Abstract
The role of interferon regulatory factor 3 (IRF3) in the innate immune response to infection has been well studied. However, less is known about IRF3 signaling in shaping the adaptive T cell response. To determine the role of IRF3 in the generation and maintenance of effective antiviral T cell responses, mice deficient in IRF3 were infected with a potentially persistent virus, Theiler’s murine encephalomyelitis virus (TMEV) or with a model acute infection, influenza A virus (IAV). IRF3 was required to prevent TMEV persistence and induce robust TMEV specific effector T cell responses at the site of infection. This defect was more pronounced in the memory phase with an apparent lack of TMEV-specific memory T cells expressing granzyme B (GrB) in IRF3 deficient mice. In contrast, IRF3 had no effect on antigen specific T cell responses at the effector stage during IAV infection. However, memory T cell responses to IAV were also impaired in IRF3 deficient mice. Furthermore, addition of cytokines during peptide restimulation could not restore GrB expression in IRF3 deficient memory T cells. Taken together, IRF3 plays an important role in the maintenance of effective anti-viral T cell memory responses.
Keywords: Interferon regulatory factor-3, T cells, Theiler’s murine encephalomyelitis virus, Influenza A virus, T cell memory, Cytokines
1. Introduction
Innate immune responses slow virus replication but also contribute to development of antiviral T cell responses. In particular, IRF3 is a transcription factor that is rapidly activated downstream of the TRIF and MAVS pathways, which initiate signaling responses to viral RNA in the endosome or cytoplasm, respectively [1,2]. IRF3 controls expression of interferon-β (IFN-β) [3,4], that in turn functions by inducing an antiviral state in cells through upregulation of a large class of downstream genes known as interferon stimulated genes (ISGs). The induction of ISGs in IFN-β-stimulated cells accelerates reduction in viral replication by degrading RNA, inhibiting translation, preventing membrane budding, and inducing apoptosis [5–9]. In addition, recent evidence suggests IFN-β is important for inducing the cytolytic activity of effector and memory CD8 T cells [10,11]. IRF3 has also been implicated in expression of other cytokines such as IL-6 [12–14], IL-12 [12,15], and IL-15 [12,16,17], all of which can influence effector and memory T cell responses. For example, the induction of IL-6 by alum and monophosphoryl lipid A adjuvants was required for induction of Granzyme B (GrB) by memory CD8 T cells [18]. In addition, IL-12 promotes cell division and survival of activated CD8 T cells [19], as well as GrB expression in CD8 T cells [20]. Finally, IL-15 production by inflammatory monocytes is required to promote memory cytolytic CD8 T cells against Listeria infection [17]. In one study, the impact of IRF3 on T cell function was examined in CD8 cells, but this was limited to the context of poly I:C induced activation [21]. While these studies collectively suggest that IRF3 is important for antiviral T cell responses, the role of IRF3 in promoting effector and memory T cell responses during virus infection has not been directly addressed.
TMEV causes acute infection of the central nervous system that is cleared through innate and adaptive immune responses in resistant mouse strains, such as C57BL/6 (B6), but causes persistent infection and subsequent demyelinating disease in SJL/J mice [22]. Although this model has been used extensively to study immune clearance of a potentially persistent virus, the factors required to prevent persistence to TMEV are not fully understood. Previously, we have shown that B6 macrophages secrete IL-6 and IFN-β in response to TMEV [23]; however, in the absence of IRF3, TMEV infected macrophages express reduced levels of these key cytokines and are unable to control replication of the TMEV genome [12]. T cell responses are also involved in TMEV clearance in B6 mice by directly killing virally infected cells or through production of antiviral cytokines such as IFN-γ [24–26]. Although IRF3 deficiency confers susceptibility of macrophages to TMEV replication, its effect on adaptive T cell responses to TMEV in vivo remains unclear.
While the outcome of TMEV infection can differ in various mouse strains, low pathogenicity or low dose IAV infection is acute and is cleared by 8–10 days in most mouse strains. IAV infects epithelial cells in both the upper and lower respiratory tracts resulting in a highly inflamed lung microenvironment [27]. This highly activated innate immunity induces prolific adaptive immune responses characterized by high levels of IFN-γ secretion and the generation of cytotoxic T lymphocytes (CTL) resulting in viral clearance. Thus, viral clearance is mediated by contributions from a number of factors including CD8 CTL [28,29] as well as polyfunctional CD4 cells [30,31]. Subsequently, upon clearance of the virus, memory T cells are generated that either remain at the site of infection or circulate in secondary lymphoid tissues to protect against reinfection [32,33]. IAV infection triggers TLR-3 and downstream signaling responses similar to TMEV infection, however, the role of IRF3 in promoting effector and memory T cell responses during IAV infection has not been fully explored. Therefore comparing T cell responses in B6 and IRF3 deficient mice to both TMEV and IAV provides an excellent opportunity to gain a more complete understanding of the role of IRF3 in the generation of anti-viral T cell responses and resistance to infection.
In this study, we find that IRF3 deficiency has a minor impact in clearance of TMEV from the brain of TMEV infected mice yet leads to chronic symptoms of neurological impairments in all IRF3KO mice. Antiviral CD8 and CD4 effector T cell responses are impaired in IRF3KO mice following TMEV infection, but minimal effector T cell impairment was observed in IAV infected IRF3KO mice. Interestingly, recall responses measured by GrB re-expression of memory CD8 and CD4 T cells are diminished in IRF3KO mice infected with either TMEV or IAV. Surprisingly, individual cytokine treatments did not restore GrB expression in antigen specific memory T cell responses in IRF3KO mice. These results provide insight into the signals required for the development and maintenance of effector and memory T cells during persistent and acute viral infections.
2. Materials and methods
2.1. Mice
Male and female C57BL/6 and SJL/J mice were purchased from Harlan Sprague Dawley and used at 6–8 weeks of age. Male and female IRF3 deficient mice (IRF3KO) on the B6 background were offspring of breeder pairs obtained from Dr. Karen Mossman, originally produced by Dr. Tadatsugu Taniguchi from the University of Tokyo [34]. Experimental animal procedures using mice were approved by and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska–Lincoln and the University of Nebraska Medical Center.
2.2. TMEV infection and analysis of virus persistence
Female mice were anesthetized with isofluorane and 1 × 106 PFU of TMEV DA in 50 µl volume was injected intracranially (i.c.). Clinical symptoms were evaluated from day 133 to day 186 post infection. Scoring was determined as follows: 0.0, no clinical symptoms; 0.5 mild waddling gait and/or partial limp tail; 1.0 full waddling gait and/or full limp tail; 1.5, partial leg tuck; 2.0, full leg tuck; 3.0, severe waddling gait, hind limb paralysis (single leg). To evaluate virus persistence, brain homogenates from TMEV-infected mice were overlaid onto BHK-21 cell monolayers for 24 h and amplification of the TMEV genome was detected by qRT-PCR as previously described [12].
2.3. IAV infection and quantification of IAV titers
Male mice were anesthetized with isoflurane and 30 µl PR8 (A/PR/8/34) IAV diluted in PBS was administered intranasally (i. n.) at 1000 EIU. In some cases, weight loss and survival curves were monitored. Six days post infection, mice were sacrificed, lungs placed in RNAlater (Ambion, Austin TX) and frozen at −20 °C. Lung samples were homogenized in TRIzol (Ambion) using a Tissue Tearor homogenizer (Biospec Products Inc, Bartlesville, OK). RNA was isolated, reverse-transcribed into cDNA and the acid polymerase (PA) gene amplified by qPCR as described [30]. A known concentration of PA-containing plasmid was used to generate a standard curve in all reactions. PA copies per lung were then calculated based an initial concentration of 100 ng of cDNA as described [30] and used for amplification by quantitative real-time PCR (Step One Plus, Applied Biosystems).
