Abstract
Purpose/Aim
The primary objective was to evaluate the effect of a bupivacaine mandibular nerve block on intraoperative blood pressure (BP) and heart rate (HR) in response to surgical stimulation and the need for systemic analgesics postoperatively. We hypothesized that a mandibular nerve block would decrease the need for systemic analgesics both intraoperatively and postoperatively.
Materials and Methods
Fourteen adult male Yucatan pigs were purchased. Pigs were chemically restrained with ketamine, midazolam, and dexmedetomidine and anesthesia was maintained with isoflurane inhalant anesthesia. Pigs were randomized to receive a mandibular block with either bupivacaine (bupivacaine group) or saline (control group). A nerve stimulator was used for administration of the block with observation of masseter muscle twitch to indicate the injection site. Invasive BP and HR were measured with the aid of an arterial catheter in eight pigs. A rescue analgesic protocol consisting of fentanyl and lidocaine was administered if HR or BP values increased 20% from baseline. Postoperative pain was quantified with a customized ethogram. HR and BP were evaluated at base line, pre-rescue, 10 and 20 min post-rescue.
Results
Pre-rescue mean BP was significantly increased (p = .001) for the bupivacaine group. Mean intraoperative HR was significantly lower (p = .044) in the bupivacaine versus saline group. All other parameters were not significant.
Conclusion
Addition of a mandibular nerve block to the anesthetic regimen in the miniature pig condylectomy model may improve variations in intraoperative BP and HR. This study establishes the foundation for future studies with larger animal numbers to confirm these preliminary findings.
Keywords: bupivacaine, swine, mandibular nerve, analgesia, condylectomy
INTRODUCTION
Animal models are a priceless resource when developing new techniques and treatments for human medical application. Though miniature swine are used extensively in the development of dental, oral, and maxillofacial surgical techniques due to similarities to humans in anatomy, healing, and remodeling [1–5]. However, there is a lack of literature describing local anesthetic techniques in miniature pig models for oral and maxillofacial surgery, despite common use in human patients undergoing the same procedures [5]. As with every animal surgical model, effective anesthesia and analgesia are top priorities.
Local nerve blocks are established for effective pain control in oral and maxillofacial surgical procedures [6]. Local action, prolonged analgesia, and limited side effects make them an appealing alternative to systemic analgesics, and use of local mandibular nerve blocks are reported to provide relief from bone and soft tissue pain in humans and animals undergoing dental procedures [6–9]. Bupivacaine (0.5%) nerve blocks alone humans [10, 11]. A proximal mandibular nerve block eliminated the need for intraoperative analgesia and provided postoperative analgesia for 8 hr in a canine patient undergoing a rostral mandibulectomy [8].
Pain relief is important both for postoperative comfort and healing. Self-trauma from licking, scratching, or rubbing a surgical site in response to pain impairs healing. Stress from postoperative surgical pain has also been linked to delayed wound healing in multiple models [12, 13]. Release of glucocorticoids and catecholamines as well as a decrease in local cytokines and cellular infiltrates from stress can impair wound healing [12]. Improving postoperative analgesia can decrease discomfort and stress in animal surgical models.
This study utilized Yucatan miniature pigs as a model for mandibular condylectomy and implant surgery. The aim was to determine the efficacy of a 0.5% bupivacaine mandibular nerve block for intra-and postoperative analgesia in the Yucatan miniature pig model. The anatomy of the miniature pig mandibular nerve has not been extensively studied but is assumed to be similar to that of other mammals [14]. We hypothesized that a mandibular nerve block with bupivacaine: (1) decreases the need for systemic analgesia during mandibular condylectomy and implant surgery compared to saline in a porcine model and (2) decreases postoperative signs of pain.
MATERIALS AND METHODS
Animals and Husbandry
This study was performed at the Louisiana State University School of Veterinary Medicine, an AAALAC International accredited facility, in accordance with the Guide for the Care and Use of Laboratory Animals [15]. All experimental procedures were approved by the IACUC.
Fourteen male castrated Yucatan miniature pigs (Sus scrofa domestica) were certified to be free from common domestic swine diseases (leptospirosis, brucellosis, pseudorabies, transmissible gastroenteritis, porcine reproductive respiratory syndrome, and toxoplasmosis) and current on Mycoplasma hyopneumoniae bacterin (M + Pac) vaccine by the supplier, Sinclair Bio-Resources (Columbia, MO). All pigs were skeletally mature (mean age, 24.7 months; range, 20 to 32 months; mean body weight 50 kg; range 44 to 60 kg). They were housed in groups of 4 to 5 in an ambient temperature facility within 124 inch by 84 inch pens with rubber padded flooring covered by about 2 inches of pine shavings (S&S Farms, Inc., Franklinton, LA). A radiant heat source was supplied when ambient temperatures decreased below 50°F (10°C). Pigs were offered Mazuri mini pig active adult diet (Land O’Lakes, Inc., Saint Paul, MN) twice daily in one trough per two pigs, and they had unrestricted access to water for the duration of the study. Pigs were allowed to acclimate to their new environment for 7 days prior to initiation of the study, and they were housed singly for about 18 hr after surgery before being returned to their established social groups.
Experimental Groups
All pigs underwent left mandibular condylectomy surgery as part of an implant study. Prior to the surgical procedure each pig was randomly assigned to one of two treatment groups. One cohort (n = 7) received a bupivicaine mandibular nerve block while the other cohort (n = 7) received an equal volume of saline at the mandibular nerve block injection site. The order of surgical procedures was randomized over a two week period. Two surgeries were performed per day. Within each treatment cohort, condylectomies were performed on all pigs and implants were placed in six (one received a condylectomy only in each group).
