Abstract
Daily rhythms of activity driven by circadian clocks are expressed by many organisms, including molluscs. We initiated this study, with the nudibranch Melibe leonina, with four goals in mind: (1) determine which behaviors are expressed with a daily rhythm; (2) investigate which of these rhythmic behaviors are controlled by a circadian clock; (3) determine if a circadian clock is associated with the eyes or optic ganglia of Melibe, as it is in several other gastropods; and (4) test the hypothesis that Melibe can use extraocular photoreceptors to synchronize its daily rhythms to natural light-dark cycles. To address these goals, we analyzed the behavior of 55 animals exposed to either artificial or natural light-dark cycles, followed by constant darkness. We also repeated this experiment using 10 animals that had their eyes removed. Individuals did not express daily rhythms of feeding, but they swam and crawled more at night. This pattern of locomotion persisted in constant darkness, indicating the presence of a circadian clock. Eyeless animals also expressed a daily rhythm of locomotion, with more locomotion at night. The fact that eyeless animals synchronized their locomotion to the light-dark cycle suggests that they can detect light using extraocular photoreceptors. However, in constant darkness, these rhythms deteriorated, suggesting that the clock neurons that influence locomotion may be located in, or near, the eyes. Thus, locomotion in Melibe appears to be influenced by both ocular and extraocular photoreceptors, although the former appear to have a greater influence on the expression of circadian rhythms.
Introduction
Most organisms are typically more active at certain times of the day or night, and this tendency is controlled, in part, by endogenous circadian clocks. When these organisms are placed in constant light, or constant darkness, they no longer receive light cues about the time of day, but they continue to express daily rhythms of behavior that have a period of “about a day” and thus they are referred to as “circadian” rhythms. Circadian rhythms of behavior, along with the molecular clocks underlying these rhythms, have been studied in a wide array of animals (Takahashi, 1991, 1995; Dunlap, 1999; Hastings et al., 2007; Allada and Chung, 2010). However, the neural mechanisms by which these well-understood molecular clocks ultimately influence the expression of circadian behaviors have not been fully elucidated. To bridge this gap, it would be advantageous to study an animal with clearly defined and easily accessible neural circuitry underlying a specific behavior that is expressed with a circadian rhythm. Gastropods have been very useful model organisms for neuroethological investigations during the last several decades, and using gastropod model systems may help elucidate the connection between clocks and certain behaviors.
Circadian rhythms of locomotion have been demonstrated in six gastropods—Aplysia californica (Kupfermann, 1968; Jacklet, 1972; Lickey et al., 1977), Bulla gouldiana (Block and Davenport, 1982), Bursatella leachi plei (Block and Roberts, 1981), Helisoma trivolvis (Kavaliers, 1981), Limax maximus (Sokolove et al., 1977), and Melanoides tuberculata (Beeston and Morgan, 1979). Aplysia and Bursatella exhibit diurnal activity in light-dark regimes (Kupfermann, 1968; Jacklet, 1972; Kupfermann and Carew, 1974; Lickey et al., 1977; Block and Roberts, 1981), whereas Bulla, Helisoma, and Limax are nocturnal (Sokolove et al., 1977; Kavaliers, 1981; Block and Davenport, 1982), and Melanoides exhibits crepuscular patterns of locomotor activity (Beeston and Morgan, 1979). In Aplysia, Bursatella, and Bulla, circadian pacemakers have been localized to the eyes, which, when isolated in vitro, exhibit circadian rhythms of electrical activity in constant darkness (Jacklet, 1969; Block and Roberts, 1981; Block and Wallace, 1982). However, further studies have indicated that the eyes are not the only circadian pacemakers in Aplysia and Bulla (Lickey et al., 1977, 1983; Roberts and Xie, 1996).
While the aforementioned investigations into the circadian rhythms of locomotion in gastropods have revealed a great deal about the location and nature of the underlying circadian clocks, the link between these clocks and the neural networks controlling locomotion has yet to be determined. Thus, we have turned our attention to another species, Melibe leonina (Gould, 1852), because the central pattern generator underlying at least one form of locomotion in this nudibranch is well understood. Melibe exhibits two modes of locomotion—crawling, like most other gastropods, and swimming, via lateral body flexions (Watson et al., 2001; Lawrence and Watson, 2002). Melibe swims as an escape mechanism (Lawrence and Watson, 2002), spontaneously for reasons that are currently poorly understood, and perhaps as a form of population dispersal (Mills, 1994). Both crawling and swimming are exhibited with greater frequency at night, and preliminary evidence from animals in 36–48 h of constant darkness suggests that these forms of locomotion may be influenced by a circadian clock (Newcomb et al., 2004). Importantly, the central pattern generator underlying swimming has been characterized and consists of only eight individually identifiable neurons (Thompson and Watson, 2005; Sakurai et al., 2014). Thus, Melibe exhibits a specific behavior, controlled by a relatively small number of identified neurons, which appears to be influenced by an endogenous circadian clock.
