Abstract
Early studies regarding the function of FcεRI in dendritic cells (DCs) and monocytes have focused on its role in mediating inflammatory signaling and enhancing T cell immunity. It has been the case in part because FcεRI is the major receptor that mediates allergic inflammatory signaling in mast cells and basophils and because DCs and monocytes are antigen presenting cells capable of activating naïve and/or effector T cells. These studies have led to the general belief that FcεRI-mediated DC signaling and antigen presentation promote development and activation of Th2 cells and contribute to allergic inflammatory diseases. However, this belief has long suffered from a lack of evidence. Recently, studies have emerged that provide evidence supporting an opposing role: that FcεRI on DCs instead promotes immune homeostasis and regulation. In this review, we will update the current status of our understanding of FcεRI biology and function, with a specific focus on DCs and monocytes.
Keywords: High affinity IgE receptor, FcεRI, Dendritic cell, Monocyte, Endocytosis, Signaling, Antigen presentation
Introduction
FcεRI is constitutively expressed by mast cells and basophils, and captures IgE in its monomeric form owing to its remarkably high affinity to IgE (K d = 10−10 M). When IgE/FcεRI complexes on these cells are engaged and crosslinked by allergens, a signaling cascade is initiated that results in extracellular release of various inflammatory mediators [1, 2]. One example of such mediators is histamine, which dilates blood vessels and makes their walls abnormally permeable, creating hives. FcεRI is also expressed in dendritic cells (DCs), both conventional and plasmacytoid DCs, and monocytes in humans. DCs and monocytes are both antigen-presenting cells that endocytose antigens, and process and present them to antigen-specific T cells via antigen presenting molecules including the major histocompatibility complex (MHC) [3, 4]. The antigen presenting activity of DCs plays an important role in the development of antigen-specific immunity by activating naïve T cells and assisting in T helper cell skewing [5, 6]. DC antigen presenting activity also plays an essential role in establishing and maintaining tolerance either by deleting self-reactive T cells or by generating the immune suppressive regulatory T cells [7]. DCs and monocytes also act as innate immune cells. They produce various cytokines and chemokines in response to danger signals such as microbes and tissue damage-associated molecules [8]. In particular, plasmacytoid DCs produce copious amounts of type 1 IFN in response to viral infection signal. Sensing of these signals is mediated by a variety of receptors expressed on the surface and endosomal compartment of the cells [9, 10].
Although the expression of FcεRI in DCs and monocytes has been known for more than two decades, its functional role is not clearly understood—in part, because studies have suffered from a lack of essential reagents. Rodents are the vertebrate species most commonly used in research laboratories because of their availability, size, low cost, ease of handling and fast reproduction rate. Rodents constitutively express high levels of FcεRI in mast cells and basophils in a fashion similar to humans, and thus have served as excellent animal models and cellular sources for studying the role of FcεRI on these cell types. However, rodents do not express FcεRI in DCs and monocytes under homeostatic condition while humans do. Therefore, studies in this field have been limited to in vitro experiments using DCs and monocytes obtained from human donors. Although monocytes are available more readily than any other primary human cells, only a small fraction of the cells express FcεRI, and the level of expression is considerably reduced upon culture. DCs are not only scarce in most tissues, but are comprised of many subsets whose markers and functions are not completely understood. All these drawbacks have markedly slowed down the progress in the field.
In recent years, however, substantial advancement has been made in the characterization of human DC subsets. Subset-specific markers have been identified, which have allowed for examination of DCs in situ by microscopy in a variety of human tissues. Identification of markers has also helped with isolation and further characterization of DCs by flow cytometry and various biochemical assays. Beyond improvements in DC identification, advancements have been also made in culturing human DCs. DCs can be derived from blood monocytes or CD34+ blood precursors, and some of these cultured DCs express FcεRI in a stable manner. Finally, the advent of genetic engineering technology has led to the creation of mouse strains that express human FcεRI in DCs and/or monocytes in a pattern that resembles human. These mouse strains made it possible to examine the role of human FcεRI in vivo, and furthermore to examine the role of DCs and monocytes in FcεRI function. In this review, we will describe these advancements in detail and summarize the current understanding of FcεRI biology in DCs and monocytes. Occasionally, it will be compared with FcεRI biology in mast cells and basophils, and highlighted for its distinctiveness.
FcεRI expression specificity and heterogeneity in DCs and monocytes
FcεRI expression in DCs was first observed in the skin epidermis, where a distinct subset of DCs named Langerhans cells was found [11, 12]. Immunohistochemical and flow cytometry studies showed that epidermal skin cells labeled by the anti-CD1a antibody, a specific marker of Langerhans cells, were also labeled by an anti-FcεRIα antibody as well as IgE [11, 12]. Immunogold labeling electron microscopy study also showed that epidermal skin cells that contained Birbeck granules in the cytosol, the specific characteristics of Langerhans cells, were decorated by anti-FcεRI antibody-conjugated gold particles on their surface [12]. Subsequently, CD1a+FcεRIα+ cells were detected in many other peripheral tissues including the airway [13], oral mucosa [14], nasal mucosa [15], and gastrointestinal tract [16].
While the anti-CD1a antibody had been most frequently used to identify DCs in human peripheral tissues, a breakthrough was made in identification of human DC subsets in circulation through finding of the novel markers named blood dendritic cell antigen (BDCA) 1, 2, 3, and 4 [17]. BDCA1 is expressed in the major subset of conventional DCs that occupy approximately 80 % of circulating DCs and are homologous to mouse CD11b+ DCs [18]. BDCA3 is expressed in XCR1+ minor subset of conventional DCs that are uniquely efficient at presenting exogenous antigens to CD8+ T cells, similarly to mouse CD8+ DCs [19–22]. BDCA2 and BDCA4 are both expressed in plasmacytoid DCs, a separate group from conventional DCs. Each unique BDCA+ DC subset found in blood was also found in peripheral tissues such as lungs [23, 24]. Notably, Langerhans cells and other mucosal DCs that express CD1a also expressed BDCA1, but not all BDCA1+ DCs expressed CD1a [23, 25], implying that BDCA1 is a more encompassing marker for classical DCs than CD1a. FcεRI expression analysis using these BDCA markers demonstrated that BDCA1+ DCs expressed FcεRI at fairly high levels, in a homogeneous manner, and through many different tissue sites. Plasmacytoid DCs also express FcεRI homogeneously, but at substantially lower levels than BDCA1+ DCs. Distinctly, BDCA3+ DCs do not express FcεRI [17, 26–28].
FcεRI expression in monocytes was first characterized in atopic blood donors and later in healthy individuals [29, 30]. FcεRI expression appears to be limited only to a small subset of monocytes characterized by the expression of CD2 [31]. CD2+ monocytes are CD16− and occupy less than 5 % of the total monocytes in peripheral blood. Studies have shown that CD2+ monocytes rapidly obtain DC-like features in culture, including the expression of CD83 and the ability to activate allo-reactive T cells [32, 33]. Therefore, it is conceivable that CD2+ monocytes differentiate to DCs in the peripheral tissues, thus contributing to the pool of peripheral DCs that express FcεRI. Considering that monocytes also differentiate into macrophages, but macrophages do not express FcεRI [34], it is further tempting to consider that FcεRI+ monocytes may represent those fated to become DCs.