Anesthesia Protocol
After 12 hr of food withdrawal, pigs were chemically restrained with ketamine (10 mg/kg, Vedco Inc.; St. Joseph, MO), midazolam (0.2 mg/kg, Hospira, Inc.; Lakeforest, IL), and dexmedetomidine (2 ug/kg, Pfizer Animal Health, NY, NY) administered intramuscularly. Fifteen minutes later, anesthesia was induced with 5% isoflurane in 100% oxygen at 1.5 l/min flow via facial mask. When muscle relaxation characterized by loss of jaw tone and palpebral reflexes was observed, the trachea was intubated with a cuffed Murphy’s endotracheal tube (7–9 mm internal diameter). Anesthesia was maintained in all pigs at a vaporizer setting of 1.5% isoflurane in a circular breathing system until the end of the surgery.
Continuous heart rate (HR) and blood pressure (BP) recordings were performed perioperatively on all pigs, beginning approximately between 30 and 45 min after induction. For purposes of this study, it was assumed all animals experienced similar stress from the induction procedure and that the window of time between induction and initiation of measures was sufficient for parameters to stabilize. Blood pressure was measured in eight pigs through an arterial line placed in the auricular artery. Arterial line placement was not possible in six pigs (two from the bupivacaine group, four from the control group), so blood pressure was monitored with a noninvasive blood pressure (NIBP) cuff on the mid metatarsus. Data from the NIBP cuff was not included in the statistical analysis due to differences between invasive and noninvasive BP monitoring techniques [16–18]. Mean values and standard deviations were calculated from continuous BP and HR data within segments corresponding to 1 min before the surgical incision (baseline), 1 min prior to rescue analgesia (pre-rescue), and for 1 min at both 10 and 20 min post-rescue. These readings included only the condylectomy portion of the surgery in all pigs. Data from the implant portion of the surgery were not included in the analysis.
Nerve Block and Rescue Analgesia
After anesthesia was induced, pigs were placed in right lateral recumbency and the left jaw prepared for surgery. Following aseptic preparation, pigs were randomized to receive a mandibular block with an equal volume of either 0.5% bupivacaine (Hospira, Inc., Lakeforest, IL) (0.4 mg/kg, bupivacaine group) or saline (volume based on bupivacaine calculation, control group). 5 ml was the average volume used. Bupivacaine was selected based on the experience of the anesthesiologist and for its longer duration of action compared to lidocaine. The person administering the block was not blinded to treatment. For the mandibular block, an insulated 10 cm, 20G needle was inserted medial to the left mandibular ramus at the point of the angle of the mandible and advanced toward the lateral canthus of the ipsolateral eye with the aid of a nerve stimulator (Pajunk, MultiStim Sensor, Germany) to identify the injection site (Figure 1). The nerve stimulator was set to deliver a 1 mA impulse. When movement of the mandible was noted, signaling stimulation of the muscles of mastication, the impulse was reduced to 0.4 mA. If mandibular movement was sustained at 0.4 mA, the treatment was administered through the stimulator needle. Protocol for administration of the block was based on the anesthesiologist’s experience and expertise. After the mandibular block, pigs were assigned to two different surgical procedures involving a left mandibular condylectomy. During the surgery, a rescue analgesic protocol consisting of fentanyl (Hospira, Inc.; Lakeforest, IL) and lidocaine (Sparhawk laboratories, Inc.; Lenexa, KS) was instituted if BP or HR increased 20% above baseline values. For rescue analgesia, a bolus of fentanyl (5 mg/kg) and lidocaine (2 mg/kg) was administered followed by a continuous rate infusion (CRI) (fentanyl 7.5 µg/kg/hr and lidocaine 50 µg/kg/min) for the remainder of the procedure.
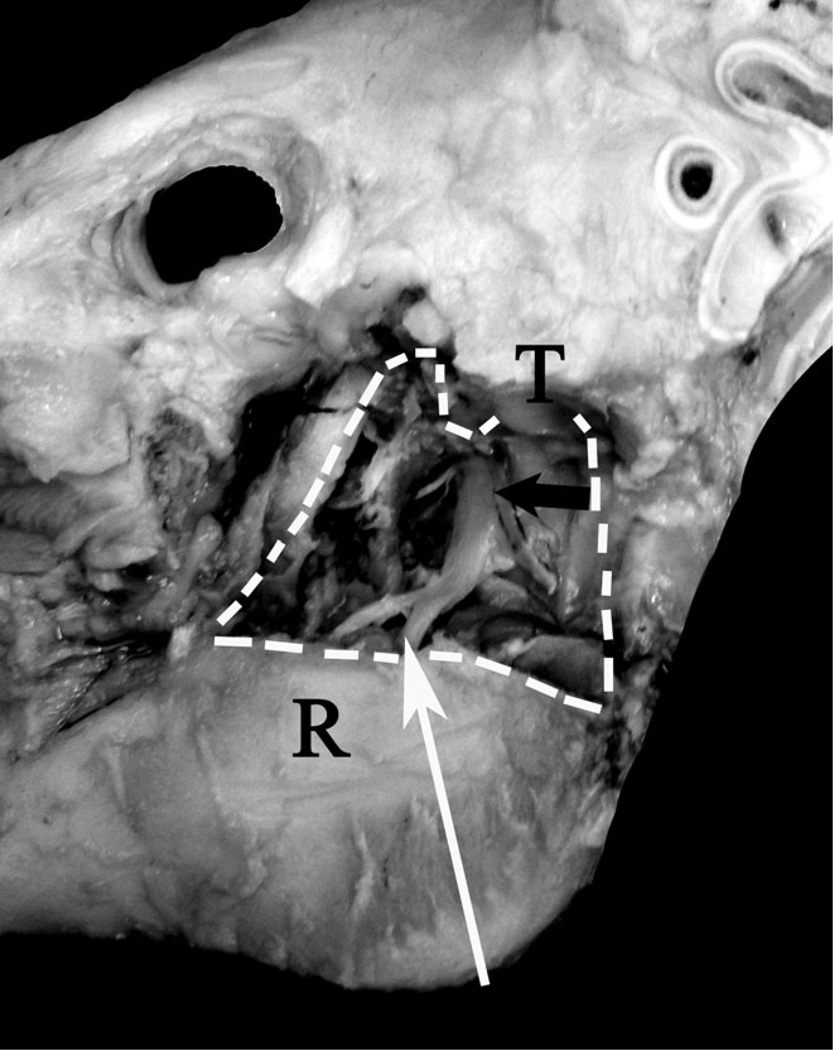
FIGURE 1.
