Abstract
Introduction
Immunophilin ligands such as FK506 (FK) preserve erectile function (EF) following cavernous nerve injury (CNI), although the precise mechanisms are unclear. We examined whether the thioredoxin (Trx) and glutathione (GSH) redox systems mediate this effect after CNI.
Aim
Investigate the roles of Trx reductase 2 (TrxR2) and S-Nitrosoglutathione reductase (GSNOR) as antioxidative/nitrosative and antiapoptotic mediators of the neuroprotective effect of FK in the penis after CNI.
Methods
Adult male rats, wild-type (WT) mice, and GSNOR deficient (GSNOR −/−) mice were divided into four groups: sham surgery (CN exposure only) + vehicle; sham surgery + FK (5mg/kg/day/rat or 2mg/kg/day/mouse, for 2 days, subcutaneous); CNI + vehicle; and CNI + FK. At day 4 after injury, electrically stimulated changes in intracavernosal pressure (ICP) were measured. Penes were collected for Western blot analysis of TrxR2, GSNOR and Bcl-2 and for immunolocalization of TrxR2 and GSNOR.
Main Outcome Measures
EF assessment represented by maximal ICP and total ICP in response to electrical stimulation. Evaluation of protein expression levels and distribution patterns of antioxidative/nitrosative and antiapoptotic factors in penile tissue.
Results
EF decreased after CNI compared with sham surgery values in both rats (p<0.01) and WT and GSNOR −/− mice (p<0.05). FK treatment preserved EF after CNI compared with vehicle treatment in rats (p<0.01) and WT mice (p<0.05) but not in GSNOR −/− mice. In rats, GSNOR (p<0.01) and Bcl-2 (p<0.05) expressions were significantly decreased after CNI. FK treatment in CN-injured rats restored expression of GSNOR and upregulated TrxR2 (p<0.001) and Bcl-2 (p<0.001) expressions compared with vehicle treatment. Localizations of proteins in the penis were observed for: TrxR2 (endothelium, smooth muscle) and for GSNOR (nerves, endothelium, smooth muscle).
Conclusions
The neuroprotective effect of FK in preserving EF after CNI involves antioxidative/nitrosative and antiapoptotic mechanisms mediated, to some extent, by Trx and GSH systems.
INTRODUCTION
With even the most highly developed nerve sparing techniques, traction, contusion and incision of the cavernous nerves (CN) may occur inadvertently at the time of radical prostatectomy. Trauma to these nerves negatively affects erectile tissue health leading to decreased postoperative erectile responses. In animal models of CN injury (CNI), increased apoptosis, tissue hypoxia, fibrosis, and oxidative/nitrosative stress as well as decreased smooth muscle to collagen ratios have been reported [1–5]. Recently, several treatment modalities have been investigated with proposals that they are involved in the regeneration and recovery of injured CN [6–8].
Immunophilin ligands such as FK506 (FK) have been shown to be neuroregenerative and neuroprotective in both central and peripheral nerves [9–11]. Previous studies have shown that FK promotes the recovery of erectile function (EF) in rats following both unilateral and bilateral CNI [2, 12, 13]. The molecular mechanisms underlying this effect remain unclear although current studies suggest that immunophilin drugs exert beneficial effects following CNI through antioxidative/nitrosative and/or antiapoptotic pathways [2, 12].
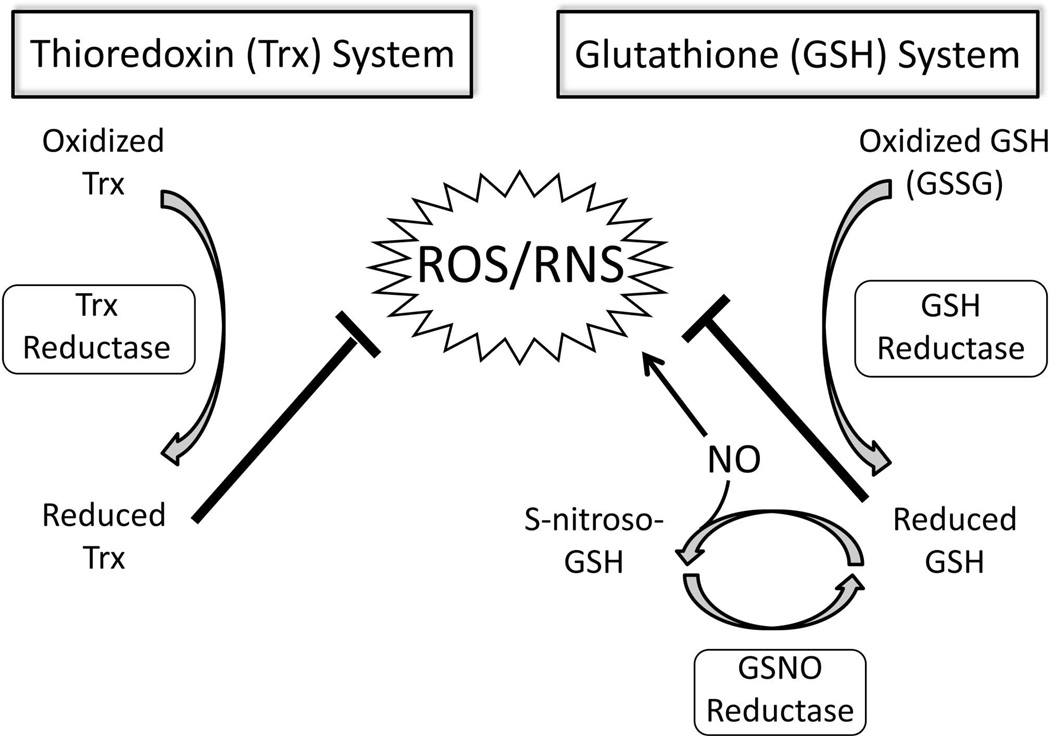
Oxidative/nitrosative mechanisms are believed to play a major pathophysiologic role in injured peripheral nerves. In this setting, an inflammatory response occurs and reactive metabolites such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) are generated [14, 15]. Oxidative stress damage occurs when reactive metabolites are created in excess of the cell’s capacity to neutralize or remove them and prolonged exposure to oxidative stress can lead to cellular apoptosis [16, 17]. Redox defense systems such as thioredoxin (Trx) and glutathione (GSH) systems are present within cells to limit neuronal cell damage caused by oxidative/nitrosative stress phenomena (Figure 1) [18].