Unlike monocytes, DCs can be differentiated from precursor cells in vitro. For example, culturing whole blood monocytes with IL-4 and GM-CSF leads to the expression of DC markers such as CD1a [35]. However, this culturing leads to the down-regulation and almost complete loss of FcεRI expression by differentiated DCs although the degree of down-regulation is modest when the monocytes are originated from atopic donors [36]. Interestingly, this down-regulation is inhibited by adding reducing agents or IgE to cultures [36, 37]. As an alternative to monocyte differentiation, DCs can be also differentiated from CD34+ blood progenitor cells by culturing with GM-CSF, TNF-α, SCF, Flt3L, and TGF-β [38, 39]. Differentiated DCs express CD1a and FcεRI, and interestingly, the level of FcεRI expression is regulated by the concentration of TGF-β in culture [40].
FcεRI structure in DCs and monocytes
FcεRI in DCs and monocytes is comprised of an α-subunit and two γ-subunits [27, 29, 41]. The α-subunit is a transmembrane protein that is necessary and sufficient for IgE binding [42]. The two domains of its extracellular portion adopts the shape of an inverted ‘v’, the second of which binds one dimeric IgE-Fc molecule asymmetrically through interactions at two sites—thus forming a 1:1 protein complex of IgE:FcεRI [43]. Upon binding to FcεRIα, IgE adopts a unique bent conformation and this conformational change contributes to the remarkably slow dissociation rate from FcεRI [44]. The α-subunit is also a glycoprotein. The glycosylation does not seem to be required for IgE binding, as unglycosylated recombinant FcεRIα made in E. coli is capable of binding IgE without a significant loss in affinity [45]. However, proper glycosylation is required for the receptor to effectively fold in the endoplasmic reticulum (ER) and traffic to the plasma membrane [46, 47]. Interestingly, a recent SDS-PAGE analysis has shown that glycosylated FcεRIα in DCs migrates a little faster than that in basophils [48], implicating FcεRIα may be modified by distinct glycan moieties depending on cell type of its expression, similarly to FcγRIIIα [49].
The γ-subunit of FcεRI is a transmembrane protein that acts as a common adaptor molecule for various Fc receptors such as FcγRI (CD64), and FcγRIIIA (CD16A) [50, 51]. It associates as a homodimer formed via a disulfide bond linked between N-terminal cysteine amino acids. The N-terminus also bears an immunoreceptor tyrosine-based activation motif (ITAM), which is phosphorylated upon FcεRI engagement and plays an essential role in transmitting activation signals in mast cells and basophils [52]. A similar role is believed to be played in DCs and monocytes.
It is worth of mentioning that in mast cells and basophils, some, if not all, FcεRI molecules associate with an additional protein subunit, FcεRIβ. The FcεRIβ subunit is a membrane protein composed of four transmembrane and two cytoplasmic domains, one of which contains an ITAM. This additional ITAM is not required for, but markedly amplifies, signal transduction triggered by FcεRI engagement [53]. Expression of FcεRIβ also enhances FcεRIα transport to the plasma membranes [54]. FcεRIβ expression has been reported in Langerhans cells; they demonstrated the presence of FcεRIβ mRNA by RT-PCR after enrichment of Langerhans cells from epidermal cell suspension [12]. However, a later study that used highly purified Langerhans cells (>98 %) showed the lack of FcεRIβ in these cells [55]. Thus, it is possible that early samples may have contained contaminating mast cells from the Langerhans cell purification process. Additional DC subsets including BDCA1+ DCs, plasmacytoid DCs, and monocytes have been examined for the expression of FcεRIβ, but none of these cells were found to express it. Thus, DCs and monocytes, distinct from mast cells and basophils, appear to express FcεRI only in its trimeric form.
FcεRI intracellular trafficking in DCs and monocytes
Secretory trafficking
Upon synthesis, the FcεRI α-subunit is inserted to the ER membranes and glycosylated at its luminal sites. This process is dependent on cotranslational assembly with the γ-subunits [56]. The γ-subunit also facilitates the ER exit of FcεRIα by masking an ER retention/retrieval signal present in the α-subunit cytoplasmic tail [57]. Consequently, the level of γ-subunit expressed in cells makes a substantial impact on the level of FcεRI found on the plasma membrane. For example, Langerhans cells hardly express FcεRIγ while expressing substantial levels of FcεRIα [58]. Because of their FcεRIγ deficiency, FcεRIα in these cells fails to move to the plasma membranes and instead accumulates in the ER. Similarly, DCs derived from monocytes in vitro also lack FcεRIγ, and thus express little or no FcεRI at the plasma membrane [36]. However, DCs derived from monocytes of patients with atopic dermatitis express detectable levels of FcεRI on their surface. An attractive explanation for these differences lies in the finding that DCs derived from atopic donors expressed FcεRIγ, which would have promoted FcεRIα exit from ER and led to the transport of trimeric receptor to the plasma membranes [36].
BDCA1+ DCs isolated from blood express both FcεRIα and FcεRIγ, and the level of their surface FcεRI expression is fairly high. In these cells, FcεRIα is not detected in the ER or in the Golgi, nor is the immature species of FcεRIα detected, defined as FcεRIα that still contains its high mannose carbohydrates attached in ER [48]. This high mannose glycans are later modified to a more complex form of glycans in the Golgi—a process referred to as FcεRIα maturation [56]. Thus, the absence of immature FcεRIα species together with the absence of FcεRI in ER and Golgi strongly suggest that FcεRI efficiently matures and traffics to the plasma membranes in BDCA1+ DCs. Considering that immature FcεRIα is readily detected in human basophils, both by microscopy and western blot analysis [48, 59], it is conceivable that the efficiency of secretory trafficking of FcεRI might be higher in DCs than basophils. Unlike basophils, however, mast cell FcεRI is not detected in the ER, but instead found in the Golgi [60]. It will be interesting to directly determine and compare the efficiency of FcεRI secretory trafficking between these cell types, as it may be relevant to the unique functional roles played by FcεRI in these cells.
Endocytic trafficking
Treating blood DCs with anti-FcεRI antibodies or anti-NP IgE/NP-BSA complexes, which would synchronously crosslink FcεRI molecules on the surface of the cells, results in approximately 90 % of FcεRI disappearing from plasma membrane within 10 min [61]. Microscopy studies revealed that crosslinked surface FcεRI enters the cells and reaches the lysosomes where FcεRI-bound IgE is degraded over time [61]. This finding indicates that FcεRI in DCs is rapidly endocytosed and transported to the lysosomes upon crosslinking. In fact, similar crosslinking-dependent endocytosis of FcεRI has been also described in mast cells, in which crosslinking initiates ubiquitination of FcεRI β- and γ-subunits, which leads FcεRI to clathrin coated pits where the physical process of endocytosis occurs [62].
