Abstract
Purpose
More than 50,000 Americans were diagnosed with kidney and renal pelvis cancer in 2010. The National Program of Cancer Registries and SEER (Surveillance, Epidemiology and End Results) combined data include cancer incidences from the entire United States. Our study presents updated incidence data, evaluates trends and adds geographic distribution to the literature.
Materials and Methods
We examined invasive, microscopically confirmed kidney and renal pelvis cancers diagnosed from 2001 to 2010 that met United States Cancer Statistics reporting criteria for each year, excluding cases diagnosed by autopsy or death certificate. Histology codes classified cases as renal cell carcinoma. Rates and trends were estimated using SEER*Stat.
Results
A total of 342,501 renal cell carcinoma cases were diagnosed. The renal cell carcinoma incidence rate increased from 10.6/100,000 individuals in 2001 to 12.4/100,000 in 2010 and increased with age until ages 70 to 74 years. The incidence rate in men was almost double that in women. The annual percent change was higher in women than in men, in those 20 to 24 years old and in grade III tumors.
Conclusions
The annual percent change incidence increased from 2001 to 2010. Asian/Pacific Islanders and 20 to 24-year-old individuals had the highest annual percent change. While some increase resulted from localized disease, the highest annual percent change was in grade III tumors, indicating more aggressive disease. Continued monitoring of trends and epidemiological study are warranted to determine risk factors.
Keywords: kidney; carcinoma, renal cell; young adult; SEER program; epidemiology
More than 50,000 individuals in the U.S. were diagnosed with cancer of the kidney and renal pelvis in 2010.1 RCC, an adenocarcinoma of the renal parenchyma, accounts for more than 80% of kidney cancer in adults.2 Associated risk factors for RCC are cigarette smoking,3 obesity4 and hypertension.5 RPC arises from transitional cells of the urinary collecting system and accounts for less than 20% of cases.6 The primary risk factors for RPC include cigarette smoking7 and the use of phenacetin, an analgesic that was taken off the market in the early 1980s due to this association.8 Previous studies showed that the RCC incidence has been increasing steadily in the last 2 decades2,9 but the RPC incidence appears to be decreasing.9
Cancer incidence data combined from NPCR and SEER cover the entire U.S. population.10 This expanded coverage captures more kidney cancer cases than SEER alone, allowing for more detailed examination of cancer rates and trends by age group, race, ethnicity and geographic region than previous studies.2,9 We present updated incidence data on RCC and characterize trends in RCC incidence by key demographic and clinical factors. We were particularly interested in determining whether the previous increase in the RCC incidence continued and if so whether the increase in the incidence differed by demographic and clinical factors, and geographic location.
METHODS
We used USCS data from population based registries participating in the CDC (Centers for Disease Control and Prevention) NPCR and National Cancer Institute SEER program. These data are collected and reported using standardized collection methods and are meant to include all cancers diagnosed in the U.S. Cancer primary site and histology were coded using the ICD-O-3.11
We examined all invasive, microscopically confirmed cases of cancer of the kidney and renal pelvis (ICD-O-3 primary site codes C64.9) diagnosed from 2001 to 2010 with known patient age which met USCS reporting criteria for each year from 2001 to 2010.12 A total of 43 states and the District of Columbia, covering 91.3% of the U.S. population, met USCS reporting criteria for each year from 2001 to 2010. We excluded cases diagnosed by autopsy or death certificate only. RCC cases were defined as histology codes 8010 to 8051 and 8131 to 8719. The clinical features of each cancer were described by SEER Summary stage 2000 and SEER Derived Summary stage 2000 (localized, regional, distant and unstaged) and grade was defined as well differentiated (grade I) to undifferentiated, anaplastic (grade IV).13,14 Hispanic ethnicity included men and women of all race categories who were identified as Hispanic in the medical records or by use of a validated Hispanic/Latino Identification Algorithm.15 Racial groups examined included white, black, AIAN and API. Race and ethnicity were not mutually exclusive. Regional rates were calculated by aggregating the data for each state into the 4 U.S. Census regions.
