Abstract
Serotonin (5-HT) influences locomotion in many animals, from flatworms to mammals. This study examined the effects of 5-HT on locomotion in the nudibranch mollusc Melibe leonina (Gould, 1852). M. leonina exhibits two modes of locomotion, crawling and swimming. Animals were bath-immersed in a range of concentrations of 5-HT or injected with various 5-HT solutions into the hemolymph and then monitored for locomotor activity. In contrast to other gastropods studied, M. leonina showed no significant effect of 5-HT on the distance crawled or the speed of crawling. However, the highest concentration (10−3 mol l−1 for bath immersion and 10−5 mol l−1 for injection) significantly increased the time spent swimming and the swimming speed. The 5-HT receptor antagonist methysergide inhibited the influence of 5-HT on the overall amount of swimming but not on swimming speed. These results suggest that 5-HT influences locomotion at the behavioral level in M. leonina. In conjunction with previous studies on the neural basis of locomotion in M. leonina, these results also suggest that this species is an excellent model system for investigating the 5-HT modulation of locomotion.
5-HT is a ubiquitous chemical messenger within the animal kingdom. Among its many functions, it appears to be highly conserved as a modulator and initiator of locomotor systems in both vertebrates (1) and invertebrates (2). In gastropods, 5-HT increases the frequency of pedal cilia beating (3), increases the frequency of muscular waves in the foot (4, 5), and initiates and modulates swimming (6–9). This current study examines the role of 5-HT in locomotion (specifically crawling and swimming) in the nudibranch mollusc Melibe leonina. While M. leonina is a well-studied model organism for neurophysiology, nothing is yet known about the effects of serotonin at the behavioral level.
M. leonina crawls using a combination of muscular contractions of the foot and beating of pedal cilia (pers. obs.), and it swims by alternating lateral body flexions (10). In this animal, swimming is an important behavior that it uses for many purposes, including to escape from predators (10). The central pattern generator (CPG) responsible for the swimming behavior consists of only two types of interneurons, swim interneuron 1 (Si1) and swim interneuron 2 (Si2) (11). These swim interneurons synapse on follower neurons in the pedal ganglia that carry the neural signal to the periphery (12). The relative simplicity of this CPG makes it an excellent system for studying the neural basis of behavior. The neural control of crawling, unlike that of swimming, has yet to be determined in M. leonina.
In addition to swim interneurons, putative serotonergic neurons have been identified in the central nervous system of M. leonina (13). About 175 neurons in the brain are serotonergic, but these do not include Si1 or Si2 (13, 14). However, in isolated brain preparations 5-HT elicits swim motor patterns in the swim CPG and accelerates ongoing swim motor patterns (14). Thus, in M. leonina, 5-HT may have a role in locomotion similar to that in other gastropods, but this has not been tested at the behavioral level.
This study tests the effects of 5-HT on locomotion in intact, freely behaving animals. Two methods were used: immersion of animals in a solution containing 5-HT and injection of 5-HT into the hemolymph.
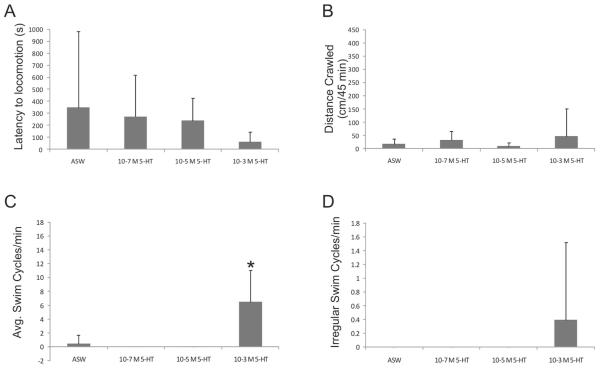
The mean latency to locomotion (i.e., length of time after exposure to 5-HT before the animal started crawling or swimming) gradually decreased as a result of immersion in increasing concentrations of 5-HT—10−7 mol l−1 to 10−3 mol l−1—chosen on the basis of prior research (4, 6, 8, 14; Fig.1A). However, these differences were not significant. There was also no significant effect of 5-HT on the distance crawled (Fig. 1B). These results suggest that 5-HT does not have a general excitatory effect on locomotion or crawling.