Interestingly, a recent study has shown that FcεRI in DCs and monocytes is endocytosed and transported to the lysosomes in a constitutive manner. Microscopic study of human blood DCs and monocytes revealed that a large fraction of FcεRI in these cells resides in the lysosomes under homeostatic condition and that these cells contain appreciable amounts of IgE inside the cells [48]. While there could presumably be a physiologic FcεRI-crosslinker in the blood, monomeric IgE added to DCs in culture was also spontaneously internalized [48]. Thus, it is likely that DCs constantly internalize FcεRI in a crosslinking-independent manner, and IgE bound to the FcεRI is also internalized during this process. Importantly, no such constitutive internalization of IgE was observed in mast cells or basophils [48]. This finding strongly suggests that DCs and monocytes operate a unique mechanism by which FcεRI is constitutively endocytosed and sorted to the lysosomes.
Endocytosis of FcεRI bound by IgE draws a particular attention because previous literatures have extensively described the role of IgE in inhibiting FcεRI endocytosis. Repetitive injection of IgE into mice results in a substantial increase in the surface expression of FcεRI in mast cells and basophils [63, 64]. Conversely, mast cells and basophils from IgE-deficient mice have significantly reduced FcεRI compared to those from wild type mice [63, 64]. In vitro, addition of IgE to cultures of FcεRI-expressing cells also increases FcεRI surface levels [63–66]. Mechanism studies have shown that this increase in plasma membrane expression of FcεRI upon IgE binding is not by the increase in FcεRI synthesis but by the decrease in FcεRI endocytosis [65, 67], leading the conclusion that IgE binding stabilizes FcεRI at the plasma membrane by inhibiting its internalization and degradation. However, these previous studies were performed using human basophils [66, 67], bone marrow-derived mouse mast cells [64] and hFcεRI-transfected cell lines of either hematopoietic or non-hematopoietic origin expressing either trimeric or tetrameric FcεRI [65], but not DCs or monocytes isolated from human tissues. In fact, a similar study performed using human Langerhans cells has shown that FcεRI surface levels decrease during ex vivo culture, and this decrease is not prevented by addition of IgE [58]. A recent study using blood DCs in children has shown no correlation between FcεRI in conventional DCs and serum IgE unless serum IgE levels reached certain threshold [68]. More recently, FcεRI expression in blood DCs and basophils in adults were examined in reference to a broad range of serum IgE. While FcεRI in basophils sharply increased in correlation with serum IgE levels, FcεRI in DCs remained fairly flat [48], suggesting that DCs control FcεRI surface levels distinctly from basophils in a manner independent of extracellular IgE.
FcεRI function in DCs and monocytes
Signaling: immune stimulatory or suppressive
FcεRI-mediated signaling has been extensively studied in mast cells. Cross-linking of IgE bound to FcεRI with multivalent antigen triggers a series of biochemical events that culminate in multiple mast cell effector functions [69–71]. Signaling is initiated through the phosphorylation of ITAMs in the cytoplasmic tails of the FcεRIβ and FcεRIγ subunits by the Src protein tyrosine kinases such as Lyn, which are recruited upon clustering of FcεRI. The phosphorylated ITAMs then recruit the kinase Syk, which mediates activation of the adaptor molecules LAT and SLP76, resulting in calcium mobilization. Ultimately, FcεRI aggregation results in initiation of the allergic inflammatory process by eliciting mast cell degranulation with a rapid release of preformed vasoactive amines like histamine and the de novo synthesis of proinflammatory cytokines like TNF-α and IL-6.
Comparatively little is known of the signaling events following FcεRI engagement in DCs and monocytes. Particularly, early signaling events have been poorly characterized; no studies have directly shown that the ITAMs of the FcεRIγ subunit are phosphorylated following FcεRI crosslinking in DCs or monocytes. In mast cells and basophils, FcεRI proximal signaling is largely dependent on the FcεRIβ subunit, which markedly reduces the extent of FcεRI crosslinking required for initiation of signaling [53]. Since monocytes and DCs lack FcεRIβ, FcεRI signaling in these cells may require heavy crosslinking of FcεRI, which may be hard to achieve due to its constitutively endocytosis. Nevertheless, FcεRI crosslinking has been shown to induce protein tyrosine phosphorylation and calcium mobilization in Langerhans cells [55]. It also induces activation of NF-κB in Langerhans cells, monocytes, and monocyte-derived DCs [72]. The NF-κB activation is preceded by serine phosphorylation of IκB-α. The activated NF-κB complexes contain p50 and p65 subunits, and leads to the synthesis and release of TNF-α and MCP-1 (CCL-2) from monocytes and monocyte-derived DCs [72].
Consistent with the role of NF-κB in increasing transcription of proinflammatory mediators, FcεRI crosslinking in monocytes and DCs, results in the production of TNF-α and IL-6 [73–76]. Blood conventional DCs also produce CCL-28, a chemokine that recruits T and B cells and eosinophils [77]. Inflammatory DCs cultured from monocytes were shown to produce IL-12 and IL-18, which are both potent proinflammatory and IFN-γ producing cytokines [78]. These studies suggest that FcεRI signaling in DCs and monocytes may promote inflammation by upregulating the production of cytokines and chemokines that activate and recruit a variety of types of inflammatory cells.
However, studies also suggest an anti-inflammatory and immune suppressive role of FcεRI signaling. FcεRI crosslinking in monocytes and DCs has been shown to induce high amounts of the anti-inflammatory cytokine IL-10, which subsequently acts in an autocrine fashion and negatively regulates production of TNF-α in these cells [74–76, 79]. Additionally, a high-throughput analysis has been performed to identify genes regulated by FcεRI signaling in a non-biased manner. This study has revealed that at relatively later time points following FcεRI-crosslinking, monocytes upregulate the expression of indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in the catabolism of tryptophan [80]. IDO production from macrophages and DCs, and consequent depletion of tryptophan, have been shown to inhibit T cell proliferation in part by activating a cellular stress response or by inducing activation and regulatory function of functionally quiescent regulatory T cells [81, 82]. Indeed, FcεRI-crosslinked monocytes markedly suppressed proliferation of T cells in vitro and this suppression was reverted when the culture was supplemented with tryptophan or an IDO inhibitor [80].
Additional data further support the role of FcεRI signaling in immune-suppression. Toll-like Receptor (TLR) 7 and TLR 9 mediate activation of PDCs during viral infection resulting in the production of type 1 IFNs such as IFN-α and -β. Several studies have shown that FcεRI aggregation significantly reduced the production of IFN-α and IFN-β by plasmacytoid DCs stimulated with CpG-A (a ligand of TLR9), influenza virus, or gardiquimod (a TLR 7 agonist) [76, 83, 84]. This down-regulation in IFN responses was accompanied by a concomitant reduction in the transcription of TLR 7 or TLR 9 [76, 84]. More recently, it was shown that FcεRI crosslinking in monocyte-derived DCs reduces production of CCL-2 following stimulation with lipopolysaccharide, a ligand of TLR 4 [85], suggesting that FcεRI signaling interferes with TLR signaling not only in plasmacytoid DCs but also monocyte-derived DCs and possibly conventional DCs.
