, an upstream transcriptional activator of the BIM gene(19, 20). In total, Axl inhibition in CLL B-cells resulted in modulation of two key cell survival signaling axes and subsequent reduction of anti-apoptotic proteins and upregulation of a pro-apoptotic protein, a situation that would favor the robust apoptosis induction in CLL B-cells.
CLL BMSCs play a key role in the protection of leukemic B-cells from spontaneous as well as drug induced apoptosis(12, 13, 22, 23). To that point our findings suggest that TP-0903 is able to overcome CLL BMSC mediated protection of CLL B-cells in co-culture without affecting the stromal cells, suggesting that the use of TP-0903 as a treatment option for CLL could be highly effective. In addition we also explored whether TP-0903 could be combined with BTK inhibitors including ibrutinib.
Ibrutinib provides an effective treatment option for previously treated, relapsed/drug-refractory CLL patients however; as a single agent ibrutinib often induces only a partial response and resistance develops, and some patients have transformed to Richter’s Syndrome(24). As Axl regulates upstream kinases of BTK, Lyn/Syk(4), elevated levels and aberrant activation of Axl in CLL B-cells may also cause resistance to BTK inhibition. BTK-deficient neutrophils showed activation of NADPH oxidase resulting in increased production of reactive oxygen species (ROS)(45). Related to this, a previous report demonstrates that elevated levels of ROS activate Axl in vascular smooth muscle cells(46). Thus, one possible mediator of such resistance development to ibrutinib therapy might be activation of NADPH oxidase in response to chronic BTK inhibition resulting in elevated ROS levels in CLL B-cells; suggesting that NADPH oxidase inhibitors(47) may block compensatory Axl activation. Given these clinical issues we explored the possibility whether combining TP-0903 with ibrutinib could augment apoptosis levels in CLL B-cells. We found that the in vitro combination treatment generated additive/synergistic effects only in 4 of 11 CLL samples with the majority (remaining 7) samples demonstrating antagonistic effects. To test whether other BTK-inhibitors produced similar combination effects, we tested a reversible BTK inhibitor, TP-4216 which binds a region on BTK different than ibrutinib (courtesy of Tolero Pharmaceuticals). Indeed, concurrent in vitro administration of TP-0903 and TP-4216 produced synergistic/additive cytotoxic effects on CLL B-cells from 6 of 10 CLL patients while TP-4216 did not show any significant impact on CLL B-cell survival when used alone.
We further studied whether concurrent BCR stimulation sensitizes CLL B-cells to BTK inhibitors in vitro based on the conception that BTK inhibitor efficacy heavily relies on stimulation of BCR through in vivo mechanisms(48, 49). Indeed tissue microenvironment plays critical role in activating the BCR signal in CLL B-cells(50). However we did find an increase of apoptosis levels, albeit at variable levels, in CLL B-cells exposed to BTK inhibitors (ibrutinib or TP-4216) under in vitro BCR stimulation as compared to that seen in unstimulated cells (Supplementary Fig. 1). Thus, in agreement with previous reports these observations suggest that status of in vivo BCR stimulation likely plays a determining role to the efficacy of BCR-targeted agents in CLL.
In summary, we have found that CLL B-cells not only express constitutively active Axl but also increased levels of Tyro3, another member of the TAM RTK family and that, Tyro3 remains as constitutively phosphorylated. However, it is not clear why CLL B-cells express two constitutively phosphorylated RTKs from the same family of RTKs. A possible explanation could be formation of a heterodimer between Axl and Tyro3 stabilizing the TAM survival signals.
