Abstract
Background
To analyze patient reported outcomes (PROs) in GOG 240, the practice-changing, randomized phase 3 trial that concluded that chemotherapy (cisplatin-paclitaxel or topotecan-paclitaxel) plus bevacizumab significantly improves overall survival (OS), progression-free survival (PFS), and response rates compared to chemotherapy alone in advanced cervical cancer. Trial registration number: NCT00803062.
Methods
Patients were assessed pre-cycle 1, 2, and 5 and at 6 and 9 months post-cycle 1 with the Functional Assessment of Cancer Therapy-Cervix Trial Outcome Index (FACT-Cx TOI), and items from the FACT/GOG-Neurotoxicity (Ntx) subscale, and a worst pain item from the Brief Pain Inventory (BPI). Differences in FACT-Cx TOI scores were assessed using a linear mixed model adjusting for baseline score and age. A mixed effects mixed distributions model was fitted to evaluate treatment differences of likelihood to report neurotoxicity and pain, and severity of these symptoms, once reported. The association between baseline health-related quality of life and survival was analyzed using Cox proportional hazards models.
Findings
Among 390 evaluable patients, PRO completion rates declined from 96% (baseline) to 63% (9 months post-cycle 1). Completion rates were not statistically different among treatment regimens (p=0.67). Patients receiving chemotherapy plus bevacizumab reported 1.2 points lower on average (98.75% CI: −4.1, 1.7; p=0.30) in the FACT-Cx TOI scores than those with chemotherapy alone. Patients treated with chemotherapy plus bevacizumab were less likely to report neurotoxicty (overall odds ratio: 0.58; 98.75% CI: 0.17, 0.98; p=0.01). Severity of neurotoxic symptoms did not differ between the two groups (p=0.69). Both groups had similar odds of complaining of pain (odds ratio=0.96; 95% CI: 0.39, 1.52; p=0.78) and reported similar severity of pain (p=0.1). For the entire population, the baseline FACT-Cx TOI score was significantly associated with OS (HR 0.80; 95% CI 0.74, 0.87; p<0.001) and PFS (0.88; 95% CI: 0.83, 0.95; p<0.001).
Interpretation
Improvements in OS and PFS attributed to the incorporation of bevacizumab in the treatment of advanced cervical cancer were not accompanied by any significant deterioration in health-related quality of life. Study supported by NIH funding.
Keywords: Cervical cancer, quality of life, bevacizumab
INTRODUCTION
Throughout the world there are approximately 500,000 new cases of cervical cancer each year and 250,000 deaths. (1) Although screening with cytology and/or high risk human papillomavirus (HPV) DNA testing has decreased the incidence and mortality of this disease, women who lack access to healthcare as well as those living in resource-poor areas remain at high risk for death by cervical cancer. Prophylactic HPV vaccination is an important preventative tool but one that also requires access to healthcare. Although early stage and locally advanced disease can be cured with radical surgery and chemoradiation, respectively, women with metastatic and non-operable recurrent disease have previously had limited treatment options. (1) Platinum-based chemotherapy in this setting is palliative and associated with median overall survival (OS) rates of 8 to 12 months. (2–4)
Vascular endothelial growth factor (VEGF) has emerged as an important therapeutic target in many solid tumors. (5) Gynecologic Oncology Group (GOG) protocol 240 was a randomized phase III clinical trial which demonstrated that compared to chemotherapy alone, chemotherapy plus bevacizumab (a monoclonal antibody that binds VEGF) significantly increased OS from 13.3 to 17.0 months (hazard ratio (HR) 0.71; 98% CI 0.54 to 0.95; P=0.004) with advanced cervical cancer. (6) The triplet regimens used in the study (cisplatin-paclitaxel-bevacizumab and topotecan-paclitaxel-bevacizumab) were relatively well tolerated but associated with a 6% incidence of fistula and 8% incidence of thromboembolism. Importantly, on August 14, 2014, the United States Food and Drug Administration approved both bevacizumab-containing triplet regimens for the treatment of advanced cervical cancer. This regulatory milestone occurred under the auspices of the FDA’s Priority Review program, underscoring the agency’s commitment to make available to patients promising therapies expeditiously.
In the advanced cervical cancer setting, it is essential to measure QOL in order to understand the balance of potential toxicity with the hope that disease-related symptoms may improve. However, this is a balance which is considered important while simultaneously evaluating the lengthening of PFS and OS. Prior to GOG 240, PFS and OS benefits in cancer treatments were modest, with little benefit or difference in HRQoL. (7–10) Given the limited prognosis for this population, we strive to identify treatments which prolong life but do not create additional toxicities which would further compromise quality of life. With the typically marginal benefit of the addition of new agents to combination chemotherapy, it was anticipated that short of an overall survival benefit, clincial benefit would have to be demonstrated by the additional benefit of PROs to a PFS advantage. Therefore, a major objective of GOG 240 was to determine whether the addition of bevacizumab to chemotherapy affected HRQoL. Determining whether baseline HRQoL in this population was associated with survival was considered an exploratory endpoint, where earlier literature has indicated that quality of life at study entry is prognostic for survival.
MATERIALS AND METHODS
Treatment
The trial was conducted through the GOG and the Spanish cooperative group, Grupo Español de Investigation en Cancer de Ovario (GEICO) with NIH funding and NCI-supplied bevacizumab (NSC #704865, IND #113912), with central IRB approval and registration (NCT00803062). Primary endpoints were OS and the frequency and severity of toxicity, with progression-free survival (PFS), and tumor response as secondary endpoints. Eligibility required primary Stage IVB or recurrent/persistent carcinoma of the cervix with measurable disease and GOG performance status 0–1. Participants were randomized to one of four regimens: paclitaxel (135 mg/m2 IV over 24 hrs or 175 mg/m2 IV over 3 hrs) with cisplatin 50 mg/m2 IV with or without bevacizumab 15 mg/kg IV, or paclitaxel 175 mg/m2 over 3 hrs on day 1 with topotecan 0.75 mg/m2 over 30 minutes days 1–3 with or without bevacizumab 15 mg/kg IV. Cycles were repeated every 21 days to disease progression or unacceptable toxicity. To reduce the risk of neurotoxicity paclitaxel infusions could also be given on non-platinum days.