Antigen presentation: immunogenic or tolerogenic
Dendritic cells and monocytes are professional antigen presenting cells in humans. Therefore, the identification of FcεRI expression in these cells naturally prompted the question of whether this receptor might facilitate presentation of IgE-bound antigens. Studies using multivalent antigens that bind and crosslink IgE/FcεRI complexes have shown that these antigens were rapidly internalized by DCs and monocytes, processed by lysosomal proteases, loaded onto MHC II, and presented to antigen-specific CD4+ T cells, resulting in their proliferation [41, 61]. IgE loading was crucial to efficient antigen presentation because antigens added to cells in the absence of IgE were presented at a 100 to 1000-fold lower efficiency [41]. These findings suggest that FcεRI facilitates presentation of IgE-bound antigens, such as allergens, to T cells—implicating its contribution to T cell-mediated inflammation in allergic diseases. More recently, plasmacytoid DCs loaded with Bet v1 allergen-IgE immune complexes were shown to induce activation of autologous naïve CD4 T cells producing higher amounts of IL-4 and lower levels of IFN-γ than T cells cocultured with untreated plasmacytoid DCs [27]. A similar response has been also observed with naïve T cells cocultured with blood BDCA1+ DCs loaded with Der p1 allergen-IgE immune complexes [86]. These studies suggest that FcεRI in DCs not only enhances activation of effector CD4 T cells but also mediates priming of Th2 cells.
Recently, the role of FcεRI in antigen presentation by DCs was further examined in vivo using transgenic mice [85, 87]. This strain expresses the human FcεRIα under the control of the DC-restricted, constitutively active CD11c promoter. The human FcεRIα expressed in DCs forms a complex with mouse FcεRIγ, transports to the plasma membranes, and binds circulating murine IgE albeit at a reduced affinity compared to human IgE. Two independent groups used these mice and examined whether DCs of this mouse present IgE-bound antigens better than DCs of wild type mouse, and if so, whether the antigen presentation results in Th2 cell priming [85]. While both studies concluded that FcεRI greatly enhances DC presentation of IgE-bound antigens in vivo, they reached contradictory conclusions regarding Th2 cell priming; one study found improved Th2 cell generation [87], but the other found no evidence of any improvement [85]. The reason behind this contradiction is not completely clear, but additional studies remain to be seen to clearly answer this long-standing question.
Another recent study examined the role of FcεRI in DC antigen presentation using a unique hFcεRIα-Tg mouse strain in a distinct experimental setting [88]. This study used mice that express hFcεRIα under the control of human FcεRIα promoter [89]. Accordingly, this mouse expresses hFcεRIα in cell types where it is also expressed in humans, including mast cells, basophils, monocytes, and DCs, both conventional and plasmacytoid DCs. In this study, the mice were injected with a recombinant protein consisting of the human IgE Fc domain covalently linked to a specific peptide antigen, and examined for the presentation of the antigen to antigen-specific CD4+ T cells that had been adoptively transferred into the mice. Thus, this study examined the role of FcεRI in mediating presentation of IgE-bound monovalent antigen. The antigen was effectively presented by DCs to naïve T cells, resulting in T cell proliferation. However, the proliferation was transient and was followed by systemic deletion of the antigen-specific T cells from the mice. Furthermore, the mice became resistant to developing T cell immune responses against the antigen at later challenges. These findings suggest that FcεRI facilitates DC presentation of IgE-bound antigens and that this presentation results in antigen-specific T cell tolerance as long as the IgE-bound antigens do not crosslink FcεRI. Although such occasion remains to be identified in physiologic conditions, it may pertain to the production of natural IgE following tissue damage and its potential role in immune regulation. For example, helminth infection causes massive damage in the intestines and lungs, and is followed by production of high amounts of IgE antibodies, which are not specific for parasites but are polyclonal in nature [90, 91]. In addition, surgery and burns, both involving potentially severe tissue damage, are also accompanied by the production of considerable amounts of polyclonal IgE [92, 93]. Although the specificity of these IgE molecules remains to be determined, they are believed to be natural IgE, which is of low affinity and poly-specific against various self-antigens including phosphatidylcholine [94]. This natural IgE may promote DC recognition of self antigens released during tissue damage via FcεRI—but this recognition is not likely to cause FcεRI crosslinking because the likelihood of that an antigen-specific IgE molecule to be adjacent to the same antigen-specific IgE molecule is extremely low. In addition, IgE bound to FcεRI will be rapidly internalized by DCs via constitutive FcεRI endocytic trafficking, which will further minimize the likelihood of FcεRI crosslinking. Consequently, the IgE-bound self-antigens would be internalized in the absence of FcεRI crosslinking, subsequently presented to self-reactive T cells, which in turn would be deleted. Thus, IgE generated following tissue damage may play an important role in preventing autoimmunity from developing against self-antigens released during the damage by focusing the antigens to FcεRI-expressing DCs, which induces deletional T cell tolerance against those antigens. In this context, FcεRI in DCs would contribute to immune tolerance to self-antigens.
IgE clearance
IgE has the shortest serum half-life (approximately 2 days) among five immunoglobulin isotypes [95, 96]. In an interesting mathematical study of immunoglobulin metabolism, it was found that IgE differs from other immunoglobulins in that it undergoes substantial catabolism at extravascular sites [96]. The study proposed that the extravascular catabolism is part of a unique mechanism specific for IgE, which is related to unique interactions of IgE with cells expressing FcεRI in the peripheral tissues. This interaction was considered to exert two potential effects on overall IgE survival; on one hand, it may result in sequestration and persistence of IgE molecules in local tissue sites. On the other hand, it may be followed by subsequent catabolism of IgE by the cells. Interestingly, they concluded that IgE catabolism after interaction with cells is a quantitatively more significant phenomenon than IgE sequestration.
Nevertheless, the role of FcεRI-expressing cells in IgE catabolism has been difficult to prove experimentally. IgE bound to FcεRI on mast cells persists as long as the cells are alive, which suggests that IgE bound to mast cells is stabilized rather than catabolized. Furthermore, mast cell-deficient mice were found to clear IgE at a similar rate to wild type mice [97]. FcεRI-deficient mice also cleared IgE similarly to wild type mice [98]. These findings have provided strong evidences supporting the hypothesis that FcεRI-expressing cells do not contribute to IgE catabolism. However, humans differ from mice; in this case, in that human also express FcεRI in monocytes and DCs. The role of these cells in IgE catabolism has not been examined until recently.
The recent finding that FcεRI is constitutively endocytosed and transported to the lysosomes in human monocytes and DCs invited the question of whether IgE bound to FcεRI in these cells would be also endocytosed and transported to the lysosomes, and possibly degraded by lysosomal proteases. Indeed, IgE bound to DCs but not basophils were rapidly endocytosed, transported to the lysosomes, and degraded [48]. To take it a step further, this study was extended to determine the role of monocytes and DCs in IgE catabolism in vivo [48]. The aforementioned hFcεRIα-Tg mice that express human FcεRIα under the human promoter were used in this study because these mice recapitulated not only the cell-type specific expression of FcεRI but also the unique cell type-specific intracellular trafficking of the receptor; human FcεRI was localized in endolysosomes in transgenic murine DCs and monocytes while it was localized in the plasma membranes in mast cells and basophils [48]. Human IgE injected to these mice was internalized by DCs and monocytes but not by mast cells and basophils. Furthermore, the transgenic mice cleared IgE at a much faster rate than non-transgenic control mice, and the rate of clearance correlated with the prevalence of monocytes and DCs. This study reveals that FcεRI-mediated constitutive internalization of IgE by DCs and monocytes promotes serum IgE clearance, implicating its role in IgE homeostasis.
