Abstract
Importance
Among youths with conduct problems, callous-unemotional (CU) traits are known to be an important determinant of symptom severity, prognosis, and treatment responsiveness. But positive correlations between conduct problems and CU traits result in suppressor effects that may mask important neurobiological distinctions among subgroups of children with conduct problems.
Objective
To assess the unique neurobiological covariates of CU traits and externalizing behaviors in youths with conduct problems and determine whether neural dysfunction linked to CU traits mediates the link between callousness and proactive aggression.
Design, Setting, and Participants
This cross-sectional case-control study involved behavioral testing and neuroimaging that were conducted at a university research institution. Neuroimaging was conducted using a 3-T Siemens magnetic resonance imaging scanner. It included 46 community-recruited male and female juveniles aged 10 to 17 years, including 16 healthy control participants and 30 youths with conduct problems with both low and high levels of CU traits.
Main Outcomes and Measures
Blood oxygenation level–dependent signal as measured via functional magnetic resonance imaging during an implicit face-emotion processing task and analyzed using whole-brain and region of interest–based analysis of variance and multiple-regression analyses.
Results
Analysis of variance revealed no group differences in the amygdala. By contrast, consistent with the existence of suppressor effects, multiple-regression analysis found amygdala responses to fearful expressions to be negatively associated with CU traits (x = 26, y = 0, z = −12; k = 1) and positively associated with externalizing behavior (x = 24, y = 0, z = −14; k = 8) when both variables were modeled simultaneously. Reduced amygdala responses mediated the relationship between CU traits and proactive aggression.
Conclusions and Relevance
The results linked proactive aggression in youths with CU traits to hypoactive amygdala responses to emotional distress cues, consistent with theories that externalizing behaviors, particularly proactive aggression, in youths with these traits stem from deficient empathic responses to distress. Amygdala hypoactivity may represent an intermediate phenotype, offering new insights into effective treatment strategies for conduct problems.
Externalizing behaviors and conduct problems are among the primary reasons youths in the United States are referred to psychiatric care.1 However, there remains a dearth of effective risk assessment and treatment strategies, partly owing to heterogeneity among children and adolescents with antisocial behavior.2
Youths with conduct problems can be distinguished by the presence or absence of callous-unemotional (CU) traits, which include reduced empathy and remorse and shallow affect3 and are associated with more severe, persistent, and treatment-refractory externalizing behaviors.4 Conduct problems in youths with and without CU traits are thought to emerge from distinct etiological trajectories.5 However, because conduct problems and CU traits are positively correlated, statistical suppressor effects may impede understanding of the unique neurobiological correlates of these variables. This has led to an increasing emphasis on the importance of treating these variables as continuously varying traits and using analyses that simultaneously model both to account for their covariance.6,7 The present study assessed whether patterns of neurobiological functioning among youths with conduct problems who vary in CU traits are better captured by analyses that simultaneously model both externalizing behaviors and CU traits as continuous variables than by analyses that dichotomize these variables. It also assessed whether such analyses can demonstrate that specific patterns of neuro biological dysfunction mediate the relationship between CU traits and the characteristic behavioral phenotype of proactive aggression that is associated with these traits.
Callous-unemotional traits in children with conduct problems are consistently linked to disrupted functioning of the amygdala, particularly reduced responses to socioaffective cues such as fearful expressions.8-11 Because fearful expressions elicit empathy and inhibit aggression in adults and typically developing youths,12,13 reduced responsiveness to these cues is thought to mediate the increase in proactive, or goal-directed, aggression observed in youths with CU traits.12,14 However, this causal pathway has not been directly tested.
In contrast to youths with elevated CU traits, youths with conduct problems (particularly adolescent-onset conduct problems) and unspecified levels of CU traits typically exhibit elevated activity in the amygdala, insula, and striatum in response to socioaffective stimuli.15,16 This is consistent with observations of primarily reactive aggression in these youths14,17,18 and with hypotheses that externalizing behaviors not linked to high levels of CU traits are more likely to reflect emotional dysregulation and elevated threat sensitivity than reduced empathy.2 Thus, although CU traits are positively correlated with externalizing behaviors, these variables are, respectively, negatively and positively correlated with amygdala responses to socioaffective stimuli. This pattern can result in statistical suppressor effects, which occur when 2 correlated predictors exhibit opposite relationships with a criterion variable.6
Despite this pattern and growing consensus regarding the importance of modeling CU traits as continuous variables,19,20 most neuroimaging research in youths with conduct problems and CU traits treats these variables dichotomously.8-10,21 Among the limitations of this approach is that it may not be sufficiently sensitive for detecting group differences when suppressor effects are present.7 We aimed to directly compare the efficacy of this approach and a regression-based approach capable of accounting for suppressor effects in a study that identified neurobiological correlates of CU traits and externalizing behaviors using functional magnetic resonance imaging (fMRI). We scanned healthy control participants and youths with conduct problems who varied in CU traits; we hypothesized that regression-based analyses would more effectively capture predicted patterns of amygdala responsiveness than would analysis of variance (ANOVA). We also hypothesized that regression-based analyses would find that decreased amygdala activation in response to fearful expressions mediates the relationship between CU traits and proactive aggression, suggesting that amygdala hypoactivation serves as an intermediate pheno type.
Methods
Participants
Following study approval by the Georgetown University institutional review board, youths aged 10 to 17 years were recruited from the Washington, DC, community via fliers, brochures, and advertisements. Written informed consent and assent were obtained from parents/guardians and participants, respectively, and children and parents completed a battery of assessments.