Nerve stimulation and block technique. This dissection of a pig skull demonstrates the pathway of the mandibular nerve and the point at which the block is expected to be administered. The mandibular nerve (black arrow) runs cranial ventral from the foramen ovale toward the mandibular foramen. The needle attached to the electronic nerve stimulator was advance from the angle of the mandible toward the lateral canthus of the ipsolateral eye (white arrow) and medial to the ramus of the mandible. The white dotted line indicates the edges of the removed portion of the ramus (R). The temporomandibular joint is also labeled (T).
Surgical Procedure
Two pigs received condylectomy only (one in each treatment group) and 12 received condylectomy and implant (six in each treatment group). Briefly, a #10 blade was used to make a 5 cm skin incision in the left submandibular area. The incision was carried through the platysma, cervical fascia, and masseter muscle with electrocautery using blunt and sharp dissection. A periosteal elevator was used to expose the ramus of the mandible from the mandibular angle to the condyle. A premeasured osteotomy from the sigmoid notch was carried ventrally to the middle of the ramus where a horizontal osteotomy was created through the caudal border with a reciprocating saw with copious irrigation. This segment of bone containing the condyle and a portion of the ramus was then removed after dissecting the medial musculature. Bone implants similar in size and shape to the removed segment were secured in the space previously occupied by the native ramuscondyle unit with Stryker-CMF miniplates and screws. Following copious sterile saline irrigation, the masseter muscle, platysma muscle, and subcutaneous tissue were closed with #2-0 synthetic monofilament absorbable suture (Biosyn, Covidien; Mansfield, MA) in respective layers. Skin edges were apposed with #2 nylon (Oasis; Mettawa, IL). All surgical procedures were performed by oral and maxillofacial surgeons with the assistance of a boarded veterinary surgeon.
Postoperative Pain Assessment
During recovery, pigs were monitored for signs of pain or discomfort (Table 1). Heart rate (direct auscultation), respiratory rate (visual observation), appetite, urination, defecation, ambulation, vocalization, nonpurposeful movement, and mentation were recorded every 15 min for 2 hr and then at 4 hr post-extubation with first recording made at the time of extubation. Subjective assessments (vocalization, ambulation, etc.) were assigned a numeric value for statistical analysis. Time from end of surgery to extubation varied due to the need for postoperative computer tomography scans and based on duration of recovery from anesthesia. Assessment of all pigs was performed by a veterinarian who was blinded to treatment. If pigs were noted to be painful by the observer, a dose of buprenorphine (Reckitt Benckiser Pharmaceuticals Inc.; Richmond, VA) (30 ug/kg) was administered intramuscularly and then every 8 hr as needed. The dose was selected for maximum analgesia and minimum side effects. Administration of buprenorphine was based on continual observation by the attending veterinarian and unrelated to the ethogram score.
TABLE 1.
Ethogram for postoperative pain assessment
| Behavior | Score | |||||
|---|---|---|---|---|---|---|
| Appetite | 0 | 1 | 2 | 3 | ||
| None | Mild | Moderate | Aggressive | |||
| Urination | 0 | 1 | ||||
| None | Urine | |||||
| Defecation | 0 | 1 | ||||
| None | Feces | |||||
| Ambulation | 0 | 1 | 2 | 3 | 4 | |
| Recumbent | Uncoordinated | Sluggish | Normal | Hyperactive | ||
| Vocalization | 0 | 1 | 2 | 3 | ||
| None | Grunt | Whine | Squeal | |||
| Non-purposeful movement | 0 | 1 | 2 | 3 | ||
| None | Mild | Moderate | Violent | |||
| Mentation | 0 | 1 | 2 | 3 | 4 | 5 |
| Nonresponsive | Obtunded | Dull | Quiet | Bright | Hyper-responsive | |
Note: A custom ethogram was used to assess clinical pain in pigs postoperatively. Assessments were completed every 15 min for the first 2 h after extubation with a final reading at 4 h after extubation.