Randomization and Masking
Web-based permuted block randomization was conducted by the GOG Statistical and Data Center, utilizing a 2×2 factorial design. Randomization ratio was 1:1 for each level of each factor, or 1:1:1:1 for each group. Random assignment to one of the four groups was balanced within disease status (recurrent/persistent versus stage IVB primary), performance status (0 versus 1), and prior platinum therapy as a radiation sensitizer (e.g. no prior cis-RT versus prior cis-RT). Treatment assignment was concealed at randomization and became open-labelled when the patient was registered to the trial. Overall and progression-free survival were determined from date of randomization. [HH1]
PROs Assessment Time Points
In all regimens, patient reported outcomes (PROs) were assessed at five time points: baseline (prior to randomization), before cycles 2 and 5, and at 6 and 9 months post-cycle 1.
PROs Measures
The HRQL instruments used in this trial were the Functional Assessment of Cancer Therapy-Cervix (FACT-Cx) Trial Outcome Index (TOI), for which a higher score indicates better HRQL; the FACT/GOG-Neurotoxicity 4-item Subscale (FACT/GOG-Ntx-4), for which a higher score indicates less neurotoxicity; and the Brief Pain Inventory (BPI) single item evaluating worst pain in the past 24 hours, for which a higher score indicates more pain. These measures were selected based on prior justification that quality of life, neurotoxicity and pain are all sensitive to change in advanced cervical cancer randomized clinical trials. All instruments are available in English and Spanish and both language versions were used in this trial. (11, 12)
The Functional Assessment of Cancer Therapy – Cervix (FACT-Cx)
The FACT-Cx is the FACT-G plus a cervix cancer-specific subscale. (11) The FACT-G is a 27-item self report quality of life (QOL) measure that includes 4 subscales (physical well-being, social well-being, functional well-being and emotional well-being). Each scale produces a score, and scores can be summed to produce a total QOL score. The FACT-Cx endpoint for this trial focuses on the aspects of HRQL that are most sensitive and responsive in clinical trials. This “Trial Outcome Index” of FACT-Cx (FACT-Cx TOI), is the summation of the Physical Well Being, Functional Well Being and Cervix Cancer Subscales.
FACT/GOG-Ntx 4-item Scale
Four items from the 11-item FACT/GOG-Neurotoxicity (FACT/GOG-Ntx) subscale were included to evaluate this important side effect associated with many of the chemotherapy agents in this trial. These 4 questions also explain more than 50% of the variation in the total Ntx score. (12)
The Brief Pain Inventory (BPI)
The BPI is a 23-item, self-report instrument designed to assess pain in cancer and other diseases. (13) To limit patient burden, we selected the most commonly-employed endpoint from the BPI which is the single item assessing “worst pain” in the past 24 hours, on a 0–10 scale.
The Trial Outcome of Index of the Functional Assessment of Cancer Therapy (FACT-Cx TOI) was selected as the primary QOL endpoint and consists of two subscales from the FACT-G: Physical Well Being (7 items) and Functional Well Being (7 items), plus the Cervix Cancer-specific subscale (15 items). (11, 14) Chemotherapy-induced peripheral neuropathy was measured with 4 previously validated items from the 11-item FACT/GOG-Neurotoxicity subscale (Ntx). Each item in the FACT-Cx TOI and FACT/GOG-Ntx4 subscale are scored using a 5-point scale (0=not at all; 1=a little bit; 2=somewhat; 3=quite a bit; 4=very much). The range of possible scores is 0–116 for the FACT-Cx TOI and 0–16 for FACT/GOG-Ntx4 subscale. For all FACIT PRO scales a larger score indicates better QOL or fewer symptoms/toxicity. We also used the Brief Pain Inventory (BPI) single item: 0 (no pain) to 10 (worst pain) to measure the worst pain patients experienced in the last 24 hours. Collection of the later PRO data, at 6 and 9 months post cycle #1, were required independent of whether participants had experienced PD, and participants were required to fill out instruments on their own. Validating the prognostic significance of PROs in this trial was an exploratory endpoint.
Statistical Analysis
Patients who completed baseline and at least one follow-up PROs assessments were considered evaluable for this final analysis.
The association between baseline FACT-Cx TOI score and OS and PFS was explored with a proportional hazards model stratified with prognostic factors, assignment of bevacizumab, and treatment with cisplatin or topotecan. (15) The median follow-up for survival was 20.8 months.
The presence of neurotoxic symptoms was defined as Ntx score <16. The severity of reported neurotoxic symptoms was determined with the mean Ntx score that less than 16. The distribution of the Ntx subscale score tended to clumping at 16 (no neurotoxicity) and skewed to left. The distribution of BPI score tended to cluster at zero and skewed to right. To analyze these data, a mixed-effects mixed-distribution (MEMD) model were considered. This MEMD model contains two components. The first component consists of a logistic model to estimate the odds of reporting a nonzero value (<16 for Ntx scores) and the second component models the possibly truncated distribution of the nonzero scores (<16 for Ntx scores). Random effects are used to account for the correlation of repeating measures within an individual. This model also allows for a correlation of the random effects from the two components of the model. (16)
All analyses were undertaken on the whole intention-to-treat population using SAS/STAT Software 9.4. The difference in FACT-Cx TOI was assessed with the linear mixed model adjusting for patient’s pretreatment score, performance status, assignment of bevacizumab, treatment with cisplatin or topotecan, and age at enrollment. The assessment time points were treated as categorical since they are not equally spaced. The covariance matrix is assumed to be unstructured. To reflect the observed covariance pattern of the TOI scores, the ‘empirical’ variance was used in estimating the precision of parameter estimates. The denominator degrees of freedom was approximated as described by Kenward and Roger. (17) The independence effect of two factors on QOL (interaction between bevacizumab and topotecan/cisplatin) was tested first and showed not significant (p=0.31). Then the interactions between assessment time points and treatment assignments were tested first for differential effects of treatments on TOI scores over time. If the interaction effect was statistically significant, the treatment differences were estimated for each assessment time points. Otherwise the overall treatment effect was estimated by a weighted average of estimates from each time point.
