Abstract
OBJECTIVE
Critically ill patients with pulmonary hypertension (PH) pose additional challenges due to the existence of right ventricular (RV) dysfunction. The purpose of this study was to assess the impact of hemodynamic factors on the outcome.
METHODS
We reviewed the records of patients with a diagnosis of PH admitted to the intensive care unit. In addition to evaluating traditional hemodynamic parameters, we defined severe PH as right atrial pressure >20 mmHg, mean pulmonary artery pressure >55 mmHg, or cardiac index (CI) <2 L/min/m2. We also defined the RV functional index (RFI) as pulmonary artery systolic pressure (PASP) adjusted for CI as PASP/CI; increasing values reflect RV dysfunction.
RESULTS
Fifty-three patients (mean age 60 years, 72% women, 79% Blacks), were included in the study. Severe PH was present in 68% of patients who had higher Sequential Organ Failure Assessment (SOFA) score (6.8 ± 3.3 vs 3.8 ± 1.6; P = 0.001) and overall in-hospital mortality (36% vs 6%; P = 0.02) compared to nonsevere patients, although Acute Physiology and Chronic Health Evaluation (APACHE) II scores (19.9 ± 7.5 vs 18.5 ± 6.04; P = 0.52) were similar and sepsis was more frequent among nonsevere PH patients (31 vs 64%; P = 0.02). Severe PH (P = 0.04), lower mean arterial pressure (P = 0.04), and CI (P = 0.01); need for invasive ventilation (P = 0.02) and vasopressors (P = 0.03); and higher SOFA (P = 0.001), APACHE II (P = 0.03), pulmonary vascular resistance index (PVRI) (P = 0.01), and RFI (P = 0.004) were associated with increased mortality. In a multivariate model, SOFA [OR = 1.45, 95% confidence interval (C.I.) = 1.09–1.93; P = 0.01], PVRI (OR = 1.12, 95% C.I. = 1.02–1.24; P = 0.02), and increasing RFI (OR = 1.06, 95% C.I. = 1.01–1.11; P = 0.01) were independently associated with mortality.
CONCLUSION
PH is an independent risk factor for mortality in critically ill patients. Composite factors rather than individual hemodynamic parameters are better predictors of outcome. Monitoring of RV function using composite hemodynamic factors resulting in specific interventions is likely to improve survival and needs to be studied further.
Keywords: pulmonary hypertension, intensive care unit, hemodynamic factors, right ventricular dysfunction, mortality, lung
Introduction
Pulmonary hypertension (PH) may be encountered in the intensive care unit (ICU) as an acute rise of pulmonary artery pressure or as a preexisting condition.1–3 Many of these patients have important right ventricular dysfunction or may be adversely affected hemodynamically in the ICU even by standard therapies such as fluid resuscitation or mechanical ventilation.4,5 Accordingly, the RV functional state may be a critical determinant of outcome.6 There is no well-defined hemodynamic parameter that reliably reflects RV function or predicts survival among critically ill patients with PH.6 Pulmonary hypertension is defined as mean pulmonary artery pressure (PAP) >25 mmHg, but high pulmonary pressures may not always accurately reflect the functional state of the right ventricle (RV).7 In patients with pulmonary arterial hypertension, an increasing pulmonary artery systolic pressure (PASP) is a reflection of the ability of the RV to maintain stroke volume in presence of increasing pulmonary vascular resistance (PVR), albeit at increased workload. However, as the RV function declines, PASP may decrease with decreasing stroke volume. Therefore, compared to a very high PASP with a relatively preserved cardiac index (CI), a PASP that is abnormal but not very high may be worse in terms of prognosis in presence of low CI. This is supported by findings of a recent study on patients with chronic heart failure, which showed that prognosis for patients with high PAP and preserved right ventricular ejection fraction (RVEF) was similar to those with normal PAP. Reduced RVEF did not increase the risk if PAP was normal, but the prognosis was worse if patients had a combination of high PAP and reduced RVEF.8 This study emphasizes the need to assess composite hemodynamic parameters that take into account pulmonary pressures as well as RV function. Dwelling on this concept further, we postulated that PASP adjusted for CI as PASP/CI would produce a linear index in which increasing values reflect RV dysfunction. A high value may be the result of increasing PASP, decreasing CI, or both, indicative of worsening RV function. We hypothesized that in critically ill patients with PH, hemodynamic factors such as right ventricular functional index (RFI), measured as PASP/CI, can predict outcome. We studied the feasibility of using various known hemodynamic parameters as well as RFI to predict the outcome of patients with PH admitted to ICU.