TP-0903 was able to induce massive apoptosis in CLL B-cells by targeting Axl without inhibiting P-Tyro3, suggesting that Axl is likely the predominant RTK of the TAM family regulating CLL B-cell survival. Further studies are underway to define more completely the role of Tyro3 in regulation of CLL B-cell survival. Our current studies on the oral Axl inhibitor TP-0903 show that it is highly specific for Axl, induces robust cell death in CLL B-cells by reducing expression levels of the critical anti-apoptotic proteins Bcl-2, XIAP and Mcl-1 and upregulating the pro-apoptotic protein BIM via activation of FOXO3a, and appears to be effective even in the presence of stromal cells. Finally, combination studies demonstrate that use of TP-0903 with a reversible BTK inhibitor is more effective than the single agents. Given these in vitro findings, we are encouraged to conduct clinical trials using TP-0903 either as a single agent or in combination for CLL patients.
Supplementary Material
Statement of Translational Relevance.
Despite aggressive treatment regimen B-Cell Chronic Lymphocytic Leukemia (CLL) is still incurable. Previously we defined the role of a novel receptor tyrosine kinase (RTK) Axl in CLL B-cell survival. In this study we demonstrated the impact of Axl inhibition on CLL B-cell survival using a high-affinity Axl inhibitor, TP-0903. Results suggest that TP-0903 is highly effective in inducing apoptosis in primary CLL B-cells via inhibition of AKT/Src signaling pathways, downstream of Axl, with nanomolar range LD50 doses which overcome bone marrow stromal cell protection of the leukemic B-cells. Finally, combined treatment of CLL B-cells with TP-0903 and a reversible Bruton’s tyrosine kinase (BTK) inhibitor induces apoptosis more efficiently with additive/synergistic effects. These findings underscore the usage of the Axl inhibitor as a single agent or in combination with BTK inhibitors to more effectively treat CLL patients.
Acknowledgments
This work was supported by a research fund from the National Cancer Institute CA170006 and research fund from Mayo Clinic internal grants to AKG. It was also partly supported by an ASH Bridge grant to NEK. Collection, processing and deposition of CLL samples into the CLL tissue bank were supported by the Predolin Foundation grant. We also wish to acknowledge Tolero Pharmaceuticals for providing the inhibitors to Axl and BTK with relevant information, and the excellent secretarial help of Ms. Tammy Hughes.
Footnotes
Authors declare no potential conflict of interest
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine & growth factor reviews. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, et al. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood. 2011;117:1928–37. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boysen J, Sinha S, Price-Troska T, Warner SL, Bearss DJ, Viswanatha D, et al. The tumor suppressor axis p53/miR-34a regulates Axl expression in B-cell chronic lymphocytic leukemia: implications for therapy in p53-defective CLL patients. Leukemia. 2014;28:451–5. doi: 10.1038/leu.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Deglesne PA, Chevallier N, Letestu R, Baran-Marszak F, Beitar T, Salanoubat C, et al. Survival response to B-cell receptor ligation is restricted to progressive chronic lymphocytic leukemia cells irrespective of Zap70 expression. Cancer Res. 2006;66:7158–66. doi: 10.1158/0008-5472.CAN-06-0085. [DOI] [PubMed] [Google Scholar]
- 8.Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. The New England journal of medicine. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115:1755–64. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch”. Leuk Res. 2007;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–96. [PubMed] [Google Scholar]
- 14.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Mollard A, Warner SL, Call LT, Wade ML, Bearss JJ, Verma A, et al. Design, Synthesis and Biological Evaluation of a Series of Novel Axl Kinase Inhibitors. ACS Med Chem Lett. 2011;2:907–12. doi: 10.1021/ml200198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE, et al. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res. 2005;65:9294–303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol. 2009;71:89–101. doi: 10.1016/j.critrevonc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, et al. Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mammalian genome: official journal of the International Mammalian Genome Society. 2001;12:163–8. doi: 10.1007/s003350010242. [DOI] [PubMed] [Google Scholar]
- 19.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. The Journal of cell biology. 2003;162:613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–31. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 21.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:729–39. [PubMed] [Google Scholar]