Figure 1.
Immersion in serotonin (5-HT) increased swimming but not crawling in Melibe leonina. Animals were placed individually in a small, clear plastic container containing artificial seawater (ASW). The container had a 1-cm grid pattern drawn on the bottom and sides to track the distance that animals crawled. Each animal was allowed to acclimate to the container for at least 30 min before the experimental solution was added. One hundred milliliters of 5-HT solution (Sigma-Aldrich, St. Louis, MO), or ASW, was added to the container such that the final concentration of 5-HT in the container was 10−3 mol l−1, 10−5 mol l−1, or 10−7 mol l−1. Animals were then visually monitored for at least 45 min. (A) Increasing concentrations of 5-HT resulted in a trend toward decreased latency to locomotion (i.e., the amount of time before the animal exhibited locomotor behavior). However, this trend was not significant (P = 0.29, F3,44 = 1.30). (B) Serotonin had no significant effect (P = 0.34, F3,44 = 1.14) on the distance crawled, which was calculated as the number of centimeters crawled in 45 min. (C) In contrast, the highest concentration of 5-HT significantly increased the average number of swim cycles observed during the experiment (P < 0.001, F3,44 = 22.25). A swim cycle was determined as a bend to one side, followed by a bend to the other side, and then a return to the central longitudinal axis. (D) In the highest concentration of 5-HT, some animals exhibited some irregular swim behavior, which typically consisted of incomplete swim cycles. However, the frequency of these irregular swim cycles was not significantly different between bath solutions (P = 0.23, F3,44 = 1.50). Forty-eight animals were used, with 12 in each treatment group. Error bars indicate standard deviation. Statistical comparisons of means here and elsewhere were made in InStat ver. 3 (GraphPad Software, San Diego, CA) with ANOVAs and Tukey post hoc analysis. For all statistical tests, a P value less than 0.05 was considered significant.
In contrast, 5-HT did significantly increase the number of swim cycles (Fig. 1C). In artificial seawater (ASW) and the two lowest concentrations of 5-HT, the prevalence of swimming was nearly zero. However, in 10−3 mol l−1 5-HT, a prevalence of 6.5 ± 4.5 swim cycles/min (mean ± standard deviation) was observed, which was significantly greater than in the other bath solutions. In ASW and the two weaker 5-HT concentrations, there were no irregular swim cycles. In contrast, irregular swim cycles (typically incomplete swim cycles) occurred with a mean frequency of 0.4 ± 1.1 cycles/min in the highest concentration of 5-HT (Fig. 1D). However, this was not statistically different than in the less concentrated 5-HT bath solutions or ASW.
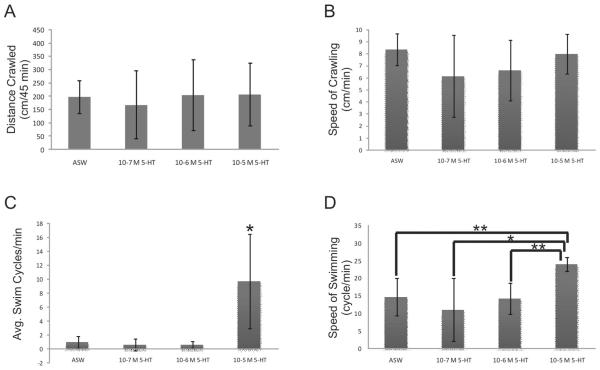
As with bath immersion, injection with increasing concentrations of 5-HT had no significant effect on the distance crawled during the observation period (Fig. 2A) or on the speed of crawling when it occurred (Fig. 2B). However, injection with the highest concentration of 5-HT (10−5 mol l−1) significantly increased the amount of swimming observed during the experiment (9.73 ± 6.32 cycles/min) when compared to other 5-HT concentrations and ASW (Fig. 2C). Furthermore, when swimming did occur, animals that had been injected with 10−5 mol l−1 5-HT exhibited a significantly higher frequency of swimming (24.23 ± 1.9 cycles/min) than animals injected with lower concentrations of 5-HT or with ASW (Fig. 2D). This confirms previous experiments demonstrating excitatory effects of 5-HT on the swim motor program in isolated brains of M. leonina (14).