Methods
Study subjects
We identified all patients with a diagnosis of PH admitted to the ICU between January 1, 2006 and December 31, 2008, in a tertiary care teaching hospital. From this group, we selected patients who had PH confirmed by right heart catheterization (RHC) with a mean PAP of >25 mmHg.7 We excluded patients who had terminal cancer or were admitted to the ICU after cardiopulmonary arrest (due to dismal prognosis), for initiation of prostacyclin therapy, or for monitoring purposes only. The study was approved by the human investigation committee (Institutional Review Board) of Wayne State University.
Data definitions and analysis
We reviewed the medical records and collected the following data: demographics, ICU admission diagnosis, presence of sepsis, Sequential Organ Failure Assessment (SOFA), Acute Physiology and Chronic Health Evaluation (APACHE) II score, basic laboratory data on day 1 of ICU admission, and the use of vasopressors and invasive ventilation during ICU stay. Patients were assigned to the World Health Organization (WHO) Group for PH based on the predominant risk factor.9
Hemodynamic parameter evaluation
We included data from the procedure most recent in relation to the ICU admission (median interval 1.6 months). Echocardiography data included right ventricular systolic pressure (RVSP), ejection fraction (EF), and the presence of chamber dilation of right atrium and ventricle. From the RHC data, we collected the following measured variables: PASP and pulmonary artery diastolic pressures (PADP), mean PAP, right atrial pressure (RAP), CI, and pulmonary artery occlusion pressure (PAOP). Pulmonary vascular resistance index (PVRI) was derived as 79.9 × (mean PAP – PAOP)/CI. We defined severe PH as the presence of one of the following: RAP >20 mmHg, CI <2 L/min/m2, or mean PAP >55 mmHg.10 We defined the RV functional index (RFI) as PASP adjusted for CI as PASP/CI as described above. Sepsis, severe sepsis, and septic shock were defined according to the ACCP/SCCM (American College of Chest Physicians/Society of Critical Care Medicine) consensus conference.11
Statistical analysis
We used chi-square tests for categorical variables and Student’s t-test for continuous variables to evaluate the differences between survivors and nonsurvivors. Univariate variables that were significantly associated with mortality were included in the multivariate logistic regression analysis to determine the independent predictors of mortality. We compared the discriminatory power of the different hemodynamic parameters of interest by estimating the area under a receiver operating characteristic (ROC) curve.12 The normality of the data was assessed using Kolmogorov–Smirnov test, which confirmed the normality of the data (D = 0.13239602, Pr > D 0.021). A two-tailed P-value <0.05 was considered significant. All analyses were performed using the SAS software (version 9.1, SAS Institute).