All participants had an estimated full-scale IQ of at least 80 measured by the Kaufman22 Brief Intelligence Test–2 and reported no history of head trauma or neurological disorder. All youths were medication free at the time of scanning, with the exception of 5 youths with conduct problems and 1 control participant for whom psychiatric medications (including aripiprazole, divalproex sodium, fluoxetine, guanfacine, methylphenidate, quetiapine fumarate, risperidone, and trazo done) could not be withheld prior to scanning; the control participant was excluded from all analyses. Data from 4 participants (1 healthy control and 3 with conduct problems) were excluded owing to excessive movement during scanning. Qualified participants (N = 46) included 30 youths with conduct problems and 16 healthy control participants (Table 1). For all group-based analyses, youths with conduct problems were divided into 2 groups post hoc–those with low CU traits (n = 16) and those with high CU traits (n = 14) following a median split (median = 44) on maximum scores on the Inventory of Callous-Unemotional Traits (ICU).
Table 1. Demographic and Clinical Characteristics of Healthy Control Individuals and Participants With Conduct Problemsa.
| Participant Characteristic | Healthy Controls (n = 16) | Conduct Problems (n = 30) | P Value | Low CU (n = 16) | High CU (n = 14) | P Value |
|---|---|---|---|---|---|---|
| Demographic variable, mean (SD) | ||||||
| Male:female ratio | 10:6 | 16:14 | .76 | 9:7 | 7:7 | >.99 |
| Age, y | 12.75 (2.41) | 14.74 (2.46) | .01b | 14.09 (2.69) | 15.50 (2.00) | .12 |
| Cognitive intelligence score | 112.69 (15.38) | 98.10 (9.73) | <.001b | 100.56 (10.94) | 95.29 (7.54) | .14 |
| Behavioral measure, mean (SD) | ||||||
| CBCL/DSM scalec | 43.38 (9.47) | 72.70 (4.94) | <.001b | 71.50 (5.11) | 74.07 (1.30) | .16 |
| Affective | 0.81 (1.33) | 6.93 (4.93) | <.001b | 7.19 (5.44) | 6.64 (4.47) | .77 |
| Clinical threshold, No. | 0 | 13 | .002b | 8 | 5 | .48 |
| Anxiety | 0.94 (1.34) | 3.23 (3.07) | .01b | 3.56 (3.16) | 2.93 (3.05) | .58 |
| Clinical threshold, No. | 0 | 8 | .04b | 5 | 3 | .69 |
| ADHD | 1.06 (1.29) | 8.13 (3.42) | <.001b | 7.81 (3.43) | 8.50 (3.50) | .59 |
| Clinical threshold, No. | 0 | 10 | .01b | 3 | 7 | .12 |
| Alcohol use, No.d | 0 | 3 | .54 | 1 | 2 | .59 |
| Drug use, No.d | 0 | 5 | .15 | 2 | 3 | .64 |
| ICUe | 25.50 (6.66) | 44.28 (8.31) | <.001b | 38.13 (5.35) | 51.32 (4.58) | <.001b |
| Reactive aggressionf | 6.12 (4.29) | 12.47 (4.46) | <.001b | 12.56 (4.230) | 12.36 (4.58) | .90 |
| Proactive aggressionf | 0.88 (1.09) | 6.40 (5.54) | <.001b | 5.44 (4.70) | 7.50 (6.19) | .31 |
| fMRI task accuracy, % | 92.79 (6.69) | 93.21 (4.99) | .81 | 91.91 (5.98) | 94.67 (3.15) | .14 |
| fMRI task reaction time, ms | 914.92 (116.05) | 900.12 (132.53) | .71 | 920.49 (138.36) | 876.85 (126.49) | .38 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; CU, callous-unemotional; fMRI, functional magnetic resonance imaging; ICU, Inventory of Callous-Unemotional Traits.
Cognitive intelligence is the full-scale IQ from the Kaufman Brief Intelligence Test–2.
Significant group differences at P < .05, 2-tailed, measured with t tests except for Fisher exact test for categorical variables.
CBCL scores are the age- and sex-standardized t scores of externalizing behavior (items measuring aggression and rule breaking). CBCL/DSM scores are from the CBCL/DSM-oriented scales and the number of participants who meet the age- and sex-normed cutoffs that indicate symptoms in the clinical range are given below each scale.
Drug and alcohol use are derived from parent reports indicating frequent use.
ICU scores are derived from the maximum score on each item from the parent and youth versions.
Reactive and proactive aggression are subscores from the Reactive-Proactive Aggression Questionnaire.
Assessment Instruments
Parents completed the Strengths and Difficulties Questionnaire11,23 and the Child Behavior Checklist (CBCL)24 to assess conduct problems. Youths who met the criteria for clinically significant conduct problems on both the Strengths and Difficulties Questionnaire and CBCL were included in the conduct problems group, and age- and sex-normalized scores on the externalizing behavior subscales of the CBCL were used as estimates of conduct problems. Healthy control participants did not meet criteria for conduct problems on either measure.
Participants and parents separately completed the ICU.25 For each item, the highest numeric response given by either the youth or parent was selected and summed as a measure of CU traits.7,9 Rather than a summative or averaging approach, this scoring method is recommended to account for reporter bias and discrepancies.26
Aggressive behavior was assessed using the youth-completed Reactive-Proactive Aggression Questionnaire.27 Analyses of internal consistency found CBCL, ICU, and Reactive-Proactive Aggression Questionnaire scales to exhibit strong reliability (Cronbach α, 0.77-0.95; see eTable 1 in Supplement for correlation matrix and Cronbach α of assessment variables).
fMRI Task
During scanning, participants completed an implicit face-emotion processing task used in previous studies of children with conduct problems and CU traits.8 Stimulus images included 10 men and women from the Pictures of Facial Affect series28 displaying positive-neutral, fearful, and angry expressions. Positive-neutral stimuli were morphs of neutral and happy expressions (25% happiness). Fearful and angry expressions included both full-intensity natural expressions and morphed expressions of 50% and 150% intensity levels. Hair, ears, neck, and clothing were masked and faces appeared against a black background in randomized order for 2 seconds, followed by a 1-second fixation cross. The task featured an implicit processing paradigm such that participants indicated the sex of each face rather than the expression via button press.8,9,29 The task included four 5.5-minute consecutive runs, each containing 80 face trials and 20 jittered interstimulus interval trials.