Statistics
Mean values and standard deviations were calculated from continuous BP and HR data within segments corresponding to 1 min before the surgical incision (baseline), 1 min prior to rescue analgesia (pre-rescue), and for 1 min at 10 and 20 min post-rescue (10 min post-rescue and 20 min post-rescue, respectively). Repeated measures analysis of variance was used to compare hemodynamic parameters between treatment groups and time points (NCSS 8 Version 8.0.13, NCSS LLC, Kaysville, UT). Tukey’s post hoc analysis was performed to assess statistically significant differences. Time to rescue and postoperative pain scores were analyzed using a second statistical package (MYSTAT Version 12.02.00, Chicago, IL). Student’s t test was used to compare times to rescue and postoperative pain scores. Significance was set at p < .05.
RESULTS
For purposes of this study variations in BP and HR were used to represent responses to painful stimuli [9, 19, 20]. All pigs were maintained on 1.5% isoflurane throughout the procedure, received a constant intravenous fluid rate of 5 ml/kg/hr, and had minimal blood loss.
Time to Rescue
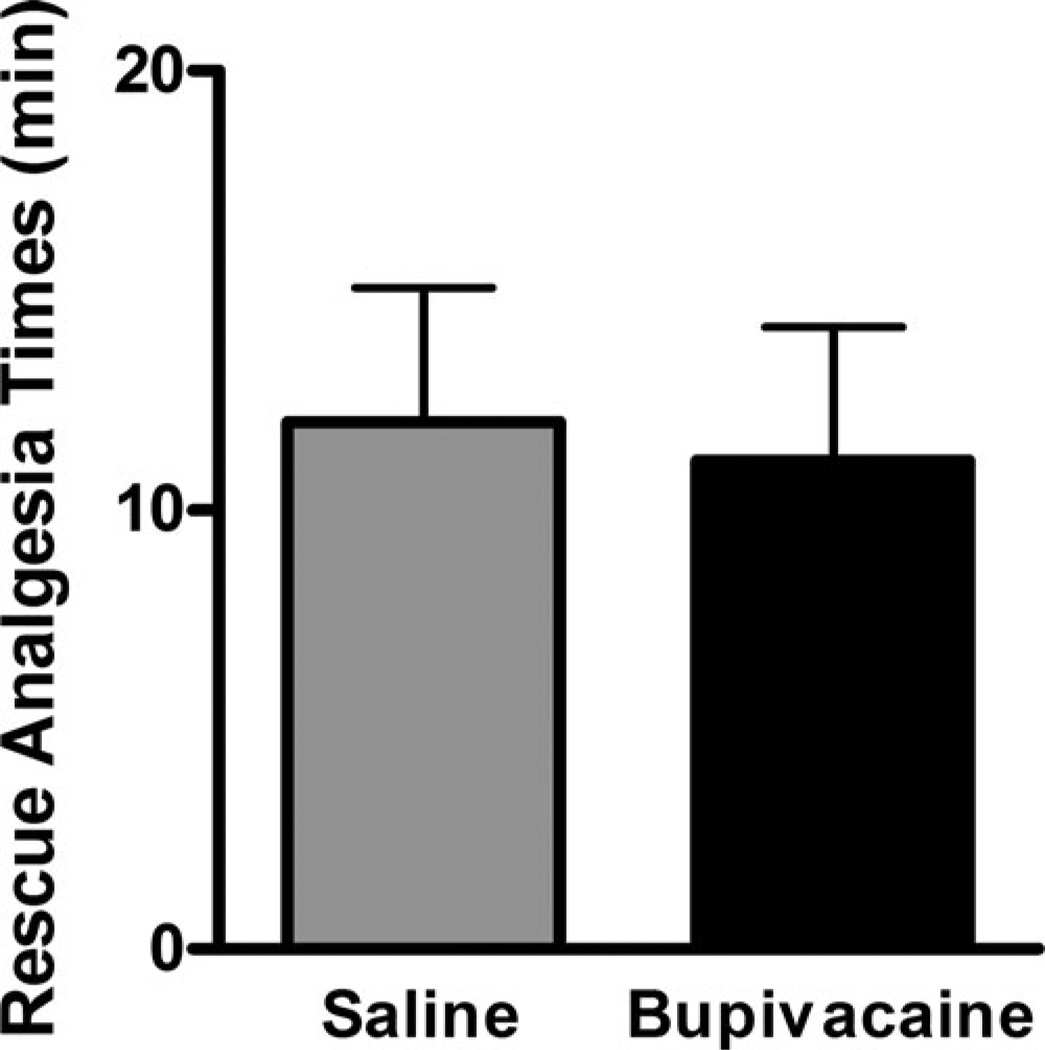
A total of eight pigs were included in the statistical analysis (n = 5 bupivacaine cohort; n = 3 saline cohort), all of which required rescue analgesics intraoperatively. There was no difference in time until rescue (p = .84) between the bupivacaine and saline groups (Figure 2).
FIGURE 2.
Mean ± SEM time from start of surgery until rescue analgesics (fentanyl/lidocaine) were administered based on predetermined BP and HR changes. Differences between treatment groups were not significant (p = .84).