Figure 2.
Serotonin injected through the ventral surface of the foot increased swimming but not crawling in Melibe leonina. Solution concentrations based on animal volume were injected such that final internal hemolymph concentrations were as indicated on the graphs. Injection with ASW served as a negative control. Serotonin had no effect on the distance crawled (A) (P = 0.97, F3,27 = 0.08) or the speed of crawling when it did occur (B) (P = 0.22, F3,27 = 1.57). (C) However, the highest concentration of 5-HT significantly increased the average swimming rate (P < 0.001, F3,27 = 15.78). (D) 5-HT at 10−5 mol l−1 also caused a significant increase in the speed of swimming during bouts of this behavior (*P < 0.001, **P < 0.05, F3,27 = 6.86). Thirty-two animals were used, with 8 in each treatment group.
The number of swim cycles exhibited by animals in the presence of ASW or relatively dilute concentrations of 5-HT was very low. This is probably due to the fact that these animals are generally nocturnal (15) and these experiments were purposely done during the daytime to limit normal locomotion. However, immersion or injection with the highest concentration of 5-HT resulted in a sharp increase in the number of swim cycles. This result is similar to responses in Aplysia brasiliana (6), another opisthobranch that, like M. leonina, uses swimming as more than just an escape behavior. Serotonin may be acting as a trigger for initiation of swimming. This would coincide with neurophysiological studies in M. leonina that suggest that the serotonergic CeSP-A neurons are triggers for swimming (14).
Injection of animals with 10−5 mol l−1 5-HT had some additional noticeable effects. Five of the eight animals injected with this concentration of 5-HT autotomized at least two cerata (dorsal outgrowths), suggesting that 5-HT may also play a role in this defensive response. Three of the eight animals also exhibited some instances of atypical crawling behavior. Instead of crawling with the whole foot attached to the substrate, the rear half of the foot was detached from the substrate and moved in a motion similar to swimming while the front half of the foot remained attached and exhibited the muscular contractions typical of crawling. In other words, the animals appeared to be exhibiting elements of both crawling and swimming simultaneously. These effects may be due to specific activation of particular circuits and actions, or they could be the result of a 5-HT-induced increase in general arousal (16, 17).
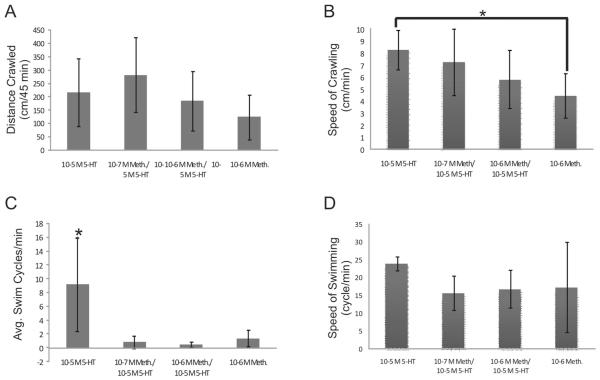
Additional experiments were done using the 5-HT receptor antagonist methysergide (Meth), which has been successfully used in M. leonina (14) and other opisthobranchs (6, 8) to inhibit the effects of serotonin. When Meth was injected either alone or along with 5-HT (at two different concentrations), there were no significant effects on the distance crawled during the experiment (Fig. 3A). This is not surprising, considering that 5-HT itself had no effect on crawling distance (Fig. 2A). Although there was also no effect of Meth on the speed of crawling in animals with exogenously applied 5-HT, the speed of crawling in animals injected only with Meth was significantly less than in animals injected only with 5-HT (Fig. 3B). This suggests that even though endogenous 5-HT may not be involved with the prevalence of crawling (Fig. 3A), it may be important in the speed of crawling.