Association with diseases of FcεRI-expressing DCs and monocytes
Atopic dermatitis
Atopic dermatitis, or eczema, is an inflammatory skin disease characterized by red and itchy dry rashes on the skin. The lesions are infiltrated by inflammatory cells and accompanied by keratinocyte metaplasia, and are often associated with high levels of total IgE and allergen-specific IgE. Blood monocytes from atopic dermatitis patients express significantly higher levels of FcεRI than those from healthy individuals, implicating them in this disease. Crosslinking of FcεRI in monocytes promotes differentiation of the monocytes to histamine receptor 1 (H1R)-expressing macrophages with high proinflammatory properties and increased histamine biosynthesis in vitro [99]. In agreement with these in vitro findings, H1R-expressing CD68+ macrophages were also found in high amounts in the dermal compartment of AD skin lesions. Considering that histamine is one of the most important mediators of allergic inflammatory reactions, FcεRI signaling in monocytes may contribute to the generation of macrophages that initiate the inflammatory activities of histamines, aggravating skin inflammation in atopic dermatitis.
In addition to macrophages, the skin lesions of atopic dermatitis are infiltrated by a large numbers of DCs; namely, IDECs (inflammatory dendritic epidermal cells), which express distinctively high levels of FcεRI on their surface [100]. Culturing monocytes with IL-4 and GM-CSF under reducing conditions generates DCs with similar phenotypes to IDEC, including high levels of FcεRI [37]. These IDEC-like DCs produce inflammatory cytokines and chemokines including IL-12, and IL-18 upon FcεRI crosslinking, which are enriched in lesion of atopic skin [78]. This finding suggests that FcεRI signaling in IDEC may be a significant contributor to the skin inflammation in this disease.
Asthma
Asthma is a chronic inflammatory lung disease characterized by airway hyper-reactivity and excessive mucus production. Similar to atopic dermatitis, a major fraction of asthma is associated with high levels of total IgE and allergen-specific IgE in serum. It is also associated with both an increased expression of FcεRI in DCs and monocytes and an infiltration of airways with FcεRI-expressing DCs [13]. This infiltration by FcεRI-expressing DCs is associated with a specific subset of asthma characterized by a Th2 immune profile, namely Th2 high asthma [28, 101]. This subset of asthma is also accompanied by a more severe form of eosinophilia and subepithelial fibrosis compared to Th2 low asthma [101]. Thus, FcεRI signaling in DCs may be a contributing factor to Th2 inflammation and associated asthma pathologies.
The role of FcεRI-expressing DCs in Th2 asthma pathologies has been also implicated in studies that used wild type mice as the animal models. While wild type mice do not express FcεRI in DCs at steady state, mouse lung DCs do express FcεRI following infection by the mouse paramyxovirus Sendai virus [102]. This virus induces acute Th1 antiviral immune responses but also triggers a later switch to persistent Th2 inflammation, mucous cell metaplasia, and airway hyper-reactivity, reminiscent of asthma associated with respiratory viral infection. As no previous studies have shown FcεRI expression in mouse DCs, this study thoroughly tested FcεRI expression on the DCs using multiple methods including flow cytometry, western blot analysis, and RT-PCR. These methods consistently pointed to the induction of FcεRI expression in lung DCs following the viral infection. This study further showed that crosslinking FcεRIα on lung DCs of the mice resulted in the production of the T cell chemoattractant CCL-28. Furthermore, FcεRIα-deficient mice had decreased CCL-28 and decreased recruitment of IL-13 producing CD4 T cells to the lung after viral infection. However, these decreases were reverted by transfer of DCs from wild type but not from FcεRI-deficient mice. Thus, this study suggests that FcεRI expressed in DCs contributes to allergic inflammation in the lung by recruiting Th2 cells that promote mucous cell metaplasia by mediating production of CCL-28.
In addition to mice infected with Sendai virus, mice intranasally challenged with house dust mite have been shown to express FcεRI in a distinct subset of DCs namely inflammatory DCs [103]. However, this finding was solely dependent on detection of FcεRI by flow cytometry using an anti-FcεRI monoclonal antibody, MAR-1. Although the MAR-1 antibody binds mouse FcεRI and has been frequently used to identify basophils and mast cells in mice, the degree of its specificity has not been extensively characterized. In fact, others and we have found that MAR-1 binds a subset of monocytes in FcεRIα-deficient mice under inflammatory conditions. Additionally, no functional role has been reported in association with FcεRI expressed by these inflammatory mouse DCs. Thus, further studies are required to verify FcεRI expression by these mouse inflammatory DCs as well as its functional significance.
The role of FcεRI expressed in DCs in asthma has been also examined using the CD11c-hFcεRIα-Tg mice that express hFcεRIα under control of the DC-specific CD11c promoter. One study showed that these mice produced a greater amount of IL-4 in the airways and developed a more severe form of lung eosinophilia compared to wild type mice, and that both phenotypes were completely dependent on IgE [87]. This finding suggests that FcεRI on DCs promotes asthma. However, another study showed that these mice develop a comparable level of airway inflammation to wild type mice indicated by similar amounts of TNF-α, IL-6, IL-13, IL-5 produced in the airways and a similar degree of eosinophil recruitment [85]. In addition, the mice failed to develop airway hyper-reactivity while wild type mice did [85]. These findings suggest that FcεRI on DCs may instead play a regulatory role in asthma. The apparent contradiction between these studies is not clearly explainable. It may be attributed to different microbiota in animals due to different housing facilities. Alternatively, it may be attributed to different asthma models used. The former employed an acute asthma model largely independent of mast cells while the latter adopted a chronic asthma model that significantly depends on mast cells. In fact, the latter study has shown that a smaller number of mast cells are recruited to the airways in the CD11c-hFcεRIα-Tg mice than wild type mice. Similar observation was made in a mast cell-dependent food allergy model [85]. Based on these results, perhaps FcεRI on DCs may specifically regulate mast cell-associated allergic inflammation.