2.4. Analysis of T cell responses following infection
Female B6 or IRF3KO mice were infected intraperitoneally (i. p.) with 1 × 106 PFU TMEV DA strain. Seven days post infection (p. i.), mice were sacrificed and peritoneal exudate cells were obtained by washing the peritoneal cavity with 2 ml DMEM media. Total spleen cells were also isolated by dissociating the spleen through 70 µM mesh followed by red blood cell lysis. PEC or splenocytes were incubated with fluorochrome labeled anti-CD4, anti-CD 8 and anti-CD43 (activation isoform 1B11) (eBiosciences, San Diego, CA). Cells were then fixed in 4% paraformaldehyde followed by intracellular staining with fluorochrome labeled anti-human GrB antibody (Life Technologies, Grand Island, NY) in 0.25% saponin (Sigma–Aldrich, St. Louis, MO) buffer.
For analysis of cytokine synthesis, peritoneal exudate cells (PEC) or splenocytes were incubated with the class I peptide VP2121–130 [35] and class II peptide VP2201–220 [36] (New England Peptide, Gardner, MA) for 6 h (effectors) or 48 h (memory) adding 10 µg/ml Brefeldin A (Sigma–Aldrich) during the last 2 or 12 h respectively. Cell suspensions were incubated with fluorescently labeled anti-CD4 and anti-CD8 as above, followed by incubation with fluorescently labeled anti-IL-17 and anti-IFN-γ antibodies (eBiosciences) in saponin buffer.
For analysis after IAV infection, male B6 or IRF3KO mice were sacrificed at the indicated time points with a lethal dose of tribromoethanol i. p. Lungs and splenocytes were processed as described [31] for flow cytometric analysis and peptide restim-ulation. Briefly, lungs were perfused with PBS, treated with collagenase D, and filtered through a 70 µm mesh filter. Splenocytes were dissociated into single cell suspensions using 70 µm filters followed by red cell lysis. Cell suspensions were stained with fluorochrome-conjugated anti-CD4, anti-CD8, anti-NKG2A/C/E, or anti-CD44 (eBioscience), fixed in 4% paraformaldehyde and stained with anti-GrB in 0.25% saponin buffer as described above. For intracellular cytokine assays, lung cells or splenocytes were restimulated with class I IAV peptide nucleoprotein (NP) NP366–374 or class II peptide NP261–275 (New England Peptide) for 6 h with 10 µg/ml Brefeldin A added in the last 2 h of incubation. For memory responses, cells were restimulated for 48 h with Brefeldin A added during the last 12 h of incubation [37]. Cells were then stained with for intracellular cytokine expression as described above. All samples were analyzed using a Becton Dickinson FACSCalibur and data analyzed using FlowJo software (Treestar, Ashland, OR).
2.5. In vitro cytokine reconstitution experiments
B6 or IRF3KO mice were infected with 1 × 10 PFU TMEV DA i.p. or 1000 EIU IAV A PR8 i.n. for 30 days. Splenocytes from individual mice were restimulated with TMEV VP2 peptide, IAV NP peptide or no peptide for 48 h. During restimulation, 200 U/ml IFN-β, 10 ng/ml IL-12, 10 ng/ ml IL-6, or 10 ng/ml IL-15 were added for 48 h (with Brefeldin A added for the last 12 h for cytokine analysis) and then processed for flow cytometric analysis as described.
2.6. Statistics
Statistical significance between experimental groups was determined by two-tailed Student’s t test, one way ANOVA (Fig. 1) or one way ANOVA with Tukey’s multiple comparisons test (Fig. 5 and Fig. 6) using Prism 4.0 (Graph Pad Software). Kaplan–Meier survival curves were analyzed by the log rank test.
3. Results
3.1. IRF3 is required to prevent TMEV persistence and chronic disease symptoms
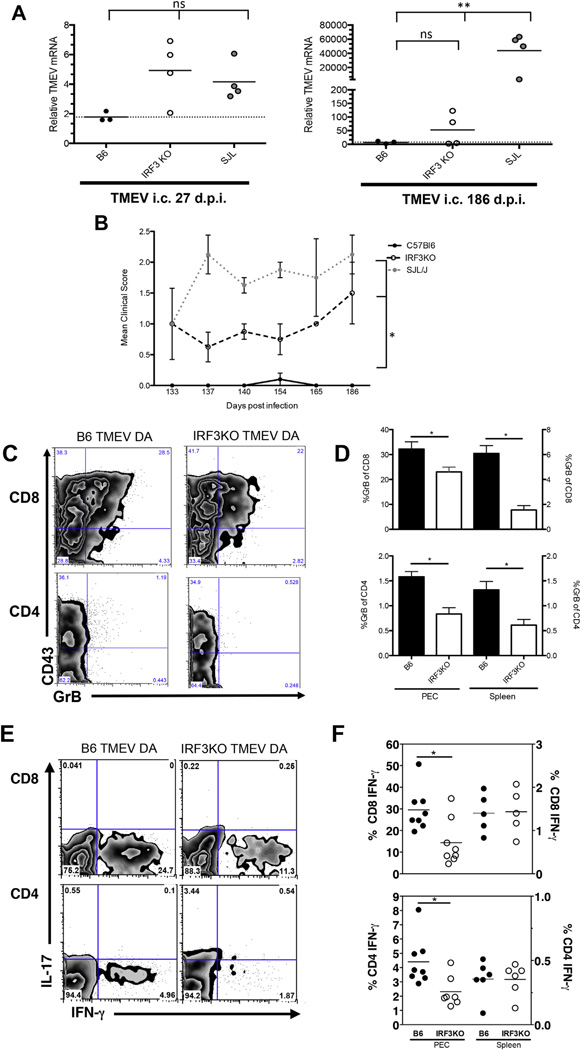
Previously, we showed that deletion of the IRF3 gene is sufficient to confer susceptibility of B6 macrophages to TMEV replication [12]. Because virus replication in macrophages is required for persistence of TMEV in the CNS [38], we sought to determine if IRF3KO mice develop a persistent infection with TMEV. In order to detect persistent low levels of infectious virus, brain homogenates from TMEV-infected mice were overlaid onto BHK-21 cell monolayers for 24 h and amplification of the TMEV genome was detected by qRT-PCR (Fig. 1A). TMEV genome was detected from brains of infected IRF3KO mice at both 27 and 186 d.p.i. (Fig. 1A). However, the differences between B6 and IRF3KO mice at 186 d.p.i were not statistically significant and only 2 of 4 IRF3KO mice exhibited TMEV genome in the brain, while TMEV genome was not detected in any brains of infected B6 mice. Infected SJL/J mice, which served as a positive control for TMEV persistence, showed levels of TMEV genome comparable to IRF3KO mice at 27 d.p.i., but had much greater levels of TMEV genome than IRF3KO mice at 186 d.p.i.
