Abstract
Background
While schizophrenia is generally considered a neurodevelopment disorder, our basic understanding of the biochemical processes involved in disease etiology and/or progression is limited. One class of biochemical mediators that has been suggested to play a role in the development of schizophrenia is N-acyl ethanolamine metabolites of N-acylphosphatidylethanolamines. However, no investigations of N-acylphosphatidylserines or their N-acylserine metabolites have been published.
Methods
We undertook a targeted postmortem lipidomics analysis of N-acylphosphatidylserines (NAPS) and N-acylserines (NAS) in gray matter of the frontal cortex of schizophrenia subjects.
Results
Our data are the first to demonstrate that NAPS and NAS are present in human brain. Furthermore, NAPS and their bioactive metabolites, N-acylserines (NAS), were found to be significantly elevated in the frontal cortex of schizophrenia subjects.
Conclusions
Elevated levels of NAPS lipid pools in schizophrenia may result in complex alterations in the structural function of neuronal membranes while increases in NAS may alter signal transduction pathways.
Keywords: schizophrenia, N-acylserines, N-acylphosphatidylserines, frontal cortex, lipidomics
Introduction
N-Acylphosphatidylethanolamines (NAPE) function as structural components of membranes, with acylation of the polar phosphoethanolamine head group resulting in a lipophilic substitution that folds back into the hydrophobic interior of membranes, thereby stabilizing membranes [1]. In this regard, brain NAPE levels are augmented in models of neuronal injury [2–4].
In contrast, NAE which are cleaved from NAPE by phospholipase D (PLD) serve complex signal transduction functions [4, 5]. NAE have been investigated in schizophrenia and been shown to be elevated in CSF [6] but decreased in postmortem prefrontal cortex, hippocampus, and cerebellum [7].
However, in addition to NAE there are a number of N-acyl amino acids generated in the brain and other tissues [8, 9]. An example is N-acylserines (NAS) which are the metabolic products of N-acylphosphatidylserines (NAPS) via PLD [9]. NAPS were first demonstrated in sheep red blood cells [10] and subsequently in pig brain (0.1% of total lipids) and mouse RAW264.7 macrophage cells [11]. The NAS, N-oleoyl serine and N-arachidonyl serine have also been isolated from mouse bone [12] and bovine brain [13], respectively. N-Arachidonyl serine also has been shown to be a potent modulator of calcium-activated potassium channels [14] and of N-type calcium channels [15].
Based on potential roles of NAPS in membrane function and NAS in modulation of synaptic function, we investigated these complex lipids in the postmortem frontal cortex of control and schizophrenia subjects.
Materials and Methods
Patient Brain Samples
Brain samples were provided by the UCLA brain bank. Schizophrenia patients were diagnosed based on the Structural Interview for Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV). The demographics of the donors are presented in Table 1.
Table 1.
Demographics of the donors for the frontal cortex samples.
| Diagnosis | Age | Gender | PMI (Hr.) | Neuropathology |
|---|---|---|---|---|