Hemodynamic Parameters
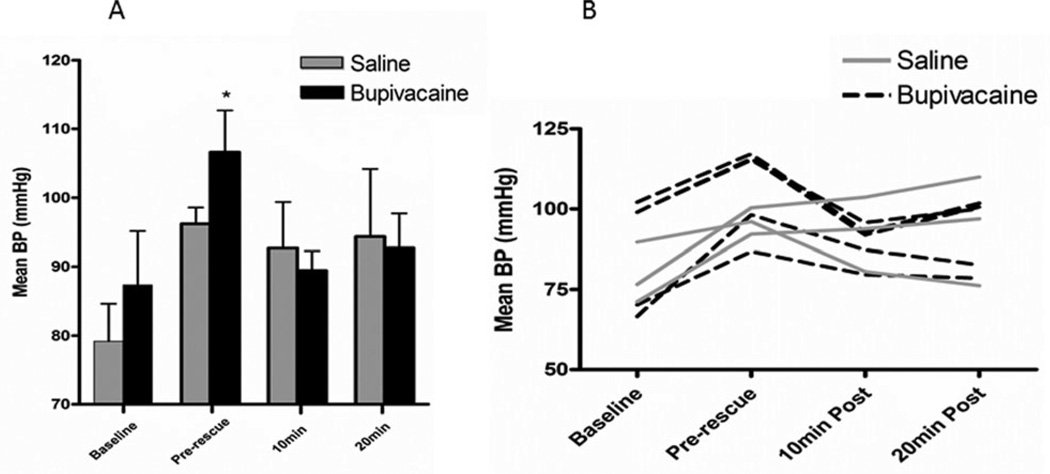
A total of eight pigs were included in the statistical analysis (n = 5 bupivacaine cohort; n = 3 saline cohort). Mean BP differed significantly over time (p = .002) with the difference occurring between the baseline and the pre-rescue time points for all pigs. No difference was seen between the treatment groups overall. Within the bupivacaine group, the mean pre-rescue BP was significantly higher than baseline, 10 min post, and 20 min post (p = .001) (Figure 3). No differences were demonstrated between time points for the saline group.
FIGURE 3.
Perioperative blood pressure. (a) Mean ± SEM BP for the two treatment groups at each of four time points. (b) Perioperative changes in BP by individual pig (bupivacaine group, n = 5; saline group, n = 3). Pre-rescue BP (*) was significantly higher from other time points within the bupivacaine group (p = .001).
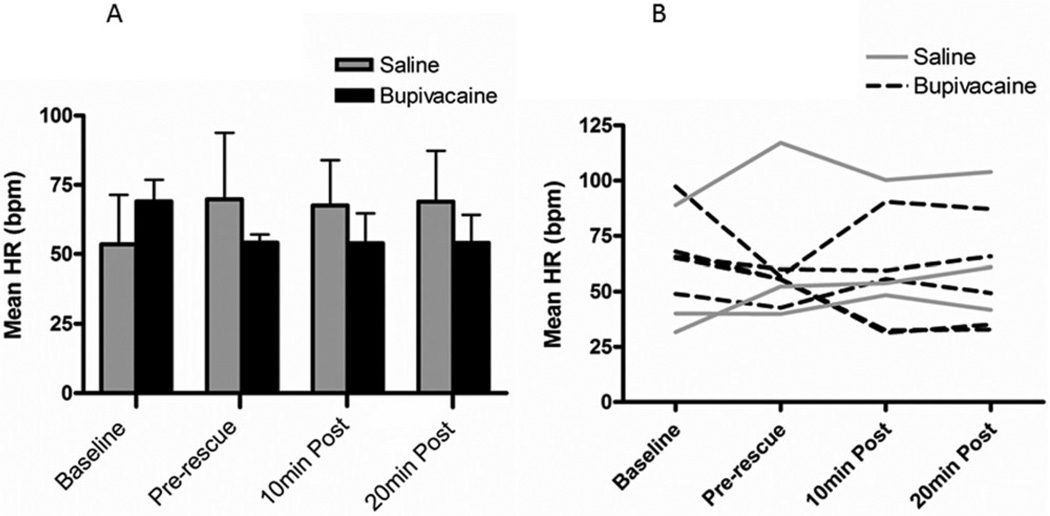
On a percentage change basis, mean HR differed significantly between treatment groups (p = .044) with the bupivacaine group having a lower HR than the saline group (Figure 4). Post hoc analysis revealed no differences between time points within treatment groups.
FIGURE 4.
Perioperative heart rate. (a) Mean ± SEM percent change in HR from baseline at each of three time points for the two treatment groups. (b) Perioperative changes in HR by individual pig (bupivacaine group, n = 5; saline group, n = 3). The HR was significantly lower in the bupivacaine treatment group overall (p = .044) but not at any individual time point.