Because chronic infection of SJL/J mice with TMEV DA leads to chronic disease that manifests as waddling gait and hind limb paralysis [39], we determined if TMEV-infected IRF3KO mice developed symptoms of chronic neurological disease. Indeed, infection of IRF3KO mice led to significantly greater chronic symptoms of neurological disease in comparison to B6 mice, which did not exhibit any symptoms (Fig. 1B). However, clinical signs in IRF3KO mice during chronic TMEV infection were less severe and distinct from those observed in SJL/J mice, with flaccid tail paralysis being the prominent symptom in IRF3KO mice (Fig. 1B). Thus, although TMEV is persistently maintained in SJL/J mice causing clinically measurable demyelinating disease, IRF3KO mice show an intermediate phenotype with 50% of mice harboring TMEV infection in the CNS and a lower clinical score. Nevertheless, these results indicate that IRF3 is essential for complete clearance of TMEV from the CNS and prevention of disease due to chronic infection.
3.2. IRF3 deficiency impacts development of TMEV specific effector T cell responses
TMEV DA resistant strains of mice clear virus from the CNS and prevent chronic disease via antigen specific CD8 CTL and IFN-γ expressing CD4 cells [24–26,40]. Moreover, these anti-viral T cell responses could be influenced by production of IFN-β [10,11], IL-6 [41], IL-12 [19,42], or IL-15 [17], all of which require IRF3 for maximum expression [12,43,44]. To test our hypothesis that IRF3 impacts anti-TMEV T cell responses, splenic and PEC mononuclear cells from i. p.-infected TMEV B6 or IRF3KO mice were isolated and analyzed for effector function in activated CD43+ T cell populations. Fig. 1C shows that accumulation of activated CD4 and CD8 T cells in the peritoneal cavity at day 9 post infection was similar between B6 and IRF3KO mice. However, the level of GrB expression in activated CD8 and CD4 T cells in the peritoneal cavity from TMEV infected IRF3KO mice was significantly less compared with B6 mice (Fig. 1D). Approximately 30% of CD8 T cells express GrB in the peritoneal cavity of B6 mice, while only 20% of CD8 T cells express GrB in the peritoneal cavity of IRF3KO mice (Fig. 1D). This pattern was seen in splenic CD8 cells as well as in CD4 T cells from the peritoneal cavity and the spleen in TMEV infected mice (Fig. 1D). Thus, IRF3 deficiency significantly impairs the expression of GrB in effector T cells during TMEV infection.
Fig. 1.
IRF3 deficiency leads to TMEV persistence and reduced TMEV specific effector T cell responses. (A) B6, IRF3KO, or SJL/J mice were infected with 1 × 106 PFU TMEV DA i.c. and sacrificed at 27 or 186 days post infection. Brain homogenates were overlaid onto BHK-21 monolayers and TMEV genome assessed by qRT-PCR as described in Materials and Methods. Shown is the relative amount of TMEV mRNA in brain homogenates (**p = 0.04 by one way ANOVA with Tukey’s multiple comparisons test). (B) In a parallel experiment, mice were infected as in (A) and monitored for signs of clinical disease. Mean clinical score of 4 mice per group is shown, defined as in Materials and Methods (*p < 0.001 by one way ANOVA). In both experiments, SJL mice were used as a positive control for viral persistence and clinical symptoms of paralysis. (C–F) B6 or IRF3KO mice were infected with 1 × 106 PFU TMEV DA i.p. and peritoneal exudate cells (PEC) and spleen were isolated at 7 days p. i. Representative plots of GrB and CD43 expression in CD4 and CD8 T cells is shown in (C) and the mean percentage GrB+ +/− SEM of 5–8 mice per group from two independent experiments in CD4 and CD8 T cells is shown in (D). PEC and splenocytes were also restimulated with VP2 peptides as described in Materials and Methods and intracellular levels of IFN-γ determined by flow cytometry. Representative FACS plots are shown (E) and the percentage IFN-γ+, of 5–8 mice per group from two independent experiments is shown in (F). *p < 0.05 between indicated groups determined by student’s t test. An additional independent experiment using 4 mice per group was performed with similar results.
To analyze cytokine expression, PEC and splenocytes from mice infected with TMEV DA were restimulated with TMEV VP2 specific peptides and intracellular IFN-γ in CD4 and CD8 T cells was measured. Because IL-17 producing CD4 T cells are thought to contribute to pathogenesis in TMEV susceptible strains [35], we also sought to determine if IRF3 deficiency leads to elevated IL-17 expression (Fig. 1E). In the peritoneal cavity, the percentage of antigen specific, IFN-γ expressing CD4 and CD8 cells was significantly less in IRF3KO mice compared to B6 mice (Fig. 1E and F). In contrast, B6 and IRF3KO mice had similar levels of IFN-γ producing CD4 and CD8 T cells in the spleen (Fig. 1F). IL-17 expression was not robust in CD4 or CD8 T cells from either B6 or IRF3KO mice (Fig. 1E). These results indicate that IRF3 deficiency impairs TMEV-specific IFN-γ production by T cells at the site of infection but not in peripheral lymphoid sites such as the spleen.
3.3. IRF3 deficiency does not affect IAV specific effector T cell responses
IAV is a single stranded, negative sense RNA virus and like TMEV, can trigger endosomal and intracellular nucleic acid sensors including TLR-3 [45] and RIG-I [45–47]. Downstream of these pattern recognition receptors, IAV induced signals converge to activate NF-κB and IRF3 to induce proinflammatory cytokines such as IL-6 and type I IFNs [45]. However, unlike chronic TMEV infection of SJL/J, IAV infection of mice results in an acute infection that is cleared (low pathogenicity strains or low dose infection) or an infection in which mice succumb (high pathogenicity strains or high dose infection). Thus, we sought to determine the impact of IRF3 on CD4 and CD8 T cell responses following an acute IAV infection.