Concluding remark
In this review, we have summarized the current knowledge of FcεRI biology on human DCs and monocytes. FcεRI is homogeneously and constitutively expressed by the major human DC subset, the BDCA1+ conventional DCs, and also by BDCA2+ plasmacytoid DCs throughout various tissue sites. FcεRI is also constitutively expressed in Langerhans cells, the distinct skin DC subset. Exceptions are the BDCA3+ conventional DCs that lack FcεRI. Unlike DCs, monocytes express FcεRI in a heterogeneous and more regulated manner. In both monocytes and DCs, FcεRI is effectively transported to the plasma membranes but also effectively endocytosed. This endocytosis is not inhibited by IgE binding, thereby resulting in FcεRI-bound IgE entering the cells, reaching the lysosomes, and becoming subject to degradation (Fig. 1a). This distinct FcεRI trafficking may render monocytes and DCs an IgE cleaner as opposed to an IgE effector. When antigens come into the picture, FcεRI endocytosis reinforces the ability of DCs to internalize, process, and present antigens by focusing these activities toward antigens bound to IgE. The consequences of this antigen presentation may differ depending on whether or not FcεRI was crosslinked during engagement with antigens; in the absence of crosslinking, the antigen presentation results in deletional T cell tolerance whereas in the presence of crosslinking, it may result in development of effector T cells (Fig. 1b, c). Besides antigen presentation, FcεRI mediates intracellular signaling upon crosslinking (Fig. 1c). This signaling could either promote or inhibit immune stimulation depending on environmental factors including tissue sites, neighboring cells, and cytokines. Based on its ability to promote antigen presentation as well as to transmit signaling, FcεRI in DCs and monocytes has been suggested to contribute to allergic diseases by enhancing T cell immunity and inflammation. However, recent studies using murine models raise the possibility that it may instead function as the regulator of allergic inflammation.
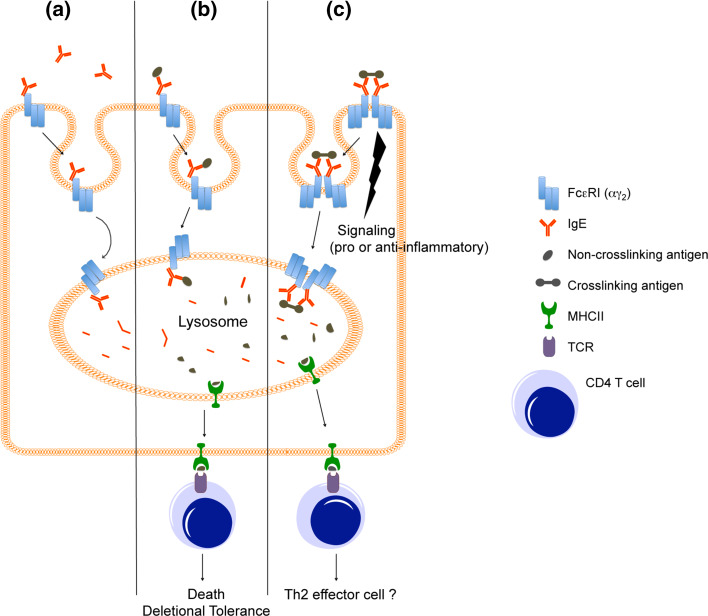
Fig. 1.
Role of FcεRI in DCs and monocytes. a IgE clearance. FcεRI on DCs and monocytes captures extracellular IgE and takes it to the lysosome resulting in the intracellular degradation [48]. b T cell tolerance. FcεRI takes IgE-bound antigen to the lysosomes resulting in the generation of antigenic peptides. These peptides are loaded onto MHCII, transported to cell surface, and presented to antigen-specific CD4+ T cells. This function is not dependent on FcεRI crosslinking; non-crosslinking antigens as well as crosslinking antigens are internalized and presented to CD4+ T cells [61, 88]. When antigen presentation is made in the absence of FcεRI crosslinking, the antigen-recognizing T cells undergo apoptosis resulting in development of T cell tolerance [88]. c Pro- or anti-inflammation. FcεRI transmits intracellular signaling upon crosslinking that results in production of cytokines and chemokines of pro- or anti-inflammatory potential [72–78, 80, 102]. Crosslinked FcεRI takes IgE-bound antigens to the lysosomes, where the antigens are processed and loaded onto MHCII before being presented to CD4+ T cells [61]. The functional outcome of this presentation is unclear as some studies suggest development of Th2 effector T cells [27, 86, 87] while others do not [85]
There is no question that additional studies are required to clarify the functional role of FcεRI in DCs and monocytes. The recently emerged animal models are expected to continue serving useful reagents to the field. However, caution is urged, as they express the chimeric receptor composed of the human FcεRIα and the mouse FcεRIγ, which do not exist in either humans or mice. In addition, mouse DCs and monocytes may not behave the same as human counterparts. Therefore, studies using human cells will need to be accompanied with mouse models, and studies using mice will need to be thoroughly assessed for their validity and relevance to human physiology or disease. Alternatively, one may utilize a humanized mouse strain in which their immune cells were derived from human hematopoietic stem cells. In fact, a human PBMC-engrafted murine model of gut allergic inflammation has been recently developed [104]. This model depends on human FcεRI-expressing cells; thus, it could serve a tool to examine the role of FcεRI-expressing DCs or monocytes in gut allergic inflammation. It is clear that the field is moving fast forward, and a major progress is to be seen in the near future.
Acknowledgments
This work is supported by the UCSF Sandler Asthma Basic Research Center and the American Heart Association.