Postoperative Pain Assessment
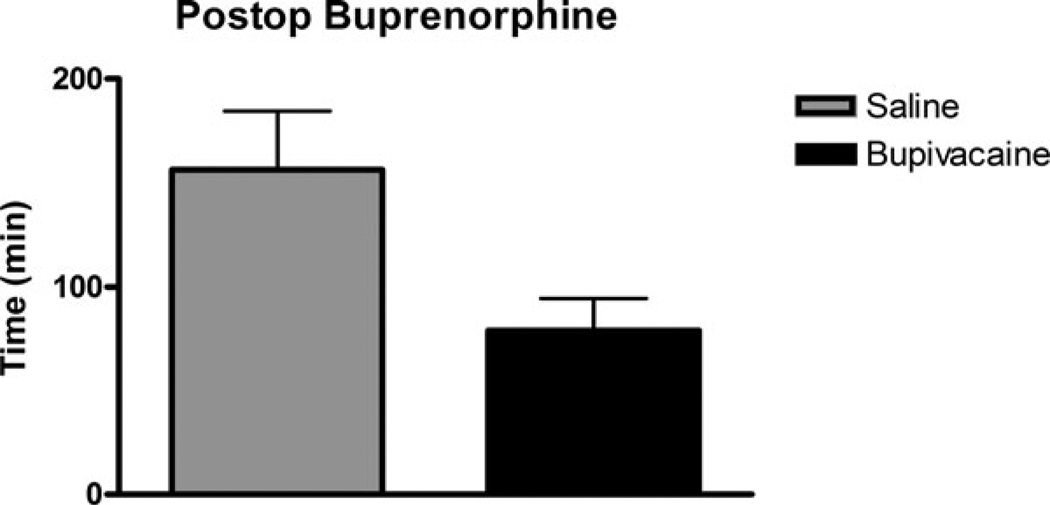
No difference was observed in postoperative parameters leading to buprenorphine injection for pain (p = .13). Four pigs (57%) in the saline group and six pigs (86%) in the bupivacaine group received buprenorphine injections within 3 hr of extubation (Figure 5). The only sign of pain displayed by the pigs was an unwillingness to eat and frequent motion of the mandible not associated with chewing. The two pigs that did not receive implants (split evenly between treatment groups) began eating normally within 2 hr of extubation. All pigs ate normally within 24 hr of extubation.
FIGURE 5.
Time to buprenorphine injection after extubation. Differences between treatment groups were not significant (p = .13).
DISCUSSION
The current study was designed to assess the efficacy of a mandibular nerve block as part of an anesthetic regimen for porcine mandibular condylectomy surgery. Addition of a bupivacaine mandibular nerve block resulted in significantly lower HR compared to a saline control at an identical level of inhalant isoflurane anesthesia and systemic fentanyl analgesia. BP was significantly reduced in the bupivacaine group after initiation of rescue analgesia while no difference was noted in the saline control. As indicated in methods, BP and HR values up to the end of the condylectomy procedure were used for statistical analysis. These findings support our first hypothesis that a bupivacaine mandibular nerve block may decrease the need of systemic analgesics during mandibular condylectomy surgery. Our second hypothesis of decreased postoperative pain was not supported by the results. Based on the combined results of this study and the common use of local blocks in human oral and maxillary surgery [6], a mandibular nerve block is justified to improve patient comfort during surgery and thereby reduce potential necessity for higher dosages of systemic analgesics.
The porcine model for oral maxillofacial surgical procedures is well established [2, 14, 21, 22]. Miniature pig jaw structure closely resembles that of the human jaw in terms of bone anatomy, morphology, and healing [23]. In the study reported here, the Yucatan miniature pig condylectomy model was selected for evaluation of a therapeutic intervention for temporomandibular joint disease. Animal models optimized to represent the human clinical scenario contribute to information with the highest translational value. Local anesthesia through nerve blocks of maxillary, mandibular, superficial cervical plexus, trigeminal, and sphenopalatine, among others, are commonly used for human oral and maxillofacial surgery [6]. Benefits in humans include improved perioperative analgesia, reduced preoperative opioids, and lower postoperative systemic analgesics [24–26]. Based on this information, we sought to improve the porcine mandibular condylectomy model with a more effective anesthetic protocol. The results achieved by addition of the block to this porcine model are similar, with exception of postoperative pain management, to those reported for human patients using standard assessments of blood pressure and heart rate.
There are limitations to every animal model typically associated with size, conformation, and behavior [23]. While the Yucatan pig generally has optimal size, they do differ significantly from humans in several ways. Perhaps the most important are slight differences in jaw anatomy and very thick skin that are most evident when trying to identify nerve block sites. This obstacle was overcome by the use of a nerve stimulator. The use of nerve stimulation for accurate nerve localization is a feasible option for most animal research surgical facilities. Based on the knowledge that identical isoflurane administration does not equate to identical planes of anesthesia among animals, all measures were assessed relative to baseline.
The pig’s stoic nature can make postoperative pain assessment challenging. Observational signs of pain are often useful clinically but can be difficult to assess statistically. The lack of verified pain assessment signs for swine in oral and maxillofacial studies makes this process even more challenging. A custom ethogram was created specifically for the patients in this study and was based on input from multiple veterinarians with swine experience. The ethogram used did not detect a difference in behavior between groups. Increased jaw motion and reduced food consumption were the only behaviors interpreted as evidence of pain. In the future, the facial tremors observed in some animals may be added to the ethogram if they are determined to be sufficiently outside of the direct surgical site. Many of the behaviors being evaluated may have been affected by other variables besides pain itself. All variables in observational pain studies should be controlled in order to eliminate the possibility of confounding results. Unavoidable individual variation in time between the nerve block and extubation complicated differentiation of pain versus stages of anesthetic recovery. The reported postoperative observations and associated statistical analyses are intended to be informative within the context of subjective assessments
It is standard practice to control postoperative pain with non-steroidal anti-inflammatory drugs (NSAIDs). However, NSAIDs are controversial in research surrounding bone formation and stem cell osteogenic capabilities since the anti-inflammatory properties have been shown to delay bone healing and stem cell differentiation [27–30]. Other analgesics like opioids require frequent administration which may cause increased stress and make the animals averse to handling. Long acting regional anesthesia is a potential alternative, but there is limited published information on this approach in swine. The local nerve block seemed to improve pain control under anesthesia in combination with lidocaine/fentanyl CRI. Local nerve blocks are commonly used in human medicine for surgical procedures on the mandible and maxilla and they have been shown to improve postoperative pain management [6, 24, 25, 31–33]. The increasing use of swine models for oral and maxillofacial surgical research justifies further investigation into the effectiveness of local nerve blocks.