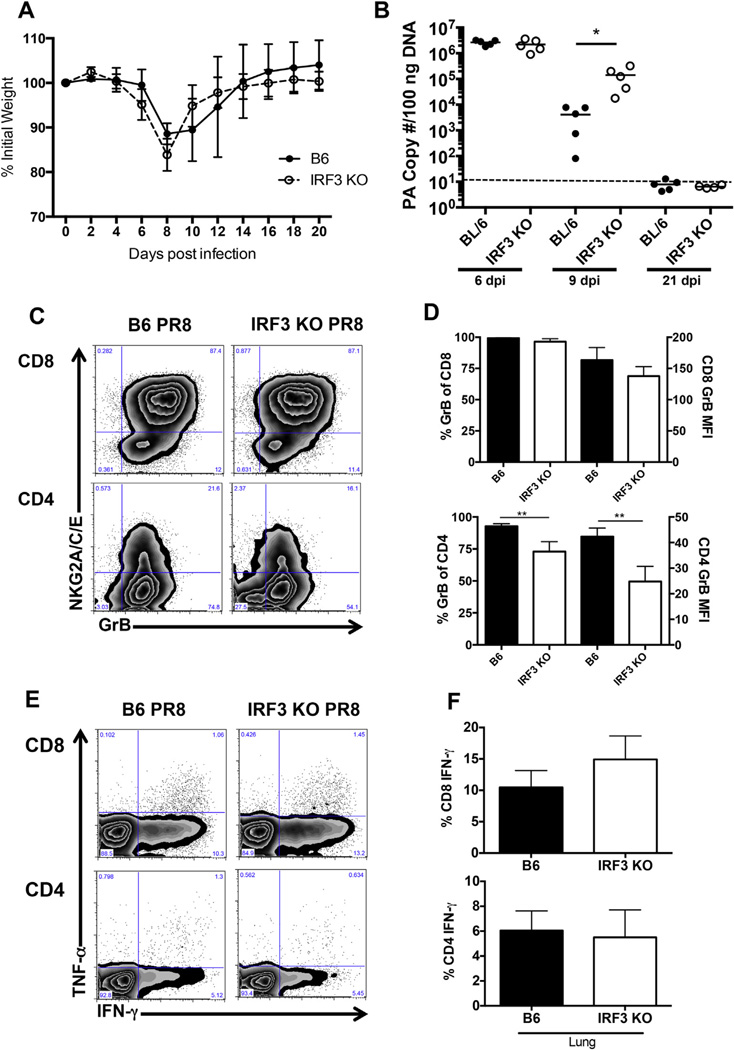
First, to determine if IRF3 conferred any survival advantage, mice were infected with a sublethal dose of IAV PR8. Interestingly, IRF3 deficiency had no impact on weight loss (Fig. 2A) or survival (data not shown) following IAV infection. Although weight loss was unaffected, IRF3KO mice showed delayed clearance of IAV at day 9 post infection but complete clearance by day 21 post infection (Fig. 2B). Next, T cell responses after IAV infection were examined by analyzing GrB expression in lung resident CD8 and CD4 T cells at day 9 post infection (Fig. 2C). As shown in Fig. 2D, similar percentages of CD8 cells in the lung express GrB in B6 and IRF3KO mice, while a significant decrease in the percentage of CD4 cells that express GrB was observed in IRF3KO mice. The level of GrB expression was also decreased in CD4 cells, but not CD8 cells in IRF3KO mice compared to B6 mice (Fig. 2D, MFI). Cytokine production in T cells after ex vivo restimulation with IAV specific peptides was also analyzed. IFN-γ was the predominant cytokine produced by both CD4 and CD8 cells, with modest levels of TNF-α expressed (Fig. 2E). However, no differences in cytokine production by lung resident CD4 or CD8 T cells were observed between B6 and IRF3KO mice (Fig. 2F). In addition, the absolute numbers of CD4 and CD8 cells expressing GrB or IFN-γ were not different between IRF3KO and B6 mice after IAV infection. Thus, in contrast to the impaired effector T cell responses observed during TMEV infection of IRF3 deficient mice, IRF3 deficiency does not impact primary effector T cell responses following IAV infection.
Fig. 2.
IRF3 deficiency does not affect IAV specific effector T cell responses. (A–F) B6 or IRF3KO mice were infected intranasally with 1000 EIU PR8 and followed over time for weight loss (A) or sacrificed at day 6, 9 or 21 p. i. for viral titers (B) or at day 9 p. i. for analysis of T cell responses (C–F). A) Weight loss is expressed as the mean percentage of initial weight at day 0, +/− SD for 5 mice per group. This experiment was repeated with similar results. B) RNA was extracted from whole lungs at day 6, 9 or 21 p. i. and IAV PA gene copy number was determined by qPCR. Shown is the average PA copy number per 100 ng of lung RNA in five individual mice per group, per timepoint. *p < 0.05 and the dotted line represents the limit of detection of the assay. (C) Representative GrB FACS plots for CD4 and CD8 cells 9 days p. i. in B6 or IRF3KO mice (n = 5 mice per group). (D) Mean percentage, +/− SD of GrB+ cells and mean fluorescence intensity (MFI), +/− SD in the CD4 or CD8 cell populations in the lungs of 5 infected mice per group (**p < 0.001). (E) Representative FACS plots showing intracellular IFN-γ and TNF-α cytokine expression gated on CD8 or CD4 T cells in the lung after peptide restimulation. (F) Mean percentage, +/− SD of IFN-γ+ in lung resident CD4 or CD8 cells (n = 5 mice per group). This experiment was repeated with an additional 5 mice per group at day 9 with similar results.
3.4. IRF3KO mice fail to develop memory T cell responses following TMEV infection
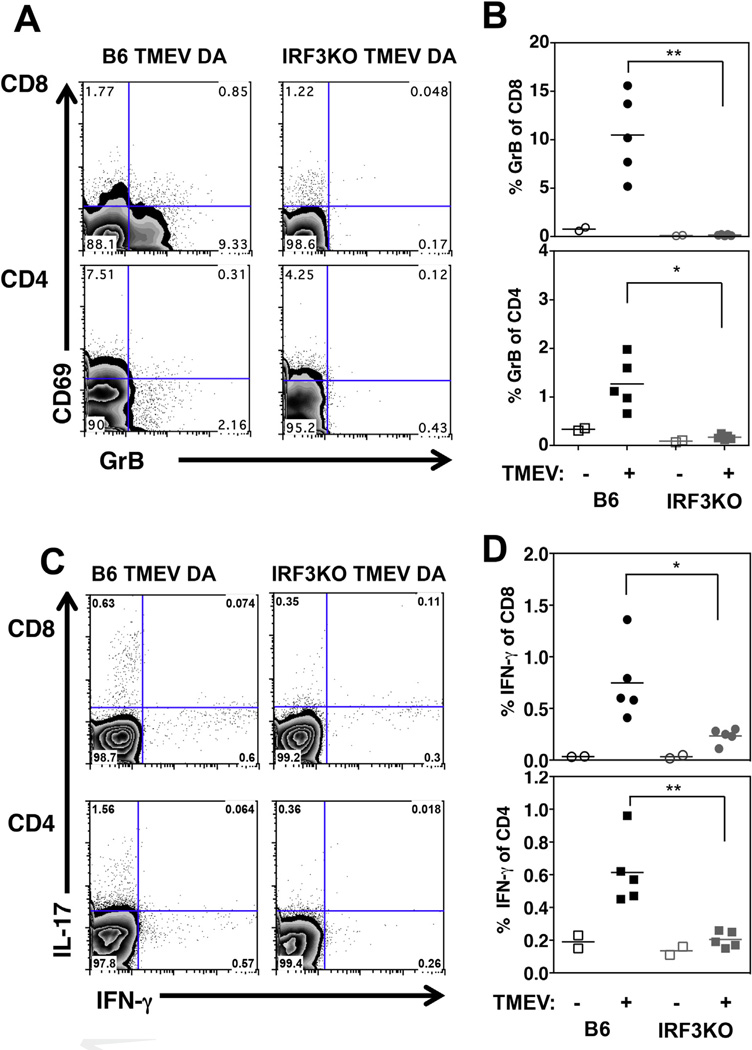
Our results so far show that IRF3 deficiency impacts the generation of effector T cell responses against TMEV during the acute infection. However, these results do not indicate whether IRF3-dependent signals could also be important for developing and sustaining T cell memory. Therefore, we sought to determine a role for IRF3 in the development of antigen specific memory T cells following TMEV infection by analyzing GrB and cytokine expression in splenocytes at > 15 weeks after TMEV infection, when IRF3 deficient mice show neurological symptoms (Fig. 1B). As shown in Fig. 3A and B, IRF3KO mice showed a significant impairment in peptide-specific GrB expression in both CD8 and CD4 T cells in the spleen. Antigen specific GrB recall responses in CD8 and CD4 T cells from IRF3KO mice are similar to uninfected mice, indicating the lack of a responsive memory T cell population (Fig. 3B). Similarly, significantly less CD4 and CD8 T cells from IRF3KO mice expressed IFN-γ than those from B6 mice (Fig. 3C and D). Although more antigen specific CD4 T cells from B6 mice tended to express IL-17 than those from IRF3KO mice (Fig. 3C), these differences were not significant. GrB or IFN-γ expression was not readily detectable in uninfected mice (Fig. 3B and D) or in the absence of peptide restimulation (data not shown). To confirm these results in our model of TMEV induced acute encephalitis (Fig. 1A and B), B6 or IRF3KO mice were infected with TMEV DA i.c. and antigen specific splenic T cell recall responses were measured after >20 weeks. As expected, significantly more CD8 and CD4 T cells expressed GrB in TMEV infected B6 mice compared to IRF3KO mice (data not shown). These results indicate that IRF3 is critical for developing and sustaining memory T cells following TMEV infection, regardless of the infection route.