Results
During the study period, out of 4,233 admissions to the ICU, 285(6.7%) admissions for 251 patients had a diagnosis of PH. Only 87 had undergone diagnostic RHC, and of these 6 were excluded because of insufficient data. Of the remaining 81 patients, 2 had mean PAP <25 mmHg, 1 had terminal cancer, 6 were admitted after cardiorespiratory arrest, 18 were admitted for monitoring only or for elective initiation of prostacyclin therapy, and 1 was transferred to another hospital; these patients were excluded. The remaining 53 patients (mean age 60 years, 72% women, 79% Blacks) were included in the study. Based upon predominant risk factors, patients were classified into PH groups (I = 32%, II = 45%, III = 19%, and V = 4%). The baseline characteristics of patients are shown in Table 1. The immediate reasons for admission to ICU were respiratory failure in 43%, hypotension 20%, arrhythmia 11%, severe sepsis 9%, and other causes 15%; 25% were postoperative surgical patients. Sepsis was diagnosed in 42% patients, 51% required invasive mechanical ventilation, and 55% required vasoactive medication. Twenty percent patients were receiving specific therapy for PH prior to admission to the ICU, and only 4% additional patients received PH-specific therapy in the ICU. For the survivors, the average length of stay in the ICU and hospital was 6 ± 9 days and 14 ± 11 days, respectively.
Table 1.
Characteristics of patients and comparison between survivors and nonsurvivors.
| VARIABLE | OVERALL (n = 53) | SURVIVORS (n = 39) | NONSURVIVORS (n = 14) | P-VALUE |
|---|---|---|---|---|
| Age (in years)* | 60 ± 15 | 59 ± 15 | 59 ± 15 | 0.30 |
| Men | 28% | 28% | 29% | 0.99 |
| Ethnicity | 0.49 | |||
| African Americans | 79% | 82% | 71% | |
| Whites | 17% | 13% | 29% | |
| Asians and Hispanics | 4% | 5% | 0% | |
| Hypertension | 70% | 75% | 57% | 0.23 |
| Coronary artery disease | 51% | 44% | 71% | 0.07 |
| Diabetes mellitus | 42% | 41% | 43% | 0.90 |
| Chronic obstructive pulmonary disease | 41% | 42% | 36% | 0.67 |
| Chronic renal failure | 30% | 30% | 21% | 0.74 |
| Obstructive sleep apnea | 15% | 15% | 14% | 0.92 |
| Cirrhosis | 8% | 8% | 7% | 0.94 |
| Interstitial lung disease | 8% | 10% | 0% | 0.21 |
| Cancer | 6% | 5% | 7% | 0.78 |
| Sickle cell disease | 4% | 3% | 7% | 0.44 |
| Sepsis | 42% | 39% | 50% | 0.45 |
| Vasopressor use | 55% | 39% | 100% | <0.001 |
| Invasive ventilation | 51% | 41% | 79% | 0.02 |
| Heart rate, beats/minute* | 100 ± 23 | 97 ± 23 | 109 ± 21 | 0.08 |
| Mean arterial pressure, mmhg* | 84 ± 26 | 89 ± 25 | 72 ± 23 | 0.03 |
| APACHE II* | 19.4 ± 7.0 | 18.2 ± 6.7 | 23 ± 7 | 0.03 |
| Sequential organ failure assessment* | 5.8 ± 3.2 | 4.8 ± 2.6 | 8.5 ± 3.3 | 0.001 |
Note:
Mean ± standard deviation.
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation.
Overall mortality was 26% (14/53); comparison of demographics, comorbidities and severity of illness, and organ failure among survivors and nonsurvivors is summarized in Table 1. There was no difference between survivors and nonsurvivors in the variables measured by echocardiography (Table 2), but nonsurvivors had higher RAP (P = 0.06), mean PAP (P = 0.02), PVRI (P = 0.02), and RFI (P = 0.004) and lower CI (P = 0.001).
Table 2.