For RCC we present total counts and incidence. Incidence rates were standardized to the 2000 U.S. Standard Population (Census P25-1130) and are shown per 100,000 individuals. The CI limit was 95% and CIs were based on the gamma methods using the modification described by Tiwari et al.16 Age adjusted incidence rates are reported for gender, age, race, ethnicity, region, stage, grade and diagnosis year. Trends in incidence were calculated by the APC, that is the annual rate of change in incidence. Statistical significance in trend was assessed at p ≤0.05. All analysis was done using SEER*Stat, version 8.1.2 (http://seer.cancer.gov/seerstat/).17
RESULTS
RCC Incidence
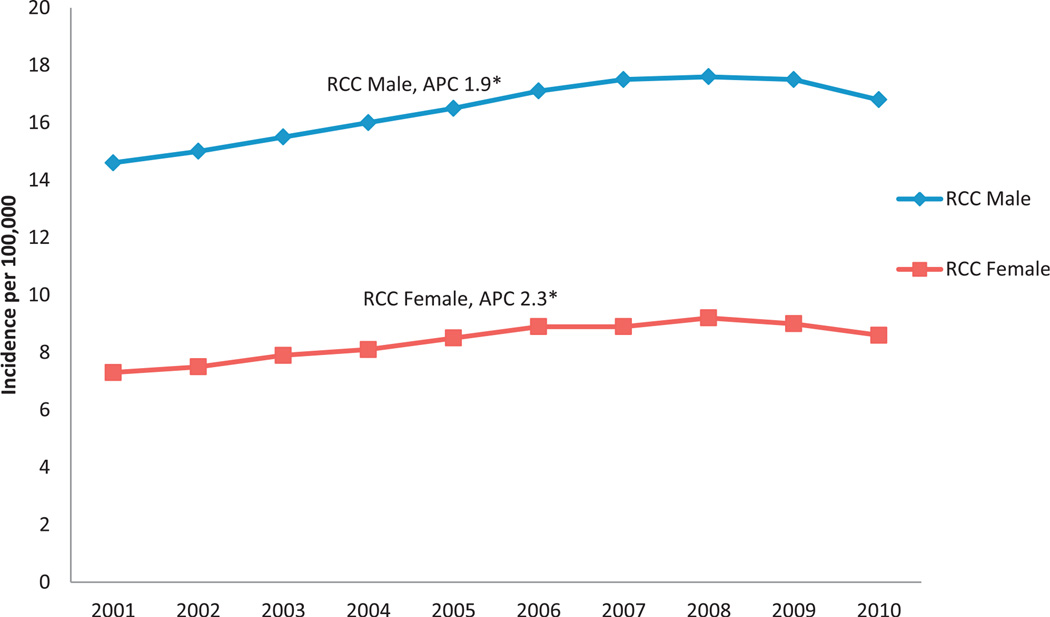
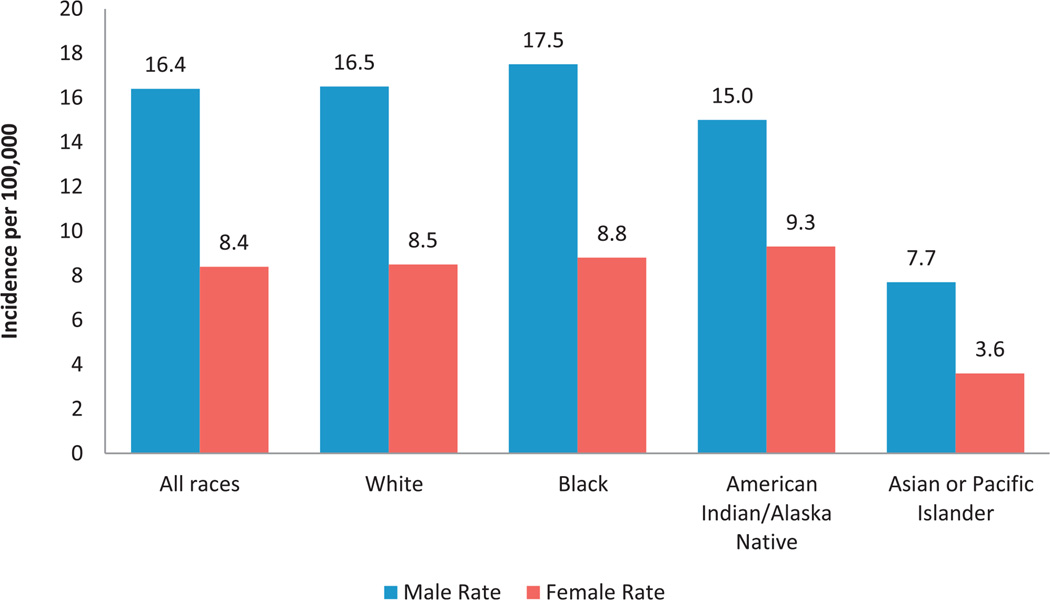
A total of 342,501 RCC cases were diagnosed from 2001 to 2010 with an age adjusted incidence rate of 12.1 cases per 100,000 population (see table). RCC incidence rates increased with age until ages 70 to 74 years with a peak incidence of 52.8. The RCC incidence rate in men was almost double that in women (16.4 vs 8.4). Most RCCs were diagnosed as localized disease (67.0%), followed by regional (14.5%) and distant (13.7%). Almost 38% of tumors were grade II and 20.3% were grade III while grade was unknown in 25.1%. Only 11.6% of tumors were diagnosed as grade I and 5.1% were grade IV. From 2001 to 2010 the age adjusted incidence rate of RCC increased significantly from 10.6/100,000 individuals in 2001 to 12.4/100,000 in 2010. During this period the RCC incidence rate increased significantly in each gender (fig. 1). Overall black individuals had the highest incidence rate (12.6), followed by white (12.2) and AIAN individuals (11.9) while API individuals had the lowest incidence rate (5.4). Further analysis by gender and race showed that black men had the highest overall RCC incidence rate, almost double that of black women. This twofold difference in incidence rate by gender and race persisted in all races except AIAN (fig. 2). AIAN men had the third highest incidence rate (15.0) but AIAN women had the highest incidence by race among women (9.3).