Figure 1. Model of the regulation of antioxidant redox mechanisms.
The Trx and GSH redox systems limit oxidative/nitrosative stress caused by ROS/RNS and function via Trx reductase and GSH reductase enzyme activities. NO may directly cause increased RNS production (via peroxynitrite formation) although NO may also interact with GSH leading to GSNO formation through S-nitrosylation. GSNOR is the major enzyme involved in GSNO metabolism and protects against increased ROS/RNS. (GSSG, glutathione disulfide; S-nitroso-GSH, GSNO; ROS, reactive oxygen species; RNS, reactive nitrogen species; NO, nitric oxide)
The Trx system is essential for cell proliferation and survival and is involved in protection against oxidative/nitrosative stress and in the regulation of mitochondrial apoptosis signaling [19–21]. Overexpression of Trx, using transgenic mice and gene therapy, increases expression of the antiapoptotic factor Bcl-2 [22, 23]. Trx exists in two different forms, cytosolic (Trx1) and mitochondrial (Trx2) [24]. In knockdown experiments of both TrxR1 and TrxR2 in cultured endothelial cells, TrxR2 knockdown produced a significant increase in hydrogen peroxide (H2O2), a common ROS, suggesting that TrxR2 may play a bigger role in antioxidation than TrxR1 [25]. Thioredoxin reductase 2 (TrxR2) is an NADPH dependent oxidoreductase responsible for maintaining Trx2 in its reduced state thereby conserving its antioxidative and antiapoptotic function [26]. The Trx system has also been shown to provide neuroprotection after injury in rat optic and hypoglossal nerves [18, 27].
GSH is capable of scavenging both ROS and RNS, and its redox status is characterized by the ratio of reduced GSH to oxidized glutathione disulfide (GSSG) [28, 29]. This antioxidant system is induced after hypoglossal and sciatic nerve injury [18, 30], and depletion of GSH leaves neurons susceptible to deleterious reactive metabolites and apoptosis [31]. One way in which GSH exerts its function is by serving as an endogenous nitric oxide (NO) reservoir of S-nitrosoglutathione (GSNO) [32]. GSNO reductase (GSNOR) is an enzyme that controls tissue levels of GSNO, and mice deficient in this enzyme display increased nitrosative stress, tissue damage and increased mortality [33].
In this study, we sought to characterize the molecular basis for the neuroprotective effect of FK in the preservation of EF after CNI. Our objective was to investigate the potential antioxidant/nitrosative and antiapoptotic capacity of this pharmacological agent in penile tissue, in light of the putative pathophysiologic phenomena encountered by the penis after CNI. Specifically, we proposed that the promotion of EF recovery after CNI by FK involves Trx and GSH redox defense mechanisms. Our studies feature the use of the GSNOR-deficient (GSNOR −/−) mutant mouse as an experimental tool to evaluate the role of the GSH system [33].
METHODS
Chemicals
A rabbit polyclonal antibody for TrxR2 and a goat polyclonal antibody for GSNOR were purchased from Abcam (Cambridge, MA, USA). A rabbit polyclonal Bcl-2 antibody was purchased from Cell Signaling Technology (Beverly, MA, USA), and a mouse monoclonal β-actin antibody was purchased from Sigma Chemical (St. Louis, MO, USA). FK was purchased from LC Laboratories (Woburn, MA, USA) and diluted in a mixture of 50% saline, 40% ethanol, and 10% dimethyl sulfoxide.
Animals and Treatment
Adult male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA, USA) weighing 300–350 g and young adult (8–10 week old) male homozygous GSNOR −/− mice with age-matched wild-type (WT) mice (C57 BL/6, The Jackson Laboratory, Bar Harbor, ME, USA) were used. Animals were cared for and housed under strict guidelines and all procedures were approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee. Animals were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) plus xylazine (10 mg/kg). In rats, the right CN was identified on the posterolateral aspect of the prostate and crushed 2–3 mm distal to the major pelvic ganglion with an ultra fine hemostat (Fine Science Tools, Foster City, CA, USA) using full closure for three minutes [13]. In mice, nerve injury was produced using Dumont #5 forceps (Fine Science Tools, Foster City, CA, USA) that were held closed two times for 15 seconds each, causing a moderate injury, as described previously [2]. Sham surgeries were completed by simply exposing the CN but not manipulating it. To limit variability, all surgeries were completed by the same investigator. Animals were randomly divided into four groups (n=5–8/group): sham surgery + vehicle, sham surgery + FK, CNI + vehicle; and CNI + FK. FK was administered subcutaneously at 5mg/kg/day in rats or 2mg/kg/day in mice immediately after CNI and then on the day after injury (2 day treatment). We chose this timeframe to measure acute functional and morphological changes in penile tissue following CNI based on previous reports showing increased apoptosis in the penis as soon as two days after CN denervation [4].