The sample size was determined by the primary clinical objective. (6) Because the original study design tested two independent hypotheses (impact of bevacizumab; substitution of topotecan for cisplatin), to control the overall type 1 error at 5% the significance level was set to 0.0125 for the FACT-Cx TOI and the FACT/GOG-Ntx subscale, and 0.05 for the BPI worst pain score for exploratory purpose. According to previous studies, the correlation between repeated measures made on the same subject was ranged 0.3 to 0.8. Assuming at least 80% of eligible patients were evaluable for the analysis (completed baseline and at least one follow-up assessments), the study was expected to have approximately at least 86% power to detect an effect size of 0.35 in FACT-Cx TOI scores between treatment arms. Treatment effect size was calculated as the ratio of the treatment difference to the baseline standard deviation in the control group. The minimum clinically important difference (MCID) for FACT-Cx TOI is considered to range from 5.8 to 8.7 points, perceived by patients as important, and by clinicians to require a change in the patients’ management. (18)
Every effort was made to avoid missing data and the reasons for missing assessments were collected at each assessment time points and documented in the analysis. The assessment compliances were compared across assigned groups using generalized estimating equation (GEE). In addition, multiple imputation was conducted for missing values by those still living at assessment time. The results from multiple imputation were consistent with the original analysis.
Role of the funding source
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517) and NRG Oncology Group Grant 1 U10 CA180822. The sponsors were not involved in the study design, conduct, analysis, interpretation of the data, or writing of the report. Only the statistician (HQH) had access to the raw data, per GOG policy. The corresponding author had final responsibility to submit for publication.
RESULTS
Accrual and PROs assessment completion
Between April 6, 2009 and January 3, 2012, a total of 452 patients were enrolled. Ninety-six percent (436/452) of patients completed baseline QoL assessment. Instrument compliance rates (including those who had progressive disease as patients were followed for survival) dropped to 84% (372/443) at pre-cycle 2 assessment, 78% (322/412) at pre-cycle 5 assessment, 67% (245/368) at six months, and 63% (193/307) at nine months post cycle 1 follow-up. Compliance rates were not significantly different among treatment regimens (p=0.78) and reasons for noncompliance were similar among treatment groups (Table 1). Nearly one-third of enrolled patients completed all the scheduled QoL assessments and 14 patients completed none. In addition, 12 patients did not complete baseline QoL assessments and 36 patients did not complete any follow-up assessments. These 62 patients were not evaluable for PROs and therefore were not included in the final analysis.
Table 1.
Status of QOL assessment completion
| Assessment Time | Status | Reasons for noncompliance |
CP N=114 |
CP+Bev N=115 |
TP N=111 |
TP+Bev N=112 |
|---|---|---|---|---|---|---|
| Pre-cycle 1 | Received | 110 | 112 | 108 | 106 | |
| Noncompliance | Insufficient answer | 0 | 0 | 0 | 1 | |
| Illness or toxicities | 1 | 0 | 1 | 1 | ||
| Patient Refusal | 1 | 1 | 1 | 1 | ||
| Administrative error | 2 | 0 | 0 | 2 | ||
| Other | 0 | 2 | 1 | 1 | ||
| Pre-cycle 2 | Death | 1 | 1 | 4 | 3 | |
| Received | 95 | 95 | 89 | 93 | ||
| Noncompliance | Insufficient answer | 1 | 0 | 0 | 0 | |
| Illness or toxicities | 6 | 5 | 2 | 1 | ||
| Patient Refusal | 1 | 4 | 4 | 2 | ||
| Administrative error | 8 | 5 | 11 | 10 | ||
| Lost to follow-up | 1 | 3 | 1 | 0 | ||
| Other | 1 | 2 | 0 | 3 | ||
| Pre-cycle 5 | Death | 11 | 8 | 12 | 9 | |
| Received | 82 | 82 | 77 | 81 | ||
| Noncompliance | Illness or toxicities | 7 | 1 | 1 | 4 | |
| Patient Refusal | 4 | 6 | 4 | 5 | ||
| Administrative error | 5 | 9 | 8 | 4 | ||
| Lost to follow-up | 3 | 3 | 4 | 4 | ||
| Other | 2 | 6 | 5 | 5 | ||
| 6 months post cycle 1 | Death | 24 | 16 | 25 | 19 | |
| Received | 61 | 66 | 54 | 64 | ||
| Noncompliance | Illness or toxicities | 7 | 2 | 6 | 2 | |
| Patient Refusal | 4 | 9 | 5 | 5 | ||
| Administrative error | 6 | 10 | 6 | 9 | ||
| Lost to follow-up | 7 | 6 | 7 | 8 | ||
| Other | 5 | 6 | 8 | 5 | ||
| 9 months post cycle 1 | Death | 39 | 30 | 43 | 33 | |
| Received | 48 | 49 | 43 | 53 | ||
| Noncompliance | Illness or toxicities | 7 | 2 | 3 | 5 | |
| Patient Refusal | 4 | 9 | 5 | 4 | ||
| Administrative error | 6 | 9 | 4 | 5 | ||
| Lost to follow-up | 4 | 8 | 6 | 6 | ||
| Other | 6 | 8 | 7 | 6 | ||
| Evaluable for PROs | 99 | 98 | 95 | 98 | ||
| Inevaluble for PROs | Baseline incomplete | 7 | 6 | 5 | 8 | |
| Follow-ups incomplete | 8 | 11 | 11 | 6 | ||
Patient characteristics
Clinical features among 390 evaluable patients were similar in groups treated with chemotherapy with and without bevacizumab, and in those treated with cisplatin or topotecan (Table 2). Overall, 39% (153/390) were performance status 1, 83% (322/390) had recurrent/persistent disease, and 73% (286/390) had received prior platinum. This study encouraged all living patients to complete the PROs regardless of their progression status. At 9 months, 68% (104/153) of patients without disease progression vs. 64% (83/129) of those with disease progression completed PROs.
Table 2.