RCC incidence rates and tumor characteristics in the U.S. from 2001 to 2010
| No. Pts (%) | Adjusted Incidence Rate (95% CI)* | APC (95% CI)† | ||||
|---|---|---|---|---|---|---|
| Overall | 342,501 | (100.0) | 12.1 (12.0–12.1) | 2.2 | (1.2–3.1)‡ | |
| Male | 214,943 | (62.8) | 16.4 (16.4–16.5) | 1.9 | (1.1–2.8)‡ | |
| Female | 127,558 | (37.2) | 8.4 | (8.4–8.5) | 2.3 | (1.2–3.4)‡ |
| Age: | ||||||
| 0–19 | 456 | (0.1) | 0.1 | (0.1–0.1) | 3.9 | (−0.3–8.3) |
| 20–24 | 652 | (0.2) | 0.3 | (0.3–0.4) | 7.9 | (4.6–11.4)‡ |
| 25–29 | 1,695 | (0.5) | 0.9 | (0.9–1.0) | 6.3 | (4.0–8.6)‡ |
| 30–34 | 3,954 | (1.2) | 2.2 | (2.1–2.2) | 7.5 | (5.6–9.4)‡ |
| 35–39 | 8,199 | (2.4) | 4.3 | (4.2–4.4) | 6.7 | (6.1–7.3)‡ |
| 40–44 | 15,782 | (4.6) | 7.8 | (7.7–7.9) | 4.1 | (3.0–5.2)‡ |
| 45–49 | 26,439 | (7.7) | 13.1 (12.9–13.2) | 3.2 | (2.4–4.0)‡ | |
| 50–54 | 37,053 | (10.8) | 19.9 (19.7–20.1) | 2.1 | (1.1–3.1)‡ | |
| 55–59 | 45,334 | (13.2) | 28.6 (28.4–28.9) | 1.5 | (0.6–2.3)‡ | |
| 60–64 | 47,805 | (14.0) | 38.2 (37.9–38.5) | 1.2 | (0.0–2.4) | |
| 65–69 | 47,983 | (14.0) | 49.2 (48.7–49.6) | 1.9 | (0.8–3.0)‡ | |
| 70–74 | 42,557 | (12.4) | 52.8 (52.3–53.3) | 1.9 | (0.8–3.0)‡ | |
| 75–79 | 34,751 | (10.1) | 51.2 (50.6–51.7) | 1.5 | (0.1–2.8)‡ | |
| 80–84 | 20,953 | (6.1) | 41.1 (40.6–41.7) | 1.7 | (0.1–3.3)‡ | |
| 85+ | 8,888 | (2.6) | 20.0 (19.6–20.5) | 0.9 | (−1.0–2.8) | |
| Race:§ | ||||||
| White | 294,483 | (86.0) | 12.2 (12.2–12.3) | 2.2 | (1.2–3.1)‡ | |
| Black | 35,759 | (10.4) | 12.6 (12.4–12.7) | 2.6 | (1.6–3.6)‡ | |
| AIAN | 2,768 | (0.8) | 11.9 (11.4–12.4) | 1.6 | (−0.2–3.3) | |
| API | 6,342 | (1.9) | 5.4 | (5.3–5.6) | 2.7 | (1.1–4.3)‡ |
| Ethnicity: | ||||||