The purpose of this study was fourfold. First, we aimed to more rigorously determine if Melibe exhibits clear daily rhythms of specific behaviors (crawling, swimming, feeding, and feeding while crawling) in normal light-dark (LD) conditions. Second, once we identified behaviors with daily rhythms, our goal was to determine if they persisted, as circadian rhythms, in constant darkness (DD). Third, we tested the hypothesis that the circadian clock of Melibe is located in, or near, the eyes, as in several other molluscs. Finally, we sought to determine if Melibe is capable of synchronizing its activity to ambient light changes in the absence of its eyes, using extraocular photoreceptors. Ultimately, these studies will provide a foundation for further investigations of how clocks interact with the neural circuitry underlying a behavior that is expressed with a circadian rhythm.
Materials and Methods
Animal collection and housing
Specimens of Melibe were obtained during 2011–2013 from two areas. Some animals were collected in southern California by Marinus Scientific (Newport Beach, CA) and the Monterey Abalone Company (Monterey, CA), while others were obtained from the waters surrounding San Juan Island and Shaw Island, Washington. Animals used in 2011–2012 were shipped to New England College (NEC) and the University of New Hampshire (UNH) within 24 h of collection and housed in tanks filled with either artificial seawater (Carolina Biological) or natural seawater from the UNH Coastal Marine Laboratory (New Castle, NH). The seawater was maintained at temperatures between 10 and 15 °C and salinities of 29–32 ppt. Animals were typically fed Artemia nauplii 2–3 times per week prior to experiments. Lighting conditions matched those used during subsequent LD experiments—either 10 h of light and 14 h of darkness (10:14 LD) or 12:12 LD. Light was provided with 40–60-W compact fluorescent bulbs, which typically resulted in daytime light levels of 215–290 lux.
The animals collected and used in the winter and spring of 2013 (n = 18) were housed in outdoor flow-through seawater tanks at the Friday Harbor Laboratories (FHL; Friday Harbor, WA), less than 2 km from where they were collected. At this time of year, the ambient water temperature ranged from 8–10 °C. These animals fed ad libitum on prey carried into their tanks via the flow-through seawater system, and they were subjected to ambient LD cycles. As indicated in subsequent figures, the light intensity during the day was typically 375–800 lux.
Activity experiments
Individuals were each placed in a circular plastic container (20–30 cm in diameter) with screened openings in the sides to allow adequate flow of fresh seawater through each container. At NEC, containers were placed inside temperature-controlled aquaria; at UNH they were inside larger containers filled with seawater and located in a cold room held at 10–12 °C; and at FHL they were located in a sea table continuously supplied with ambient seawater. This arrangement made it possible to provide adequate filtering and aeration of the seawater while maintaining a calm environment inside each container, which facilitated subsequent analysis of the videos. Animals were subjected to a 10:14 or 12:12 LD regimen in New Hampshire or ambient lighting at FHL for the initial 3–7 days of the trial, followed by DD for 5–10 days. During the periods of darkness for all experiments, an infrared light was used to illuminate the arenas; preliminary electrophysiological data indicate that Melibe cannot perceive light wavelengths in the far red to infrared range. Water temperature and light levels were monitored with a HOBO Temperature/Light Pendant logger (Onset Computer, MA), set to obtain a reading every 10 min.
Behavior was monitored with an infrared-sensitive camera, and its output was digitized and captured at a rate of one frame per second. Both Gawker 8.0 (Phil Piwonka) and HandyAVI 4.3 (AZcendant) software were used to create the digital time-lapse videos, which were stored as separate files every 24 h. These videos were further compressed using HandyAVI or Stomp 1.24 (Neil Clayton) and then merged into a single video that encompassed the entire length of the experiment with either Quicktime (Apple) or Windows Movie Maker (Microsoft). Videos analyzed with Ethovision ver. 8.0 tracking software (Noldus, see below) were first converted to AVI format with either Format Factory (Free Time) or Quicktime.
Eyeless animal experiments
Eyes were surgically removed from 10 animals to determine if these structures, which are located on the brain, are necessary to entrain activity to a LD cycle or are the location of the circadian clock or clocks. After the animals recovered for 3 days, their activity was monitored using the same experimental protocol as outlined above. For the surgery, an incision was made in the dorsal integument behind the oral hood, above the brain where the eyes are located. Eyes were then excised and the wound was closed with a suture. Sham surgeries involved the cutting and sealing of the integument, but the eyes were left intact. Surgeries both for experimental animals (10) and sham controls (4) were completed in less than 15 min. All animals were given at least 3 days to recover before they were used in an experiment. All of the animals subjected to these surgical procedures survived for the duration of the experiments.
Data analysis
Locomotion was quantified either visually or using Ethovision software. The Ethovision software tracked individual animals once the user defined the amount of contrast and the general shape of the animal. The Ethovision data were exported into an Excel spreadsheet in terms of distance traveled per unit of time (cm/min). In videos analyzed by eye, the amount of time that animals spent crawling during each 10-min bin (of real time) was recorded. This approach was necessary in cases where the contrast was poor, and it was also used to calibrate the Ethovision system. Videos analyzed using both methods produced similar results. Therefore, the percentage of activity during day or night and tau values from actograms (see below) were ultimately pooled, regardless of the method of analysis.