| Schizophrenia Bipolar | 33 | M | 195 | Incidental choroid plexus papilloma |
| Schizophrenia Bipolar | 46 | M | 21.7 | Mildly shrunken CA1 cells |
| Schizophrenia Bipolar Pancreatic cancer | 52 | F | 15.6 | None |
| Schizophrenia Bipolar Lung cancer | 53 | M | 20.5 | None |
| Schizophrenia Anxiety Depression | 55 | M | 10.7 | None |
| Schizophrenia Stroke | 62 | F | 12.2 | Cerebral infarction |
| Schizophrenia | 72 | F | 13 | Old perivascular hemorrhages |
| Schizophrenia Bipolar CHF | 77 | F | 14.7 | None |
| Schizophrenia Alzheimers | 83 | F | 17 | Plaques & tangles |
| Schizophrenia Depression | 85 | F | 11.5 | None |
| Seizure disorder COPD | 35 | F | 11.8 | None |
| CHF | 59 | M | 20.2 | None |
| Colon cancer | 63 | M | 12 | None |
| Myocardial infarction | 68 | F | 23.7 | None |
| CAD Leukemia | 70 | M | 11.8 | None |
| Pancreatic cancer Diabetes | 73 | F | 18.5 | None |
| Stomach cancer COPD Renal failure | 84 | M | 11.8 | None |
| CHF | 87 | M | 9.3 | None |
| Pancreatic cancer | 90 | M | 19.2 | None |
| Uterine cancer | 92 | F | 23.3 | None |
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease
High Resolution Mass Spectrometry
We undertook a high-resolution mass spectrometric shotgun analysis of N-acylphosphatidylserines (NAPS) and N-acylserines (NAS) in postmortem gray matter of the frontal cortex from patients suffering from schizophrenia. Tissue samples were processed utilizing tert-butylmethylether and methanol for extraction of lipids [16, 17]. The extraction solution contained [2H8] arachidonic acid, [13C16] palmitic acid, [2H31] PtdEtn 34:1, [2H54] PtdEtn 28:0, NAPS 36:2-N-19:0, and bromocriptine as internal standards. Extracts were dried by centrifugal vacuum evaporation prior to dissolution in isopropanol: methanol: chloroform (4:2:1) containing 7.5 mM ammonium acetate. Shotgun lipidomics were performed utilizing high-resolution (140,000 at 200 amu) data acquisition from 260 to 1450 amu, with sub-millimass accuracy on an orbitrap mass spectrometer (Thermo Q Exactive, Thermo Scientific) in negative ion mode [16,17]. Samples were infused for 1 min at 5 μL/min. followed by successive 500 μL washes of the infusion line with methanol and hexane/ethyl acetate (3:2) to minimize ghost effects. In negative ion ESI (3.2 kV, capillary temp. of 320°C, sheath gas of 10), the anions of NAPS, NAS, and internal standards were monitored.
Lipid identities were validated by MS2. In negative ion mode utilizing a collision energy of 10, the phosphatidic acid resulting from the loss of NAS was monitored [11]. With a collision energy of 25 the fatty acids of the phosphatidic acid were monitored [11]. This allowed for full elucidation/verification of the structure of each NAPS. Similarly, in negative ion mode with a collision energy of 25, the structure of NAS could be verified [8]. This included the loss of [-CH2O], [-CH2O-H2O], [-H2O-CO2], and [-CH3O-COOH].
Data Analyses
Data are presented as R values (ratio of the endogenous lipid to the peak area of an appropriate internal standard), corrected for tissue wet weight, in bar graphs ± SEM [17]. Data were analyzed by the Student’s t-test.
Results
NAPS
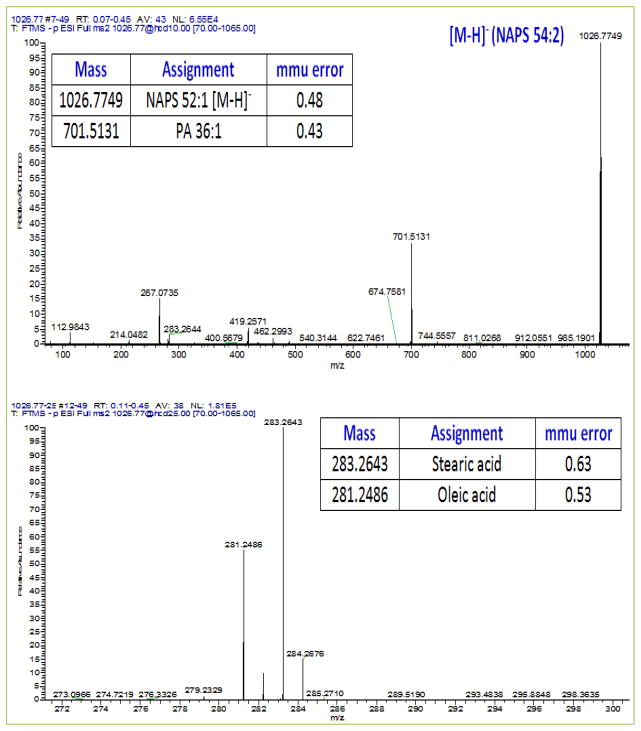
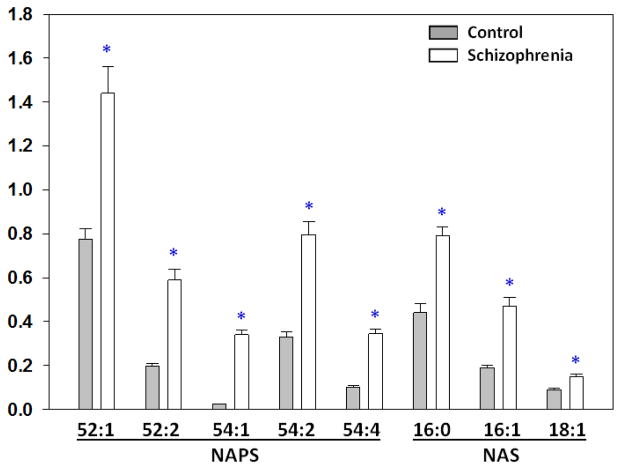