In Vivo Erection Physiology Studies
Four days after CNI, EF was evaluated in all animals. To monitor mean arterial pressure (MAP) in rats, the right carotid artery was cannulated with polyethylene (PE) tubing filled with heparinized saline (200U/ml). To monitor changes in intracavernosal pressure (ICP), the penis was denuded of skin and fascia and a 23-gauge needle (rats) or 30-gauge needle (mice) connected via PE tubing to a pressure transducer was inserted into the right crus. Previously injured or sham-treated nerves were placed on a bipolar electrode attached to a Grass Instruments S48 stimulator (Quincy, MA, USA) and stimulated at 4 volts, 16 Hz with a 5 millisecond square-wave duration for 1 minute. ICP was recorded using the DI-190 system (Dataq Instruments, Akron, OH, USA) from the start of electrical stimulation until 60 seconds after stimulation ended. In rats, EF was represented by the normalized maximal ICP/MAP (maximal ICP) and total area under the curve/MAP (total ICP). Since we did not measure MAP in mice (to reduce surgical trauma and morbidity), we calculated ICP responses to electrical stimulation of the right crushed nerve as percent ICP response of the stimulation of the left sham nerve, as described previously [34, 35]. Results were analyzed using the MATLAB program (Mathworks, Natick, MA, USA).
Western Blot Analysis
At the conclusion of experiments, penes were removed and immediately snap frozen in liquid nitrogen, and animals were sacrificed by a lethal intracardiac injection of saturated potassium chloride. Penes were homogenized, as described previously [36]. Thirty µg protein was loaded on either 4–20% or 10% Tris HCl gels (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to a polyvinylidene fluoride membrane. After blocking the membranes, sample-transferred membranes were incubated at 4 °C overnight with the following antibodies (dilutions indicated): TrxR2 (1:1000), GSNOR (1:1000), Bcl-2 (1:1000) and β- actin (1:10,000). Blots were scanned and quantified using NIH Image J software and standardized to β-actin. The ratio was determined in terms of arbitrary units and expressed relative to the ratio for sham surgery + vehicle treated animals.
Immunohistochemistry
Penes from the sham + vehicle treated group were also collected for immunolocalization of TrxR2 and GSNOR. Tissue was immediately fixed with 10% formalin, paraffin wax-embedded and sectioned. Sections (10 µm) were baked at 60 °C for 1 h, deparaffinized and boiled in a target retrieval solution for 20 min. They were then quenched in 3% hydrogen peroxide/phosphate buffered saline (PBS) solution for 10 minutes, blocked with 1.5% normal goat serum in PBS for 1 hour and incubated overnight at 4°C in PBS containing 2% bovine serum albumin with TrxR2 (1:1000) and GSNOR (1:50) antibodies. Staining was visualized with the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) using diaminobenzidine (Sigma Aldrich, St. Louis, MO) as the chromagen. A tissue section omitting primary antibody was used as a negative control. Microscopy was performed using a Nikon Eclipse E400 biological microscope and photographed using a Nikon DS –Fi1 color camera.
Statistical Analysis
The data are expressed as the mean ± SEM. Statistical analyses were performed using one-way ANOVA, followed by Newman-Keuls multiple comparison or by Student's t-test. P less than 5% was considered significant.
RESULTS
FK Treatment Upregulates TrxR2, GSNOR and Bcl-2 in the Penis after CNI
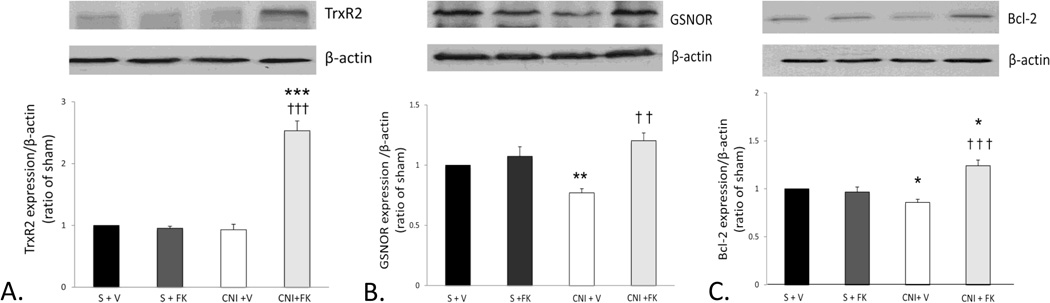
Densitometry results show TrxR2 expression was unchanged after CNI + vehicle treatment compared with sham treatment group levels. FK treatment significantly increased TrxR2 expression after CNI compared with CNI + vehicle (p< 0.001) and sham (p< 0.001) treatment group levels (Figure 2a). GSNOR (p<0.01) (Figure 2b) and Bcl-2 (p<0.05) (Figure 2c) expressions were significantly decreased after CNI compared to sham treatment group levels. Treatment with FK in CN-injured rats restored expression of GSNOR and significantly increased Bcl-2 (p<0.001) expression level compared with vehicle treatment. Bcl-2 expression after FK treatment was also significantly (p<0.05) increased compared with sham treatment group levels. These data indicate that the mediatory effect of FK in preserving EF after CNI is associated with preservation and upregulation of antioxidant/nitrosative and antiapoptotic factors. Data from the sham + vehicle group were similar to the sham + FK group suggesting that immunophilin ligand treatment does not influence neuroregulated erectile responses or protein expressions in the absence of CNI.