Swimming and feeding were quantified only visually. Swimming was noted when animals detached from the wall or floor of the container and began flexing from side-to-side (Lawrence and Watson, 2002). Feeding involved a very noticeable opening and closing of the large oral hood (Hurst, 1968; Watson and Trimarchi, 1992). In some cases, videos were analyzed in 5-min intervals (of real time) and scored with a “1” if the behavior occurred during the period, or a “0” if the behavior did not occur. In other cases, the amount of time spent doing either swimming or feeding during 10-min bins (of real time) was recorded. Videos analyzed with both methods produced similar results, so data regarding the percentage of activity during day or night and tau values from actograms (see below) were considered together.
To determine whether animals expressed a given behavior more in the day versus the night, the percentage of time doing a given behavior during 10-min time bins was averaged for 5 consecutive days and nights, omitting the hour just before and just after both sunset and sunrise. A paired Student’s t-test (Instat, GraphPad) was then used to determine if there were statistically significant differences.
To determine the presence of circadian rhythms, most of the data were visualized as actograms in ClockLab 2.72 (Actimetrics), and then Lomb-Scargle periodograms were used to determine endogenous circadian periods (tau) of locomotion in the range of 20–28 h. A P-value of less than 0.01 (standard for this type of actogram analysis) was considered statistically significant. All variance values were reported as standard error of the mean.
Results
Daily patterns of behaviors
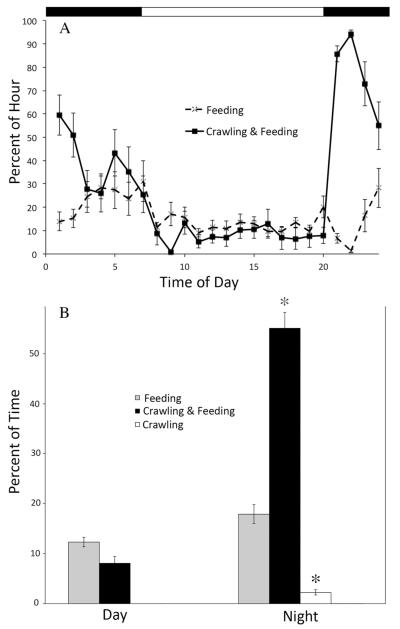
The dominant behaviors expressed by this species are swimming, crawling, and feeding. In addition, animals often feed while crawling. It is not clear whether they are actually capturing food during this activity or simply sampling the water in a search of planktonic prey. This feeding while crawling activity was actually the most common behavior expressed by animals in our experiments, followed by feeding alone, crawling alone, and then swimming. In normal LD conditions, animals fed, without crawling, at a consistently low level, regardless of the time of day. In contrast, crawling occurred significantly more frequently at night (P < 0.05; Fig. 1a, b; a statistical test was not performed for swimming because the animals used for this analysis swam only at night). For this reason, we focused our circadian analyses on locomotion.
Figure 1.
Locomotion, but not feeding, was expressed rhythmically in LD. (A) A comparison of the percentage of each hour Melibe (n = 3) spent engaged in two different behaviors, feeding alone vs. simultaneously crawling and feeding. Values represent the average for five consecutive days. Error bars indicate standard error of the mean (SEM). Note that the rate of feeding was fairly consistent throughout the day and night, while simultaneous crawling and feeding occurred more often at night. Black bars at top indicate periods of darkness. (B) A comparison of the mean percentage of time (± SEM) spent on three activities between day and night demonstrates significantly more (*) crawling and crawling while feeding during the night than the day, while there was no significant difference in the amount of feeding (when not exhibited with any locomotion) between day and night.
Locomotion in laboratory experiments
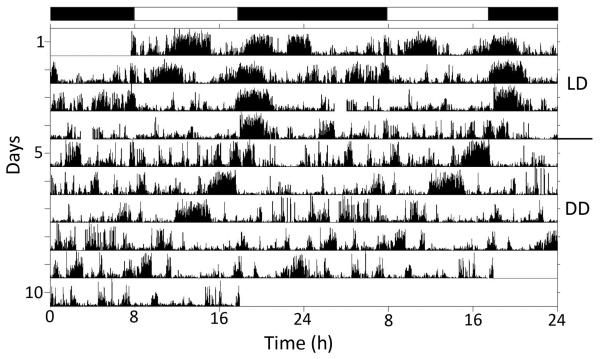
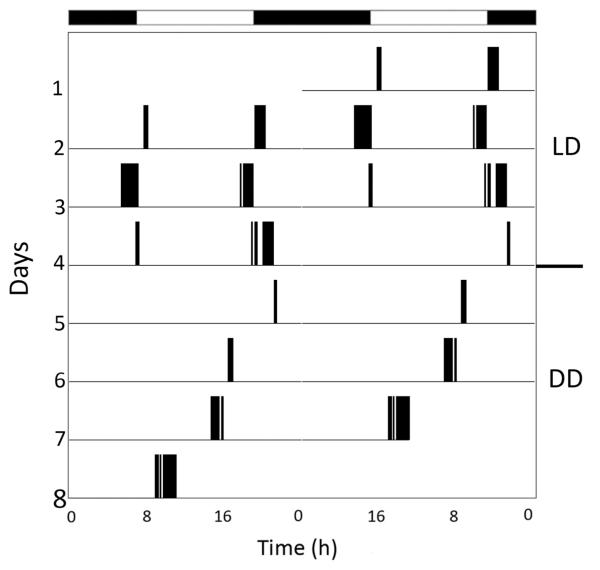
Time-lapse videos lasting at least 10 full days and nights were obtained in 2011–2012 from 12 animals at NEC and 35 individuals at UNH. Animals were typically subjected to an artificial LD cycle for 5–10 days, followed by DD for an additional 5–10 days. The majority (19/35 or 54% at UNH, and 9/12 or 75% at NEC) of these animals expressed a daily rhythm of locomotor activity in LD (the remainder were arrhythmic), and all of those with a daily rhythm preferred to be most active at night. This tendency held true for both crawling (Fig. 2) and swimming (Fig. 3), with the most activity typically occurring right after sunset. Animals swam significantly more often during the night (P < 0.0001), with 83.6% ± 3.2% of swim episodes occurring at night, compared to 16.4% ± 3.2% of swim episodes during the day. When animals that exhibited daily rhythms of activity in LD were subsequently exposed to DD, the majority of them (45% at UNH and 67% at NEC) continued to express an ~24 h, or circadian, rhythm (Figs. 2, 3), with mean periods (tau) of 23.2 ± 1.1 h for crawling and 23.5 ± 0.7 h for swimming.