Fig. 3. IRF3KO mice fail to develop memory T cell responses following TMEV infection. B6 or IRF3KO mice were infected with 1×106 PFU TMEV DA i.p. One hundred and fifty days p. i., mice were sacrificed and splenocytes restimulated for 48 h with CD4 and CD8 specific TMEV VP2 peptide (A–D). Representative FACS plots for GrB are shown in A and percentage of GrB+ cells in TMEV infected (+) or uninfected (−) individual mice is shown in B (**p = 0.001 and *p < 0.02). Representative FACS plots of intracellular IFN-γ and IL-17 after VP2 restimulation (C). Shown are percent IFN-γ+ cells in TMEV infected (+) or uninfected (−) mice (D) (**p = 0.001 and *p < 0.02). This experiment was repeated at 160 days post infection with similar results.
3.5. IRF3KO mice fail to mount robust memory T cell responses following IAV infection
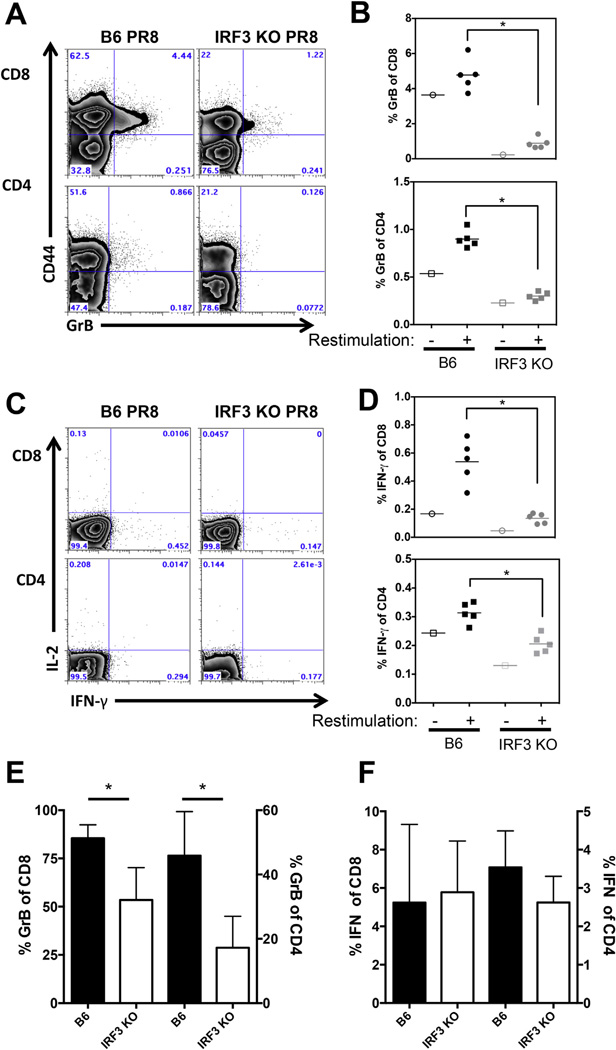
Though IRF3 deficiency had no impact on IAV specific T cell effector function, the lack of memory responses observed in TMEV infected mice prompted us to determine if the memory response to IAV infection was also altered. Subsequently, we examined the role of IRF3 in recall responses in memory T cells at > 4 weeks post IAV infection. Shown are representative flow cytometry plots of memory marker CD44 and GrB expression after gating on CD8 or CD4 T cells (Fig. 4A). Similar to memory responses to TMEV, the percent, as well as absolute numbers (data not shown)of IAV specific CD8 and CD4 memory cells expressing GrB was significantly diminished in IRF3KO mice (Fig. 4B). Recall cytokine responses were also measured and reveal that the percentage and absolute numbers (data not shown) of CD8 and CD4 T cells expressing IFN-γ was significantly decreased in IRF3KO mice compared to B6 mice (Fig. 4C and D). The percent of T cells producing IL-2 upon IAV peptide restimulation was also decreased in IRF3KO mice (Fig. 4C and data not shown). Thus, in contrast to the primary effector response, IRF3 impacts the recall capacity of IAV specific memory T cells. To determine whether IRF3 deficiency impacted in vivo recall responses, B6 and IRF3KO mice were infected with HKx31 H3N2 virus and 28 days later, challenged with PR8 IAV Five days post challenge mice were analyzed for GrB expression and IFN-g expression after NP peptide restimulation (Fig. 4E and F). Similar to results shown in Fig. 4A and B, IRF3KO mice showed significantly impaired production of GrB in lung resident CD8 and CD4 T cells compared to B6 mice (Fig. 4E). However, IFN-γ responses in lung resident CD8 and CD4 cells were equivalent between IRF3KO and B6 mice (Fig. 4F). Thus, IRF3 impacts memory (Fig. 4B) and secondary effector (Fig. 4E) CD8 and CD4 production of GrB, but does not impact the ability of secondary effectors to produce IFN-γ upon challenge (Fig. 4F).
Fig. 4.
IRF3KO mice fail to mount robust memory T cell responses following IAV infection. B6 or IRF3KO mice (5 mice/group) were infected with 1000 EIU PR8 for 28 days. Splenocytes were restimulated for 48 h with CD4 and CD8 specific IAV NP peptide (A–D). Representative FACS plots for GrB are shown in A and percentage of GrB+ cells in individual mice shown in B (*p < 0.05). Representative FACS plots of intracellular IFN-γ and IL-2 after NP restimulation (C). Shown is percent IFN-γ+ cells after restimulation with NP peptide (+) or in the absence of peptide (−) (D). *p < 0.05. This experiment was repeated with an additional 5 mice per group. In panels E and F, B6 or IRF3KO mice (5 per group) were infected with 1000 EIU ×31 (H3N2) virus for 28 days followed by challenge with 1000 EIU PR8. Five days post challenge, mice were sacrificed, lungs isolated and analyzed for GrB expression (E) or restimulated with NP peptides for intracellular IFN-γ expression (F). Shown is percent GrB+ +/− SD of CD8 or CD4 populations (E) *p < 0.05 or percent IFN-γ+ cells +/− SD of CD8 or CD4 populations (F). This experiment was repeated twice with similar results.