Figure 3.
The serotonin receptor antagonist methysergide (Meth; Sigma-Aldrich, St. Louis, MO), blocked some of 5-HT’s excitation of swimming in Melibe leonina. Meth was injected into animals either alone (negative control) or with 5-HT. Serotonin injections without Meth were also used as a positive control. (A) There was no significant difference between any of the groups in the distance crawled during the experiments (P = 0.18, F3,21 = 1.81). (B) Meth did not significantly affect the speed of crawling in the presence of exogenously applied 5-HT, but when injected alone, did significantly reduce the speed of crawling compared to 5-HT alone (P = 0.05, F3,21 = 3.08). (C) Meth was able to significantly block the overall excitatory effect of 5-HT on swimming (P = 0.0004, F3,21 = 9.37) but not the rate of body flexions during swimming bouts (D) (P = 0.16, F3,21 = 1.90). Thirty-two animals were used, with 8 in each treatment group.
Meth also influenced swimming. When injected alone or with 5-HT (at two different concentrations), the number of swim cycles was significantly lower than when just 5-HT was used (Fig. 3C). This suggests that Meth was capable of blocking 5-HT’s influence on the prevalence of swimming. Interestingly, Meth was not able to significantly block the effects of 5-HT on swimming speed (Fig. 3D). This may be the result of only partial inhibition of 5-HT receptors but still contradicts results in isolated brain preparations of M. leonina where Meth significantly reduced 5-HT’s ability to increase the cycle frequency of ongoing swim motor patterns (14). However, in intact A. brasiliana, Meth does not alter the frequency of 5-HT-induced swimming (6). It should also be noted that swimming in the current study still occurred after injection with just Meth (right bars in Fig. 3C, D). This suggests that 5-HT was not necessary for swimming to occur (i.e., it was only important for modulation).
In contrast to studies in other molluscs, our study found that exogenous 5-HT did not influence crawling in M. leonina. Pharmacological application of similar concentrations of 5-HT excite pedal cilia in the relatively closely related nudibranch Tritonia diomedea, both in isolated patches of foot and in intact animals (3). The opisthobranch sea hare Aplysia californica crawls in a manner similar to M. leonina, and 5-HT increases the speed of muscular waves in the foot (4). Even the more distantly related pulmonate gastropod Helix lucorum increases its crawling speed (muscular waves) after injection of 5-HT (5). However, it is notable in the current study that Meth alone did significantly reduce the speed of crawling (Fig. 3B), suggesting that endogenous serotonin release may play a role in this aspect of crawling even though exogenous application of 5-HT did not increase the speed further. Considering that serotonergic innervation of the foot has been demonstrated in a number of gastropods (2, 18–20), it would be informative to examine whether there is serotonergic innervation of the foot in M. leonina as well.
In regard to swimming, 5-HT appears to be important in eliciting this behavior as well as in its speed. It is likely that these two effects of 5-HT are produced by distinct neural mechanisms, especially considering that Meth reduced the number of swimming episodes but not the swimming speed (Fig. 3C, D). Similar to exogenous application of 5-HT in this study, stimulation of the serotonergic CeSP-A neurons in M. leonina elicits a swim motor program in quiescent preparations (14). Therefore, the CeSP-A neurons may be at least part of the endogenous serotonergic system responsible for influencing the likelihood of swimming. However, stimulation of these same neurons during an ongoing swim motor pattern has no effect on burst frequency of swim interneurons (14). Thus, the neural mechanisms underlying the speed of swimming noted in this study remain to be determined.
Serotonin has similar excitatory roles in locomotion in other invertebrates, such as the flatworm Dendrocoelum lacteum (2), the nematode Caenorhabditis elegans (21), the freshwater mussel Anodonta cygnea (2), and the arthropod Drosophila melanogaster (22). Serotonin also elicits swim motor programs in isolated nerve cords of the leech Hirudo medicinalis (23), similar to the results in the current study on M. leonina. The excitatory effects that 5-HT has on locomotion in invertebrates is similar to that in vertebrates, such as the zebrafish Danio rerio (24), the frog Xenopus laevis (25), neonatal mice (26), and spinalized cats (27). Serotonin can also elicit locomotor patterns of activity in rabbits (28) and rats (29). Thus, 5-HT influences locomotion in at least six phyla, suggesting that this is a highly conserved or convergent feature of nervous systems.