Figure 2. FK increased expressions of TrxR2, GSNOR and Bcl-2 in rat penile tissue after unilateral CNI.
A.) TrxR2 expression (56 kDa) did not change after CNI + vehicle compared to the sham treated groups while FK treatment caused TrxR2 expression to increase significantly after CNI compared to both the sham and CNI + vehicle treated groups. B.) GSNOR expression (40 kDa) decreased after CNI compared to the sham treated groups. FK treatment prevented this decrease, and GSNOR expression levels in the CNI + FK group were similar to sham values. C.) Bcl-2 expression (26 kDa) decreased after CNI compared to the sham treated groups. FK treatment prevented this decrease, and expression increased significantly in the CNI + FK group compared with both the CNI + vehicle and sham treatment groups.
* p<0.05, ** p<0.01, *** p<0.001 vs sham + vehicle. †† p<0.01, ††† p<0.001 vs. CNI + vehicle. Top panels: representative immunoblots; bottom panel: densitometry data; bars represent mean ± SEM of 7–8 rats per group. All protein expressions were normalized to β-actin and expressed as a ratio of sham values. (S +V = sham + vehicle, S + FK = sham + FK treatment, CNI +V = unilateral injury + vehicle, CNI + FK = unilateral injury + FK treatment).
TrxR2 and GSNOR Are Differentially Localized in Penile Tissue
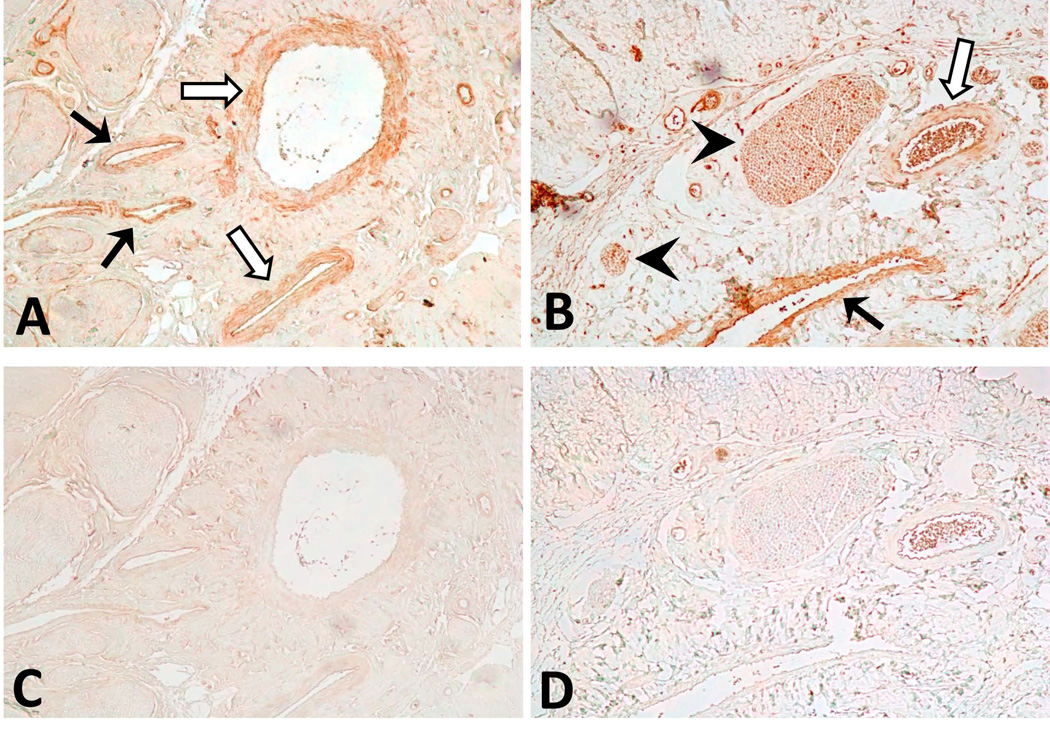
We investigated the distribution pattern of TrxR2 and GSNOR in penile tissue (Figure 3). TrxR2 was localized to vascular endothelial and smooth muscle cells lining the dorsal vein and dorsal arteries. GSNOR was also localized to vascular endothelial and smooth muscle cells in penile blood vessels but in addition was localized to penile nerves. These data indicate that the cellular sites of action of antioxidative/nitrosative and antiapoptotic mechanisms refer particularly to blood vessels and nerves in the penis.
Figure 3. TrxR2 and GSNOR immunoreactivities in rat penis.