| NonHispanic | 312,231 | (91.2) | 12.1 (12.1–12.1) | 2.2 | (1.3–3.2)‡ | |
| Hispanic | 30,270 | (8.8) | 12.1 (12.0–12.3) | 1.6 | (0.4–2.9)‡ | |
| Region: | ||||||
| Northeast | 74,546 | (21.8) | 12.4 (12.3–12.5) | 1.9 | (0.6–3.1)‡ | |
| Midwest | 82,020 | (23.9) | 12.9 (12.8–12.9) | 2.4 | (1.3–3.5)‡ | |
| South | 115,091 | (33.6) | 12.4 (12.4–12.5) | 2.0 | (1.1–2.9)‡ | |
| West | 70,844 | (20.7) | 10.6 (10.5–10.7) | 2.6 | (1.8–3.5)‡ | |
| SEER Summary stage: | ||||||
| Localized | 229,545 | (67.0) | 8.1 | (8.1–8.1) | 4.3 | (2.7–5.9)‡ |
| Regional | 49,579 | (14.5) | 1.7 | (1.7–1.8) | 2.0 | (0.3–3.7)‡ |
| Distant | 46,967 | (13.7) | 1.6 | (1.6–1.7) | 0.7 | (−0.6–2.0) |
| Unstaged | 16,410 | (4.8) | 0.6 | (0.6–0.6) | −20.8 | (−27.4–−13.6)‡ |
| Grade: | ||||||
| I (well differentiated) | 39,599 | (11.6) | 1.4 | (1.4–1.4) | 1.5 | (−0.4–3.3) |
| II (moderately differentiated) | 129,769 | (37.9) | 4.6 | (4.6–4.6) | 5.1 | (2.8–7.3)‡ |
| III (poorly differentiated) | 69,480 | (20.3) | 2.4 | (2.4–2.5) | 5.5 | (3.3–7.9)‡ |
| IV (undifferentiated, anaplastic) | 17,517 | (5.1) | 0.6 | (0.6–0.6) | 3.7 | (0.9–6.5)‡ |
| Unknown | 86,136 | (25.1) | 3.1 | (3.0–3.1) | −4.5 | (−6.6–−2.3)‡ |
| Yr: | – | |||||
| 2001 | 27,415 | (8.0) | 10.6 (10.5–10.7) | |||
| 2002 | 28,733 | (8.4) | 10.9 (10.7–11.0) | |||
| 2003 | 30,559 | (8.9) | 11.3 (11.2–11.5) | |||
| 2004 | 32,200 | (9.4) | 11.7 (11.6–11.9) | |||
| 2005 | 33,953 | (9.9) | 12.2 (12.0–12.3) | |||
| 2006 | 36,023 | (10.5) | 12.7 (12.5–12.8) | |||
| 2007 | 37,383 | (10.9) | 12.9 (12.7–13.0) | |||
| 2008 | 38,720 | (11.3) | 13.0 (12.9–13.2) | |||
| 2009 | 39,203 | (11.4) | 12.9 (12.8–13.1) | |||
| 2010 | 38,312 | (11.2) | 12.4 (12.3–12.5) | |||
Per 100,000 individuals, age adjusted to 2000 U.S. Standard Population (19 age groups, Census P25-1130) standard, 95% CIs for rates with Tiwari modification16 and trends, and data covering about 91.3% of U.S. population.