Image Acquisition
Magnetic resonance images were acquired with a 3-T Siemens Tim Trio scanner (Siemens Medical Solutions) and 8-channel phased-array head coil. Functional data were collected using a T2*-weighted echoplanar imaging sequence with the following parameters: repetition time = 3000 milliseconds, echo time = 30 milliseconds, 3.0 mm3 voxels, 56 slices, 64 × 64 matrix, and field of view = 192 mm. A high-resolution T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo structural scan was collected for each participant (repetition time = 1900 milliseconds, echo time = 5.52 milliseconds, 1.0 mm3 voxels, 176 slices, 246 × 256 matrix, and field of view = 250 mm).
Image Analysis
Data were processed using SPM8 (Wellcome Trust Department of Cognitive Neurology). Prior to preprocessing, the first 4 volumes of each functional run were excluded, yielding 106 volumes for each of the 4 functional runs. Echoplanar images were slice-time corrected, realigned, coregistered, normalized into Montreal Neurological Institute space, smoothed with an 8-mm Gaussian kernel, and written out to 2-mm3 isometric voxels. Following previous findings that normalizing brain volumes from age 7 to 8 years onward does not create significant age-related distortions in localization or signal time course in event-related fMRI,30 participants’ anatomical scans were individually registered to the Montreal Neurological Institute brain atlas.31 Realignment parameters were examined to ensure head movement did not exceed 1 voxel per repetition time, and, as previously noted, 4 participants were excluded from analysis owing to excessive motion. All preprocessed images were visually inspected for image quality. At the individual level, a design matrix was created for each participant that included regressors for each stimulus type (eg, fear at full intensity and anger at full intensity), incorrect/nonresponse trials, and 6 motion parameters. Contrast images were generated for each stimulus type over implicit baseline for group-level analyses.
At the group level, we conducted both region of interest (ROI) and whole-brain analyses. Using MarsBaR,32 we constructed a 6-mm spherical right amygdala ROI centered at coordinates x = 24, y = −5, and z = −13 obtained from a recently published study on juvenile conduct problems.7 To identify the roles of externalizing behaviors and CU traits in the patterns of amygdala activation in response to full-intensity fearful expressions, we conducted both ANOVA and multiple-regression analyses in SPM8 using the ROI as a mask to constrain the results, which were thresholded at P < .05, family-wise error (FWE) corrected (preliminary analyses raised concerns about the ecological validity of the morphed expressions—particularly at 150% intensity—which were not analyzed at the group level as a result). To assess the sensitivity of our regression approach compared with previous approaches that created subgroups of individuals with conduct problems, we conducted a 1-way ANOVA on 3 groups (healthy control, conduct problems/low CU traits, and conduct problems/high CU traits), with age and cognitive intelligence included as covariates to account for significant group differences in these variables. Multiple-regression analyses using the ROI mask were also conducted to assess amygdala activation both across the entire sample (again including age at testing and cognitive intelligence scores as covariates) and separately for only children with conduct problems.
In addition, we conducted 2 whole-brain multiple-regression analyses, one that included healthy control participants and children with conduct problems and another that included only children with conduct problems. Analyses including all participants were conducted following results of a Shapiro-Wilk W test of normality confirming that ICU scores were normally distributed across the entire sample (W = 0.98, P = .61). Both externalizing behaviors and CU traits were simultaneously modeled as continuous variables following emerging consensus that this analytic strategy most effectively captures the latent structure of both phenomena and would allow us to identify the unique contributions of each variable.7,33 Age at testing and cognitive intelligence scores were included as covariates of no interest to account for group differences in these variables. Analyses were thresholded at P < .005 and k = 10 voxel cluster extent, a combination that has been demonstrated to produce a desirable balance between type I and type II errors as is more appropriate for investigating complex cognitive and emotional effects than other correction methods.10,34
Finally, to interrogate the role of the amygdala as a potential mediator of proactive aggression, amygdala parameter estimates averaged across the independent spherical ROI were extracted from each contrast and tested as potential mediator variables. We performed a bootstrap-mediation analysis using the INDIRECT macro-implemented in SPSS version18.35 This procedure uses nonparametric resampling to test mediation and does not require normality assumptions; this approach has been recommended over other types of mediation analysis, such as the Sobel test, causal steps, and distribution of the product approaches, owing to high power, good control over type I error rates, and the ability to be used in small samples.36,37 Rather than providing formal P values, this method uses bootstrap confidence intervals of the indirect effect to test significance, whereby confidence intervals that do not contain zero are indicative of mediation.
Results
Behavioral Responses
All participants performed above chance on the sex discrimination task performed inside the scanner. Mean (SD) total accuracy rates were 92.79% (6.69%) for healthy control participants and 93.21% (4.99%) for youths with conduct problems, which were not significantly different (t44 = 0.24, P = .81). Within the conduct-problems group, accuracy was also not significantly different (t28 = 0.24, P = .14) when comparing those with low and high CU traits,who scored a mean(SD)of 91.93% (5.98%) and 94.67% (3.15%), respectively. There were no group differences for individual emotions or significant correlations with age or cognitive intelligence (all P > .05). Incorrect and nonresponse trials were omitted from fMRI analyses.