The major NAPS detected in human frontal cortex were NAPS 52:1, NAPS 52:2, and NAPS 54:2. Minor species included NAPS 54:1 and NAPS 54:4. MS/MS experiments verified that NAPS 52:1 was PS 18:0/18:1-N-16:0, NAPS 52:2 was PS 18:1/18:0-N-16:1, and NAPS 54:2 was PS 18:0/18:1-N-18:1 (Fig. 1). In the frontal cortex from schizophrenia subjects all NAPS were significantly elevated (Fig. 2).
Fig 1. Negative ESI MS2 spectra for NAPS 52:1 at a collision energy of 10 (upper plot) and at 25 (lower plot) in human frontal cortex lipid extracts.
Low collision energies produced phosphatidic acid (PA 36:1) as a result of the loss of N-acyl serine (palmitoylserine). Higher collision energies generated the 2 fatty acid substituents of the PA, namely stearic acid (18:0) and oleic acid (18:1).
Fig 2. Postmortem levels of NAPS and NAS in the frontal cortex of control and schizophrenia subjects.
Levels are expressed as the ratio to an appropriate internal standard. Mean ± SEM (N= 9–10). *p< 0.05 vs. control.
NAS
The major NAS detected in human frontal cortex were NAS 16:0, NAS 16:1, and NAS 18:1. These N-acylserines were validated by MS/MS experiments (Fig. 3). In the frontal cortex from schizophrenia subjects the NAS also were significantly elevated (Fig. 2).
Fig 3. Negative ESI MS2 spectrum for the NAS, palmitoylserine at a collision energy of 25 in human frontal cortex lipid extracts.
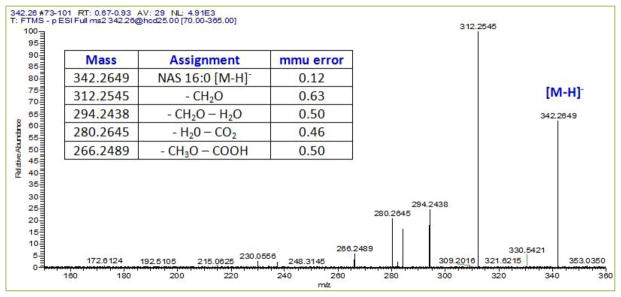
The positive ESI MS2 spectrum was dominated by the [M+H]+ ion for serine 106.0501 (0.22 mmu error; data not shown) further confirming the identity of palmitoylserine.
Discussion
Despite intensive research efforts to identify biomarkers for early detection of schizophrenia, our knowledge gap in schizophrenia remains large. It is imperative to increase our understanding of the neurodevelopmental factors that contribute to abnormal oligodendrocyte and synaptic function in the limbic cortices of schizophrenia patients [18–23]. Current concepts involve hyper- and hypo-function in both mesolimbic dopaminergic and cortical glutamatergic pathways [18–23]. In this regard, excessive glutamatergic neurotransmission has been demonstrated to increase levels of NAPE and NAE after ischemia in the rat brain [24, 25], after ischemia in the mouse brain [26], with glutamate toxicity in cultured mouse neocortical cells [27], and in the rat hippocampus with in vivo kainate-induced neuronal injury [2]. Increased levels of NAPE are hypothesized to represent a neuroprotective mechanism activated by neuronal injury [4]. Therefore, our observations of elevated NAPS and NAS in the frontal cortex of schizophrenia subjects may also be a biomarker of an endogenous neuroprotective mechanism since N-arachidonyl serine is already known to be neuroprotective [9]. Augmentation of NAPS and NAS may represent biochemical responses to excessive glutamatergic neurotransmission [21] and/or altered neuronal development in the cortex [28]. Alterations in PLD which metabolizes NAPS [4, 11] and fatty acid amide hydrolases (FAAH1, FAAH2) which metabolize NAS [29] also need to be investigated for their potential roles in altered NAS levels (Fig. 4). In this regard FAAH has been demonstrated to be a postsynaptic enzyme in the rat hippocampus, cerebellum, and amygdala [30].