Percent change calculated using 1 year for each end point and APC calculated using weighted least squares method.
APC significantly different from zero (p <0.05).
Categories do not sum to total due to other unspecified and unknown race counts.
Figure 1.
RCC incidence and APC by gender and year in U.S. from 2001 to 2010. Data cover about 91.3% of U.S. population. Incidence rates are shown per 100,000 population, age adjusted to 2000 U.S. Standard Population (19 age groups, Census P25-1130) standard. Percent changes were calculated using 1 year per end point and APCs were calculated using weighted least squares method. Asterisk indicates APC significantly different from zero (p <0.05).
Figure 2.
RCC incidence by gender and race in U.S. from 2001 to 2010. Data cover about 91.3% of U.S. population. Incidence rates are shown per 100,000 population, age adjusted to 2000 U.S. Standard Population (19 age groups, Census P25-1130) standard.
RCC Incidence Trends
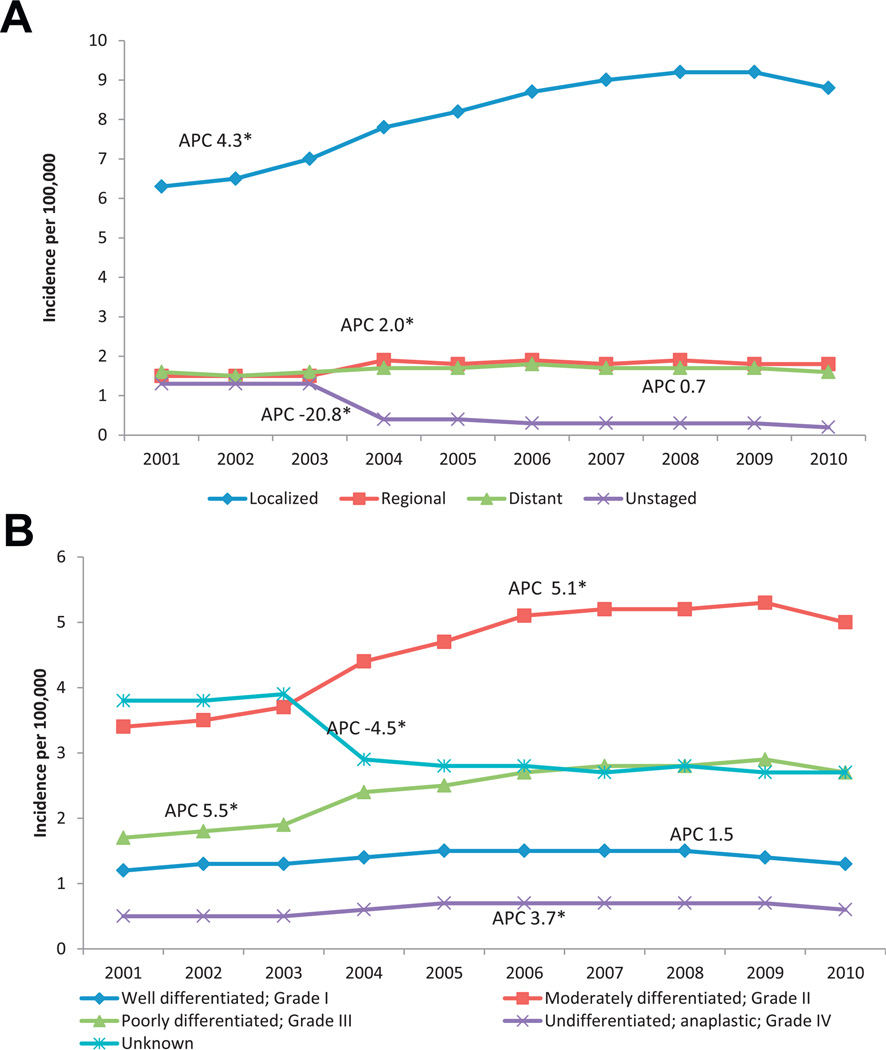
Given an observed increase in RCC (fig. 1), we focused on temporal trends in RCC, and demographic and clinical factors (see table). The APC was statistically significant in each gender but it was higher in women than in men (2.3 vs 1.9, see table). By age group the APC was higher in 4 younger groups (20 to 24, 25 to 29, 30 to 34 and 35 to 39 years) than in any of the other age groups. API showed the highest annual percent change at 2.7. When looking at differences by region, the West had the highest APC at 2.6. Localized stage showed the largest APC (4.3) and distant disease showed the lowest APC (0.7). APC was highest for grade III tumors (5.5), followed by grade II (5.1) and grade IV (3.7). When evaluating RCC for trends the increased incidence of RCC from 2001 to 2010 appeared to be primarily due to a steady increase in localized stage disease. However, these cases did not appear to be only low grade tumors since the largest increase was seen for grade II and III tumors (fig. 3).