Comparison of echocardiographic and hemodynamic variable among survivors and nonsurvivors.
| VARIABLE | OVERALL (n = 53) | SURVIVORS (n = 39) | NONSURVIVORS (n = 14) | P-VALUE |
|---|---|---|---|---|
| Echocardiography | ||||
| Left ventricular ejection fraction | 51.3 ± 16 | 49.2 ± 16 | 43.1 ± 16 | 0.22 |
| Right ventricular systolic pressure (mmHg) | 54.8 ± 30 | 52.1 ± 25 | 63.2 ± 42 | 0.24 |
| Right atrial dilatation† | 53% | 46% | 71% | 0.10 |
| Right ventricular dilatation† | 51% | 44% | 71% | 0.07 |
| Right heart catheterization | ||||
| Right atrial pressure (mmHg)† | 17.3 ± 8.8 | 15.9 ± 8.8 | 21 ± 8.2 | 0.06 |
| Pulmonary artery systolic pressure (mmHg) | 67.1 ± 20.7 | 64.1 ± 21.8 | 75.4 ± 15.1 | 0.08 |
| Pulmonary artery diastolic pressure (mmHg) | 30.4 ± 12.4 | 28.9 ± 12.3 | 34.8 ± 12.2 | 0.15 |
| Mean pulmonary artery pressure (mmHg) | 45.0 ± 14.9 | 42.6 ± 15.4 | 51.6 ± 11.6 | 0.02 |
| Cardiac index (L/min/m2) | 2.1 ± 0.8 | 2.3 ± 0.8 | 1.7 ± 0.5 | 0.01 |
| Pulmonary vascular resistance index (dynes s−1 cm−5 m−2) | 1087.5 ± 870 | 891.4 ± 709 | 1635.8 ± 1057 | 0.01 |
| Pulmonary artery occlusion pressure (mmHg)† | 20.3 ± 9 | 18.7 ± 8 | 22.2 ± 10 | 0.32 |
| Severe pulmonary hypertension | 67% | 23% | 93% | 0.02 |
| Right ventricular functional index | 36.5 ± 18.9 | 31.7 ± 16.7 | 50.0± 19.0 | 0.004 |
| Right ventricular functional index >35 | 22% | 28% | 71% | 0.01 |
Notes:
Variables with missing values – EF, RA, RV dilatation (6); RVSP (8); RAP (2), PCW P (1).
Thirty-six (68%) patients had severe PH and required vasopressors more often (63 vs 35%, P = 0.05), but needed invasive mechanical ventilation (53 vs 47%, P = 0.70) similar to nonsevere PH patients. Compared to patients with nonsevere PH, severe PH patients had higher SOFA score (6.8 ± 3.3 vs 3.8 ± 1.6, P = 0.001) and higher overall in-hospital mortality (36% vs 6%, P = 0.02), although the APACHE II scores (19.9 ± 7.5 vs 18.5 ± 6.04, P = 0.52) were similar. Incidentally, sepsis was more common among nonsevere PH patients (31 vs 64%, P = 0.02).
In univariate analysis (Table 3), the following variables were associated with mortality: severe PH (P = 0.04), lower mean arterial pressure (P = 0.04) and CI (P = 0.01), need for invasive ventilation (P = 0.02) and vasopressors (P = 0.03), higher SOFA (P = 0.001), APACHE II (P = 0.03), PVRI (P = 0.01), as well as RFI (P = 0.004) (Table 3). There was no association of age, sex, race, comorbidity, reason for admission, PH group, PH-specific therapy, presence of sepsis, left ventricular EF, PAOP, or transpulmonary gradient with in-hospital mortality.
Table 3.
Univariate predictors of mortality.