A.) TrxR2 immunoreactivity was observed in smooth muscle and endothelium of dorsal veins (solid arrows) and arteries (open arrows). B.) GSNOR immunoreactivity was also observed in smooth muscle and endothelium of dorsal veins (solid arrows) and arteries (open arrows) as well as in nerves (arrowhead). Negative controls without primary antibody for TrxR2 (C) and GSNOR (D) did not show staining. Magnification ×100.
GSNOR Deletion Limits FK-dependent EF Recovery after CNI
In rats, both maximal and total ICP were significantly (p<0.01) reduced after CNI compared with that of sham treated animals (Figure 4). FK treatment significantly (p<0.01) preserved both maximal and total ICP compared with that of vehicle treatment. These data are consistent with previous experiments from our lab showing neuroprotective effects of FK on EF after CNI in rats [2, 13, 34, 37].
Figure 4. FK treatment preserved EF 4 days after CNI in rats.
A.) Maximal ICP was significantly decreased after CNI compared with that of both sham groups. FK treatment increased maximal ICP compared with that of CNI + vehicle. B.) Total ICP was also significantly decreased after CNI compared with that of both sham groups. FK treatment increased total ICP compared with that of CNI + vehicle. ** p<0.01 vs. sham + vehicle. †† p<0.01 vs. CNI + vehicle. (S +V = sham + vehicle, S + FK = sham + FK treatment, CNI +V = unilateral injury + vehicle, CNI + FK = unilateral injury + FK treatment).
In mice (both WT and GSNOR −/−), maximal and total ICP values were also significantly (p<0.05) reduced after CNI compared with sham + vehicle treatment levels (Figure 5). FK significantly preserved EF in WT mice (p<0.05) while in GSNOR −/− mice, FK treatment did not produce an effect that differed from that of CNI alone (p>0.05). In GSNOR−/− mice, maximal and total ICP values in the CNI + FK treatment group were also significantly decreased compared with that of the sham + vehicle treatment group (p<0.05). We observed that ICP data were variable when computing ICP response of the right nerve as percent stimulation of the left nerve among animals. Overall, these data provide direct genetic evidence that after CNI, GSNOR is essential for the protection of EF by FK.
Figure 5. FK treatment preserved EF 4 days after CNI in WT mice but not in GSNOR −/− mice.
A.) In WT mice, maximal ICP significantly decreased after CNI compared with sham treatment while FK treatment increased maximal ICP compared with CNI + vehicle. B.) Total ICP was also significantly decreased after CNI compared with that of sham treatment in WT mice while FK treatment increased total ICP compared with CNI + vehicle. C.) In GSNOR −/− mice, maximal ICP significantly decreased after CNI compared with sham treatment while FK treatment had no effect. D.) Total ICP was also significantly decreased with CNI while FK treatment had no effect. * p<0.05 vs. sham + vehicle, † p<0.05 vs. CNI + vehicle (S +V = sham + vehicle, S + FK = sham + FK treatment, CNI +V = unilateral injury + vehicle, CNI + FK = unilateral injury + FK treatment).
DISCUSSION
This study broadens current knowledge regarding the reputed neuroprotective effect of FK on EF after CNI [2, 12, 13, 34] by exploring molecular mechanisms involved in the drug’s prevention of oxidative/nitrosative stress and apoptosis in the penis. Our current results showed that after CNI regulatory proteins involved in oxidation and apoptosis were altered and FK treatment attenuated these changes. Specifically, although TrxR2 expression was unchanged and GSNOR and Bcl-2 expressions were decreased after CNI, following FK treatment expression levels of all of these antioxidative/nitrosative and antiapoptotic factors were greatly increased suggesting that FK exerts its protective effects by their upregulations and actions. Our results showing that FK treatment was ineffective in CN-injured GSNOR −/− mutant mice further substantiates this conclusion. Our immunolocalization results indicate the effect potentially involves both vascular and neurologic systems.
Although the exact mechanism through which FK is protective after CNI is unclear, FK has been shown to be integral in several diverse mechanisms. In vitro studies in human neuroblastoma and glioma cells as well as rat hippocampal neurons and hepatocytes demonstrated FK’s antioxidative properties are linked to increased glutathione and superoxide dismutase levels and decreased ROS production [38–40]. These results add to our previous work showing enhanced expression of glutathione peroxidase (an antioxidant enzyme) and decreased expression of nitrotyrosine (a marker of peroxynitrite and oxidative stress) after CNI with FK treatment [2]. Our current results are in line with these findings and support the contention that antioxidative/nitrosative and antiapoptotic factors mediate FK’s effect. One proposed mechanism of FK-induced neuroprotection involves FK directly binding to cellular proteins known as immunophilins (FKBPs). The FK/FKBP complex binds to calcineurin and inhibits its activity leading to decreased NO production and decreased harmful RNS production [41]. FK may also indirectly involve the Ras/Raf/MAP kinase signaling pathway or rely on rapid de novo protein synthesis of antioxidative and antiapoptotic molecules [42, 43]. Our current results with FK treatment suggest possible roles of these mechanisms in the nerve-damaged penis but until more studies are done this remains an open question. Further studies may also be performed to examine molecular changes of these target proteins directly in the CN following CNI.