Duration of action of local nerve blocks varies with the use of different local anesthetic drugs [34]. Indwelling catheters have even been placed in humans to provide a continuous block for control of mandibular pain [35, 36]. Bupivacaine reportedly provides local analgesia for 2 to 12 hr depending on the species [10, 11, 37, 38]. It has been shown to be effective in relieving mandibular pain for up to 12 h between infusions in humans when given through an indwelling catheter [35, 36]. The lack of pain control postoperatively in this study may have been due to lack of duration, variation in time lapse from block to extubation, the low animal numbers, discomfort from implant, or failure of block to provide postoperative pain. The addition of drugs such as epinephrine has been shown to increase local anesthetic duration [37, 39]. By adding epinephrine or utilizing a long acting local anesthetic such as liposomal bupivacaine, the duration of the local block may increase and be evident in the postoperative period.
The mandibular nerve block is a simple addition to the anesthetic regimen in the miniature pig condylectomy model that may improve analgesia. While locally blocking the mandibular nerve improved surgical analgesia in this study, a bupivacaine nerve block should not be the sole means of pain management. Systemic analgesics are required for proper pain control. Further studies and larger animal numbers are needed to better assess the intraoperative and postoperative effects of a mandibular nerve block in the porcine mandibular condylectomy model.
Acknowledgments
FUNDING Funding for this study was provided by the Partnership Fund for New York City.
Footnotes
Declaration of interests: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Ruehe B, Niehues S, Heberer S, et al. Miniature pigs as an animal model for implant research: bone regeneration in critical-size defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):699–706. doi: 10.1016/j.tripleo.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Papadaki ME, Troulis MJ, Glowacki J, et al. A minipig model of maxillary distraction osteogenesis. J Oral Maxillofac Surg. 2010;68(11):2783–2791. doi: 10.1016/j.joms.2010.06.179. [DOI] [PubMed] [Google Scholar]
- 3.Herring SW, Decker JD, Liu ZJ, et al. Temporomandibular joint in miniature pigs: anatomy, cell replication, and relation to loading. Anat Rec. 2002;266(3):152–166. doi: 10.1002/ar.10049. [DOI] [PubMed] [Google Scholar]
- 4.Schlegel KA, Rupprecht S, Petrovic L, et al. Preclinical animal model for de novo bone formation in human maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):e37–e44. doi: 10.1016/j.tripleo.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Olopade JO, Okandeji ME. A study of some rostrofacial indices related to regional anaesthesia of the porcine: implications as an animal model for dental research. Niger J Physiol Sci. 2010;25(2):159–164. [PubMed] [Google Scholar]
- 6.Kanakaraj M, Shanmugasundaram N, Chandramohan M, et al. Regional anesthesia in faciomaxillary and oral surgery. J Pharm Bioallied Sci. 2012;4(Suppl 2):S264–S269. doi: 10.4103/0975-7406.100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trullenque-Eriksson A, Guisado-Moya B. Comparative study of two local anesthetics in the surgical extraction of mandibular third molars: bupivacaine and articaine. Med Oral Patol Oral Cir Bucal. 2011;16(3):e390–e396. doi: 10.4317/medoral.16.e390. [DOI] [PubMed] [Google Scholar]
- 8.Carotenuto AM, Ravasio G, Fonda D, et al. Proximal mandibular nerve block, using electrolocation, for rostral mandibulectomy in a geriatric dog. Can Vet J. 2011;52(5):515–518. [PMC free article] [PubMed] [Google Scholar]
- 9.Mamiya H, Ichinohe T, Kaneko Y. Effects of block analgesia on attenuating intraoperative stress responses during oral surgery. Anesth Prog. 1997;44(3):101–105. [PMC free article] [PubMed] [Google Scholar]
- 10.Lizarraga I, Janovyak E, Beths T. Comparing lidocaine, bupivacaine and a lidocaine-bupivacaine mixture as a metacarpal block in sheep. Vet J. 2013;197(2):515–518. doi: 10.1016/j.tvjl.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Chahar P, Cummings KC., 3rd Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257–264. doi: 10.2147/JPR.S27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouin JP, Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Crit Care Nurs Clin North Am. 2012;24(2):201–213. doi: 10.1016/j.ccell.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walburn J, Vedhara K, Hankins M, et al. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009;67(3):253–271. doi: 10.1016/j.jpsychores.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki R, Watanabe Y, Yamato M, et al. Surgical anatomy of the swine face. Lab Anim. 2010;44(4):359–363. doi: 10.1258/la.2010.009127. [DOI] [PubMed] [Google Scholar]
- 15.Research IfLA, editor. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academic Press; 2011. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. [PubMed] [Google Scholar]
- 16.Holt TR, Withington DE, Mitchell E. Which pressure to believe? A comparison of direct arterial with indirect blood pressure measurement techniques in the pediatric intensive care unit. Pediatr Crit Care Med. 2011;12(6):e391–e394. doi: 10.1097/PCC.0b013e3182230f43. [DOI] [PubMed] [Google Scholar]