| VARIABLE | ODDS RATIO | 95% C.I. | P-VALUE |
|---|---|---|---|
| Sepsis | 1.60 | 0.47–5.5 | 0.45 |
| Invasive ventilation | 5.27 | 1.27–21.96 | 0.02 |
| Vasopressor use | ** | ** | 0.03 |
| Sodium | 1.10 | 0.99–1.21 | 0.06 |
| Blood urea nitrogen | 1.02 | 0.99–1.05 | 0.06 |
| Sequential organ failure assessment | 1.51 | 1.17–1.96 | 0.001 |
| APACHE II | 1.11 | 1.01–1.23 | 0.03 |
| Mean arterial pressure | 1.03 | 1.002–1.062 | 0.04 |
| Pulmonary artery systolic pressure | 1.03 | 0.99–1.07 | 0.06 |
| Pulmonary artery diastolic pressure | 1.04 | 0.99–1.09 | 0.15 |
| Mean pulmonary artery pressure | 1.04 | 0.99–1.09 | 0.06 |
| Transpulmonary gradient | 1.03 | 0.99–1.08 | 0.11 |
| Cardiac index | 5.55 | 1.43–21.48 | 0.01 |
| Pulmonary vascular resistance index | 1.001 | 1.00–1.002 | 0.01 |
| Severe pulmonary hypertension | 9.04 | 1.07–76.22 | 0.04 |
| Right ventricular function index | 1.06 | 1.02–1.10 | 0.004 |
Note:
As all nonsurvivors had vasopressor use, so odds ratio could not be calculated.
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation.
In the multivariate regression analysis adjusted for all significant univariate predictors, the association with all-cause mortality remained significant for PVRI (P = 0.02) and RFI (P = 0.01). SOFA on day 1 of ICU was independently associated with all-cause mortality in the models (Table 4). Further analysis stratifying RFI suggested that an RFI value of more than 35 was strongly associated with all-cause mortality (OR = 5.62; 95% C.I.: 1.47–21.6, P = 0.01) with sensitivity and specificity of 79% and 70%, respectively. We compared the independent C-statistics of the individual hemodynamic parameters (RFI and PVRI) using ROC curves to evaluate the strength of their association with all-cause mortality without any additional covariates (Fig. 1). The AUC for RFI was 0.77 (95% C.I. = 0.72–0.82) and that for PVRI was 0.70 (95% C.I. = 0.64–0.76, P = 0.03). The P-value for the difference in these two AUC values was 0.03.
Table 4.
Multivariate logistic regression analysis for all-cause mortality*.
| PARAMETER | ODDS RATIO | 95% C.I. | P-VALUE |
|---|---|---|---|
| Model A | |||
| Right ventricular function index | 1.06 | 1.01–1.11 | 0.01 |
| Sequential organ failure assessment (SOFA) | 1.45 | 1.09–1.93 | 0.01 |
| Model B | |||
| Pulmonary vascular resistance index | 1.12 | 1.02–1.24 | 0.02 |
| SOFA | 1.51 | 1.12–2.03 | 0.01 |
| Model C | |||
| Cardiac index | 0.33 | 0.06–1.72 | 0.19 |
| SOFA | 1.51 | 1.17–1.96 | 0.001 |
Note:
Every model is adjusted for univariate predictors (P < 0.05) including mean arterial pressure, ventilator use, and APACHE II.
Figure 1.
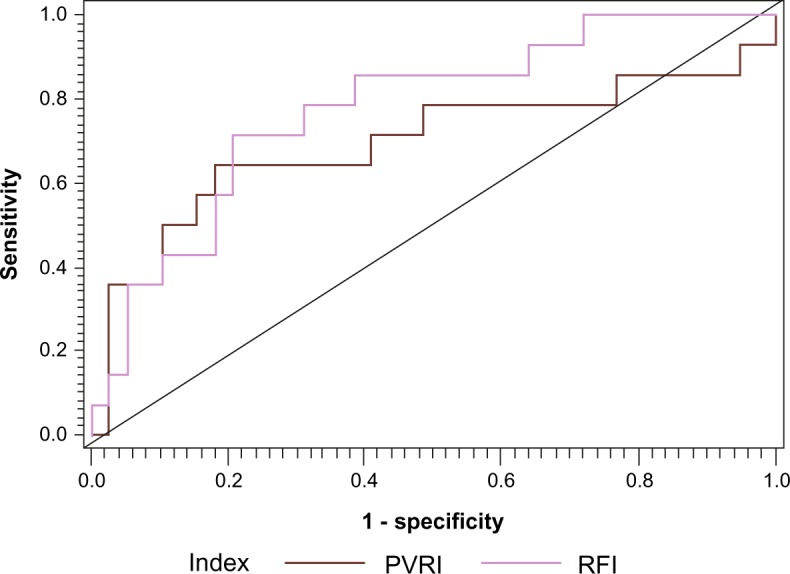
Comparison of the receiver operative characteristic curves of right ventricular functional index and pulmonary vascular resistance index for prediction of risk of mortality.