Figure 3.
RCC incidence in U.S. from 2001 to 2010. A, by stage at diagnosis. B, by grade at diagnosis. Data cover about 91.3% of U.S. population. Incidence rates are shown per 100,000 population, age adjusted to 2000 U.S. Standard Population (19 age groups, Census P25-1130) standard. Percent changes were calculated using 1 year per end point. APCs were calculated using weighted least squares method. Asterisk indicates APC significantly different from zero (p <0.05).
DISCUSSION
To our knowledge this study is the first to use the USCS database to examine high quality incidence data on kidney cancer covering approximately 91% of the U.S. population. Previous studies, including the pioneering study in 1999 by Chow et al,2 examined the SEER database, which comprises less than 25% of the U.S. population.
Our study shows that the RCC incidence rate continued to increase from 2001 to 2010 with the largest APC increase in young adults, API individuals, and localized stage and grade III tumors. Although the highest incidence rates were seen in men, the greatest increase in incidence was seen in women. Our study also revealed that while the highest RCC incidence rates were in those 70 to 74 years old, the fastest increase occurred in the younger age groups, especially those 20 to 24 years old. This increase in incidence was present for decades2 but only recently are we clearly seeing that younger age groups have the highest APC.
Our study provides a broader population based perspective supported by other recent findings. Using SEER data on 1975 to 2006 Nepple et al found a continuing increase in the RCC incidence.18 They noted no difference in the incidence rate between the genders. Our study showed a particular, marked increase in women, especially younger women, perhaps because it was based on almost the entire U.S. population. Another important contrast between our study and that by Chow et al, who looked at 1975 to 1995, was that we observed no difference in incidence rates between white and black individuals while their data demonstrated significant differences.2
The current results are of interest in a global context. The recently released GLOBOCAN 2012 results show a worldwide increase in kidney cancer incidence rates with time compared to the GLOBOCAN 2008 and 2002 results.19 While the kidney cancer rate in some countries such as Sweden or Finland may be stabilized or possibly decreasing with time, the kidney cancer rate in countries such as the United Kingdom and China continues to increase. A potential explanation may lie in the increasing obesity rate in many countries, including the U.S. and United Kingdom, and in the increasing smoking rate in countries such as China.20
Some of the observed increase in RCC incidence such as the increase in localized stage may be related to an increase in advanced imaging, including computerized tomography, magnetic resonance imaging and positron emission tomography.21 However, we found an increasing incidence, especially in women and those 20 to 24 years old. More research is needed to determine whether either gender or any age group undergoes more imaging to explain in part the increased diagnosis of localized tumors. Likewise, if early detection is occurring through increased imaging, one would simultaneously expect to see an increase in the localized cancer rate and a decrease in the number of regionally advanced or unknown stage cases because tumors are being diagnosed at an earlier stage. This was not observed and similar increases were not seen for RPC, which would also be diagnosed more often as a result of increased imaging. The number of cases of regionally advanced disease did not change.