Mean (SD) reaction times for healthy control participants (914.72 [116.05] milliseconds) and participants with conduct problems (900.12 [132.53] milliseconds) were not significantly different (t44 = 0.37, P = .71) nor were mean (SD) response times for participants with conduct problems and low (920.43 [138.36] milliseconds) vs high (876.85 [126.49] milliseconds) CU traits (t28 = 0.90,P = .38). There were no group differences for individual emotions or correlations with cognitive intelligence. Across stimuli, reaction time was negatively correlated with age (r = −0.42, P = .003).
ROI Analyses
The results of our 3-group 1-way ANOVA found no significant effect of group in the amygdala following FWE correction. The results of a second ANOVA, including the severity of externalizing behaviors as an additional covariate of no interest, again found no significant main effect of group. Because of the conservative nature of the ANOVA F test and our directional pre-dictions, we also conducted independent-sample t tests com paring healthy control cases with participants with conduct problems and, among those with conduct problems, participants with low CU traits and high CU traits to further investigate group differences. We found no significant group differences in right amygdala responses to fear expressions, even at the less-conservative threshold of P < .05, FWE corrected.
By contrast, the results of a multiple-regression analysis across the full sample found that responses in the right amygdala were positively associated with externalizing behaviors (x = 24, y = 0, z = −14; k = 8) and negatively associated with CU traits (x = 26, y = 0, z = −12; k = 1) as hypothesized. Repeating this analysis while excluding the 5 participants for whom medication could not be withheld, we again found that responses in the right amygdala were positively associated with externalizing behaviors (x = 24, y = 0, z = −14; k = 16) and negatively associated with CU traits (x = 26, y = 0, z = −12; k = 17).
A second regression restricted to only youths with conduct problems found similar results: right amygdala activity was positively associated with externalizing behavior (x = 26, y = −4, z = −12; k = 47) and negatively associated with CU traits (x = 26, y = 0, z = −12; k = 11) (Figure 1). Excluding the 5 participants taking medication, we once again found that right amygdala activity was positively associated with externalizing behavior (x = 24, y = 0, z = −10; k = 9) and negatively associated with CU traits (x = 26, y = 0, z = −14; k = 35).
Figure 1. Results of a Regression Analysis in the Right Amygdala Region of Interest (ROI).
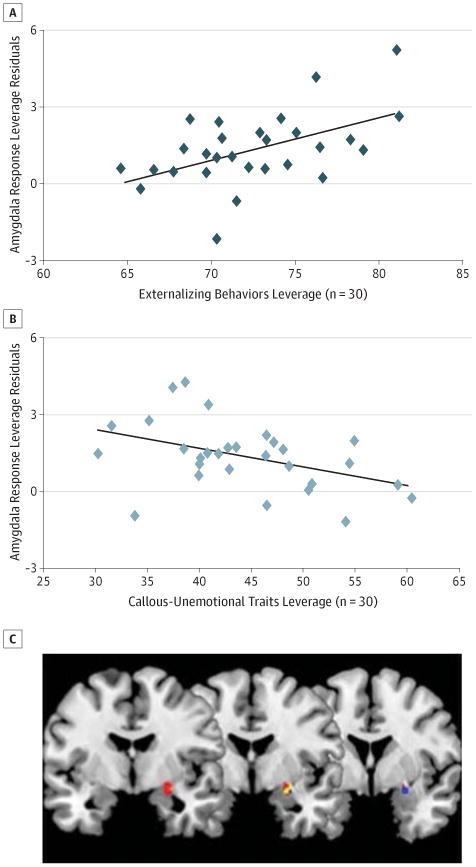
Right amygdala responses to full-intensity fearful expressions were contrasted over implicit baseline. The leverage plots show the unique association between mean beta values extracted from the entire amygdala ROI and externalizing behaviors (A) and callous-unemotional traits (B) after accounting for the variance of the other. C, Results of a multiple-regression analysis including 30 participants with conduct problems found activation restricted to the 6-mm spherical ROI was positively associated with externalizing behaviors (red voxels) and negatively associated with callous-unemotional traits (blue voxels), with overlapping areas in yellow (P < .05, familywise error).
We confirmed that these patterns of results were limited to responses to fearful expressions. Parallel ROI regression analyses examining the responses to full-intensity angry expressions and neutral expressions found no associations between right amygdala activation and either externalizing behavior or CU traits. No significant associations were found in response to neutral expressions in the full sample. However, when the sample was limited to youths with conduct problems, we found that right amygdala responses to neutral expressions were positively associated with externalizing behaviors (x = 24, y = −8, z = −16; k = 17).
Whole-Brain Analyses
Across the entire sample, the results of whole-brain multiple-regression analyses confirmed that amygdala responses to fearful expressions were positively associated with externalizing behaviors (controlling for CU traits) in clusters that included the right caudate nucleus (x = 12, y = 2, z = 20; k = 303), the putamen and amygdala (x = 26, y = 4, z = −12; k = 161), and a cluster in the right superior temporal gyrus (x = 62, y = −6, z = −10; k = 876) that extended into the orbitofrontal cortex and insula, as well as the left superior frontal gyrus (x = −22, y = 66, z = 10; k = 15). Conversely, regions negatively associated with CU traits (controlling for externalizing behaviors) included a large cluster (x = 14, y = −2, z = 18; k = 1276) containing the right caudate, putamen, and amygdala; the right inferior frontal gyrus (x = 44, y = 26, z = −14; k = 42); and several clusters bilaterally that extended into the right insula. The reported coordinates signify the location of peak voxel intensity within the cluster according to the Montreal Neurological Institute atlas (Table 2). Comparable results emerged when these results were limited to only children with conduct problems (eTable 2 in Supplement).