Patient characteristics
| Ctx N=194 |
Ctx+Bev N=196 |
CP±Bev N=197 |
TP±Bev N=193 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % | |
| Age Group | <=39 | 20 | 10.3 | 24 | 12.2 | 21 | 10.7 | 23 | 11.9 |
| 40–49 | 67 | 34.5 | 54 | 27.6 | 65 | 33.0 | 56 | 29.0 | |
| 50–59 | 49 | 25.3 | 67 | 34.2 | 53 | 26.9 | 63 | 32.6 | |
| 60–69 | 41 | 21.1 | 35 | 17.9 | 35 | 17.8 | 41 | 21.2 | |
| >=70 | 17 | 8.76 | 16 | 8.16 | 23 | 11.7 | 10 | 5.18 | |
| Race | Asian | 5 | 2.58 | 12 | 6.12 | 10 | 5.08 | 7 | 3.63 |
| Black | 21 | 10.8 | 31 | 15.8 | 26 | 13.2 | 26 | 13.5 | |
| Other | 12 | 6.19 | 8 | 4.08 | 8 | 4.06 | 12 | 6.22 | |
| White | 156 | 80.4 | 145 | 74.0 | 153 | 77.7 | 148 | 76.7 | |
| Ethnicity | Hispanic | 25 | 12.9 | 22 | 11.2 | 27 | 13.7 | 20 | 10.4 |
| Non-Hispanic | 161 | 83.0 | 165 | 84.2 | 159 | 80.7 | 167 | 86.5 | |
| Other/Unspecified | 8 | 4.12 | 9 | 4.59 | 11 | 5.58 | 6 | 3.11 | |
| PS | 0 | 121 | 62.4 | 116 | 59.2 | 119 | 60.4 | 118 | 61.1 |
| 1 | 73 | 37.6 | 80 | 40.8 | 78 | 39.6 | 75 | 38.9 | |
| Disease Status | Advanced | 36 | 18.6 | 32 | 16.3 | 35 | 17.8 | 33 | 17.1 |
| Persistent | 17 | 8.8 | 26 | 13.3 | 17 | 8.6 | 26 | 13.8 | |
| Recurrent | 141 | 72.7 | 138 | 70.4 | 145 | 73.6 | 134 | 69.4 | |