The current study indicates that 5-HT affects locomotion in Melibe leonina, adding behavioral data on 5-HT to prior experiments at the neural level. This more complete story suggests that M. leonina is therefore an excellent model system for exploring the mechanisms underlying serotonergic modulation of locomotion. Because 5-HT modulates locomotion in many phyla, determining the role of 5-HT in a relatively simple CPG, such as in M. leonina, can potentially be helpful in understanding its role in more complex neural networks.
Acknowledgments
We thank David Duggins for collecting and shipping animals, Maria Colby for ordering supplies, and Ryan Amick, Emily Baker, Lori Bergeron, Katie Cloughley, Eric Simon, and two anonymous reviewers for providing valuable feedback. This work was supported by funding from New England College and the National Institutes of Health, through a New Hampshire INBRE grant.
Abbreviations
- ASW
artificial seawater
- CPG
central pattern generator
- 5-HT
serotonin
- Meth
methysergide
- Si1
swim interneuron 1
- Si2
swim interneuron 2
Literature Cited
- 1.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 2.Sakharov DA. Integrative function of serotonin common to distantly related invertebrate animals. In: Gustafsson M, Reuter M, editors. The Early Brain: Proceedings of the Symposium “Invertebrate Neurobiology,”; Åbo, Finland: Åbo Academy Press; 1990. pp. 73–88. [Google Scholar]
- 3.Audesirk G, McCaman RE, Willows AOD. The role of serotonin in the control of pedal ciliary activity by identified neurons in Tritonia diomedea. Comp. Biochem. Physiol. C. 1979;62:87–91. doi: 10.1016/0306-4492(79)90104-7. [DOI] [PubMed] [Google Scholar]
- 4.Mackey S, Carew TJ. Locomotion in Aplysia: triggering by serotonin and modulation by bag cell extract. J. Neurosci. 1983;3:1469–1477. doi: 10.1523/JNEUROSCI.03-07-01469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlova GA. Effects of serotonin, dopamine and ergometrine on locomotion in the pulmonate mollusc Helix lucorum. J. Exp. Biol. 2001;204:1625–1633. doi: 10.1242/jeb.204.9.1625. [DOI] [PubMed] [Google Scholar]
- 6.Parsons DW, Pinsker HM. Swimming in Aplysia brasiliana: behavioral and cellular effects of serotonin. J. Neurophysiol. 1989;62:1163–1176. doi: 10.1152/jn.1989.62.5.1163. [DOI] [PubMed] [Google Scholar]
- 7.Kabotyanski EA, Milošević I, Sakharov DA. Neuronal correlates of 5-hydroxytryptophan-induced sustained swimming in Aplysia fasciata. Comp. Biochem. Physiol. C. 1990;95:39–44. [Google Scholar]
- 8.McClellan AD, Brown GD, Getting PA. Modulation of swimming in Tritonia: excitatory and inhibitory effects of serotonin. J. Comp. Physiol. A. 1994;174:257–266. doi: 10.1007/BF00193792. [DOI] [PubMed] [Google Scholar]
- 9.Satterlie RA, Norekian TP. Modulation of swimming speed in the pteropod mollusc, Clione limacina: role of a compartmental serotonergic system. Invertebr. Neurosci. 1996;2:157–165. doi: 10.1007/BF02214171. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence KA, Watson WH., III Swimming behavior of the nudibranch Melibe leonina. Biol. Bull. 2002;203:144–151. doi: 10.2307/1543383. [DOI] [PubMed] [Google Scholar]
- 11.Thompson SH, Watson WH., III Central pattern generator for swimming in Melibe. J. Exp. Biol. 2005;208:1347–1361. doi: 10.1242/jeb.01500. [DOI] [PubMed] [Google Scholar]
- 12.Watson WH, III, Newcomb JM, Thompson S. Neural correlates of swimming behavior in Melibe leonina. Biol. Bull. 2002;203:152–160. doi: 10.2307/1543384. [DOI] [PubMed] [Google Scholar]