Table 2. Regions of Activation Positively and Negatively Associated With Externalizing Behaviors and CU Traitsa.
| Anatomical Region | MNI Coordinates | ke | Z Score | P Value | ||
|---|---|---|---|---|---|---|
| X | y | z | ||||
| Positive associations with conduct problems | ||||||
| Superior frontal gyrus | −22 | 66 | 10 | 15 | 2.91 | .002 |
| Superior frontal gyrus | −16 | 42 | 48 | 14 | 2.80 | .003 |
| Superior medial gyrus | 14 | 66 | 6 | 151 | 4.49 | <.001 |
| Middle frontal gyrus | 24 | 38 | −8 | 14 | 2.71 | .003 |
| Temporal pole | −38 | 4 | −20 | 11 | 2.88 | .002 |
| Putamen | 26 | 4 | −12 | 161 | 3.45 | <.001 |
| Putamen | 12 | 2 | 20 | 303 | 4.43 | <.001 |
| Caudate | −24 | −8 | 20 | 122 | 3.23 | .001 |
| Superior temporal gyrus | 62 | −6 | −10 | 876 | 4.07 | <.001 |
| Superior temporal gyrus | 48 | −24 | −2 | 124 | 3.25 | .001 |
| Thalamus | 0 | −8 | 8 | 21 | 3.15 | .001 |
| Hippocampus | 26 | −20 | −14 | 20 | 2.82 | .002 |
| Middle cingulate cortex | 6 | −26 | 30 | 40 | 2.80 | .003 |
| Frontal lobe | −26 | −42 | 26 | 24 | 2.97 | .001 |
| Posterior cingulate cortex | −4 | −46 | 6 | 93 | 3.71 | <.001 |
| Inferior temporal gyrus | 54 | −46 | −14 | 18 | 3.30 | <.001 |
| Calcarine gyrus | 16 | −48 | 10 | 13 | 2.76 | .003 |
| Precuneus | −10 | −52 | 16 | 15 | 2.81 | .002 |
| Precuneus | 6 | −58 | 64 | 59 | 3.82 | <.001 |
| Cuneus | 6 | −74 | 32 | 30 | 2.82 | .002 |
| Negative associations with conduct problems | ||||||
| Postcentral gyrus | 66 | −12 | 30 | 47 | <.001 | |
| Middle occipital gyrus | 32 | −68 | 32 | 21 | .002 | |
| Positive associations with CU traits | ||||||
| Inferior parietal lobule | 40 | −30 | 28 | 14 | 2.94 | .002 |
| Negative associations with CU traits | ||||||
| Superior medial gyrus | 14 | 66 | 8 | 44 | 3.46 | <.001 |
| Middle frontal gyrus | −40 | 40 | 32 | 35 | 3.15 | .001 |
| Middle frontal gyrus | 36 | 14 | 40 | 11 | 2.82 | .002 |
| Inferior frontal gyrus | 52 | 40 | 0 | 125 | 3.59 | <.001 |
| Inferior frontal gyrus | 44 | 26 | −14 | 42 | 3.02 | .001 |
| Inferior frontal gyrus | 58 | 26 | 20 | 38 | 3.76 | <.001 |
| Inferior frontal gyrus | −48 | 20 | 30 | 569 | 3.80 | <.001 |
| Middle cingulate cortex | 8 | 30 | 34 | 17 | 3.15 | .001 |
| Middle cingulate cortex | −4 | −32 | 34 | 31 | 2.94 | .002 |
| Caudate | 20 | 24 | 8 | 26 | 3.00 | .001 |
| Caudate | 14 | −2 | 18 | 1276 | 3.84 | <.001 |
| Rectal gyrus | 12 | 24 | −12 | 23 | 3.26 | .001 |
| Temporal pole | 54 | 12 | −18 | 266 | 3.63 | <.001 |
| Precentral gyrus | 50 | 10 | 30 | 51 | 2.85 | .002 |
| Postcentral gyrus | −44 | −20 | 58 | 21 | 2.89 | .002 |
| Middle temporal gyrus | 58 | −40 | −10 | 158 | 3.37 | <.001 |
| Inferior parietal lobule | −28 | −50 | 38 | 46 | 3.06 | .001 |
| Precuneus | −2 | −64 | 48 | 13 | 2.76 | .003 |
| Superior parietal lobule | −26 | −70 | 46 | 154 | 3.53 | <.001 |
| Cuneus | 6 | −74 | 34 | 122 | 3.38 | <.001 |
| Inferior occipital gyrus | −48 | −74 | −14 | 253 | 3.81 | <.001 |
| Superior occipital gyrus | 26 | −80 | 34 | 47 | 3.44 | <.001 |
| Middle occipital gyrus | −24 | −84 | 24 | 114 | 3.15 | .001 |
Abbreviations: CU, callous-unemotional; MNI, Montreal Neurological Institute.
Whole-brain regression analyses in 46 participants with scores of age and cognitive intelligence as covariates of no interest. Results thresholded at P < .005, k = 10.
Mediation Analysis
We conducted bootstrap-mediation analysis restricted to youths with conduct problems, in which CU traits, right amygdala parameter estimates extracted from the full-intensity fear contrast and averaged across the independent spherical ROI, and proactive aggression were modeled as the independent, mediating, and dependent variables, respectively, with externalizing behaviors included as covariates. The results revealed a bias-corrected 95% CI for the indirect effect size that excluded zero (0.01 to 0.34), and therefore indicated a significant indirect effect of CU traits on proactive aggression through the amygdala (Figure 2).37 A parallel analysis found that amygdala activation did not mediate the relationship between CU traits and reactive aggression (bias-corrected 95% CI, −0.08 to 0.19), which is not surprising given that CU traits and reactive aggression were not significantly correlated (eTable 1 in Supplement). Nor did amygdala activity in response to fearful expressions mediate the relationship between externalizing behaviors and reactive aggression. Finally, no significant mediation effects were found for amygdala responses to angry or neutral expressions.