Figure 2.
Double-plotted actogram showing the pattern of crawling expressed by one Melibe specimen in artificial LD for 4 days, followed by 6 days in DD. One line on the actogram represents 2 days. Black bars at top indicate periods of darkness in LD. While exhibiting some activity during the day, this animal was much more active during the early portion of the evening. In DD, this individual expressed a tau value of 21.3 h. With a tau value less than 24 h, the activity clearly shifts to the left on successive days.
Figure 3.
Actogram showing the daily rhythm of swimming expressed by a Melibe specimen in artificial LD for 3 days, followed by DD for 4 days. Black bars at top of figure indicate periods of darkness in LD. In LD, this animal consistently swam around sunset and sunrise. In DD, a circadian rhythm of swimming around subjective sunset persisted, with a tau value of 21.7 h.
Locomotion in experiments with natural light-dark conditions
In 2013, behaviors were recorded and quantified for eight animals exposed to natural LD cycles and ambient seawater at FHL. As expected, 6 of the 8 animals were more active at night than during the day (Figs. 4 and 5). Locomotion in the evening increased rapidly only after it became very dark, but ceased almost immediately at the return of light in the morning (Fig. 5). Subsequently, when 4 of the 8 were exposed to DD, 3 of them continued to express a circadian rhythm of locomotion (Fig. 4), but the rhythms in DD tended to be very weak compared to their daily rhythms in LD.
Figure 4.
Actogram showing a clear nocturnal pattern of crawling by a Melibe specimen exposed to a natural LD cycle, followed by DD (tau = 22.9 h). Black bars indicate periods of darkness in LD. Note that there was a period of inactivity around the transition from LD to DD. However, this inactivity began before the shift to DD, and other animals did not exhibit such periods of inactivity around this transition, suggesting that this period of quiescence was not related to the transition to DD.
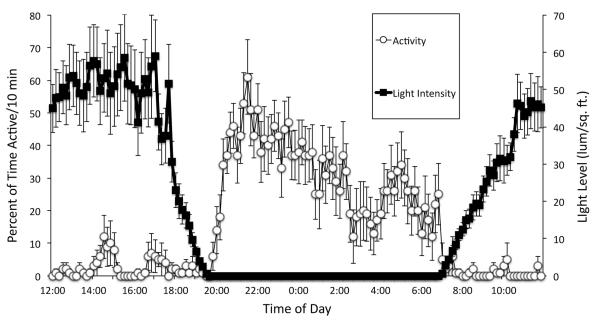
Figure 5.
The relationship between Melibe locomotion and ambient light levels. Data from 5 consecutive days are averaged and plotted (± SEM) for one sham-operated individual. Activity is calculated in terms of the percentage of each 10-min time interval that the animal was active. Note how strongly this animal’s behavior was influenced by the change in light intensity at sunset and sunrise.
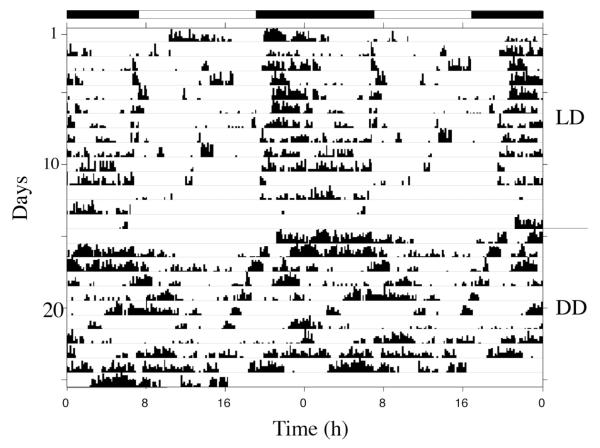
Eyeless animals
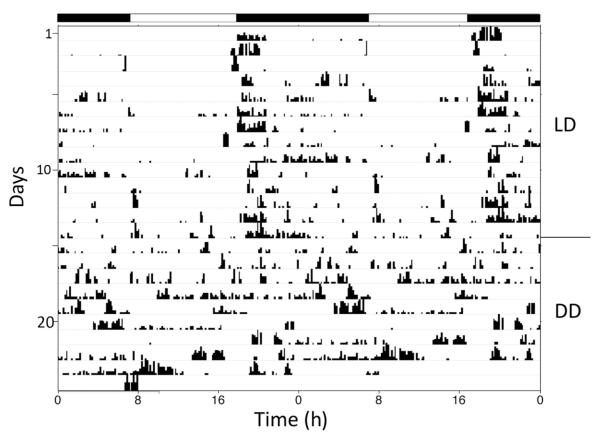
Experiments were conducted with eyeless animals to test two questions: (1) Is the circadian clock in Melibe associated with the eyes or optic ganglion? (2) Does Melibe have extraocular photoreceptors that can be used to entrain their behavioral rhythms to the LD cycle? The eyes, and associated small optic ganglia, were dissected from 10 animals and then, after at least 3 days of recovery, their behavioral rhythms were recorded first in LD (10), followed by DD (6 of the 10). Four additional animals served as sham-operated controls. While there was some individual variability in the overall levels of activity, as seen in normal animals, eyeless animals were indistinguishable from normal animals in a natural light cycle and expressed a strong tendency to be most active at night (Figs. 6 and 7). Moreover, like normal animals, their shifts in activity were very closely linked to changes in ambient light (Fig. 7). This suggests that they have extraocular photoreceptors. However, in DD, eyeless animals did not appear to express a circadian rhythm, suggesting that their circadian clocks might be located in, or close to, their eyes (Fig. 6).