Antioxidant systems such as the Trx system play a critical role in the ability of injured neurons to manage oxidative stress and apoptosis [20]. The antioxidative and antiapoptotic properties of Trxs are mediated by TrxRs, which function to reduce oxidized Trxs [18]. In this study we focused on TrxR2, commonly expressed in mitochondria, because it is believed that mitochondria are the major source of ROS during pathological conditions such as inflammation, a common side effect of nerve injury [44]. We showed four days after CNI that TrxR2 expression was significantly increased with FK treatment. This suggests that the protective effect of FK on erection physiology may be attributed to its antioxidative and/or antiapoptotic capability. Our FK treatment results are consistent with previous studies suggesting Trx2 overexpression has protective effects against oxidative stress-induced apoptosis after optic nerve transection [27]. Our observation that TrxR2 did not change after CNI with vehicle treatment may be explained due to the fact that the CNI was unilateral and perhaps a more significant injury, bilaterally or via CN transection, would have changed its expression. Another explanation could be that the timeframe of the experiment of four days after injury was relatively early to observe changes in TrxR2 expression with CNI alone. Additional studies may be done to further evaluate this concern. Given that TrxR2 expression only changed after CNI + FK, it is likely that additional mechanisms are involved in FK’s protective action and further studies may be done to examine possible roles of Trx1/TrxR1 and other redox systems.
Prolonged exposure to oxidative stress can lead to cellular apoptosis [17]. Previous studies have shown increased apoptosis in rat penile tissue following both unilateral and bilateral CNI [4, 45]. Our current results support this finding and show that after CNI, Bcl-2 expression was decreased. Furthermore, we show that Bcl-2 was upregulated after CNI with FK treatment. Taken together, these results suggest that apoptosis resulting from CNI may be attenuated by the administration of FK. Although we did not measure apoptosis directly, these data coincide with previous data from our lab showing downregulated antiapoptotic pathways such as PI3K/Akt up to 7 days following unilateral CNI [2]. Our results are consistent with prior research indicating that Bcl-2 expression is decreased after optic and facial nerve injury [46, 47]. The finding that FK treatment increased Bcl-2 expression also coincides with other nerve injury studies suggesting that overexpression of Bcl-2 is neuroprotective and that FK administration upregulates Bcl-2 expression [48–50].
Penile erection requires relaxation of cavernosal tissue controlled by the release of NO from nerves and endothelial cells within the corpus cavernosum and is part of a unique biochemical cascade involving production of the second-messenger molecule cyclic guanosine monophosphate (cGMP) [51, 52]. However, additional cGMP-independent mechanisms for NO bioactivity such as protein S-nitrosylation have recently been investigated. S-nitrosylation refers to the addition of an NO group to the thiol moiety of a cysteine residue consequently leading to S-nitrosothiol (SNO) formation [53]. The most common non-protein SNO, GSNO, is generated when NO interacts with GSH and is subsequently denitrosylated by the enzyme GSNOR. GSNOR plays important roles in the regulation of protein S-nitrosylation and in protection against nitrosative stress [33, 54, 55]. The protective effect of FK seen in WT mice, unlike the GSNOR −/− mutant mice, is conceivably mediated, at least in part, by unaltered GSNO regulation. Thus, GSNOR appears to be critical for FK’s effect on EF preservation after CN injury. Although the mechanism is unknown it could be a direct effect of FK on GSNOR activity or expression. FK may also afford protection through GSNOR by indirectly affecting S-nitrosylation/denitrosylation of factors involved in oxidative/nitrosative stress and apoptosis. Further studies are needed to elucidate the mechanism by which GSNOR mediates the protective effect of FK on EF.
Besides their plausible roles in countering oxidative/nitrosative phenomena directly, Trx and GSH systems may also exert protective effects via denitrosylation [32]. S-nitrosylation describes a physiologic mechanism by which NO-based signaling regulates proteins involved in apoptosis, inflammation and cellular degeneration [53, 56]. Denitrosylation, the antithesis of NO-based pathobiologic regulation, may represent an alternative mechanism for FK’s protective effect. Additional studies may further clarify the biologic consequences of S-nitrosylation/denitrosylation on the regulation of target proteins involved in penile erection.
CONCLUSION
Following radical prostatectomy CN functional recovery is gradual, and it is understood that the cavernosal tissue deteriorates in the absence of functional innervation. Thus, it is important to investigate pharmacological modalities that may protect these injured nerves and counter apoptosis in the penis. Our study suggests that immunophilin ligands such as FK may serve to preserve EF after CNI through upregulation of proteins (TrxR2, GSNOR and Bcl-2) involved in reduction of oxidative/nitrosative stress and apoptosis.