Abbreviations: PVRI, Pulmonary vascular resistance index; RFI, Right ventricular functional index.
Discussion
Among patients with preexisting PH admitted to ICU, severe PH was associated with worse organ failure and high overall mortality as compared with patients with nonsevere PH. Severe organ failure, high PVRI, and increasing RFI were independent risk factors for in-hospital mortality. This study indicates that PH and RV function plays a critical role in the outcome of critically ill PH patients, thus lending credence to earlier assumptions that therapeutic intervention to improve right ventricular function in critically ill patients with PH may improve outcome and merits further investigation.6 Our findings suggest that a composite hemodynamic measure combining PASP and CI is a better predictor of mortality than either parameter alone. Therefore, invasive hemodynamic monitoring in ICU may be beneficial in a subset of critically ill patients with elevated pulmonary pressures. Alternatively, derivation and application of similar composite indices obtained through noninvasive means and institution of therapies aimed to improve these indices could improve outcome in such patients.
The increased mortality in patients with severe PH is due to the presence of right ventricular dysfunction; it could not be attributed to the underlying disease, severity of illness, sepsis, or presence of comorbid conditions, as there was no difference in the frequency of comorbid conditions or mean APACHE II scores between severe and nonsevere PH patients. Given that the steady-state output from the RV must equal that of the LV, as well as the small volume of blood in the pulmonary veins, RV is critical for overall hemodynamic function.5,13 Further increase in PVR due to hypoxemia or increased intrathoracic pressure during positive pressure ventilation increases the RV workload. Increase in the RV end diastolic volume due to failing RV or due to fluid resuscitation may decrease left ventricular (LV) stroke volume due to bulging of the septum as well as to reduced RV stroke volume.14 Low tissue perfusion secondary to reduced LV stroke volume and hypotension is a precursor of multiple organ failure and poor outcome, as seen in our patients.
Our study shows that increasing RFI indicating RV dysfunction is independently associated with in-hospital mortality. There are no well-defined guidelines or definitions for monitoring or assessment of RV function.6 Previous attempts to define RV failure have focused on the relation between central venous pressure and PAOP.15,16 These definitions are based on an all-or-none phenomenon or cut-off points rather than a range, exclude patients who may have LV dysfunction, and are dependent on derivation of PAOP, which is technically difficult and unreliable in many patients with severe PH.17,18 In our study, individual hemodynamic parameters such as high mean PAP, RAP, and CI were unfavorable among nonsurvivors but were not independently associated with outcome in the multivariate analysis. Recent data have questioned the value of individual hemodynamic factors such as mean PAP, as this may be misleading due to the inability of a dysfunctional RV to generate high pressures.6,7 Based on the pathophysiology of PH and RV dysfunction, a composite hemodynamic factor that reflects worsening obstruction to RV as well as RV function is likely to predict the outcome better than a single hemodynamic factor.8
Our study demonstrated a strong association between RFI and mortality, with a fivefold increased mortality in patients with RFI values above 35. Our data corroborate the findings of study by Ghoi et al, which emphasized the necessity of combining the right heart hemodynamic variables with a functional evaluation of the RV to ascertain the risk among patients with chronic heart failure.8 The study was aimed at evaluating the effect of stratification of patients based on a combination of PAP and RV function on prognosis. In this study, the patients with chronic heart failure were divided into four groups: patients with normal PAP + preserved RVEF; normal PAP + low RVEF; high PAP + preserved RVEF; and high PAP + low RVEF. Patients with high PAP + low RVEF had the worst prognosis compared to other groups, whereas there was no significant difference in prognosis between the other groups. Patients with normal PAP and low RVEF or normal RVEF and high PAP had similar prognosis. This study shows that elevated PAP may not always be associated with RV dysfunction. Instead of grouping patients, we developed a linear index that would take into account both PAP and RV function simultaneously, which was highest for those with high PAP and reduced RV function followed by rest of the patients with different combinations of PAP and RV function. Our results show that increased RFI is associated with increased risk of mortality
Our definition does not exclude patients with concomitant LV dysfunction; the presence of PH in patients with LV dysfunction is associated with increased morbidity and mortality.19 The derivation of this type of index is not unique and has been used successfully. The ratio of partial pressure of oxygen to fraction of inspired oxygen and the rapid shallow breathing index are widely used successfully in day-to-day clinical practice.20,21 A trend of RFI in acute as well as chronic conditions can be a valuable tool to assess prognosis as well as response to therapy.