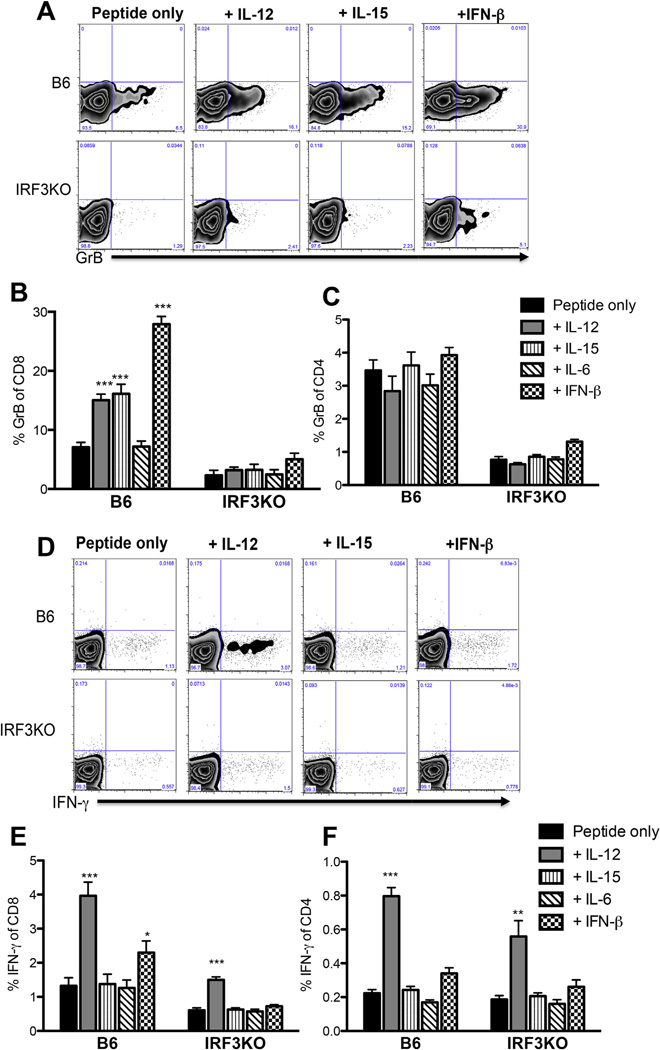
3.6. IL-12 restores IFN-γ expression, but not GrB expression in IRF3 deficient, TMEV specific memory T cells
We and others have shown that IRF3 is involved in expression of IFN-β [44,48], IL-6 [13,14,44], IL-12 [44], and IL-15 [44]. Because recent studies implicate these cytokines in GrB expression in memory CD8 CTLs [10,17,18], we hypothesized that deficiencies in these cytokines were responsible for the impaired recall responses of memory T cells from IRF3KO mice. Thus, cytokine supplementation might restore expression of antigen specific GrB and IFN-γ in CD8 and possibly CD4 memory cells. In order to test this hypothesis, B6 or IRF3KO mice were infected with TMEV as described and 4 weeks later, splenocytes were restimulated with TMEV VP2 peptides with or without IL-6, IL-12, IL-15 or IFN-β for 48 h. As others have shown [10,17,20], IL-12, IL-15, and IFN-β treatment of B6 memory T cells enhanced GrB expression in CD8 T cells (Fig. 5A and B). In contrast, treatment of CD8 cells with IL-6 had no effect on GrB expression. However, none of the aforementioned cytokines influenced GrB expression in CD4 T cells (Fig. 5C). Surprisingly, treatment of IRF3KO memory CD8 T cells with IL-12, IL-15, or IFN-β could not restore GrB expression (Fig. 4A and B). These results show that individual cytokines are not sufficient to restore GrB expression in memory CD8 or CD4 T cells from IRF3KO mice, suggesting that IRF3-dependent signals are required during the effector T cell response in order to generate optimal T cell memory.
Fig. 5.
IL-12 restores IFN-γ expression, but not GrB expression in IRF3 deficient, TMEV specific memory T cells. B6 or IRF3KO mice (5 mice/group) were infected with 1 × 106 PFU TMEV DA i.p. for 30 days. Splenocytes were restimulated with TMEV VP2 peptide (or no peptide) +/− 200 U/ml IFN-β, 10 ng/ml IL-12, 10 ng/ml IL-6, or 10 ng/ml IL-15 for 48 h (with Brefeldin A added for the last 12 h) and analyzed for GrB (A–C) or IFN-γ (D–F) expression by flow cytometry. Representative flow cytometry plots of GrB+ in CD8 T cells are shown (A). Bar graphs represent the mean percent GrB +/− SEM in CD8 (B) or CD4 (C) T cells with or without the addition of cytokines. Representative flow cytometry plots of IFN-γ+ in CD8 T cells are shown (D). Bar graphs are representative of mean percent IFN-γ +/− SEM in CD8 (E) or CD4 (F) T cells with or without the addition of cytokines. *p < 0.05, **p < 0.01, ***p < 0.0001, by one way ANOVA with Tukey’s multiple comparisons test.
Next, we sought to determine if treatment with IRF3-induced cytokines could restore the peptide specific IFN-γ deficiency in IRF3KO memory T cells. As others have shown [20], IL-12 significantly enhanced IFN-γ expression in memory CD4 and CD8 T cells from B6 mice (Fig. 5D – F). In addition, IL-12 was able to restore IFN-γ expression in memory CD4 and CD8 T cells from IRF3KO mice (Fig. 5D – F). Although IFN-β was able to moderately enhance IFN-γ expression in CD8 T cells from B6 mice, only IL-12 was able to have any impact on IFN-γ expression in IRF3KO memory T cells (Fig. 5D – F).
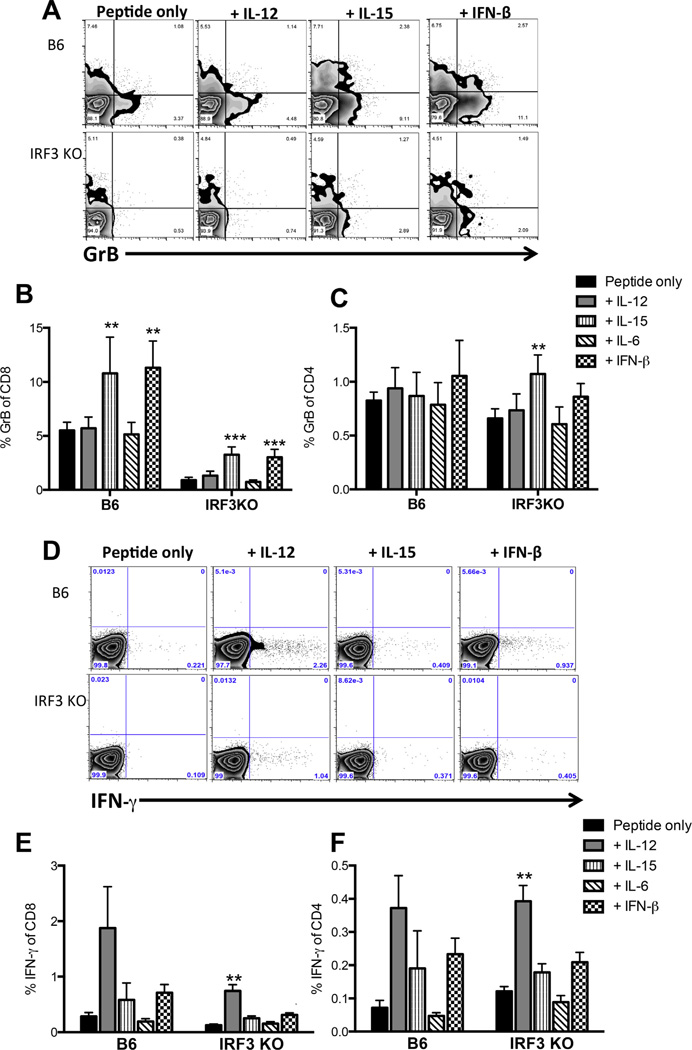
3.7. IL-12 restores IFN-γ expression, but not GrB expression in IRF3 deficient, IAV specific memory T cells
To determine whether cytokines could restore the defects in IRF3KO memory T cell populations following IAV infection, IL-12, IL-6, IL-15 or IFN-β were added to splenocyte cultures 4 weeks post IAV infection. IL-15 and IFN-β increased GrB expression in CD8 T cells from B6 mice as well as in IRF3KO CD8 T cells. Although IL-15 and IFN-β significantly enhanced GrB expression in IRF3KO CD8 cells, these cytokines could not completely restore GrB expression to the levels observed in B6 cells (Fig. 6B). Similar to the response observed in TMEV infected mice, addition of IL-12 partially restored the IFN-γ defect in IRF3KO CD8 cells and enhanced IFN-γ in B6 and IRF3KO CD4 cells (Fig. 6D – F). For CD4 cells, IL-12 enhanced IFN-γ production in both B6 and IRF3KO cells compared to peptide only restimulation, although the recall responses of CD4 cells were generally not robust (Fig. 6F). These results indicate that deficiencies in IFN-γ expression by IRF3KO memory CD4 and CD8 T cells may be due to deficient IL-12 production during peptide-specific recall responses. In contrast, no cytokines tested could restore peptide specific GrB re-expression suggesting IRF3 deficiency has a greater impact on cytotoxic potential rather than cytokine secretion. Altogether, these results demonstrate that IRF3-dependent signals play a significant role in the generation of effector and memory T cell responses in addition to their well-established innate role in controlling virus replication.