| Prior Platinum | Yes | 139 | 71.6 | 147 | 75.0 | 144 | 73.1 | 142 | 73.6 |
| No | 55 | 28.4 | 49 | 25.0 | 53 | 26.9 | 51 | 26.4 | |
PS: Performance Status
Effects of Bevacizumab on FACT-Cx TOI
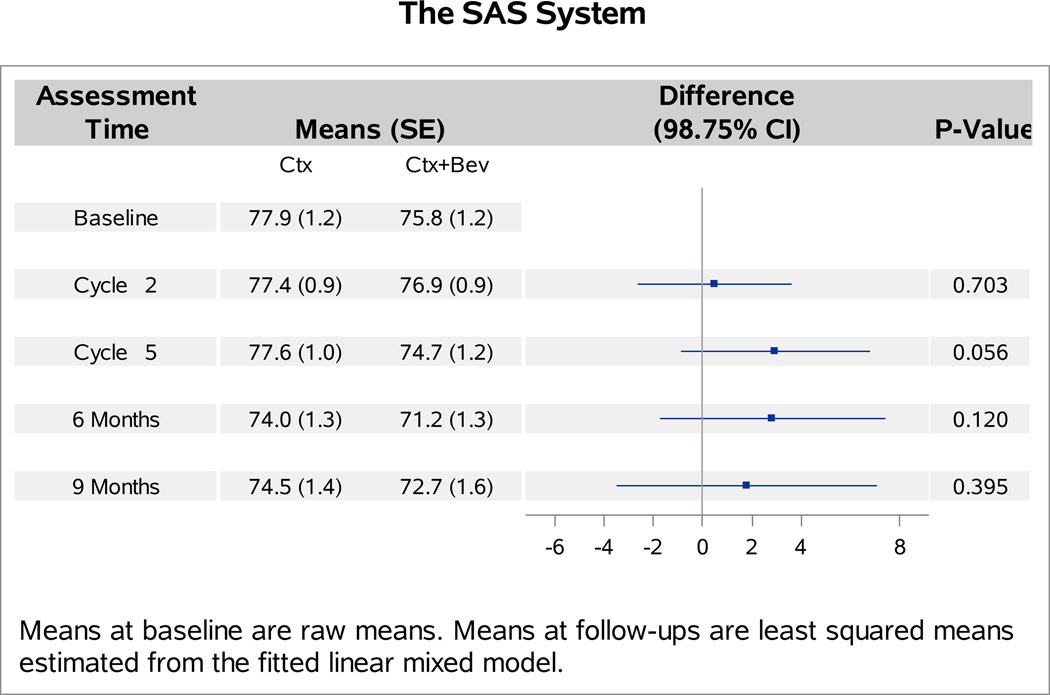
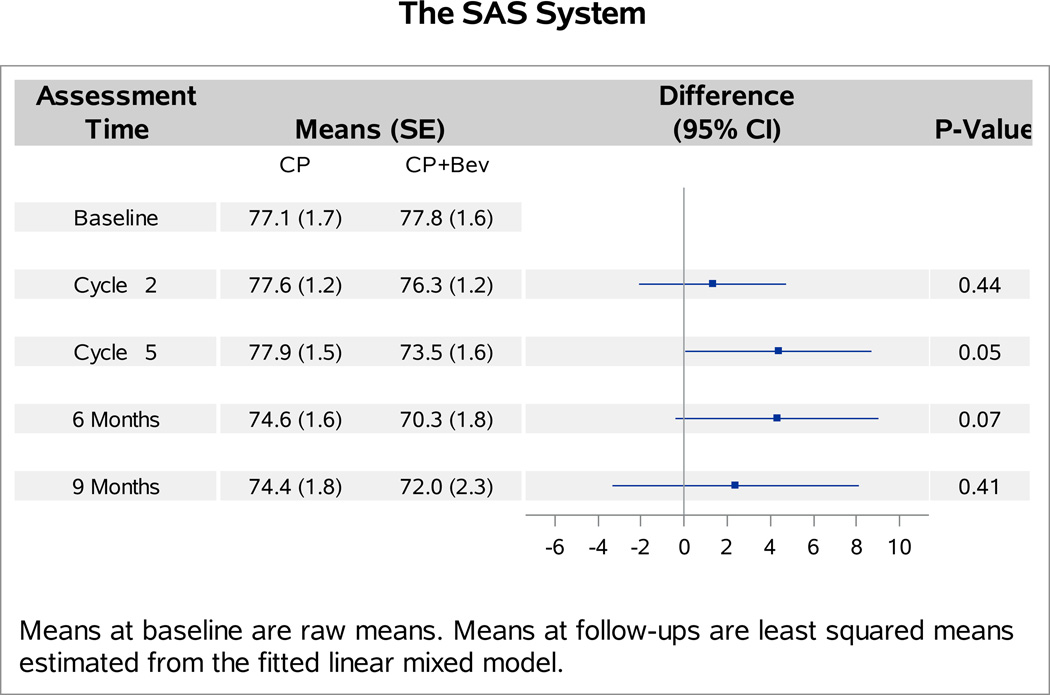
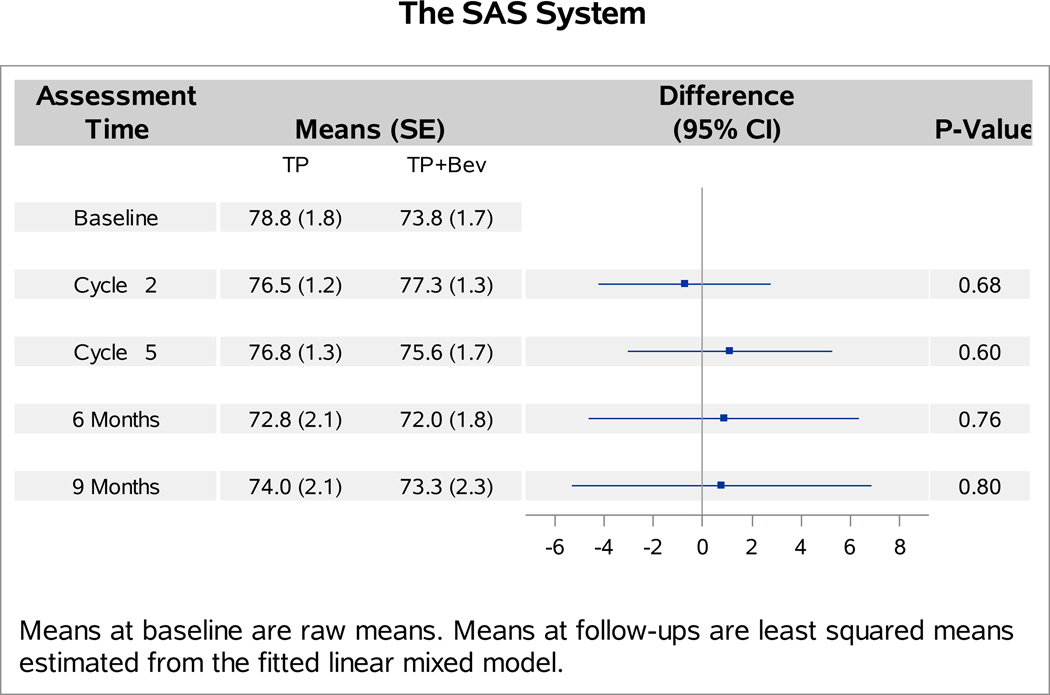
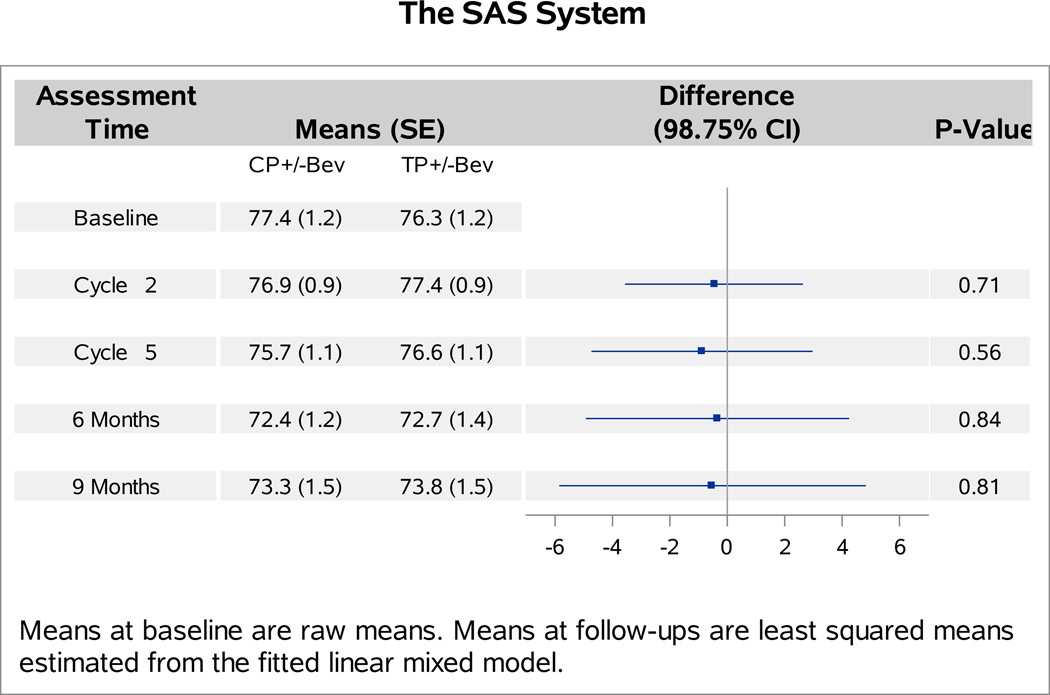
The baseline FACT-Cx TOI scores were not significantly different between patients with versus without bevacizumab (p=0.27). Analyses of follow-up FACT-Cx TOI scores indicated no interaction between bevacizumab and topotecan (p=0.31), or between assessment time and assignment of bevacizumab (p=0.29). Patients receiving bevacizumab reported 1.2 points (98.75% CI: −4.1,1.7; p=0.30) lower on average in the FACT-Cx TOI scores than those receiving chemotherapy alone (Figure 1A). After adjustment for baseline score and patients’ age, those treated with cisplatin and paclitaxel plus bevacizumab reported 2.1 points lower (95% CI: −1.2, 5.3; p=0.20) on the FACT-Cx TOI than those treated with cisplatin and paclitaxel (Figure 1B). Patients treated with topotecan and paclitaxel plus bevacizuamb reported 0.1 points higher (95% CI: −3.1, 3.2; p=0.96) than those treated with topotecan and paclitaxel (Figure 1C).