References
- 1.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7(5):365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 2008;20(1):52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 6.Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14(4):420–431. doi: 10.1016/S0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, van Vliet SJ, Engering A, Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 10.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcγ receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14(2):94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 11.Bieber T, et al. Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (Fc epsilon RI) J Exp Med. 1992;175(5):1285–1290. doi: 10.1084/jem.175.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, et al. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. 1992;175(5):1353–1365. doi: 10.1084/jem.175.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tunon-De-Lara JM, et al. Dendritic cells in normal and asthmatic airways: expression of the alpha subunit of the high affinity immunoglobulin E receptor (Fc epsilon RI -alpha) Clin Exp Allergy. 1996;26(6):648–655. doi: 10.1111/j.1365-2222.1996.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 14.Allam JP, et al. Characterization of dendritic cells from human oral mucosa: a new Langerhans’ cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol. 2003;112(1):141–148. doi: 10.1067/mai.2003.1607. [DOI] [PubMed] [Google Scholar]
- 15.Allam JP, et al. Comparative analysis of nasal and oral mucosa dendritic cells. Allergy. 2006;61(2):166–172. doi: 10.1111/j.1398-9995.2005.00965.x. [DOI] [PubMed] [Google Scholar]
- 16.Bannert C, et al. Fc-epsilon-RI, the high affinity IgE-receptor, is robustly expressed in the upper gastrointestinal tract and modulated by mucosal inflammation. PLoS ONE. 2012;7(7):e42066. doi: 10.1371/journal.pone.0042066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dzionek A, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165(11):6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 18.Robbins SH, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9(1):R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207(6):1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207(6):1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulin LF, et al. Characterization of human DNGR-1+BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207(6):1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crozat K, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207(6):1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol. 2005;32(3):177–184. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- 24.Yu CI, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity. 2013;38(4):818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112(6):1132–1138. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Novak N, et al. Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2004;114(2):364–370. doi: 10.1016/j.jaci.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 28.Greer A, et al. Accumulation of BDCA1+ dendritic cells in interstitial fibrotic lung diseases and Th2-high asthma. PLoS ONE. 2014;9(6):e99084. doi: 10.1371/journal.pone.0099084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurer D, et al. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med. 1994;179(2):745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh N, Kraft S, Wessendorf JH, Bieber T. The high-affinity IgE receptor (FcepsilonRI) blocks apoptosis in normal human monocytes. J Clin Invest. 2000;105(2):183–190. doi: 10.1172/JCI6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng YX, et al. CD2 identifies a monocyte subpopulation with immunoglobulin E-dependent, high-level expression of Fc epsilon RI. Clin Exp Allergy. 2006;36(11):1436–1445. doi: 10.1111/j.1365-2222.2006.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Pucchio T, et al. CD2+/CD14+ monocytes rapidly differentiate into CD83+ dendritic cells. Eur J Immunol. 2003;33(2):358–367. doi: 10.1002/immu.200310010. [DOI] [PubMed] [Google Scholar]
- 33.Takamizawa M, et al. Dendritic cells that process and present nominal antigens to naive T lymphocytes are derived from CD2+ precursors. J Immunol. 1997;158(5):2134–2142. [PubMed] [Google Scholar]
- 34.Segura E, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38(2):336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak N, et al. Evidence for a differential expression of the FcepsilonRIgamma chain in dendritic cells of atopic and nonatopic donors. J Clin Invest. 2003;111(7):1047–1056. doi: 10.1172/JCI200315932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak N, et al. A reducing microenvironment leads to the generation of FcepsilonRIhigh inflammatory dendritic epidermal cells (IDEC) J Invest Dermatol. 2002;119(4):842–849. doi: 10.1046/j.1523-1747.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- 38.Riedl E, Strobl H, Majdic O, Knapp W. TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol. 1997;158(4):1591–1597. [PubMed] [Google Scholar]
- 39.Strobl H, et al. flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90(4):1425–1434. [PubMed] [Google Scholar]
- 40.Allam JP, Klein E, Bieber T, Novak N. Transforming growth factor-beta1 regulates the expression of the high-affinity receptor for IgE on CD34 stem cell-derived CD1a dendritic cells in vitro. J Invest Dermatol. 2004;123(4):676–682. doi: 10.1111/j.0022-202X.2004.23428.x. [DOI] [PubMed] [Google Scholar]
- 41.Maurer D, et al. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154(12):6285–6290. [PubMed] [Google Scholar]
- 42.Hakimi J, et al. The alpha subunit of the human IgE receptor (FcERI) is sufficient for high affinity IgE binding. J Biol Chem. 1990;265(36):22079–22081. [PubMed] [Google Scholar]
- 43.Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature. 2000;406(6793):259–266. doi: 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- 44.Holdom MD, et al. Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcvarepsilonRI. Nat Struct Mol Biol. 2011;18(5):571–576. doi: 10.1038/nsmb.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson MW. Phage and Escherichia coli expression of the human high affinity immunoglobulin E receptor alpha-subunit ectodomain. Domain localization of the IgE-binding site. J Biol Chem. 1993;268(17):12736–12743. [PubMed] [Google Scholar]
- 46.Albrecht B, Woisetschlager M, Robertson MW. Export of the high affinity IgE receptor from the endoplasmic reticulum depends on a glycosylation-mediated quality control mechanism. J Immunol. 2000;165(10):5686–5694. doi: 10.4049/jimmunol.165.10.5686. [DOI] [PubMed] [Google Scholar]
- 47.Letourneur O, Sechi S, Willette-Brown J, Robertson MW, Kinet JP. Glycosylation of human truncated Fc epsilon RI alpha chain is necessary for efficient folding in the endoplasmic reticulum. J Biol Chem. 1995;270(14):8249–8256. doi: 10.1074/jbc.270.14.8249. [DOI] [PubMed] [Google Scholar]
- 48.Greer AM, et al. Serum IgE clearance is facilitated by human FcepsilonRI internalization. J Clin Invest. 2014;124(3):1187–1198. doi: 10.1172/JCI68964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeck A, Pohlentz G, Schlothauer T, Peter-Katalinic J, Regula JT. Cell type-specific and site directed N-glycosylation pattern of FcγRIIIa. J Proteome Res. 2011;10(7):3031–3039. doi: 10.1021/pr1012653. [DOI] [PubMed] [Google Scholar]
- 50.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 51.Ra C, Jouvin MH, Blank U, Kinet JP. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989;341(6244):752–754. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- 52.Paolini R, Jouvin MH, Kinet JP. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- 53.Lin S, Cicala C, Scharenberg AM, Kinet JP. The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell. 1996;85(7):985–995. doi: 10.1016/S0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 54.Donnadieu E, Jouvin MH, Kinet JP. A second amplifier function for the allergy-associated Fc(epsilon)RI-beta subunit. Immunity. 2000;12(5):515–523. doi: 10.1016/S1074-7613(00)80203-4. [DOI] [PubMed] [Google Scholar]
- 55.Jurgens M, Wollenberg A, Hanau D, de la Salle H, Bieber T. Activation of human epidermal Langerhans cells by engagement of the high affinity receptor for IgE, Fc epsilon RI. J Immunol. 1995;155(11):5184–5189. [PubMed] [Google Scholar]
- 56.Fiebiger E, Tortorella D, Jouvin MH, Kinet JP, Ploegh HL. Cotranslational endoplasmic reticulum assembly of FcepsilonRI controls the formation of functional IgE-binding receptors. J Exp Med. 2005;201(2):267–277. doi: 10.1084/jem.20041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Letourneur F, Hennecke S, Demolliere C, Cosson P. Steric masking of a dilysine endoplasmic reticulum retention motif during assembly of the human high affinity receptor for immunoglobulin E. J Cell Biol. 1995;129(4):971–978. doi: 10.1083/jcb.129.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraft S, Wessendorf JH, Hanau D, Bieber T. Regulation of the high affinity receptor for IgE on human epidermal Langerhans cells. J Immunol. 1998;161(2):1000–1006. [PubMed] [Google Scholar]