Figure 6.
Representative actogram from a Melibe specimen that had its eyes removed, showing activity both in LD and DD. Black bars indicate periods of darkness in LD. This eyeless animal’s activity in LD was entrained to sunset, indicating an ability to still detect light. The pattern of activity became more arrhythmic in DD.
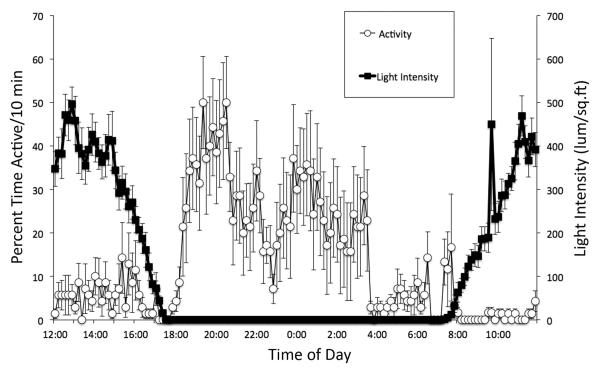
Figure 7.
The relationship between changes in ambient light levels and the activity of an eyeless Melibe specimen. The percentage of each 10-min time interval that a single animal was active, from 5 consecutive days, was averaged along with light data, for the same time intervals. Error bars represent SEM. Data were obtained in February 2013, so the days were relatively short. Note how nocturnal activity does not increase until after sunset is complete, and how sedentary this animal became after sunrise, indicating that the animal still coordinated its locomotor activity to light levels.
Discussion
Melibe exhibits nocturnal locomotion
In LD, animals exhibited higher levels of locomotor activity at night than during the day. Individuals were not always active all night, with many animals exhibiting the majority of their locomotion during the first few hours after sunset. This pattern was present regardless of whether animals were subjected to sudden light transitions in laboratory experiments or more gradual light changes in outdoor tanks. This suggests that this post-sunset peak in activity was not simply an artifact of a sudden change in light intensity. Nocturnal activity is exhibited by some gastropods (e.g., Helisoma and Limax [Sokolove et al., 1977; Kavaliers, 1981]), while diurnal and crepuscular patterns have been observed in other species (e.g., Aplysia, Bursatella, and Melanoides [Kupfermann, 1968; Jacklet, 1972; Kupfermann and Carew, 1974; Lickey et al., 1977; Beeston and Morgan, 1979; Block and Roberts, 1981]). The preference for moving around at night in Melibe may be related to the avoidance of visual predators such as the kelp crab Pugettia producta (Mauzey et al., 1968; Ajeska and Nybakken, 1976; Bickell-Page, 1991). Melibe secretes a terpenoid compound that is hypothesized to be repugnant to predators (Barsby et al., 2002). However, some potential predators, such as Pugettia, do not seem to be repelled by these secretions (Bickell-Page, 1991), and thus avoidance through nocturnal activity or escape swimming (Lawrence and Watson, 2002) may be an important survival strategy.
In contrast to locomotion, the amount of feeding did not vary on the basis of the time of day, unless it was tied to locomotion, such as when Melibe crawls and feeds simultaneously (Fig. 1). Melibe feeds differently than most other gastropods because it lacks a radula and uses a large, tentacle-lined oral hood to capture zooplankton, nauplii, and other small organisms floating by in the water column (Hurst, 1968; Watson and Trimarchi, 1992). Due to the potentially patchy distribution of prey, the combination of crawling and carrying out feeding-like movements with the oral hood might be a behavior intended to continually probe the environment for food that might drift by at any time of the day or night. Thus, this behavior might be considered more of an exploratory activity than actual feeding. When the animals are actively feeding, they are typically stationary, and the feeding behavior proceeds through all the normal phases (Hurst, 1968; Watson and Trimarchi, 1992; Watson and Chester, 1993). While animals did not spend a large amount of time just feeding in our experiments, it is likely that they apportion a greater percentage of time to this behavior in their natural habitat where food is more abundant than in the laboratory tanks.