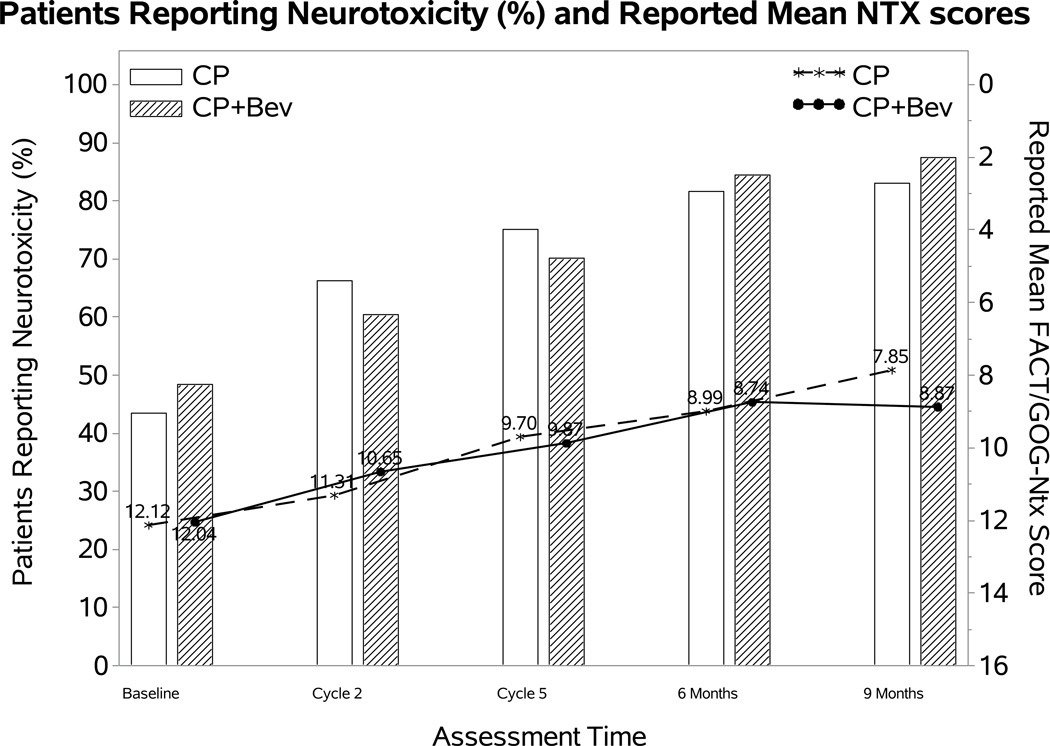
Figure 1. Patient-reported outcomes (PROs) using the Functional Assessment of Cancer Therapy – Cervix (FACT-Cx) Trial Outcome Index (TOI) by treatment group.
During the period of assessment, the mean FACT-Cx TOI scores did not significantly differ between the following treatment groups: chemotherapy alone (both backbones) vs chemotherapy (both backbones) with bevacizumab (Panel 1A); cisplatin and paclitaxel with and without bevacizumab (Panel 1B); topotecan and paclitaxel with and without bevacizumab (Panel 1C). Ctx: chemotherapy; Bev: bevacizumab; CP: cisplatin and paclitaxel; TP: topotecan and paclitaxel.
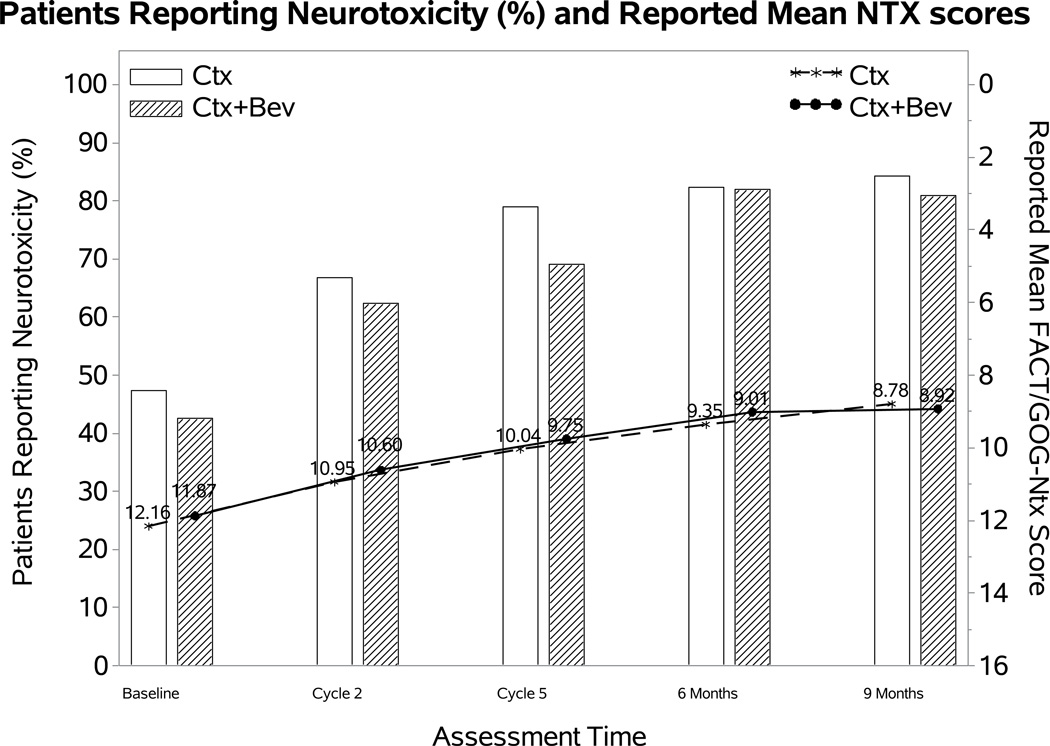
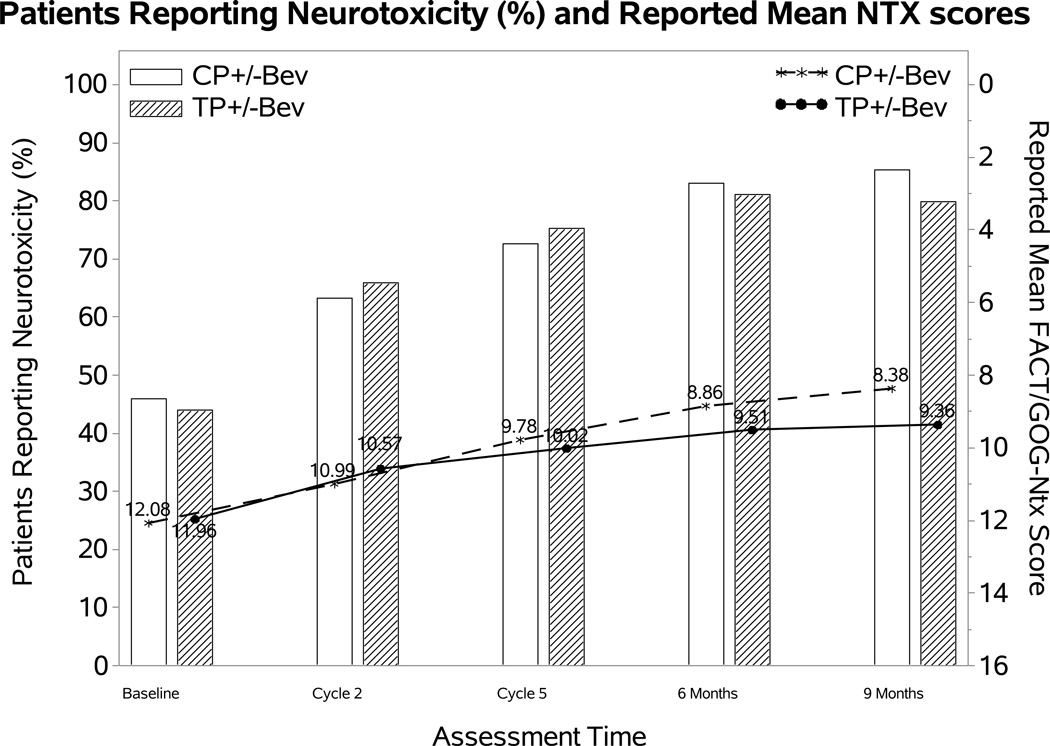
Effect of addition of bevacizumab on the FACT/GOG-Ntx score
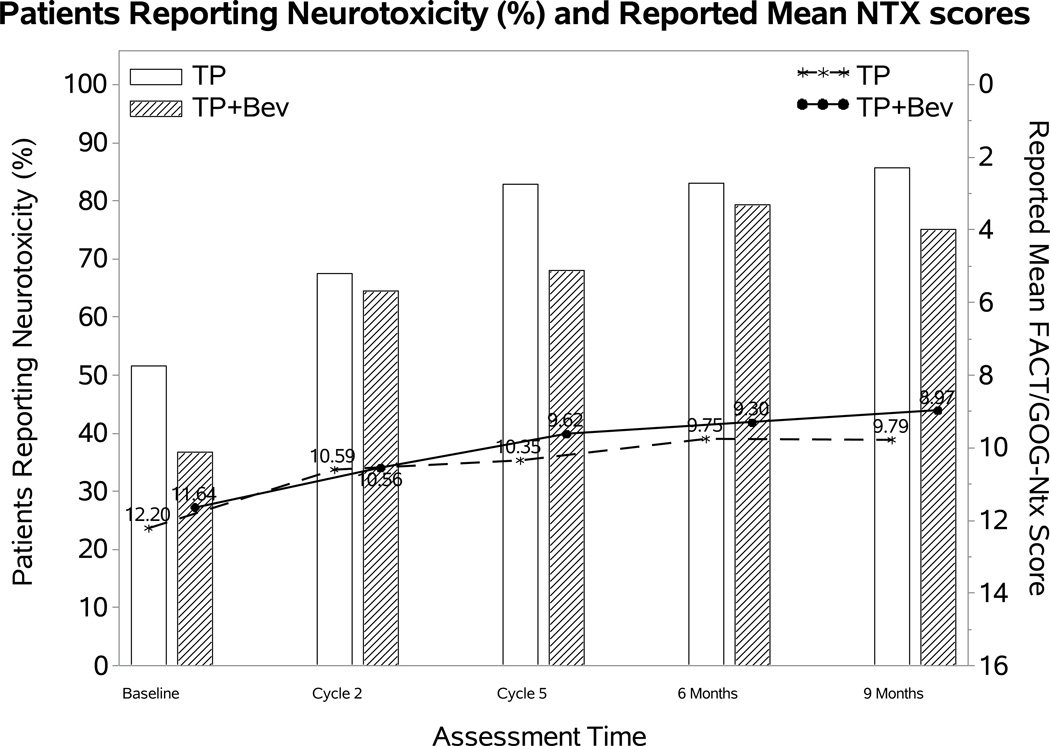
The percentage of patients reporting neurotoxic symptoms consistently increased over time in both groups. Patients receiving bevacizumab were less likely to report neurotoxic symptoms (odds ratio (OR): 0.58; 98.75% CI: 0.17, 0.98; p=0.01), however, the FACT/GOG-Ntx score when the toxicity was present was not significantly different (difference (diff): 0.23; 98.75% CI: −1.19~1.64; p=0.69) (Figure 2A). Among those treated with the cisplatin-paclitaxel backbone, incorporation of bevacizumab