- 59.Saini SS, et al. Expression and modulation of FcepsilonRIalpha and FcepsilonRIbeta in human blood basophils. J Allergy Clin Immunol. 2001;107(5):832–841. doi: 10.1067/mai.2001.114653. [DOI] [PubMed] [Google Scholar]
- 60.Rios EJ, Piliponsky AM, Ra C, Kalesnikoff J, Galli SJ. Rabaptin-5 regulates receptor expression and functional activation in mast cells. Blood. 2008;112(10):4148–4157. doi: 10.1182/blood-2008-04-152660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maurer D, et al. Fc epsilon receptor I on dendritic cells delivers IgE-bound multivalent antigens into a cathepsin S-dependent pathway of MHC class II presentation. J Immunol. 1998;161(6):2731–2739. [PubMed] [Google Scholar]
- 62.Molfetta R, Gasparrini F, Santoni A, Paolini R. Ubiquitination and endocytosis of the high affinity receptor for IgE. Mol Immunol. 2010;47(15):2427–2434. doi: 10.1016/j.molimm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Lantz CS, et al. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J Immunol. 1997;158(6):2517–2521. [PubMed] [Google Scholar]
- 64.Yamaguchi M, et al. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185(4):663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001;167(3):1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 66.MacGlashan D, Jr, et al. In vitro regulation of FcepsilonRIalpha expression on human basophils by IgE antibody. Blood. 1998;91(5):1633–1643. [PubMed] [Google Scholar]
- 67.MacGlashan D, Jr, Xia HZ, Schwartz LB, Gong J. IgE-regulated loss, not IgE-regulated synthesis, controls expression of FcepsilonRI in human basophils. J Leukoc Biol. 2001;70(2):207–218. [PubMed] [Google Scholar]
- 68.Vasudev M, et al. Expression of high-affinity IgE receptor on human peripheral blood dendritic cells in children. PLoS ONE. 2012;7(2):e32556. doi: 10.1371/journal.pone.0032556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402(6760 Suppl):B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 70.Rivera J, Olivera A. A current understanding of Fc epsilon RI-dependent mast cell activation. Current allergy and asthma reports. 2008;8(1):14–20. doi: 10.1007/s11882-008-0004-z. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez-Errico D, Lessmann E, Rivera J. Adapters in the organization of mast cell signaling. Immunol Rev. 2009;232(1):195–217. doi: 10.1111/j.1600-065X.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kraft S, Novak N, Katoh N, Bieber T, Rupec RA. Aggregation of the high-affinity IgE receptor Fc(epsilon)RI on human monocytes and dendritic cells induces NF-kappaB activation. J Invest Dermatol. 2002;118(5):830–837. doi: 10.1046/j.1523-1747.2002.01757.x. [DOI] [PubMed] [Google Scholar]
- 73.Von Bubnoff D, et al. Kinetics of gene induction after FcepsilonRI ligation of atopic monocytes identified by suppression subtractive hybridization. J Immunol. 2002;169(11):6170–6177. doi: 10.4049/jimmunol.169.11.6170. [DOI] [PubMed] [Google Scholar]
- 74.Le T, et al. Interferons modulate Fc epsilon RI-dependent production of autoregulatory IL-10 by circulating human monocytoid dendritic cells. J Allergy Clin Immunol. 2009;123(1):217–223. doi: 10.1016/j.jaci.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pyle DM, Yang VS, Gruchalla RS, Farrar JD, Gill MA. IgE cross-linking critically impairs human monocyte function by blocking phagocytosis. J Allergy Clin Immunol. 2013;131(2):491–500. doi: 10.1016/j.jaci.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. J Allergy Clin Immunol. 2008;121(2):486–491. doi: 10.1016/j.jaci.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 77.Khan SH, Grayson MH. Cross-linking IgE augments human conventional dendritic cell production of CC chemokine ligand 28. J Allergy Clin Immunol. 2010;125(1):265–267. doi: 10.1016/j.jaci.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novak N, et al. FcepsilonRI engagement of Langerhans cell-like dendritic cells and inflammatory dendritic epidermal cell-like dendritic cells induces chemotactic signals and different T-cell phenotypes in vitro. J Allergy Clin Immunol. 2004;113(5):949–957. doi: 10.1016/j.jaci.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167(2):797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 80.von Bubnoff D, et al. FcepsilonRI induces the tryptophan degradation pathway involved in regulating T cell responses. J Immunol. 2002;169(4):1810–1816. doi: 10.4049/jimmunol.169.4.1810. [DOI] [PubMed] [Google Scholar]
- 81.Mellor AL, Munn DH. Physiologic control of the functional status of Foxp3+ regulatory T cells. J Immunol. 2011;186(8):4535–4540. doi: 10.4049/jimmunol.1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 83.Schroeder JT, et al. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175(9):5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 84.Gill MA, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184(11):5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Platzer B et al (2014) Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol. doi:10.1038/mi.2014.85 [DOI] [PMC free article] [PubMed]
- 86.Sharquie IK, et al. An investigation into IgE-facilitated allergen recognition and presentation by human dendritic cells. BMC immunology. 2013;14:54. doi: 10.1186/1471-2172-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sallmann E, et al. High-affinity IgE receptors on dendritic cells exacerbate Th2-dependent inflammation. J Immunol. 2011;187(1):164–171. doi: 10.4049/jimmunol.1003392. [DOI] [PubMed] [Google Scholar]
- 88.Baravalle G, Greer AM, LaFlam TN, Shin JS. Antigen-conjugated human IgE induces antigen-specific T cell tolerance in a humanized mouse model. J Immunol. 2014;192(7):3280–3288. doi: 10.4049/jimmunol.1301751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dombrowicz D, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157(4):1645–1651. [PubMed] [Google Scholar]
- 90.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7(12):975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jarrett E, Bazin H. Elevation of total serum IgE in rats following helminth parasite infection. Nature. 1974;251(5476):613–614. doi: 10.1038/251613a0. [DOI] [PubMed] [Google Scholar]
- 92.Gleich GJ, Dunnette SL, Volenec FJ, Mani MM. Quantification of serum IgE in patients with burns. Clinical allergy. 1979;9(2):133–139. doi: 10.1111/j.1365-2222.1979.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 93.Szczeklik A, Jawien J. Immunoglobulin E in acute phase response to surgical stress. Clin Exp Allergy. 1996;26(3):303–307. doi: 10.1111/j.1365-2222.1996.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 94.McCoy KD, et al. Natural IgE production in the absence of MHC Class II cognate help. Immunity. 2006;24(3):329–339. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 95.Dreskin SC, Goldsmith PK, Strober W, Zech LA, Gallin JI. Metabolism of immunoglobulin E in patients with markedly elevated serum immunoglobulin E levels. J Clin Invest. 1987;79(6):1764–1772. doi: 10.1172/JCI113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iio A, Waldmann TA, Strober W. Metabolic study of human IgE: evidence for an extravascular catabolic pathway. J Immunol. 1978;120(5):1696–1701. [PubMed] [Google Scholar]
- 97.Watanabe N, Owhashi M, Nawa Y. Clearance of passively transferred IgE antibody from peripheral blood of mast cell-deficient W/Wv mice. Int Arch Allergy Appl Immunol. 1986;81(4):385–387. doi: 10.1159/000234170. [DOI] [PubMed] [Google Scholar]
- 98.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75(5):969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 99.Novak N, Peng WM, Bieber T, Akdis C. FcepsilonRI stimulation promotes the differentiation of histamine receptor 1-expressing inflammatory macrophages. Allergy. 2013;68(4):454–461. doi: 10.1111/all.12109. [DOI] [PubMed] [Google Scholar]
- 100.Wollenberg A, Kraft S, Hanau D, Bieber T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol. 1996;106(3):446–453. doi: 10.1111/1523-1747.ep12343596. [DOI] [PubMed] [Google Scholar]
- 101.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grayson MH, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204(11):2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hammad H, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weigmann B, et al. Allergen-induced IgE-dependent gut inflammation in a human PBMC-engrafted murine model of allergy. J Allergy Clin Immunol. 2012;129(4):1126–1135. doi: 10.1016/j.jaci.2011.11.036. [DOI] [PubMed] [Google Scholar]