Figure 2. Amygdala Response Mediates the Relationship Between Callous-Unemotional Traits and Proactive Aggression.
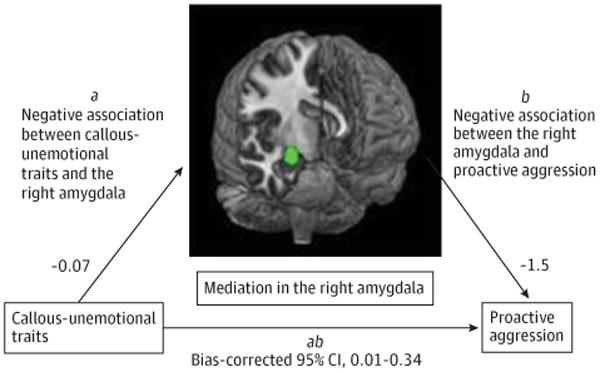
Unstandardized regression coefficients and bias-corrected 95% CI for the indirect effect from a bootstrap-mediation analysis that found that right amygdala responses to fearful expressions mediated the relationship between callous-unemotional traits and proactive aggression among 30 youths with conduct problems.
Discussion
The results of this study confirmed that amygdala responses to fearful facial expressions are differentially associated with externalizing behaviors and CU traits and that the amygdala mediates the relationship between CU traits and proactive aggression. The results also confirmed the presence of suppressor effects that reduce the efficacy of group-based dichotomous analyses (ANOVA) in identifying neurobiological distinctions among youths with conduct problems who vary in CU traits. These findings suggest that amygdala responses to fearful expressions may represent an intermediate phenotype that links CU traits in youths with conduct problems to an important behavioral phenotype, but that identifying this effect requires holding externalizing behavior constant. This research also extends prior neuroimaging research on CU traits in several important respects. To our knowledge, this is the first study of both male and female youths with conduct problems and both high and low levels of CU traits and the first to compare responses to supraliminal fearful, angry, and neutral expressions. The fact that the hypothesized effects were observed in response to fearful, but not angry or neutral, facial expressions reinforces the moderating role of responses to fearful expressions in particular in empathic and aggressive behavior.12,13
Previous studies of adolescents with conduct problems and CU traits have linked the recognition of fearful facial expressions to aggressive behaviors and empathic responsiveness,38 and they have shown that, relative to healthy control individuals, adolescents with elevated conduct problems and CU traits exhibit amygdala hypoactivation to these cues.8-10 However, these studies were limited by their inability to disentangle the neurobiological correlates of externalizing behaviors and CU traits. More recent studies have assessed adolescents with varying levels of CU traits and found that these traits—rather than conduct problems—predict amygdala hypoactivation.7,11 Although the importance of simultaneously modeling CU traits and externalizing behaviors has been described,7 to our knowledge, no previous study has simultaneously modeled CU traits and externalizing behaviors in response to fearful expressions and compared the efficacy of this technique to that of group-based analysis. In addition, no prior study has linked the resulting patterns of neural activation to aggressive behavior. The present findings reinforce the importance of considering both CU traits and externalizing behaviors simultaneously and suggest studies of undifferentiated conduct disorder may fail to capture critical distinctions among subpopulations of affected youths.
The amygdala plays a critical role in aggressive and violent behavior. Chronic hyperactivity in the amygdala and other components of the neural fear circuit as a result of environmental and genetic factors is implicated in reactive aggression, defined as an angry or aggressive response to provocation.17,39 Responses to perceived threat follow a gradient as a function of threat severity and proximity.40 Elevated threat responsiveness results in individuals who are hypersensitive to fear-relevant cues engaging in aggressive behavior in response to even mildly threatening or ambiguous stimuli. The observed positive relationship between externalizing behaviors and amygdala responses to both fearful and neutral expressions in the present study may reflect the tendency of externalizing youths to overreact to fear-relevant or ambiguous socioaffective cues. However, the absence of a mediation effect reinforces the importance of other cortical and subcortical structures in adolescent reactive aggression, as well as the distinct etiological pathways underlying proactive and reactive aggression.17
In contrast to reactive aggression, proactive aggression is an emotionally cool form of aggression used to achieve instrumental goals.12 Proactive aggression is consistently linked to CU traits and empathy deficits in both adolescents and adults.12,41 It is for this reason that aberrant responses to salient victim-distress cues, such as fearful facial expressions, are thought to reliably distinguish among individuals with conduct problems with low vs high levels of CU traits because victim-distress cues normally elicit empathy and inhibit aggression in typically developing children and adults.38,42,43 But reduced amygdala activation in response to fearful expressions (a salient form of distress cue) has not previously been found to link CU traits to proactive aggression. In addition to confirming this link, we found that amygdala hypoactivation in youths with elevated CU traits uniquely predicts proactive aggression. This pattern is consistent with the hypothesis that proactive aggression results from dysfunctional empathic responses to victim distress in youths with elevated CU traits. This potential causal pathway is supported by findings from longitudinal studies showing that early behavioral and physiological indications of amygdala dysfunction predict later aggressive and criminal behavior,44,45 although longitudinal neuroimaging studies would be needed to more conclusively test this developmental progression. In addition, although our sample size did not permit examination of any possible sex differences in the observed effects, future work may want to examine sex as a moderating variable, given the significant differences in aggression and other forms of disruptive behavior across sexes.46 A targeted examination of sex differences may yield further insights into the neurobiological bases of these behavioral differences.