was not associated with either likelihood of reporting neurotoxicity (OR: 0.59; 95% CI 0.1, 1.09; p=0.11) or with the FACT/GOG-Ntx subscale (diff 0.15; 95% CI −1.54, 1.84; p=0.86) (Figure 2B). Although patients treated with bevacizumab on the topotecan-paclitaxel backbone were less likely to report neurotoxicity (OR 0.51; 95% CI 0.11, 0.91; p=0.02) among those reporting neurotoxicity, the FACT/GOG-Ntx scores were not significantly different (diff 0.17; 95% CI −1.31, 1.65; p=0.72) (Figure 2C).
Figure 2. Patient-reported outcomes (PROs) using the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group (FACT/GOG) Neurotoxicity subscale (Ntx).
During the period of assessment, patients treated with chemotherapy plus bevacizumab as well as those on the topotecan-paclitaxel backbone who received bevacizumab had less odds of reporting neurotoxicity. Among those patients reporting neurotoxcity there were no significant differences in severity of neurotoxic symptoms for the following treatment groups: chemotherapy alone (both backbones) vs chemotherapy (both backbones) with bevacizumab (Panel 2A); cisplatin and paclitaxel with and without bevacizumab (Panel 2B); topotecan and paclitaxel with and without bevacizumab (Panel 2C). Ctx: chemotherapy; Bev: bevacizumab; CP: cisplatin and paclitaxel; TP: topotecan and paclitaxel.