Fig 4. Tentative biosynthetic and degradative pathways for NAPS and NAS.
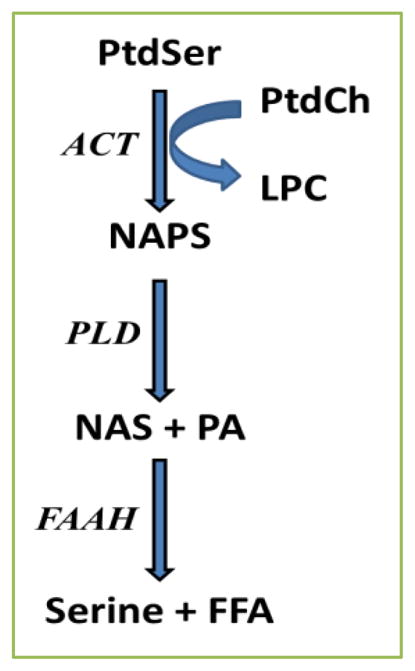
ACT, acyltransferase (fatty acid is from sn-1 of phosphatidylcholine); FAAH, fatty aid amide hydrolase; FFA, free fatty acid; LPC, lysophosphatidylcholine; NAPS, N-acylphosphatidylserine; NAS, N-acylserine; PA, phosphatidic acid; PLD, phospholipase D; PtdCh, phosphatidylcholines; PtdSer, phosphatidylserine. schizophrenia or a biomarker for the early detection of the disease process.
In summary, our targeted lipidomics analyses have revealed altered N-acyl amino acid metabolism in the frontal cortex in schizophrenia. However, the effect of sustained NAPS and NAS on neurotransmission remains to be examined since N-acyl amino acids are potent modulators of ion channel function [3, 14, 15]. It also will be important to determine if this may represent a new therapeutic target for
Acknowledgments
Frontal cortex specimens were obtained from the Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Healthcare Center, 11301 Wilshire Blvd. Los Angeles, CA 90073 which is sponsored by National Institutes of Health, National Multiple Sclerosis Society, and the US Department of Veterans Affairs. This work was funded by DeBusk College of Osteopathic Medicine, Lincoln Memorial University.
Footnotes
Conflict of interests
The author declares no competing financial interests. The funding source also had no role in these studies.