Several limitations of this study should be noted. Our study used research assessments of conduct problems and CU traits rather than clinical assessments. Previous studies using both clinical8,10 and research9,11 assessments have yielded markedly similar results, but it will be important to determine whether our findings characterize clinically assessed youths. In some cases, our groups differed in age and/or cognitive intelligence; to account for this, we included age and cognitive intelligence as covariates of no interest in our analyses. We did not exclude youths with conduct problems as a function of symptoms of frequently comorbid conditions such as attention-deficit/hyperactivity disorder (ADHD), anxiety, or depression. It has been suggested that it is particularly important for studies of children with conduct problems to consider comor bid diagnoses of ADHD when assessing the functioning of inferior frontostriatal circuits implicated in ADHD symptoms.47 However, this limitation is mitigated by the fact that the present task was not designed to assess functioning in these circuits as well as the fact that prior work has shown that ADHD is not associated with the pathological amygdala responses to fear expressions observed in children with CU traits.8 Additionally, medication in 5 adolescents with conduct problems could not be withheld prior to scanning, although previous examinations of this issue suggest that these medications are unlikely to critically influence the observed effects,48,49 a finding we confirmed by repeating our main primary analyses after excluding these participants. The results of our regression analysis in the full sample of participants—showing fear responses in the right amygdala to be negatively associated with CU traits (a finding that was significant at P < .05, FWE)—found only 1 active voxel within the ROI. Although this raises potential concerns about spurious findings, FWE correction is very conservative; in addition, when this analysis was repeated using only participants who were medication free, the results became more robust (k = 17), supporting the validity of our findings. Lastly, first-level contrast images were generated using an implicit baseline rather than contrast analyses with neutral expressions, in light of emerging evidence that neutral expressions represent unique socioaffective stimuli rather than a true affective baseline.50 Separate analyses of each face type avoid inadvertent conflation or masking of unique activation patterns to each face type, although they also cannot control as effectively for variables such as visual complexity and social salience.
Despite these limitations, our study extends previous findings regarding youths with conduct problems. We showed that consciously processed fearful expressions result in distinct patterns of amygdala activation that correspond to varying levels of CU traits and externalizing problems in a mixed-sex sample and are also related to characteristic patterns of aggressive behavior. The present findings support the use of a CU traits specifier for the assessment of children with conduct problems, and they highlight the increased sensitivity and power that result from treating clinical measures as dimensional rather than dichotomous variables.3 Although previous studies have observed group differences when dichotomizing CU traits, effects have generally emerged in the context of fairly liberal significance thresholds that introduce the risk for spurious findings.8,9 The present article demonstrates that more stringent thresholds can be maintained when more sensitive analyses are used.
Moreover, our findings may speak to the evolving debate about the nature of psychopathy as a construct. Originally described as a taxon, more recent views of psychopathy hold that this construct is less a coherent syndrome than a result of partially independent causal processes that support the CU traits and impulsive antisociality typically viewed as the 2 primary factors underlying the construct and which place individuals at particularly high risk for antisocial behaviors, particularly proactive aggression, when they co-occur.51 Although our study was not designed to directly test this model, our results are consistent with the notion of partially dissociable etiological processes among children with CU traits that place them at elevated risk for developing adult psychopathy.
Finally, to our knowledge, our findings also provide the first evidence that identified patterns of neural responses in adolescents with conduct problems who vary in CU traits apply to both male and female adolescents. This is an important issue because suggestions have been made that conduct problems may reflect distinct forms of psychopathology in males and females.52,53 Recent evidence finds similar structural abnormalities in the amygdala among both males and females with conduct disorder, and functional imaging studies using similar face-emotion tasks have identified similar patterns of dysfunction in samples of males and females8and males only9 with CU traits and conduct problems. The present research identifies patterns of atypical neural functioning in a sample of males and females similar to patterns found in previous samples of only males11; this adds to the body of evidence supporting common patterns of amygdala dysfunction in response to socioemotional cues, such as fear expressions, in males and females with CU traits.
Conclusions
Heterogeneity among youths with conduct problems has historically represented an impediment to the development of improved risk assessment, diagnosis, and treatment strategies.2 The present findings link observable patterns of aggressive behavior with specific patterns of neural dysfunction in youths with conduct problems who present with varying levels of CU traits. These findings confirm the importance of considering temperamental and personality variables in addition to behavior patterns in formulating effective, targeted treatment approaches for youths with conduct problems.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (grant R03 HD064906-01).
Role of the Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Lozier and Dr Marsh had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Marsh.
Acquisition of data: All authors.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Lozier, Cardinale, Marsh.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Lozier, VanMeter, Marsh.
Obtained funding: Marsh.
Administrative, technical, and material support: Cardinale.