- 22.Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature cell biology. 2012;14:276–86. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–50. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones JA, Byrd JC. How will B-cell-receptor-targeted therapies change future CLL therapy? Blood. 2014;123:1455–60. doi: 10.1182/blood-2013-09-453092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann Oncol. 2001;12:819–24. doi: 10.1023/a:1011126330233. [DOI] [PubMed] [Google Scholar]
- 26.Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS, et al. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002;22:1071–8. [PubMed] [Google Scholar]
- 27.Jacob AN, Kalapurakal J, Davidson WR, Kandpal G, Dunson N, Prashar Y, et al. A receptor tyrosine kinase, UFO/Axl, and other genes isolated by a modified differential display PCR are overexpressed in metastatic prostatic carcinoma cell line DU145. Cancer Detect Prev. 1999;23:325–32. doi: 10.1046/j.1525-1500.1999.99034.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun W, Fujimoto J, Tamaya T. Coexpression of Gas6/Axl in human ovarian cancers. Oncology. 2004;66:450–7. doi: 10.1159/000079499. [DOI] [PubMed] [Google Scholar]
- 29.Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70:7570–9. doi: 10.1158/0008-5472.CAN-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058–64. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264–74. doi: 10.1016/s0959-8049(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 32.Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107:1124–9. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochlitz C, Lohri A, Bacchi M, Schmidt M, Nagel S, Fopp M, et al. Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK) Leukemia. 1999;13:1352–8. doi: 10.1038/sj.leu.2401484. [DOI] [PubMed] [Google Scholar]
- 34.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–48. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–8. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 36.Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008;268:314–24. doi: 10.1016/j.canlet.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, et al. Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood. 2011;118:2211–21. doi: 10.1182/blood-2010-10-313692. [DOI] [PubMed] [Google Scholar]
- 38.Lay JD, Hong CC, Huang JS, Yang YY, Pao CY, Liu CH, et al. Sulfasalazine suppresses drug resistance and invasiveness of lung adenocarcinoma cells expressing AXL. Cancer Res. 2007;67:3878–87. doi: 10.1158/0008-5472.CAN-06-3191. [DOI] [PubMed] [Google Scholar]
- 39.Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–19. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 40.Macleod K, Mullen P, Sewell J, Rabiasz G, Lawrie S, Miller E, et al. Altered ErbB receptor signaling and gene expression in cisplatin-resistant ovarian cancer. Cancer Res. 2005;65:6789–800. doi: 10.1158/0008-5472.CAN-04-2684. [DOI] [PubMed] [Google Scholar]
- 41.Huang F, Hurlburt W, Greer A, Reeves KA, Hillerman S, Chang H, et al. Differential mechanisms of acquired resistance to insulin-like growth factor-i receptor antibody therapy or to a small-molecule inhibitor, BMS-754807, in a human rhabdomyosarcoma model. Cancer Res. 2010;70:7221–31. doi: 10.1158/0008-5472.CAN-10-0391. [DOI] [PubMed] [Google Scholar]
- 42.Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, et al. AXL Mediates Resistance to Cetuximab Therapy. Cancer Res. 2014;74:5152–64. doi: 10.1158/0008-5472.CAN-14-0294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 43.Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, et al. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2:874–85. doi: 10.18632/oncotarget.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craxton A, Jiang A, Kurosaki T, Clark EA. Syk and Bruton’s tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J Biol Chem. 1999;274:30644–50. doi: 10.1074/jbc.274.43.30644. [DOI] [PubMed] [Google Scholar]
- 45.Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, et al. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nature immunology. 2012;13:369–78. doi: 10.1038/ni.2234. [DOI] [PubMed] [Google Scholar]
- 46.Konishi A, Aizawa T, Mohan A, Korshunov VA, Berk BC. Hydrogen peroxide activates the Gas6-Axl pathway in vascular smooth muscle cells. J Biol Chem. 2004;279:28766–70. doi: 10.1074/jbc.M401977200. [DOI] [PubMed] [Google Scholar]
- 47.Munson J, Bonner M, Fried L, Hofmekler J, Arbiser J, Bellamkonda R. Identifying new small molecule anti-invasive compounds for glioma treatment. Cell Cycle. 2013;12:2200–9. doi: 10.4161/cc.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34:592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.