- 17.Lehman LW, Saeed M, Talmor D, et al. Methods of blood pressure measurement in the ICU. Crit Care Med. 2013;41(1):34–40. doi: 10.1097/CCM.0b013e318265ea46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stover JF, Stocker R, Lenherr R, Neff TA, Cottini SR, Zoller B, et al. Noninvasive cardiac output and blood pressure monitoring cannot replace an invasive monitoring system in critically ill patients. BMC Anesthesiol. 2009;9:6. doi: 10.1186/1471-2253-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King JW, Bair E, Duggan D, et al. The relationship between resting arterial blood pressure and acute postoperative pain in endodontic patients. J Orofac Pain. 2012;26(4):321–327. [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco M, Meschi M, Regolisti G, et al. The relationship between blood pressure and pain. J Clin Hypertens. 2013;15(8):600–605. doi: 10.1111/jch.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abukawa H, Shin M, Williams WB, et al. Reconstruction of mandibular defects with autologous tissue-engineered bone. J Oral Maxillofac Surg. 2004;62(5):601–606. doi: 10.1016/j.joms.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SM, Goldwasser MS, Clark SG, et al. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J Oral Maxillofac Surg. 2012;70(3):e193–e203. doi: 10.1016/j.joms.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Pearce AI, Richards RG, Milz S, et al. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 24.Van Lancker P, Abeloos JV, De Clercq CA, et al. The effect of mandibular nerve block on opioid consumption, nausea and vomiting in bilateral mandibular osteotomies. Acta Anaesthesiol Belg. 2003;54(3):223–226. [PubMed] [Google Scholar]
- 25.Plantevin F, Pascal J, Morel J, et al. Effect of mandibular nerve block on postoperative analgesia in patients undergoing oropharyngeal carcinoma surgery under general anaesthesia. Br J Anaesth. 2007;99(5):708–712. doi: 10.1093/bja/aem242. [DOI] [PubMed] [Google Scholar]
- 26.Splinter WM, Thomson ME. Somatic paravertebral block decreases opioid requirements in children undergoing appendectomy. Can J Anaesth. 2010;57(3):206–210. doi: 10.1007/s12630-009-9239-y. [DOI] [PubMed] [Google Scholar]
- 27.Teofilo JM, Giovanini GS, Fracon RN, et al. Histometric study of alveolar bone healing in rats treated with the non-steroidal anti-inflammatory drug nimesulide. Implant Dent. 2011;20(2):e7–e13. doi: 10.1097/ID.0b013e31820fbacf. [DOI] [PubMed] [Google Scholar]
- 28.Pountos I, Giannoudis PV, Jones E, et al. NSAIDS inhibit in vitro MSC chondrogenesis but not osteogenesis: implications for mechanism of bone formation inhibition in man. J Cell Mol Med. 2011;15(3):525–534. doi: 10.1111/j.1582-4934.2010.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welting TJ, Caron MM, Emans PJ, et al. Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification. Eur Cell Mater. 2011;22:420–436. doi: 10.22203/ecm.v022a31. discussion 36–37. [DOI] [PubMed] [Google Scholar]
- 30.Yoon DS, Yoo JH, Kim YH, et al. The effects of COX-2 inhibitor during osteogenic differentiation of bone marrow-derived human mesenchymal stem cells. Stem Cells Dev. 2010;19(10):1523–1533. doi: 10.1089/scd.2009.0393. [DOI] [PubMed] [Google Scholar]
- 31.Kim HS, Lee HK, Jeong HS, Shin HW. Decreased postoperative pain after reduction of fractured nasal bones using a nerve block of the anterior ethmoidal nerve. Int J Oral Maxillofac Surg. 2013;42(6):727–731. doi: 10.1016/j.ijom.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Boonsiriseth K, Sirintawat N, Arunakul K, Wongsirichat N. Comparative study of the novel and conventional injection approach for inferior alveolar nerve block. Int J Oral Maxillofac Surg. 2012;42(7):852–856. doi: 10.1016/j.ijom.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Ogle OE, Mahjoubi G. Local anesthesia: agents, techniques, and complications. Dent Clin North Am. 2012;56(1):133–148. doi: 10.1016/j.cden.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Dyhre H, Lang M, Wallin R, et al. The duration of action of bupivacaine, levobupivacaine, ropivacaine and pethidine in peripheral nerve block in the rat. Acta Anaesthesiol Scand. 1997;41(10):1346–1352. doi: 10.1111/j.1399-6576.1997.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 35.Sawhney C, Agrawal P, Soni KD. Post operative pain relief through intermittent mandibular nerve block. Natl J Maxillofac Surg. 2011;2(1):80–81. doi: 10.4103/0975-5950.85860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh B, Bhardwaj V. Continuous mandibular nerve block for pain relief. A report of two cases. Can J Anaesth. 2002;49(9):951–953. doi: 10.1007/BF03016881. [DOI] [PubMed] [Google Scholar]
- 37.Berde CB, Athiraman U, Yahalom B, et al. Tetrodotoxin-bupivacaine-epinephrine combinations for prolonged local anesthesia. Mar Drugs. 2011;9(12):2717–2728. doi: 10.3390/md9122717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanelli G, Casati A, Beccaria P, et al. A double-blind comparison of ropivacaine, bupivacaine, and mepivacaine during sciatic and femoral nerve blockade. Anesth Analg. 1998;87(3):597–600. doi: 10.1097/00000539-199809000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Sinnott CJ, Cogswell LP, Johnson A, et al. On the mechanism by which epinephrine potentiates lidocaine’s peripheral nerve block. Anesthesiology. 2003;98(1):181–188. doi: 10.1097/00000542-200301000-00028. [DOI] [PubMed] [Google Scholar]