Melibe exhibits circadian rhythms of locomotion
The locomotor patterns seen in LD tended to persist in the absence of light cues (DD) (Figs. 2–4), indicating the presence of an endogenous circadian clock. While the majority of animals exhibited this robust circadian activity, there was some notable variability between individuals, with some animals being mostly arrhythmic during the experiment. It is possible that a patchy and unpredictable source of prey, requiring frequent environmental probing for food, contributes to some of the apparent arrhythmicity of locomotion in some animals. It is interesting to consider the possibility that the potentially conflicting natural selective forces of prey availability and predator avoidance may actually push populations to express a wide variation in locomotor rhythmicity. Future investigation comparing the neural underpinnings of the circadian clock between rhythmic and arrhythmic individuals may provide insight into this common, but poorly understood, phenomenon.
For the significant number of animals that did exhibit circadian rhythms of locomotion in DD, the average tau was 23.2 ± 1.1 h for crawling and 23.5 ± 0.7 h for swimming, indicating that this clock has a free-running period that is slightly shorter than a day. The similarity of the tau values for the two behaviors suggests that they might be controlled by the same circadian clock. Nocturnal animals like Melibe often have short tau values (Aschoff, 1960), and this is the case for the nocturnal snail Helisoma (Kavaliers, 1981). However, this is not true for all nocturnal gastropods, because Limax and Bulla, which are both nocturnal, have tau values >24 h (Sokolove et al., 1977; Block and Davenport, 1982).
Eyeless animals maintain nocturnal activity in light-dark, but lose rhythmicity in constant darkness
In LD, eyeless animals maintained nocturnal activity (Figs. 6 and 7), suggesting the presence of extraocular photoreceptors. Moreover, eyeless animals exhibited the same shifts in activity during the transition from day to night, and night to day, as normal animals. Extraocular photoreceptors are quite common, and their potential role in the entrainment of circadian rhythms in many invertebrates, including gastropods, has been well documented (Page, 1982; Cronin, 1986). As with Melibe, these extraocular photoreceptors are sufficient for maintaining normal patterns of locomotor activity in LD in other gastropods whose eyes have been removed, including Aplysia (Block and Lickey, 1973; Lickey et al., 1977), Bursatella (Block and Roberts, 1981), Helisoma (Kavaliers, 1981), and Limax (Beiswanger et al., 1981). In some species, removal of the eyes can have effects on locomotion, regardless of entrainment. For instance, an eyeless individual of Aplysia, while still entrained to light and exhibiting its normal diurnal activity in LD, does exhibit additional nocturnal activity and decreased overall activity compared to intact animals (Block and Lickey, 1973; Lickey et al., 1977). Eyeless individuals of Bulla actually switch from nocturnal to diurnal activity (Block and Davenport, 1982). In our experiments, the activity of eyeless individuals of Melibe in LD was no different than that of normal animals or sham controls (which had surgery but eyes were left intact).
It remains to be determined where the extraocular receptors are located in Melibe. In other gastropods, there is evidence for both dermal (Lukowiak and Jacklet, 1972; Chase, 1979; van Duivenboden, 1982; Katagiri et al., 1990) and ganglionic (Arvanitaki and Chalazonitas, 1961; Hisano et al., 1972; Block and Smith, 1973; Brown and Brown, 1973; Pašć et al., 1975) photoreceptors. Because Melibe is largely transparent, it is possible that either ganglionic or dermal receptors (or both) could contribute to light entrainment in the absence of eyes. Preliminary evidence suggests that the isolated brain of Melibe is sensitive to light (unpubl. data, Newcomb and Watson). Furthermore, several neurons in the cerebropleural and buccal ganglia, as well as processes around the eyes and in the pedal ganglia, react with antibodies directed against cryptochrome (unpubl. data, Bixby and Watson). However, not all cryptochromes are light-sensitive (Chaves et al., 2011), and currently we do not know which type of cryptochrome is present in select neurons of Melibe.
In DD, individuals of Melibe without eyes did not continue to express the daily rhythms seen in LD. This suggests that the eyes, or the small optic ganglia adjacent to the eyes, are a crucial component in their circadian timing system. The eyes have already been demonstrated as the site of the circadian clock in some gastropods (Jacklet, 1969; Block and Roberts, 1981; Block and Wallace, 1982). As in Melibe, removal of the eyes also disrupts circadian rhythms in DD in Bulla (Block and Davenport, 1982). However, removal of the eyes in Aplysia, Bursatella, and Helisoma does not necessarily affect circadian rhythms of locomotion in DD (Block and Lickey, 1973; Lickey et al., 1977; Kavaliers, 1981), suggesting the presence of multiple clocks in these species. Thus, the locations of circadian clocks have clearly diverged in different gastropods.
In summary, we present evidence that Melibe expresses a circadian rhythm of locomotion, including swimming. The neural circuit that produces this swim behavior consists of eight individually identifiable neurons (Thompson and Watson, 2005; Sakurai et al., 2014) that are amenable to neurophysiological analysis. We are working to determine the location and nature of the circadian clock, or clocks, in the central nervous system of this animal so that we can use this model system to investigate, at the cellular level, how circadian clocks communicate with, and modulate, the neural circuits for behaviors that are expressed with a circadian rhythm.
