Abstract
[18F]fallypride was synthesized in a batch microfluidic chip with a radiochemical yield of 65±6% (n=7) and an average specific activity of 730 GBq/μmol (20 Ci/μmol) (n=4). Specific activity was ~2-fold higher than [18F]fallypride synthesized on a macroscale radiosynthesizer, despite starting with significantly less radioactivity, and thus safer conditions, in the microchip.
Microfluidic radiosynthesizers are emerging as useful platforms for PET probe synthesis due to the rapid reaction kinetics and exquisite reaction selectivity, which are highly desirable for PET radiochemistry.1a, 1b Batch microfluidic platforms, in contrast to flow-through platforms, provide additional advantages such as the ability to work at extremely low volumes (nL - μL), thus having the potential to increase the fluorination kinetics by achieving higher concentration of [18F]fluoride ion in a batch reaction. Similarly, although yet to be investigated, the reduced reagent consumption in batch microdevices has the potential to reduce cost, to simplify the downstream purification process, and to produce PET probes with higher specific activity (ratio of the amount of compound labelled with the radioisotope to the amount labelled with the stable isotope). Our group is developing an all-electronically controlled microfluidic device, known as an electrowetting-on-dielectric (EWOD)2a, 2b microdevice (Fig. S-1), for on-demand production of PET probes at individual research sites.
High specific activity radiotracer is needed for imaging low abundance of receptors in vivo, specifically in imaging the central nervous system (CNS)3, and in imaging probes that are extremely toxic.4 For regulatory reasons and for accurate kinetic modelling, the tracer concentration needs to be low to avoid any pharmacological effects.5 Based on several literature reports on specific activity, the major contamination of fluorine-19 is found in various sources ranging from the [18O]H2O, target material, tubing, reagents, solvents, and the anion exchange resins prior to the fluorination step.6a, 6b, 6c The specific radioactivity of an 18F-labelled compound is defined by:
| Eq 1 |
where A is the amount of radioactivity, t is the time elapsed since the amount of radioactivity was measured, T1/2 is the half-life of the isotope and m is the mass.7 Since typically the amount of fluorine-19 carrier far exceeds the amount of fluorine-18, the specific activity of the tracer is directly proportional to the amount of starting radioactivity, A. Herein, we demonstrate a high yielding and reliable microdroplet radiosynthesis of [18F]fallypride with higher specific activity than macroscale radiosynthesizers, despite starting with significantly less radioactivity.
[18F]Fallypride is currently used to interrogate neurological and psychiatric disorders associated with the dopaminergic system such as Parkinson's, Alzheimer's, schizophrenia and Huntington diseases.8a, 8b Due to the high specificity of [18F]fallypride as a PET radioligand for diagnosis and the development of therapy for these complex diseases, there have been a number of reports of the synthesis of [18F]fallypride in conventional systems since the first report by Mukherjee et al.9a, 9b, 9c, and also in microreactor-based platforms6c, 10. However, these platforms are all large and require extensive infrastructure and radiation shielding such as a dedicated radiochemistry facility, therefore hindering more widespread access to this tracer. This bottleneck could be alleviated with microscale technology that is sufficiently compact that production of high specific activity PET tracers on the benchtop is possible. Thus, there remains a need for a self-shielded, benchtop platform that is easy to use and is capable of producing diverse radioligands with high specific activity.
The EWOD microfluidic platform manipulates liquid droplets electronically, thus eliminating the need for bulky mechanical hardware such as syringe pumps or actuators that makes other systems so large. It is anticipated that the EWOD microdevice can readily be housed in a self- shielded unit and used in a standard laboratory.11
Based on our previous experience2, we have learned that macroscale reaction conditions are often not optimal or suitable for microscale radiosynthesis. To obtain high and reliable yield, we examined various synthesis parameters on a Teflon-glass substrate (a low-cost, electrode-less approximation of the EWOD microfluidic chip configuration). In this report, we chose a binary solvent system consisting of MeCN (bp: 82 °C) and a higher boiling point solvent, thexyl alcohol (bp: 120 °C), based on the notable report by Kim et al.12 The authors hypothesized the formation of a “flexible” fluoride complex, in which the thexyl alcohol serves as the Lewis base to the fluoride ion, and thus increases the nucleophilicity of the fluoride ion. This solvent mixture was chosen not only to assist in the nucleophilic fluorination of the tosyl-Fallypride precursor, but the higher boiling point alcohol also increases the droplet lifetime on-chip and maintains complete solvation of the reaction mixture throughout the synthesis. Through a systematic optimization study, we found that one of the most critical factors in achieving high and reliable yield is the ratio of precursor to base and the overall concentration of the reaction droplet. A 5:1 ratio of precursor to base was found to be optimal, yielding 73±7% (n=6) overall crude radiochemical yield (defined in the Supplementary Information) of 70% (Fig. 1[a]). Decreasing the ratio of precursor to 3:1 resulted in a drastic reduction in the crude radiochemical yield (51±4% (n=2)). The effect of the thexyl alcohol in the radiosynthesis was also investigated. In this study, an optimal composition of DMSO and MeCN was determined empirically such that a similar droplet size to the thexyl alcohol/MeCN system throughout the fluorination reaction was obtained, despite the differences in boiling points. In the absence of the protic alcohol as the co-solvent, the crude radiochemical yield dropped to 44±15% (n=5) (Fig. 1[a]*).