The increased RCC incidence appears to be primarily due to localized disease. However, these tumors should not be discounted. They may be clinically significant because the APC was highest for grade III tumors. Grade is a strong predictor of outcome.15 The 5-year survival rate for grade I and II tumors is 93% and 86% and there is markedly decreased survival in patients with grade III and IV tumors (66% and 42%, respectively).22 While a possible explanation is an upgrading phenomenon, which was observed for several cancers and most notably prostate cancer23 with the same prostate biopsy core upgraded with time, to our knowledge this has not been reported for kidney cancer. Thus, this increase in RCC diagnosis should not be ignored since these earlier stage tumors may also result in a large impact on morbidity and mortality. We believe that other individual risk factors such as obesity and tobacco use should also be considered.
There is a generally accepted association between RCC and obesity.4,24 Obesity in younger age groups has been increasing for several decades and childhood obesity may have contributed to the higher APC in those 20 to 39 years old.25 Also, from 1999 to 2008 the prevalence of obesity (body mass index 30 kg/m2 or greater) in American women 20 to 39 years old increased from approximately 28% to 34%.26 Adults of all races are still more likely to be obese than those in the younger age groups. However, in the last decade the prevalence of obesity has begun to stabilize in the general population while this younger age group continues to see an increase.25–27 We are uncertain whether a higher prevalence of obesity could result in a higher risk of RCC or whether obesity is instead a surrogate marker for other unmeasured confounders such as lack of physical activity, obesity driven inflammation, intake of processed or fat laden foods, or insulin resistance. While we could not investigate this potential link directly in this study, our results suggest that this warrants further investigation.
AIAN individuals had the third highest RCC incidence rates by race overall and AIAN women had the highest incidence rate by race. The literature addressing this is sparse but several potential factors are suspected to have a role.28 The cigarette smoking rate has decreased with time but a recent article showed that when grouped by race/ethnicity, nonHispanic AIAN individuals had the highest prevalence of cigarette smoking.29 Additional research is needed to identify the possible effect of social determinants and other environmental risk factors.
The primary strength of this study is that USCS is a population based data set including all NPCR and SEER registries that met USCS publication criteria for all years, covering 91.3% of the U.S. population for 2001 to 2010.30 No other published study of kidney cancer epidemiology looked at this breadth of the U.S. population, including all races and ethnicities. Our cases were based on microscopic confirmation and standardized data collection protocols. Biases may impact the collection of information on cancer stage, especially unstaged cancer since patients with unstaged disease may have economic barriers to surgery and treatment that led to cancer being unstaged. Likewise, 25% of our cases were missing information on grade and, therefore, they may represent selection bias. Also, while we believe that smoking status and obesity may have a role, this information is not collected in cancer registries. A small number of cases may have been unaccounted for in the most recent year due to reporting delay but this is not believed to have affected the results.
CONCLUSIONS
We assessed the overall burden and trends of kidney cancer in the U.S. population from 2001 to 2010. The incidence of RCC continued to increase with the largest increases in women, API individuals and those 20 to 24 years old. Likewise, while some of the increased incidence may have resulted from diagnosis of localized disease, the highest APC was seen in grade III tumors, a finding that may indicate more aggressive disease. Continued monitoring of RCC trends is warranted. Further epidemiological study of potential contributing risk factors is needed to gain a better understanding of these types of kidney cancer and identify opportunities to reverse increasing trends.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations and Acronyms
- AIAN
American Indian/Alaska Native
- APC
annual percent change
- API
Asian or Pacific Islander
- NPCR
National Program of Cancer Registries
- RCC
renal cell carcinoma
- RPC
renal pelvis carcinoma
- SEER
Surveillance, Epidemiology and End Results
- U.S.
United States
- USCS
U.S. Cancer Statistics
Footnotes
Financial interest and/or other relationship with Cancer, World Journal of Urology, American Urological Association, American Cancer Society and National Institutes of Health.