Fig. 6.
IL-12 restores IFN-γ expression in IRF3 deficient, IAV specific memory T cells. B6 or IRF3KO were infected with 1000 EIU PR8 i. n. for 30 days. Cytokines were added to splenocyte cultures as described in Fig. 5 and after 48 h, GrB and IFN-γ expression was analyzed by flow cytometry. A) Representative FACS plots showing GrB expression inCD8T cells. Mean percent GrB+ +/− SD of 5 mice/group inCD8 (B) or CD4 (C) T cells after the addition of IL-12, IL-15, IL-6, and IFN-β. D) Representative FACS plots showing percent IFN-γ expression in CD8 T cells. Mean percent IFN-γ+ +/− SD of 5 mice/group in CD8 (E) or CD4 (F) T cells after the exogenous addition of cytokines.,**p < 0.005, ***p < 0.001. This experiment was repeated with similar results.
4. Discussion
Despite the well-studied role of IRF3 in the expression of innate immune cytokines that could impact T cell responses, a role for IRF3 in promoting antiviral T cell responses has not been directly studied. Here, we demonstrate that IRF3 deficiency impairs effector T cell responses and viral clearance during infection with TMEV, a potentially persistent virus, but not with IAV, a virus that causes a strictly acute infection. In addition, we show that memory T cell responses, especially GrB expression, against both TMEV and IAV are significantly impaired in IRF3KO mice. The observed impairments in IRF3 deficient mice could be due to both T cell intrinsic and extrinsic effects of IRF3 signaling, or the relative dependence of IRF3 in pattern recognition receptor triggering.
Our first observation is that IRF3 appears to be more important for effector T cell responses against TMEV compared with those against IAV One explanation for a differential requirement of IRF3 in the development of T cell responses could be whether the infection stimulates innate immunity primarily through IRF3-dependent or IRF3-independent pathways. We have previously shown that IRF3 deficiency reduces early, but not late, expression of IFN-β and IL-6 following TMEV infection of macrophages, indicating that TMEV infection of macrophages signals through IRF3 for induction of these cytokines [12]. Similarly, during in vitro infection of human macrophages with IAV H5N1, IRF3 knockdown resulted in reduced expression of IFN-β, IL-6, and other cytokines [49]. However, while macrophages are an important target cell for TMEV replication, IAV productive infection primarily occurs in epithelial cells. This suggests that the difference in target cell during TMEV infection versus IAV infection may dictate the requirement for IRF3 in generating effector T cell responses. Alternatively, the inflammasome has been implicated in driving IAV adaptive immune responses and thus could overcome the effects of the IRF3 deficit in promoting effective anti-IAV effector responses [50].
In contrast to our observations in effector T cell responses, memory T cell responses were drastically impaired in IRF3KO mice infected with either TMEV or IAV These results suggest that IRF3-dependent signals are more involved in the generation or maintenance of antiviral T cell memory than effector T cell responses. In particular, IRF3KO mice exhibited deficits in GrB and IFN-γ expression in CD4 and CD8 T cells during peptide-specific recall responses.
One explanation for the impaired memory function in IRF3 deficient mice could be that IRF3 deficiency induces T cell exhaustion, especially in TMEV infected mice where some mice develop chronic infection. However, we did not see increased levels of PD-1 expression on CD4 or CD8 cells at day 30–34 post TMEV infection (data not shown). Furthermore, although IRF3 deficient mice showed delayed IAV clearance, all mice cleared IAV infection by day 21 yet still showed defects in memory CD8 and CD4 recall responses, without increases in PD-1 expression. Taken together, T cell exhaustion does not appear to be responsible for the differences in antigen specific memory T cell responses after infection.
A more logical explanation for differences in memory T cell responses is that IRF3 deficiency impairs expression of IRF3-dependent type I IFN, IL-12 and/or IL-15, which are needed for effective memory T cell responses to TMEV and IAV infection. We and others have shown that IRF3 promotes the production of Type I IFN [44], IL-15 [16,44] and IL-12p35 [43] in response to various inflammatory stimuli including TMEV. In turn, Type I IFN [10] and IL-15 [17] have been shown to be important for development of memory CD8 T cell responses during infection while IL-12 sustains the CD4 Th1 phenotype [42] and GrB expression in CD8 cells [19,20]. However, IRF3 signaling was shown to suppress IL-12 production in response to viral infection and IRF3 deficiency actually enhanced IL-12 production in a viral/bacterial coinfection model [51].
Altogether this suggests that multiple IRF3-dependent signals, some of which are cytokines, are involved in development of T cell memory during viral infection. Consistent with these studies, we found that treating CD8 T cells from TMEV-infected B6 mice with IFN-β, IL-12, or IL-15 during the recall response significantly enhanced GrB expression. Surprisingly, none of the aforementioned cytokines could completely restore GrB expression in IRF3KO memory T cells suggesting the defect in cytotoxic mediators may be more profound than the defect in cytokine synthesis. Consistent with this is the defect in IAV specific GrB, but not IFN-γ, upon secondary challenge with PR8 IAV (Fig. 4E and F). These results suggest that IRF3-mediated expression of IFN-β, IL-12, and IL-15 during the transition from effector to memory may be critical for developing memory CD8 T cells capable of expressing GrB upon restimulation. However, the data argue for the hypothesis that IRF3 may also be required intrinsically, downstream of cytokine binding to receptors on T cells and thus, supplementation with these cytokines cannot overcome IRF3 deficiency during the effector to memory transition. It would be interesting to see if simultaneous addition of IFN-β, IL-12, and IL-15 could restore effector T cell phenotype of IRF3KO mice following viral infection.
In contrast to cytokine requirements for GrB expression, treating with IL-12 was able to significantly enhance IFN-γ expression in memory T cells regardless of IRF3 expression in mice infected with TMEV or IAV. These findings are consistent with the accepted role for IL-12 in maintaining IFN-γ expression in T cells and would argue for the notion that in contrast to GrB expression, IRF3 is not required for T cells to respond to IL-12 with expression of IFN-γ. Specifically, these results do indicate that IFN-γ expression by anti-viral memory T cells requires IRF3-dependent cytokines either during the effector-to-memory transition or during secondary infection. Thus, impairment in IFN-γ-expressing memory T cells due to cytokine deficiencies in the primary infection can be overcome by IL-12 expression in the secondary infection.