The RV functional index is an invasive measurement requiring the presence of right heart catheterization. Over the years, the use of pulmonary artery catheters (PACs) in ICUs has declined as a result of studies showing no benefit from the use of such monitoring. It has been argued that these studies used a heterogeneous group of patients, and the lack of benefit may be due to poorly defined or selected physiological targets, incorrect use, or inappropriate patient selection.22 There is evidence to suggest that in selected patient population the use of PAC may improve outcomes.23 PH has been identified as an independent risk factor for death in other patient populations such as those with acute respiratory distress syndrome. Incidentally, the hemodynamic factor identified to correlate with mortality in this population was PVR, a composite hemodynamic factor.24 Similar to that study, our study also shows that both PVRI and RFI correlate with mortality, RFI having the advantage of excluding the need to collect PAOP data, which may not be reliable in patients with sever PH.17,18 Based on our data and on the observations by others, the use of hemodynamic monitoring needs to be re-explored in specific groups of critically ill population such as those with pulmonary hypertension, congestive heart failure, and ARDS.23–25
Our study has several limitations that affect generalizibility of the findings. These include the retrospective nature of the study and predominance of African Americans and women in the cohort. Nevertheless, sex and ethnicity did not correlate with outcome in our study. In addition, the majority of the patients in our cohort had PH secondary to LV dysfunction or lung disease (65%). Since hypertension, coronary artery disease, and COPD are more common in community compared to other conditions causing WHO group I PH, our patient group reflects the real-world scenario of PH population admitted to ICU26,27
Conclusion
PH is an independent predictor of mortality in critically ill patients. Composite hemodynamic parameters such as RFI and PVRI, which take into account pulmonary artery pressure as well as right ventricular function, are independent predictors of mortality. Hemodynamic monitoring and attention to RV function during resuscitation and ventilation as well as specific therapies aimed at improvement of right ventricular function may improve the outcome of patients with PH and need to be studied further.