Acknowledgments
This research was supported by the New Hampshire IDeA Network of Biological Research Excellence (NHINBRE) with a grant from the National Institute of General Medical Sciences (1P20GM030360), National Institutes of Health (to JMN and WHW). Additional funding was provided by New England College and the University of New Hampshire. We thank the staff at the Friday Harbor Laboratories for their help with this project, and Trevor Fay for supplying us with animals. In addition, we thank two anonymous reviewers and acknowledge the assistance of a number of undergraduates who helped with collecting and analyzing the data, including Chelsie Boroski, Jessica Bouchard, Kayla Lawlor, and Erika Moretti.
Abbreviations
- DD
constant darkness
- LD
light-dark
Literature Cited
- Ajeska RA, Nybakken J. Contributions to the biology of Melibe leonina (Gould, 1852) (Mollusca; Opisthobranchia) Veliger. 1976;19:19–26. [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitaki A, Chalazonitas N. Excitatory and inhibitory processes initiated by light and infra-red radiations in single identifiable nerve cells (giant ganglion cells in Aplysia) In: Florey E, editor. Nervous Inhibition. Pergamon Press; New York: 1961. pp. 194–231. [Google Scholar]
- Aschoff J. Cold Spring Harbor Symposia on Quantitative Biology. Vol. 25, Biological Clocks. Long Island Biological Association; Cold Spring Harbor, NY: 1960. Exogenous and endogenous components in circadian rhythms; pp. 11–28. [DOI] [PubMed] [Google Scholar]
- Barsby T, Linington RG, Andersen RJ. De Novo terpenoid biosynthesis by the dendronotid nudibranch Melibe leonina. Chemoecology. 2002;12:199–202. [Google Scholar]
- Beeston DC, Morgan E. A crepuscular rhythm of locomotor activity in the freshwater prosobranch, Melanoides tuberculata (Müller) Anim. Behav. 1979;27:284–291. [Google Scholar]
- Beiswanger CM, Sokolove PG, Prior DJ. Extraocular photoentrainment of the circadian locomotor rhythm of the garden slug Limax. J. Exp. Zool. 1981;216:13–23. [Google Scholar]
- Bickell-Page LR. Repugnatorial glands with associated striated muscle and sensory cells in Melibe leonina (Mollusca, Nudibranchia) Zoomorphology. 1991;110:281–291. [Google Scholar]
- Block GD, Davenport PA. Circadian rhythmicity in Bulla gouldiana: role of the eyes in controlling locomotor behavior. J. Exp. Zool. 1982;224:57–63. [Google Scholar]
- Block GD, Lickey ME. Extraocular photoreceptors and oscillators can control the circadian rhythm of behavioral activity in Aplysia. J. Comp. Physiol. 1973;84:367–374. [Google Scholar]
- Block GD, Roberts MH. Circadian pacemaker in the Bursatella eye: properties of the rhythm and its effect on locomotor behavior. J. Comp. Physiol. 1981;142:403–410. [Google Scholar]
- Block GD, Smith JT. Cerebral photoreceptors in Aplysia. Comp. Biochem. Physiol. 1973;46A:115–121. [Google Scholar]
- Block GD, Wallace SF. Localization of a circadian pacemaker in the eye of a mollusc, Bulla. Science. 1982;217:155–157. doi: 10.1126/science.217.4555.155. [DOI] [PubMed] [Google Scholar]
- Brown AM, Brown HM. Light response of a giant Aplysia neuron. J. Gen. Physiol. 1973;62:239–254. doi: 10.1085/jgp.62.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase R. Photic sensitivity of the rhinophore in Aplysia. Can. J. Zool. 1979;57:698–701. [Google Scholar]
- Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen L-O, van der Horst GTJ, Batschauer A, Ahmad M. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 2011;62:335–364. doi: 10.1146/annurev-arplant-042110-103759. [DOI] [PubMed] [Google Scholar]
- Cronin TW. Photoreception in marine invertebrates. Am. Zool. 1986;26:403–415. [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Gould AA. United States Exploring Expedition during the years 1838–1842. Mollusca & Shells. 1852;12:I–XV. 1–510. [Google Scholar]
- Hastings M, O’Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J. Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- Hisano N, Tateda H, Kuwabara M. Photosensitive neurones in the marine pulmonate mollusc Onchidium verruculatum. J. Exp. Biol. 1972;57:651–660. doi: 10.1242/jeb.57.3.651. [DOI] [PubMed] [Google Scholar]
- Hurst A. The feeding mechanism and behavior of the opisthobranch Melibe leonina. Symp. Zool. Soc. Lond. 1968;22:151–166. [Google Scholar]
- Jacklet JW. Circadian rhythm of optic nerve impulses recorded in darkness from isolated eye of Aplysia. Science. 1969;164:562–563. doi: 10.1126/science.164.3879.562. [DOI] [PubMed] [Google Scholar]
- Jacklet JW. Circadian locomotor activity in Aplysia. J. Comp. Physiol. 1972;79:325–341. [Google Scholar]