Our results show that IRF3 deficiency impairs effector T cell responses and viral clearance of TMEV, a potentially persistent virus, but does not impair those responses to an acute virus such as IAV However, IRF3 deficiency impairs the recall responses of memory T cells from either TMEV or IAV infected mice. These findings provide further insight into the links between innate and adaptive immune responses to virus infections and may inform future vaccine strategies against acute and persistent infections.
Acknowledgments
The authors wish to thank Michelle Hall for critical reading of the manuscript and the Nebraska Center for Virology Flow Cytometry Facility. This work was supported by Public Health Service grants P30-GM103509, R56-AI100929, P20-RR018759 (Tyler C. Moore) and T32-AI060547 (Alexander J. Vogel).
References
- 1.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 2.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 4.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 5.Feng X, Heyden NV, Ratner L. Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts. J Virol. 2003;77:13389–13395. doi: 10.1128/JVI.77.24.13389-13395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs A, Lindenmann J. Virus interference I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 7.Nilsen TW, Maroney PA, Baglioni C. Synthesis of (2′–5′)oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982;42:1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichlmair A, Lassnig C, Eberle CA, Gorna MW, Baumann CL, Burkard TR, et al. IFIT1 is an antiviral protein that recognizes 5’-triphosphate RNA. Nat Immunol. 2011;12:624–630. doi: 10.1038/ni.2048. [DOI] [PubMed] [Google Scholar]
- 9.Thomis DC, Samuel CE. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2 alpha protein kinase (PKR) expression in transfected mammalian cells. Proc Natl Acad Sci USA. 1992;89:10837–10841. doi: 10.1073/pnas.89.22.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J Immunol. 2010;185:1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore TC, Petro TM. IRF3 and ERK MAP-kinases control nitric oxide production from macrophages in response to poly-I: C. FEBS Lett. 2013;587:3014–3020. doi: 10.1016/j.febslet.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryzhakov G, Lai CC, Blazek K, To KW, Hussell T, Udalova I. IL-17 boosts proinflammatory outcome of antiviral response in human cells. J Immunol. 2011;187:5357–5362. doi: 10.4049/jimmunol.1100917. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney SE, Kimbler TB, Firestein GS. Synoviocyte innate immune responses: II. Pivotal role of IFN regulatory factor 3. J Immunol. 2010;184:7162–7168. doi: 10.4049/jimmunol.0903944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlberg A, Auble MR, Petro TM. Reduced expression of IL-12 p35 by SJL/J macrophages responding to Theiler’s virus infection is associated with constitutive activation of IRF-3. Virology. 2006;353:422–432. doi: 10.1016/j.virol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, et al. Identification of a polyI: C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–2687. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, et al. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc Natl Acad Sci USA. 2011;108:7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211:105–120. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ysebrant de Lendonck L, Tonon S, Nguyen M, Vandevenne P, Welsby I, Martinet V, et al. Interferon regulatory factor 3 controls interleukin-17 expression in CD8 T lymphocytes. Proc Natl Acad Sci USA. 2013;110:E3189–E3197. doi: 10.1073/pnas.1219221110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipton HL, Melvold R. Genetic analysis of susceptibility to Theiler’s virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- 23.Moore TC, Bush KL, Cody L, Brown DM, Petro TM. Interleukin-6 control of early Theiler’s murine encephalomyelitis virus replication in macrophages occurs in conjunction with STAT1 activation and nitric oxide production. J Virol. 2012;86:10841–10851. doi: 10.1128/JVI.01402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palma JP, Lee HG, Mohindru M, Kang BS, Dal Canto M, Miller SD, et al. Enhanced susceptibility to Theiler’s virus-induced demyelinating disease in perforin-deficient mice. J Neuroimmunol. 2001;116:125–135. doi: 10.1016/s0165-5728(01)00293-4. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez M, Zoecklein LJ, Howe CL, Pavelko KD, Gamez JD, Nakane S, et al. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler’s murine encephalomyelitis virus infection. J Virol. 2003;77:12252–12265. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi CP, McAllister A, Tanguy M, Kagi D, Brahic M. Theiler’s virus infection of perforin-deficient mice. J Virol. 1998;72:4515–4519. doi: 10.1128/jvi.72.5.4515-4519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2011;343:13–21. doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- 28.Bender BS, Small PA., Jr Influenza: pathogenesis and host defense. Semin Respir Infect. 1992;7:38–45. [PubMed] [Google Scholar]
- 29.Powell TJ, Brown DM, Hollenbaugh JA, Charbonneau T, Kemp RA, Swain SL, et al. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clin Immunol. 2004;113:89–100. doi: 10.1016/j.clim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 31.Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- 33.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, et al. CD4 memory: generation and Multi-faceted roles for CD4 T Cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 35.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohindru M, Kang B, Kim BS. Initial capsid-specific CD4(+) T cell responses protect against Theiler’s murine encephalomyelitisvirus-induced demyelinating disease. Eur J Immunol. 2006;36:2106–2115. doi: 10.1002/eji.200535785. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadzadeh M, Farber DL. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc Natl Acad Sci USA. 2002;99:11802–11807. doi: 10.1073/pnas.192263099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipton HL, Kumar AS, Trottier M. Theiler’s virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Res. 2005;111:214–223. doi: 10.1016/j.virusres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Lipton HL, Dal Canto MC. Chronic neurologic disease in Theiler’s virus infection of SJL/J mice. J Neurol Sci. 1976;30:201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- 40.Lyman MA, Myoung J, Mohindru M, Kim BS. Quantitative, not qualitative, differences in CD8(+) T cell responses to Theiler’s murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/ J mice. Eur J Immunol. 2004;34:2730–2739. doi: 10.1002/eji.200324811. [DOI] [PubMed] [Google Scholar]
- 41.Joetham A, Okamoto M, Takeda K, Schedel M, Ohnishi H, Dakhama A, et al. CD8 regulates T regulatory cell production of IL-6 and maintains their suppressive phenotype in allergic lung disease. J Immunol. 2011;186:113–120. doi: 10.4049/jimmunol.1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 43.Goriely S, Molle C, Nguyen M, Albarani V, Haddou NO, Lin R, et al. Interferon regulatory factor 3 is involved in Toll-like receptor 4 (TLR4)-and TLR3-induced IL-12p35 gene activation. Blood. 2006;107:1078–1084. doi: 10.1182/blood-2005-06-2416. [DOI] [PubMed] [Google Scholar]
- 44.Moore TC, Cody L, Kumm PM, Brown DM, Petro TM. IRF3 helps control acute TMEV infection through IL-6 expression but contributes to acute hippocampus damage following TMEV infection. Virus Res. 2013;178:226–233. doi: 10.1016/j.virusres.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, et al. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 46.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 48.Andersen J, VanScoy S, Cheng TF, Gomez D, Reich NC. IRF-3-dependent and augmented target genes during viral infection. Genes Immun. 2008;9:168–175. doi: 10.1038/sj.gene.6364449. [DOI] [PubMed] [Google Scholar]
- 49.Hui KP, Lee SM, Cheung CY, Ng IH, Poon LL, Guan Y, et al. Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J Immunol. 2009;182:1088–1098. doi: 10.4049/jimmunol.182.2.1088. [DOI] [PubMed] [Google Scholar]
- 50.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negishi H, Yanai H, Nakajima A, Koshiba R, Atarashi K, Matsuda A, et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat Immunol. 2012;13:659–666. doi: 10.1038/ni.2307. [DOI] [PubMed] [Google Scholar]