Fig. 1.
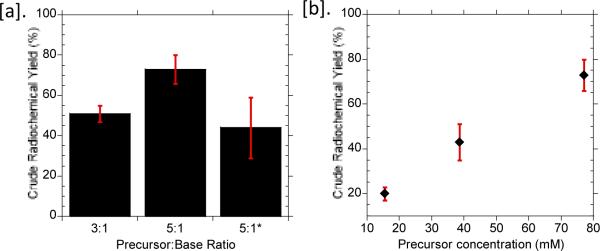
The effect of [a] precursor to base ratio and [b] reagent concentration on the crude radiochemical yield. *The second 5:1 measurement used MeCN and DMSO as the solvent, rather than MeCN and thexyl alcohol used for the other columns. The averages and standard deviations were obtained from at least three experiments.
In our preliminary investigation, we attempted to directly adapt the macroscale precursor concentration (15 mM), resulting in a low yield as shown in Fig. 1[b]. To improve the crude radiochemical yield, we investigated different reagent concentrations on the Teflon-glass substrate as an optimization platform. We found that the crude radiochemical yield increased as the reagent concentration was increased 2-fold and 5-fold from the macroscale reagent concentration (Fig. 1[b]).
In this optimization study, we have also investigated the effect of the number (1 to 3) of azeotropic distillation cycles. We found that one cycle of azeotropic distillation is sufficient to obtain an activated [18F]fluoride complex for the subsequent fluorination step in high fluorination efficiency, thereby reducing the overall synthesis time. In our attempt to further reduce the overall synthesis time, we investigated the effect of reaction volume and reaction kinetics on the fluorination efficiency of [18F]fallypride. At shorter reaction time (5 min), the fluorination efficiency was only 54±18% (n=3) (Fig. 2). We hypothesized that a smaller reaction volume would increase the overall reagent concentration as the solvent evaporates through the open sides of the EWOD chip during the reaction, thus increasing the reaction kinetics and yield. However, we found that the fluorination efficiencies at shorter reaction time and smaller droplet volume (i.e.: 2 μL) were low and unreliable in comparison to the optimized reaction condition using 4 μL droplet and 7 minutes of reaction time (Fig. 2). In the 2 μL droplet reaction, the majority of the solvent evaporated prior to the completion of the reaction, which explained the low and unreliable yield. We observed similar results in the synthesis of [18F]FDG when the low boiling MeCN was used as a solvent.2a
Fig. 2.
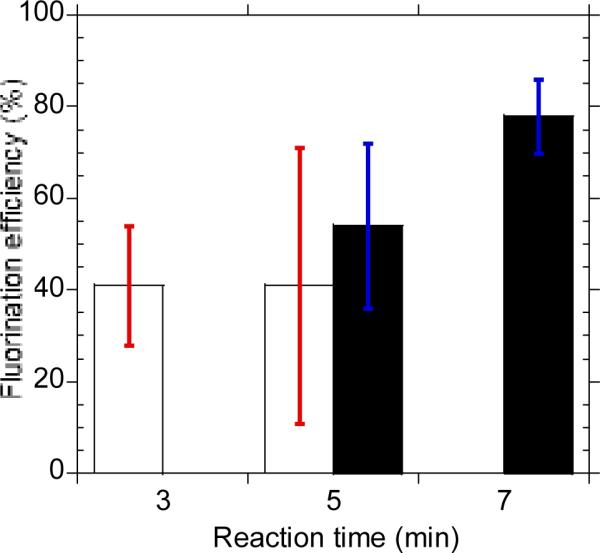
The effect of droplet size and reaction time to the fluorination efficiency. Filled bars: 4 μL reaction volume with blue error bar; unfilled bars: 2 μL reaction volume with red error bar. The fluorination efficiency and standard deviations were obtained from at least three experiments.