- Katagiri N, Hama K, Katagiri Y, Shimitani Y, Hashimoto Y, Aikawa E. High-voltage electron microscopic study on the axon of the dermal photoreceptor cell in the dorsal mantle of Onchidium verruculatum. J. Electron Microscopy. 1990;39:363–371. [Google Scholar]
- Kavaliers M. Circadian and ultradian activity rhythms of a freshwater gastropod, Helisoma trivolvis: the effects of social factors and eye removal. Behav. Neural Biol. 1981;32:350–363. doi: 10.1016/s0163-1047(81)92411-0. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. A circadian locomotor rhythm in Aplysia californica. Physiol. Behav. 1968;3:179–181. [Google Scholar]
- Kupfermann I, Carew TJ. Behavior patterns of Aplysia californica in its natural environment. Behav. Biol. 1974;12:317–337. doi: 10.1016/s0091-6773(74)91503-x. [DOI] [PubMed] [Google Scholar]
- Lawrence KA, Watson WH., III Swimming behavior of the nudibranch Melibe leonina. Biol. Bull. 2002;203:144–151. doi: 10.2307/1543383. [DOI] [PubMed] [Google Scholar]
- Lickey ME, Wozniak JA, Block GD, Hudson DJ, Augter GK. The consequences of eye removal for the circadian rhythm of behavioral activity in Aplysia. J. Comp. Physiol. A. 1977;118:121–143. [Google Scholar]
- Lickey ME, Hudson DJ, Hiaasen SO. Circadian organization in Aplysia: relations between locomotor rhythm and eye rhythms after cutting both, one or neither optic nerves. J. Comp. Physiol. A. 1983;153:133–143. [Google Scholar]
- Lukowiak K, Jacklet JW. Habituation and dishabituation: interactions between peripheral and central nervous systems in Aplysia. Science. 1972;178:1306–1308. doi: 10.1126/science.178.4067.1306. [DOI] [PubMed] [Google Scholar]
- Mauzey KP, Birkeland C, Dayton PK. Feeding behavior of asteroids and escape responses of their prey in the Puget Sound area. Ecology. 1968;49:603–619. [Google Scholar]
- Mills CE. Seasonal swimming of sexually mature benthic opisthobranch molluscs (Melibe leonina and Gastropteron pacificum) may augment population dispersal. In: Wilson SA, Stricker SA, Shinn GL, editors. Reproduction and Development of Marine Invertebrates. Johns Hopkins Press; Baltimore: 1994. pp. 313–319. [Google Scholar]
- Newcomb JM, Lawrence KA, Watson WH., III The influence of light on locomotion in the gastropod Melibe leonina. Mar. Freshw. Behav. Physiol. 2004;37:253–269. [Google Scholar]
- Page TL. Extraretinal photoreception in entrainment and photoperiodism in invertebrates. Experientia. 1982;38:1007–1013. [Google Scholar]
- Pašić M, Zećević D, Ristanović D, Popilijević G. Effects of intermittently applied light on Helix pomatia neurons. Comp. Biochem. Physiol. 1975;51A:71–74. doi: 10.1016/0300-9629(75)90414-4. [DOI] [PubMed] [Google Scholar]
- Roberts MH, Xie X. Phase relationship between ocular and behavioral circadian rhythms in Bulla gouldiana exposed to different photoperiods. Physiol. Behav. 1996;59:703–708. doi: 10.1016/0031-9384(95)02116-7. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Gunaratne CA, Katz PS. Two interconnected kernels of reciprocally inhibitory interneurons underlie alternating left-right swim motor pattern generation in the mollusc Melibe leonina. J. Neurophysiol. 2014;112:1317–1328. doi: 10.1152/jn.00261.2014. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Beiswanger CM, Prior DJ, Gelperin A. A circadian rhythm in the locomotor behaviour of the giant garden slug Limax maximus. J. Exp. Biol. 1977;66:47–64. doi: 10.1242/jeb.66.1.47. [DOI] [PubMed] [Google Scholar]
- Takahashi JS. Circadian rhythms: from gene expression to behavior. Curr. Opin. Neurobiol. 1991;1:556–561. doi: 10.1016/s0959-4388(05)80028-5. [DOI] [PubMed] [Google Scholar]
- Takahashi JS. Molecular neurobiology and genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 1995;18:531–553. doi: 10.1146/annurev.ne.18.030195.002531. [DOI] [PubMed] [Google Scholar]
- Thompson S, Watson WH., III Central pattern generator for swimming in Melibe. J. Exp. Biol. 2005;208:1347–1361. doi: 10.1242/jeb.01500. [DOI] [PubMed] [Google Scholar]
- van Duivenboden YA. Non-ocular photoreceptors and photo-orientation in the pond snail Lymnaea stagnalis (L.) J. Comp. Physiol. A. 1982;149:363–368. [Google Scholar]
- Watson WH, III., Chester CM. The influence of olfactory and tactile stimuli on the feeding behavior of Melibe leonina (Gould, 1852) (Opisthobranchia: Dendronotacea) Veliger. 1993;36:311–316. [Google Scholar]
- Watson WH, III, Trimarchi J. A quantitative description of Melibe feeding behavior and its modification by prey density. Mar. Behav. Physiol. 1992;19:183–194. [Google Scholar]
- Watson WH, III, Lawrence KA, Newcomb JM. Neuroethology of Melibe leonina swimming behavior. Am. Zool. 2001;41:1026–1035. [Google Scholar]