References
- 1.Swamy MJ, Tarafdar PK, Kamlekar RK. Structure, phase behaviour and membrane interactions of N-acylethanolamines and N-acylphosphatidyl-ethanolamines. Chem Phys Lipids. 2010;163:266–279. doi: 10.1016/j.chemphyslip.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Guan XL, He X, Ong WY, Yeo WK, Shui G, Wenk MR. Non-targeted profiling of lipids during kainate-induced neuronal injury. FASEB J. 2006;20:1152–1161. doi: 10.1096/fj.05-5362com. [DOI] [PubMed] [Google Scholar]
- 3.Wellner N, Diep TA, Janfelt C, Hansen HS. N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim Biophys Acta. 2013;1831:652–662. doi: 10.1016/j.bbalip.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Coulon D, Faure L, Salmon M, Wattelet V, Bessoule JJ. Occurrence, biosynthesis and functions of N-acylphosphatidylethanolamines (NAPE): not just precursors of N-acylethanolamines (NAE) Biochimie. 2012;94:75–85. doi: 10.1016/j.biochi.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Rahman IA, Tsuboi K, Uyama T, Ueda N. New players in the fatty acyl ethanolamide metabolism. Pharmacol Res. 2014;86C:1–10. doi: 10.1016/j.phrs.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br J Psychiatry. 2009;194:371–372. doi: 10.1192/bjp.bp.108.053843. [DOI] [PubMed] [Google Scholar]
- 7.Muguruza C, Lehtonen M, Aaltonen N, Morentin B, Meana JJ, Callado LF. Quantification of endocannabinoids in postmortem brain of schizophrenic subjects. Schizophr Res. 2013;148:145–150. doi: 10.1016/j.schres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Tan B, O’Dell DK, Yu YW, Monn MF, Hughes HV, Burstein S, et al. Identification of endogenous acylamino acids based on a targeted lipidomics approach. J Lipid Res. 2010;51:112–119. doi: 10.1194/jlr.M900198-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanuš L, Shohami E, Bab I, Mechoulam R. N-Acyl amino acids and their impact on biological processes. Biofactors. 2014;40:381–388. doi: 10.1002/biof.1166. [DOI] [PubMed] [Google Scholar]
- 10.Nelson GJ. Studies on the lipids of sheep red blood cells. IV. The identification of a new phospholipid. N-acyl phosphatidyl serine. Biochem Biophys Res Commun. 1970;38:261–265. doi: 10.1016/0006-291x(70)90706-0. [DOI] [PubMed] [Google Scholar]
- 11.Guan Z, Li S, Smith DC, Shaw WA, Raetz CR. Identification of N-acylphosphatidylserine molecules in eukaryotic cells. Biochemistry. 2007;46:14500–14513. doi: 10.1021/bi701907g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smoum R, Bar A, Tan B, Milman G, Attar-Namdar M, Ofek O, Stuart JM, et al. Oleoyl serine, an endogenous N-acyl amide, modulates bone remodeling and mass. Proc Natl Acad Sci U S A. 2010;107:17710–17715. doi: 10.1073/pnas.0912479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godlewski G, Offertáler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J, et al. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther. 2009;328:351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J, Williams DJ, Ikeda SR. N-Arachidonoyl L-serine, a putative endocannabinoid, alters the activation of N-type Ca2+ channels in sympathetic neurons. J Neurophysiol. 2008;100:1147–1151. doi: 10.1152/jn.01204.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuhmann K, Almeida R, Baumert M, Herzog R, Bornstein SR, Shevchenko A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J Mass Spectrom. 2012;47:96–104. doi: 10.1002/jms.2031. [DOI] [PubMed] [Google Scholar]
- 17.Wood PL, Shirley NR. Lipidomics analysis of postmortem interval: Preliminary evaluation of human skeletal muscle. Metabolomics. 2013;3:3. [Google Scholar]
- 18.Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkachev D, Mimmack ML, Huffaker SJ, Ryan M, Bahn S. Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia. Int J Neuropsychopharmacol. 2007;10:557–563. doi: 10.1017/S1461145706007334. [DOI] [PubMed] [Google Scholar]
- 20.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Xia M, Lai Y, Dai Z, Cao Q, Cheng Z, et al. Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophr Res. 2014;156:150–156. doi: 10.1016/j.schres.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Moesgaard B, Petersen G, Jaroszewski JW, Hansen HS. Age dependent accumulation of N-acyl-ethanolamine phospholipids in ischemic rat brain. A (31)P NMR and enzyme activity study. J Lipid Res. 2000;41:985–990. [PubMed] [Google Scholar]
- 25.Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- 26.Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, et al. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebralischemia in mice. J Neurochem. 2007;103:1907–16. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- 27.Hansen HS, Lauritzen L, Strand AM, Vinggaard AM, Frandsen A, Schousboe A. Characterization of glutamate-induced formation of N-acylphosphatidylethanolamine and N-acylethanolamine in cultured neocortical neurons. J Neurochem. 1997;69:753–756. doi: 10.1046/j.1471-4159.1997.69020753.x. [DOI] [PubMed] [Google Scholar]
- 28.Costa E, Dong E, Grayson DR, Guidotti A, Ruzicka W, Veldic M. Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics. 2007;2:29–36. doi: 10.4161/epi.2.1.4063. [DOI] [PubMed] [Google Scholar]
- 29.Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- 30.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]