The optimized reaction conditions found on the Teflon-glass substrate were then implemented in the EWOD chip to achieve [18F]fallypride with 88±6% (n=9) fluorination efficiency. The crude product was extracted using 16 μL methanol with 93±5% extraction efficiency (radioactivity collected in an Eppendorf tube divided by total radioactivity on-chip after the radiosynthesis). The crude [18F]fallypride was purified using an analytical HPLC and dried to obtain an overall radiochemical yield of 65±6% (n=7) in ~60 min. Synthesis time is expected to decrease as increase automation (e.g. of loading of reagents) is made possible by engineering developments currently underway. While we have not yet shown an improvement in synthesis time compared to the macroscale, the EWOD microfluidic chip platform provides substantial advantages such as the compactness of the device, reduced reagent consumption and the ability to synthesize PET probes with high specific activity.
Though the synthesis of [18F]fallypride has been reported by Pike et al.6c in a system based on a capillary microreactor (Advion NanoTek), the system contains many bulky fluidic components and is similarly sized to macroscale radiosynthesis systems, and thus would require operation in a hot cell. Furthermore, compared to the EWOD chip, a much higher temperature was needed for the fluorination reaction in the capillary reactor. This suggests a significant enhancement of the reaction in the EWOD chip, perhaps due to the use of a co-solvent, and possibly due to factors related to the microscale geometry of the EWOD chip.
Due to low molecular abundance of many receptors in the central nervous system (CNS), a high specific activity of tracer (i.e.: [18F]fallypride) is needed to obtain good image contrast while avoiding saturation of binding sites. This problem is particularly pronounced in preclinical research using small animals such as mice.13a, 13b To obtain sufficient specific activity for imaging low abundance of receptors, radiochemists will typically start the synthesis with high level of radioactivity to compensate for the constant amount of fluorine-19 contamination from various sources. For example, conventional macroscale radiosynthesizers start with 5.4-19 GBq of radioactivity to obtain [18F]fallypride with specific activity ranging from 37-370 GBq/μmole for small animal imaging.9a, 9b, 9c
Using significantly less radioactivity on-chip, we were able to synthesize [18F]fallypride with even higher specific activity. In order to control for the intrinsic variations in the specific activity of the [18F]fluoride ion source, we performed side-by-side radiolabeling studies on the EWOD chip and on a macroscale automated synthesizer starting with portions of the same batch of [18F]fluoride. The reaction conditions were kept exactly the same on both synthesizers except for the reaction volume and the starting radioactivity (see Supplementary Information). The reaction volume was 140× smaller in the chip, and the starting radioactivity was 25× lower. Despite starting with only ~290 MBq (~8 mCi) instead of ~7400 MBq (~200 mCi) of radioactivity, we found that the specific activity of [18F]fallypride synthesized on the EWOD chip (~730 GBq/μmol (19 Ci/μmole) was ~2-fold higher than the macroscale synthesizer (~330 GBq/μmol (~9 Ci/μmol)). In comparison, in the NanoTek capillary reactor6c the specific activity of [18F]fallypride obtained, under the most optimal condition, was still 4× lower (185 GBq/μmole or 5 Ci/μmole) despite starting with higher radioactivity (555 MBq; 15 mCi) than the EWOD chip. These results are counter-intuitive because lower radioactivity synthesis on EWOD is expected to produce lower specific activity as defined in Eq 1. This discovery could provide researchers with the flexibility of producing multiple small doses of PET radioligands of interest using the EWOD microfluidic radiosynthesizer starting with low doses of radioactivity, without jeopardizing the image quality. Due to the compact size of the EWOD radiosynthesizer and the significantly lower dose requirement for producing high specific activity of PET radioligands, radiochemists can now safely work behind several lead bricks and/or an L-block instead of requiring a bulky and expensive hot cell. With increased automation of the EWOD radiosynthesizer14, this technological platform could be highly applicable in small research laboratories and academic laboratories, where the majority of the research relies on small animal imaging.
The [18F]fallypride product from each synthesizer was subsequently used for micro-PET imaging of multiple mice. Dynamic microPET imaging was performed for 60 min (details in Supplementary Information). More comprehensive imaging studies will be performed to determine the effect of specific activity on imaging the low abundance of D2 receptors in the striatum of mice.