- 13.Newcomb JM, Fickbohm DJ, Katz PS. Comparative mapping of serotonin-immunoreactive neurons in the centralnervous systems of nudibranch molluscs. J. Comp. Neurol. 2006;499:485–505. doi: 10.1002/cne.21111. [DOI] [PubMed] [Google Scholar]
- 14.Newcomb JM, Katz PS. Different functions for homologous serotonergic interneurons and serotonin in species-specific rhythmic behaviours. Proc. R. Soc. B. 2009;276:99–108. doi: 10.1098/rspb.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcomb JM, Lawrence KA, Watson WH., III The influence of light on locomotion in the gastropod Melibe leonina. Mar. Freshw. Behav. Physiol. 2004;37:253–267. [Google Scholar]
- 16.Katz PS, Fickbohm DJ, Lynn-Bullock CP. Evidence that the central pattern generator for swimming in Tritonia arose from a non-rhythmic neuromodulatory arousal system: implications for the evolution of specialized behavior. Am. Zool. 2001;41:962–975. [Google Scholar]
- 17.Marinesco S, Wickremasinghe N, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia. II. Cellular and behavioral consequences of increased serotonergic tone. J. Neurophysiol. 2004;92:2487–2496. doi: 10.1152/jn.00210.2004. [DOI] [PubMed] [Google Scholar]
- 18.Caunce M, McKenzie JP, Tripp J, Winlow W. Serotonergic innervation of pedal epidermis of Lymnaea. In: Salànki J, S-Rózsa K, editors. Neurobiology of Invertebrates: Transmitters, Modulators and Receptors. Akadémiai Kiadó; Budapest: 1988. pp. 691–692. [Google Scholar]
- 19.Moroz LL, Sudlow LC, Jing J, Gillette R. Serotonin-immunoreactivity in peripheral tissues of the opisthobranch molluscs Pleurobranchaea californica and Tritonia diomedea. J. Comp. Neurol. 1998;382:176–188. doi: 10.1002/(sici)1096-9861(19970602)382:2<176::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg R. Serotonin-like immunoreactivity in the central and peripheral nervous systems of the interstitial acochlidean Asperspina sp. (Opisthobranchia) Biol. Bull. 2007;213:43–54. doi: 10.2307/25066617. [DOI] [PubMed] [Google Scholar]
- 21.Hardaker LA, Singer E, Kerr R, Zhou G, Schafer WR. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J. Neurobiol. 2001;49:303–313. doi: 10.1002/neu.10014. [DOI] [PubMed] [Google Scholar]
- 22.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willard AL. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J. Neurosci. 1981;1:936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brustein E, Chong M, Holmqvist B, Drapeau P. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J. Neurobiol. 2003;57:303–322. doi: 10.1002/neu.10292. [DOI] [PubMed] [Google Scholar]
- 25.Sillar KT, S.-Wedderburn JF, Simmers AJ. Modulation of swimming rhythmicity by 5-hydroxytryptamine during post-embryonic development in Xenopus laevis. Proc. R. Soc. Lond. B. 1992;250:107–114. doi: 10.1098/rspb.1992.0137. [DOI] [PubMed] [Google Scholar]
- 26.Zhong G, Díaz-Ríos M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J. Neurosci. 2006;26:6509–6517. doi: 10.1523/JNEUROSCI.1410-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- 28.Viala D, Buser P. The effects of DOPA and 5-HTP on rhythmic efferent discharges in hind limb nerves in the rabbit. Brain Res. 1969;12:437–443. doi: 10.1016/0006-8993(69)90011-0. [DOI] [PubMed] [Google Scholar]
- 29.Cazalets JR, Sqalli-Housaaini Y, Clarac F. Activation of the central pattern generator for locomotion by serotonin and excitatory amino acids in neonatal rat. J. Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]