Footnotes
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: GS discloses grants from Actelion, grants and personal fees from Gilead, and personal fees from Bayer, outside the submitted work. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: GS, AA, PM, PK, SB. Analyzed the data: GS, PM, SB. Wrote the first draft of the manuscript: GS, PM. Contributed to the writing of the manuscript: GS, AA, PM, PK, SB. Agree with manuscript results and conclusions: GS, AA, PM, PK, SB. Jointly developed the structure and arguments for the paper: GS, PM, SB. Made critical revisions and approved final version: GS, AA, PM, PK, SB. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Rubenfire M, Bayram M, Hector-Word Z. Pulmonary hypertension in the critical care setting: classification, pathophysiology, diagnosis, and management. Crit Care Clin. 2007;23:801–34. doi: 10.1016/j.ccc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Zamanian R, Haddad F, Doyle R, Weinacker A. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med. 2007;35:2037–50. doi: 10.1097/01.ccm.0000280433.74246.9e. [DOI] [PubMed] [Google Scholar]
- 3.Tsapenko MV, Tsapenko AV, Comfere TB, Mour GK, Mankad SV, Gajic O. Arterial pulmonary hypertension in non-cardiac intensive care unit. Vasc Health Risk Manag. 2008;4:1043–60. doi: 10.2147/vhrm.s3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston WE, Lin CY, Feerick AE, Spray B, Vinten-Johansen J. Volume expansion increases right ventricular infarct size in dogs by reducing collateral perfusion. Chest. 1996;109:494–503. doi: 10.1378/chest.109.2.494. [DOI] [PubMed] [Google Scholar]
- 5.Fessler HE. Heart-lung interactions: applications in the critically ill. Eur Respir J. 1997;10:226–37. doi: 10.1183/09031936.97.10010226. [DOI] [PubMed] [Google Scholar]
- 6.Hill NS, Klinger JR. Pulmonary hypertension in the intensive care unit: critical role of the right ventricle. Crit Care Med. 2007;35:2210–1. doi: 10.1097/01.CCM.0000281649.02536.61. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D340–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 10.D’Alonzo GE, Barst RJ, Ayres SM. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: the ACCP/SCCM Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 13.Magder S. More respect for the CVP. Intensive Care Med. 1998;24:651–3. doi: 10.1007/s001340050640. [DOI] [PubMed] [Google Scholar]
- 14.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–4. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 15.Osman D, Monnet X, Castelain V, et al. French Pulmonary Artery Catheter Study Group. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35:69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 16.Kopman EA, Ferguson TB. Interaction of right and left ventricular filling pressures at the termination of cardiopulmonary bypass: central venous pressure/pulmonary capillary wedge pressure. J Thorac Cardiovasc Surg. 1985;89:706–8. [PubMed] [Google Scholar]
- 17.Guillinta P, Peterson K, Ben-Yehuda O. Cardiac catheterization techniques in pulmonary hypertension. Cardiol Clin. 2004;22:401–15. doi: 10.1016/j.ccl.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli AR, Mubarak KK, Li N, Carrie R, Alnuaimat H. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest. 2011;139:115–21. doi: 10.1378/chest.10-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudiz R. Pulmonary hypertension associated with left sided heart disease. Clin Chest Med. 2007;28:233–41. doi: 10.1016/j.ccm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y-CT. Arterial blood gases. In: Hess DR, Macityre NR, Mishoe SC, Galvin WF, editors. Respiratory Care: Principles and Practice. Philadelphia, PA: W.B. Saunders Company; 2002. pp. 362–96. [Google Scholar]
- 21.Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130:1664–71. doi: 10.1378/chest.130.6.1664. [DOI] [PubMed] [Google Scholar]
- 22.Hall JB. Searching for evidence to support pulmonary artery catheter use in critically ill patients. JAMA. 2005;294:1693–4. doi: 10.1001/jama.294.13.1693. [DOI] [PubMed] [Google Scholar]
- 23.Sotomi Y, Sato N, Kajimoto K, et al. Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry. Impact of pulmonary artery catheter on outcome in patients with acute heart failure syndromes with hypotension or receiving inotropes: from the ATTEND Registry. Int J Cardiol. 2014;172:165–72. doi: 10.1016/j.ijcard.2013.12.174. [DOI] [PubMed] [Google Scholar]
- 24.Bull TM, Clark B, McFann K, Moss M. National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182:1123–8. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajaram SS, Desai NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;2:CD003408. doi: 10.1002/14651858.CD003408.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Jones D, Adams R, Brown TM, et al. Writing Group Members; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2010 update. A report from the American Heart Association. Circulation. 2010;121:e1–70. [Google Scholar]
- 27.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. Respir Care. 2002;47:1184–99. [PubMed] [Google Scholar]