Study supervision: VanMeter, Marsh.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This article was presented in part as a poster at the Annual Meeting of the Society for Neuroscience; November 15, 2011; Washington, DC.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. published correction appears in Arch Gen Psychiatry. 2005;62(7):768. [DOI] [PubMed] [Google Scholar]
- 2.Frick PJ. Developmental pathways to conduct disorder: implications for future directions in research, assessment, and treatment. J Clin Child Adolesc Psychol. 2012;41(3):378–389. doi: 10.1080/15374416.2012.664815. [DOI] [PubMed] [Google Scholar]
- 3.Frick PJ, Moffitt TE. A Proposal to the DSM-V Childhood Disorders and the ADHD and Disruptive Behavior Disorders Work Groups to Include a Specifier to the Diagnosis of Conduct Disorder Based on the Presence of Callous-Unemotional Traits. Washington, DC: American Psychiatric Association; 2010. [Google Scholar]
- 4.Lynam DR, Loeber R, Stouthamer-Loeber M. The stability of psychopathy from adolescence into adulthood: the search for moderators. Crim Justice Behav. 2008;35(2):228–243. doi: 10.1177/0093854807310153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker ED, Vitaro F, Lacourse E, Fontaine NM, Carbonneau R, Tremblay RE. Testing the developmental distinctiveness of male proactive and reactive aggression with a nested longitudinal experimental intervention. Aggress Behav. 2010;36(2):127–140. doi: 10.1002/ab.20337. [DOI] [PubMed] [Google Scholar]
- 6.Hicks BM, Patrick CJ. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. J Abnorm Psychol. 2006;115(2):276–287. doi: 10.1037/0021-843X.115.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebastian CL, McCrory EJ, Cecil CA, et al. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry. 2012;69(8):814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- 8.Marsh AA, Finger EC, Mitchell DG, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 9.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 10.White SF, Marsh AA, Fowler KA, et al. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am J Psychiatry. 2012;169(7):750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viding E, Sebastian CL, Dadds MR, et al. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am J Psychiatry. 2012;169(10):1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- 12.Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev Psychopathol. 2005;17(3):865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- 13.Marsh AA, Ambady N. The influence of the fear facial expression on prosocial responding. Cogn Emotion. 2007;21(2):225–247. doi: 10.1080/02699930600652234. [DOI] [Google Scholar]
- 14.Crowe SL, Blair RJ. The development of antisocial behavior: what can we learn from functional neuroimaging studies? Dev Psychopathol. 2008;20(4):1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- 15.Herpertz SC, Huebner T, Marx I, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 2008;49(7):781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 16.Passamonti L, Fairchild G, Goodyer IM, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67(7):729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair RJ. Considering anger from a cognitive neuroscience perspective. Wiley Interdiscip Cogn Sci. 2012;3(1):65–74. doi: 10.1002/wcs.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polier GG, Herpertz-Dahlmann B, Matthias K, Konrad K, Vloet TD. Associations between trait anxiety and psychopathological characteristics of children at high risk for severe antisocial development. Atten Defic Hyperact Disord. 2010;2(4):185–193. doi: 10.1007/s12402-010-0048-5. [DOI] [PubMed] [Google Scholar]
- 19.Edens JF, Marcus DK, Lilienfeld SO, Poythress NGJ., Jr Psychopathic, not psychopath: taxometric evidence for the dimensional structure of psychopathy. J Abnorm Psychol. 2006;115(1):131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- 20.Guay JP, Ruscio J, Knight RA, Hare RD. A taxometric analysis of the latent structure of psychopathy: evidence for dimensionality. J Abnorm Psychol. 2007;116(4):701–716. doi: 10.1037/0021-843X.116.4.701. [DOI] [PubMed] [Google Scholar]
- 21.Finger EC, Marsh AA, Blair KS, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry. 2011;168(2):152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman AS. K-BIT: Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 23.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- 25.Kimonis ER, Frick PJ, Skeem JL, et al. Assessing callous-unemotional traits in adolescent offenders: validation of the Inventory of Callous-Unemotional Traits. Int J Law Psychiatry. 2008;31(3):241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Roose A, Bijttebier P, Decoene S, Claes L, Frick PJ. Assessing the affective features of psychopathy in adolescence: a further validation of the Inventory of Callous and Unemotional Traits. Assessment. 2010;17(1):44–57. doi: 10.1177/1073191109344153. [DOI] [PubMed] [Google Scholar]
- 27.Raine A, Dodge K, Loeber R, et al. The Reactive-Proactive Aggression Questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggress Behav. 2006;32(2):159–171. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekman P, Friesen W. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- 29.Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychol Med. 2006;36(11):1563–1569. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- 30.Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 31.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 32.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox; Paper presented at: International Conference on Functional Mapping of the Human Brain; June 2-6, 2002; Sendai, Japan. [Google Scholar]
- 33.Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychol Bull. 2011;137(5):856–879. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 38.Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev. 2008;32(3):454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51(10):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 40.Gray JA, McNaughton N. The Neuropsychology of Anxiety. 2nd. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 41.Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Dawel A, O'Kearney R, McKone E, Palermo R. Not just fear and sadness: meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci Biobehav Rev. 2012;36(10):2288–2304. doi: 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Sylvers PD, Brennan PA, Lilienfeld SO. Psychopathic traits and preattentive threat processing in children: a novel test of the fearlessness hypothesis. Psychol Sci. 2011;22(10):1280–1287. doi: 10.1177/0956797611420730. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Association of poor childhood fear conditioning and adult crime. Am J Psychiatry. 2010;167(1):56–60. doi: 10.1176/appi.ajp.2009.09040499. [DOI] [PubMed] [Google Scholar]
- 45.Baker E, Shelton KH, Baibazarova E, Hay DF, van Goozen SHM. Low skin conductance activity in infancy predicts aggression in toddlers 2 years later. Psychol Sci. 2013;24(6):1051–1056. doi: 10.1177/0956797612465198. [DOI] [PubMed] [Google Scholar]
- 46.Campbell A. Sex differences in direct aggression: what are the psychological mediators? Aggress Violent Behav. 2006;11(3):237–264. doi: 10.1016/j.avb.2005.09.002.. [DOI] [Google Scholar]
- 47.Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011;69(12):e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Marsh AA, Finger EC, Fowler KA, et al. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatry. 2013;54(8):900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finger EC, Marsh AA, Mitchell DG, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- 51.Skeem JL, Polaschek DLL, Patrick CJ, Lilienfeld SO. Psychopathic personality: bridging the gap between scientific evidence and public policy. Psychol Sci Public Interest. 2011;12(3):95–162. doi: 10.1177/1529100611426706. [DOI] [PubMed] [Google Scholar]
- 52.Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry. 2000;39(12):1468–1484. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Odgers CL, Moffitt TE, Broadbent JM, et al. Female and male antisocial trajectories: from cildhood origins to adult outcomes. Dev Psychopathol. 2008;20(2):673–716. doi: 10.1017/S0954579408000333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