REFERENCES
- 1.United States Cancer Statistics. Vol. 2013. Atlanta: United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute; 2013. 1999–2009 Incidence and Mortality Web-based Report. [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JD, van der Hel OL, McMillan GP, et al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Leitzmann MF, Albanes D, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008;168:268. doi: 10.1093/aje/kwn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weikert S, Boeing H, Pischon T, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008;167:438. doi: 10.1093/aje/kwm321. [DOI] [PubMed] [Google Scholar]
- 6.Lynch C, West M, Davila J, et al. Cancers of the kidney and renal pelvis. In: Gloeckler Ries LA, Young JL, Keel GE, et al., editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001—Patient and Tumor Characteristics. chapt 23. Bethesda: SEER Program, National Cancer Institute; 2012. pp. 181–192. NIH Publication 07-6215. [Google Scholar]
- 7.Zeegers MP, Tan FE, Dorant E, et al. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Stewart JH, Hobbs JB, McCredie MR. Morphologic evidence that analgesic-induced kidney pathology contributes to the progression of tumors of the renal pelvis. Cancer. 1999;86:1576. doi: 10.1002/(sici)1097-0142(19991015)86:8<1576::aid-cncr27>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Program of Cancer Registries, Centers for Disease Control and Prevention. United States Cancer Statistics. [Accessed December 20, 2013];Technical Notes. Available at http://www.cdc.gov/cancer/npcr/uscs/technical_notes/contributors/
- 11.ICD-O-3 Coding Materials. Bethesda: National Cancer Institute, National Institutes of Health; 2012. [Google Scholar]
- 12.National Program of Cancer Registries. Technical Notes: United States Cancer Statistics Publication Criteria. Vol. 2011. Atlanta: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 13.Surveillance, Epidemiology and End Results Program: Collaborative Stage. Bethesda: National Cancer Institute; 2013. [Google Scholar]
- 14.National Cancer Institute. Fact Sheet: Tumor Grade. Bethesda: National Cancer Institute, National Institutes of Health; 2013. [Google Scholar]
- 15.NAACCR Guideline for Enhancing Hispanic/Latino Identification. Revised NAACCR Hispanic/Latino Identification Algorithm. Springfield, Illinois: North American Association of Central Cancer Registries; 2005. [Google Scholar]
- 16.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance Research Program NCI: SEER*Stat Software. Vol. 2011. Bethesda, Maryland: National Cancer Institute; 2011. [Google Scholar]
- 18.Nepple KG, Yang L, Grubb RL, 3rd, et al. Population based analysis of the increasing incidence of kidney cancer in the United States: evaluation of age specific trends from 1975 to 2006. J Urol. 2012;187:32. doi: 10.1016/j.juro.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 19.GLOBOCAN2012. Geneva: International Agency for Research on Cancer, World Health Organization; 2013. Estimated Cancer Incidence Mortality and Prevalence Worldwide in 2012. [Google Scholar]
- 20.Marugame T, Matsuda T. Comparison of time trends in kidney cancer incidence (1973–97) in East Asia, Europe and USA, from Cancer Incidence in Five Continents Vols. IV–VIII. Jpn J Clin Oncol. 2008;38:508. doi: 10.1093/jjco/hyn060. [DOI] [PubMed] [Google Scholar]
- 21.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307:2400. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun M, Lughezzani G, Jeldres C, et al. A proposal for reclassification of the Fuhrman grading system in patients with clear cell renal cell carcinoma. Eur Urol. 2009;56:775. doi: 10.1016/j.eururo.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Smith EB, Frierson HF, Jr, Mills SE, et al. Gleason scores of prostate biopsy and radical prostatectomy specimens over the past 10 years: is there evidence for systematic upgrading? Cancer. 2002;94:2282. doi: 10.1002/cncr.10457. [DOI] [PubMed] [Google Scholar]
- 24.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 25.Ogden C, Carroll M. National Center for Health Statistics Health E Stats. Atlanta: Centers for Disease Control and Prevention; 2010. Prevalence of Obesity Among Children and Adolescents: United States, Trends 1963–1965 Through 2007–2008. [Google Scholar]
- 26.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 27.Watson RA. Kidney cancer in American Indian and native Alaskan men and women—time to notice, time to care. Urology. 2008;72:726. doi: 10.1016/j.urology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Current cigarette smoking among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:889. [PubMed] [Google Scholar]
- 29.United States Cancer Statistics Working Group. 1999–2007 Cancer Incidence and Mortality Data. Atlanta: Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 30.Thoburn KK, German RR, Lewis M, et al. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109:1607. doi: 10.1002/cncr.22566. [DOI] [PubMed] [Google Scholar]