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang DY, Hyun JS, Champion HC, Abdel-Mageed AB, Hellstrom WJ. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 2.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and Sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–435. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, Cao YC, Olsson C, Shabsigh R. Apoptosis in the rat penis after penile denervation. J Urol. 1997;158:626–630. [PubMed] [Google Scholar]
- 5.Ozkara H, Alan C, Atukeren P, Uyaner I, Demirci C, Gumustas MK, Alici B. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manuplation in rat penis. Chin J Physiol. 2006;49:160–166. [PubMed] [Google Scholar]
- 6.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 7.Sezen SF, Lagoda G, Burnett AL. Role of immunophilins in recovery of erectile function after cavernous nerve injury. J Sex Med. 2009;(6 Suppl 3):340–346. doi: 10.1111/j.1743-6109.2008.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bella AJ, Lin G, Lin CS, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;(6 Suppl 3):347–352. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarikcioglu L, Demir N, Akar Y, Demirtop A. Effect of intrathecal FK506 administration on intraorbital optic nerve crush: an ultrastructural study. Can J Ophthalmol. 2009;44:427–430. doi: 10.3129/i09-071. [DOI] [PubMed] [Google Scholar]
- 10.Saxena K, Patro N, Patro I. FK506 protects neurons following peripheral nerve injury via immunosuppression. Cell Mol Neurobiol. 2007;27:1049–1057. doi: 10.1007/s10571-007-9221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildirim FB, Sarikcioglu L, Ozsoy U, Demir N, Demirtop A, Ucar Y. Effect of FK506 administration after obturator nerve injury: a functional and ultrastructural study. Acta Neurobiol Exp (Wars) 2008;68:477–483. doi: 10.55782/ane-2008-1713. [DOI] [PubMed] [Google Scholar]
- 12.Mulhall JP, Muller A, Donohue JF, Golijanin D, Tal R, Akin-Olugbade Y, Kobylarz K, Cohen-Gould L, Bennett NE, Scardino P. FK506 and erectile function preservation in the cavernous nerve injury model: optimal dosing and timing. J Sex Med. 2008;5:1334–1344. doi: 10.1111/j.1743-6109.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 13.Lagoda G, Sezen SF, Burnett AL. FK506 and rapamycin neuroprotect erection and involve different immunophilins in a rat model of cavernous nerve injury. J Sex Med. 2009;6:1914–1923. doi: 10.1111/j.1743-6109.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 14.Senoglu M, Nacitarhan V, Kurutas EB, Senoglu N, Altun I, Atli Y, Ozbag D. Intraperitoneal Alpha-Lipoic Acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj. 2009;4:22. doi: 10.1186/1749-7221-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zalba G, Beaumont J, San Jose G, Fortuno A, Fortuno MA, Diez J. Vascular oxidant stress: molecular mechanisms and pathophysiological implications. J Physiol Biochem. 2000;56:57–64. doi: 10.1007/BF03179777. [DOI] [PubMed] [Google Scholar]
- 17.Juan YS, Chuang SM, Mannikarottu A, Huang CH, Li S, Schuler C, Levin RM. Coenzyme Q10 diminishes ischemia-reperfusion induced apoptosis and nerve injury in rabbit urinary bladder. Neurourol Urodyn. 2009;28:339–342. doi: 10.1002/nau.20662. [DOI] [PubMed] [Google Scholar]
- 18.Hama I, Nakagomi S, Konishi H, Kiyama H. Simultaneous expression of glutathione, thioredoxin-1, and their reductases in nerve transected hypoglossal motor neurons of rat. Brain Res. 2010;1306:1–7. doi: 10.1016/j.brainres.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Damdimopoulos AE, Miranda-Vizuete A, Pelto-Huikko M, Gustafsson JA, Spyrou G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Hosoi F, Yamaguchi-Iwai Y, Nakamura H, Masutani H, Ueda S, Nishiyama A, Takeda S, Wada H, Spyrou G, Yodoi J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patenaude A, Ven Murthy MR, Mirault ME. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J Biol Chem. 2004;279:27302–24314. doi: 10.1074/jbc.M402496200. [DOI] [PubMed] [Google Scholar]
- 22.Koneru S, Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik N. Thioredoxin-1 gene delivery induces heme oxygenase-1 mediated myocardial preservation after chronic infarction in hypertensive rats. Am J Hypertens. 2009;22:183–190. doi: 10.1038/ajh.2008.318. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T, Iwasaki Y, Nagata K, Fushiki S, Nakamura H, Marunaka Y, Yodoi J. Thioredoxin-1 protects against hyperoxia-induced apoptosis in cells of the alveolar walls. Pulm Pharmacol Ther. 2007;20:650–659. doi: 10.1016/j.pupt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Vlamis-Gardikas A, Holmgren A. Thioredoxin and glutaredoxin isoforms. Methods Enzymol. 2002;347:286–296. doi: 10.1016/s0076-6879(02)47028-0. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama T, Michel T. Thiol-metabolizing proteins and endothelial redox state: differential modulation of eNOS and biopterin pathways. Am J Physiol Heart Circ Physiol. 2010;298:H194–H201. doi: 10.1152/ajpheart.00767.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MR, Chang HS, Kim BH, Kim S, Baek SH, Kim JH, Lee SR, Kim JR. Involvements of mitochondrial thioredoxin reductase (TrxR2) in cell proliferation. Biochem Biophys Res Commun. 2003;304:119–124. doi: 10.1016/s0006-291x(03)00547-3. [DOI] [PubMed] [Google Scholar]
- 27.Munemasa Y, Kim SH, Ahn JH, Kwong JMK, Caprioli J, Piri N. Thioredoxins 1 and 2 are neuroprotective in retinal ganglion cells after optic nerve transection and oxidative stress. Invest Ophthalmol Vis Sci. 2008:iovs.08–iovs.1716. doi: 10.1167/iovs.08-1716. [DOI] [PubMed] [Google Scholar]