Conclusions
We have successfully developed a high-yielding and reliable microdroplet radiosynthesis of [18F]fallypride on an EWOD microdevice with sufficient radioactivity and a high specific activity for imaging the striatum in the brain of mice. The observation of high specific activity despite the low starting radioactivity (which is usually associated with a low specific activity), suggests that this platform may be useful for producing high specific activity compounds without having to start with high amounts of radioactivity. More in-depth studies on the effect of microscale radiochemistry to the specific activity of PET probes are currently in progress. As the engineering and automation of the EWOD radiosynthesizer matures, researchers could safely produce diverse PET radioligands with high specific activity in a standard laboratory setting for clinical application.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
References
- 1.a Watts P, Pascali G, Salvadori PA. Nucl. Med. Biol. 2013 doi: 10.1016/j.nucmedbio.2013.04.004. In press. [DOI] [PubMed] [Google Scholar]; b Elizarov AM. Lab. Chip. 2009;9:1326–1333. doi: 10.1039/b820299k. [DOI] [PubMed] [Google Scholar]
- 2.a Keng PY, Chen S, Sadeghi S, Shah GJ, Dooraghi A, Phelps M, Satyamurthy N, Chatziioannou AF, Kim CJ, van RM. Dam, Proc. Natl. Acad. Sci. U.S.A. 2012;109:690–695. doi: 10.1073/pnas.1117566109. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee J, Moon H, Fowler J, Schoellhammer T, Kim C-J. Sensors Actuators Phys. 2002;95:259–268. [Google Scholar]
- 3.Henriksen G, Drzezga A. In: Small Animal Imaging. Kiessling F, Pichler BJ, editors. Springer; Berlin Heidelberg, Berlin, Heidelberg: 2011. pp. 499–513. [Google Scholar]
- 4.Ding Y-S, Molina PE, Fowler JS, Logan K, Volkow ND, Kuhar MJ, Carroll FI. Nucl. Med. Biol. 1999;26:139–148. doi: 10.1016/s0969-8051(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 5.Cai L, Lu S, Pike VW. Eur. J. Org. Chem. 2008;2008:2843–2843. [Google Scholar]
- 6.a Solin O, Bergman J, Haaparanta M, Reissell A. International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes. 1988;39:1065–1071. [Google Scholar]; b Berridge MS, Apana SM, Hersh JM. J Labelled Compd. Radiopharm. 2009;52:543–548. [Google Scholar]; c Lu SY, Giamis AM, Pike VW. Curr Radiopharm. 2009;21:1–13. doi: 10.2174/1874471010902010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman J, Eskola O, Lehikoinen P, Solin O. Appl. Radiat. Isot. 2001;54:927–933. doi: 10.1016/s0969-8043(00)00358-4. [DOI] [PubMed] [Google Scholar]
- 8.a Elsinga PH, Hatano K, Ishiwata K. Curr. Med. Chem. 2006;13:2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]; b Siessmeier T, Zhou Y, Buchholz H-G, Landvogt C, Vernaleken I, Piel M, Schirrmacher R, Rosch F, Schreckenberger M, Wong DF, Cumming P, Grunder G, Bartenstein P. J. Nucl. Med. 2005;46:964–972. [PubMed] [Google Scholar]
- 9.a Mukherjee J, Yang Z-Y, Das MK, Brown T. Nuc. Med. Biol. 1994;22:283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]; b Gao M, Wang M, Mock BH, Glick-Wilson BE, Yoder KK, Hutchins GD, Zheng Q-H. Appl. Radiat. Isot. 2010;68:1079–1086. doi: 10.1016/j.apradiso.2009.09.071. [DOI] [PubMed] [Google Scholar]; c Seok Moon B, Hyung Park J, Jin Lee H, Sun Kim J, Sup Kil H, Se Lee B, Yoon Chi D, Chul Lee B, Kyeong Kim Y, Eun Kim S. Appl. Radiat. Isot. 2010;68:2279–2284. doi: 10.1016/j.apradiso.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Lebedev A, Miraghaie R, Kotta K, Ball CE, Zhang J, Buschmann MS, Kolb H, Elizarov A. Lab. Chip. 2012;13:136. doi: 10.1039/c2lc40853h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keng PY, Esterby M, Van Dam M. In: Positron Emission Tomography-Current Clinical and Research Aspects. 1st edn. Hsieh C-H, editor. InTech; New York, USA: 2012. pp. 163–192. [Google Scholar]
- 12.Kim DW, Ahn D-S, Oh Y-H, Lee S, Kil HS, Oh SJ, Lee SJ, Kim JS, Ryu JS, Moon DH, Chi DY. J. Am. Chem. Soc. 2006;128:16394–16397. doi: 10.1021/ja0646895. [DOI] [PubMed] [Google Scholar]
- 13.a Hume SP, Gunn RN, Jones T. Eur. J. Nucl. Med. Mol. Imaging. 1998;25:173–176. doi: 10.1007/s002590050211. [DOI] [PubMed] [Google Scholar]; b Kung M-P, Kung HF. Nucl. Med. Biol. 2005;32:673–678. doi: 10.1016/j.nucmedbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Li Y, Lozada J, Schaffer P, Adam MJ, Ruth TJ, Perrin DM. Angew. Chem. Int. Ed. 2013;52:2303–2307. doi: 10.1002/anie.201208551. [DOI] [PubMed] [Google Scholar]
- 15.Shah GJ, Ding H, Sadeghi S, Chen S, ‘CJ’ Kim C-J, van RM. Dam, Lab. Chip. 2013;13:2785–2795. doi: 10.1039/c3lc41363b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