- 28.Aw TY. Cellular Redox: A Modulator of Intestinal Epithelial Cell Proliferation. Physiology. 2003;18:201–204. doi: 10.1152/nips.01448.2003. [DOI] [PubMed] [Google Scholar]
- 29.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 30.Guedes RP, Dal Bosco L, Araújo ASdR, Belló-Klein A, Ribeiro MFM, Partata WA. Sciatic nerve transection increases gluthatione antioxidant system activity and neuronal nitric oxide synthase expression in the spinal cord. Brain Res Bull. 2009;80:422–427. doi: 10.1016/j.brainresbull.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Rizzardini M, Lupi M, Bernasconi S, Mangolini A, Cantoni L. Mitochondrial dysfunction and death in motor neurons exposed to the glutathione-depleting agent ethacrynic acid. J Neurol Sci. 2003;207:51–58. doi: 10.1016/s0022-510x(02)00357-x. [DOI] [PubMed] [Google Scholar]
- 32.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 34.Sezen SF, Hoke A, Burnett AL, Snyder SH. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat Med. 2001;7:1073–1074. doi: 10.1038/nm1001-1073. [DOI] [PubMed] [Google Scholar]
- 35.Canguven O, Burnett A. Cavernous nerve injury using rodent animal models. J Sex Med. 2008;5:1776–1785. doi: 10.1111/j.1743-6109.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 36.Musicki B, Champion HC, Becker RE, Liu T, Kramer MF, Burnett AL. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226–232. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 37.Burnett AL, Becker RE. Immunophilin ligands promote penile neurogenesis and erection recovery after cavernous nerve injury. J Urol. 2004;171:495–500. doi: 10.1097/01.ju.0000089775.88825.ec. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K-i, Fujita N, Higashi Y, Ogawa N. Neuroprotective and antioxidant properties of FKBP-binding immunophilin ligands are independent on the FKBP12 pathway in human cells. Neurosci Lett. 2002;330:147–150. doi: 10.1016/s0304-3940(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 39.Lee KH, Won R, Kim UJ, Kim GM, Chung MA, Sohn JH, Lee BH. Neuroprotective effects of FK506 against excitotoxicity in organotypic hippocampal slice culture. Neurosci Lett. 2010;474:126–130. doi: 10.1016/j.neulet.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Tuñón MJ, Sánchez-Campos S, Gutiérrez B, Culebras JM, González-Gallego J. Effects of FK506 and rapamycin on generation of reactive oxygen species, nitric oxide production and nuclear factor kappa B activation in rat hepatocytes. Biochem Pharmacol. 2003;66:439–445. doi: 10.1016/s0006-2952(03)00288-0. [DOI] [PubMed] [Google Scholar]
- 41.Dawson TM, Steiner JP, Dawson VL, Dinerman JL, Uhl GR, Snyder SH. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klettner A, Baumgrass R, Zhang Y, Fischer G, Burger E, Herdegen T, Mielke K. The neuroprotective actions of FK506 binding protein ligands: neuronal survival is triggered by de novo RNA synthesis, but is independent of inhibition of JNK and calcineurin. Brain Res Mol Brain Res. 2001;97:21–31. doi: 10.1016/s0169-328x(01)00286-8. [DOI] [PubMed] [Google Scholar]
- 43.Price RD, Yamaji T, Matsuoka N. FK506 potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP kinase pathway. Br J Pharmacol. 2003;140:825–829. doi: 10.1038/sj.bjp.0705522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile Weight and Cell Subtype Specific Changes in a Post-Radical Prostatectomy Model of Erectile Dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 46.Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp Eye Res. 2010;91:127–134. doi: 10.1016/j.exer.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZM, Dai CF, Kanoh N, Chi FL, Li KY. Apoptosis and expression of BCL-2 in facial motoneurons after facial nerve injury. Otol Neurotol. 2002;23:397–404. doi: 10.1097/00129492-200205000-00029. [DOI] [PubMed] [Google Scholar]
- 48.Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–4194. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Most SP. Facial nerve recovery in bcl2 overexpression mice after crush injury. Arch Facial Plast Surg. 2004;6:82–87. doi: 10.1001/archfaci.6.2.82. [DOI] [PubMed] [Google Scholar]
- 50.Zhao S, Dou DD, Zeng T, Wang QS, Zhu ZP, Zhao XL, Xie KQ. Effects of tacrolimus (FK506) on heat-shock proteins 70, Bcl-2 and Bax expression in nervous tissue of acrylamide-induced rat. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010;28:255–259. [PubMed] [Google Scholar]
- 51.Burnett AL. Nitric oxide regulation of penile erection: biology and therapeutic implications. J Androl. 2002;23:S20–S26. [PubMed] [Google Scholar]
- 52.Burnett AL. Role of nitric oxide in the physiology of erection. Biol Reprod. 1995;52:485–489. doi: 10.1095/biolreprod52.3.485. [DOI] [PubMed] [Google Scholar]
- 53.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnett AL, Musicki B, Jin L, Bivalacqua TJ. Nitric oxide/redox-based signalling as a therapeutic target for penile disorders. Expert Opin Ther Targets. 2006;10:445–457. doi: 10.1517/14728222.10.3.445. [DOI] [PubMed] [Google Scholar]
- 55.Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med. 2010;2:19ra13. doi: 10.1126/scitranslmed.3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azad N, Vallyathan V, Wang L, Tantishaiyakul V, Stehlik C, Leonard SS, Rojanasakul Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J Biol Chem. 2006;281:34124–34134. doi: 10.1074/jbc.M602551200. [DOI] [PubMed] [Google Scholar]