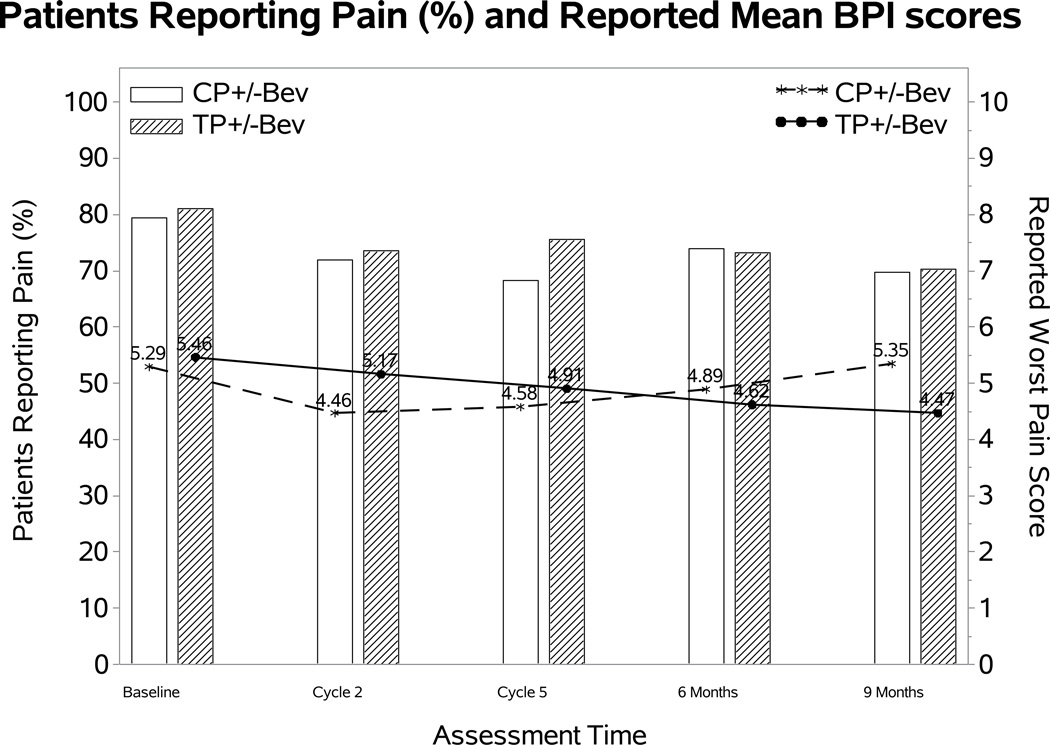
Effect of addition of bevacizumab on BPI worst pain score
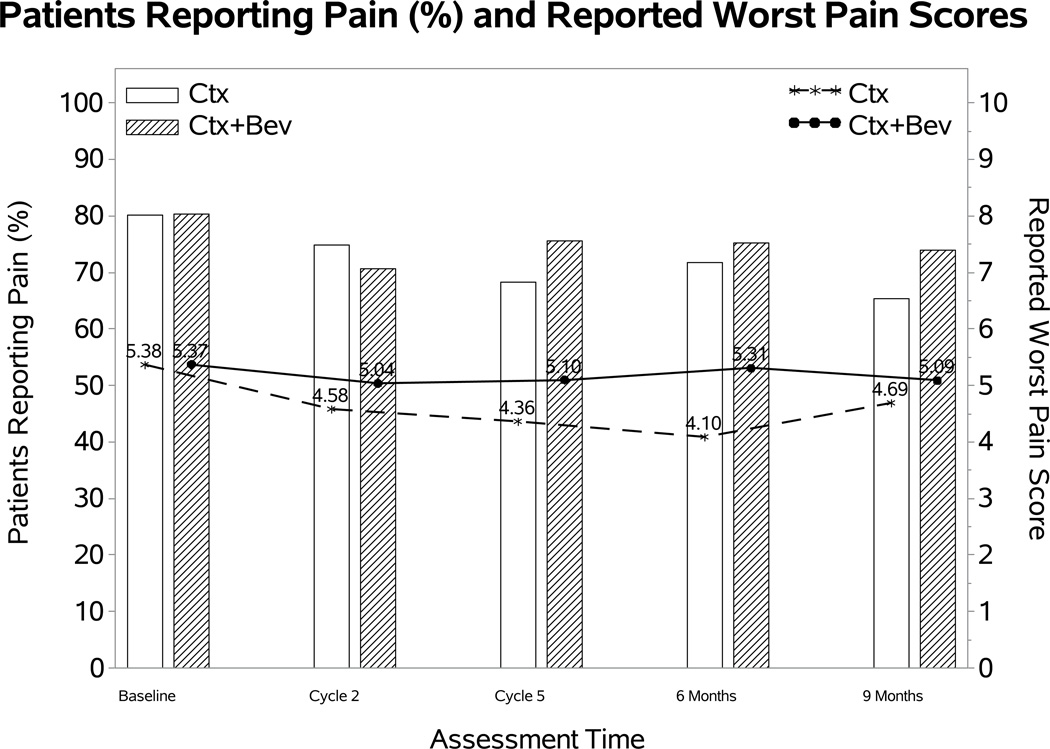
After adjusting for baseline BPI scores and the assignment of topotecan, there was no evidence that treatment differences varied significantly over time in either the odds of reporting pain or the severity of the reported worse pain score. The fitted MEMD model estimates suggested that the patients receiving chemotherapy with or without bevacizumab had similar odds of having pain (OR 0.96; 95% CI: 0.39, 1.52; p=0.78) and reported similar severity if they had pain (diff: 0.5; 95% CI: −0.14, 1.14; p=0.12) (Figure 3A).
Figure 3. Patient-reported outcomes (PROs) using the Brief Pain Inventory (BPI) single item.
During the period of assessment, there were no significant differences in the reporting of pain or in the severity of pain for the following treatment groups: chemotherapy alone (both backbones) vs chemotherapy (both backbones) with bevacizumab (Panel 3A); cisplatin and paclitaxel with and without bevacizumab (Panel 3B); topotecan and paclitaxel with and without bevacizumab (Panel 3C). Ctx: chemotherapy; Bev: bevacizumab; CP: cisplatin and paclitaxel; TP: topotecan and paclitaxel.
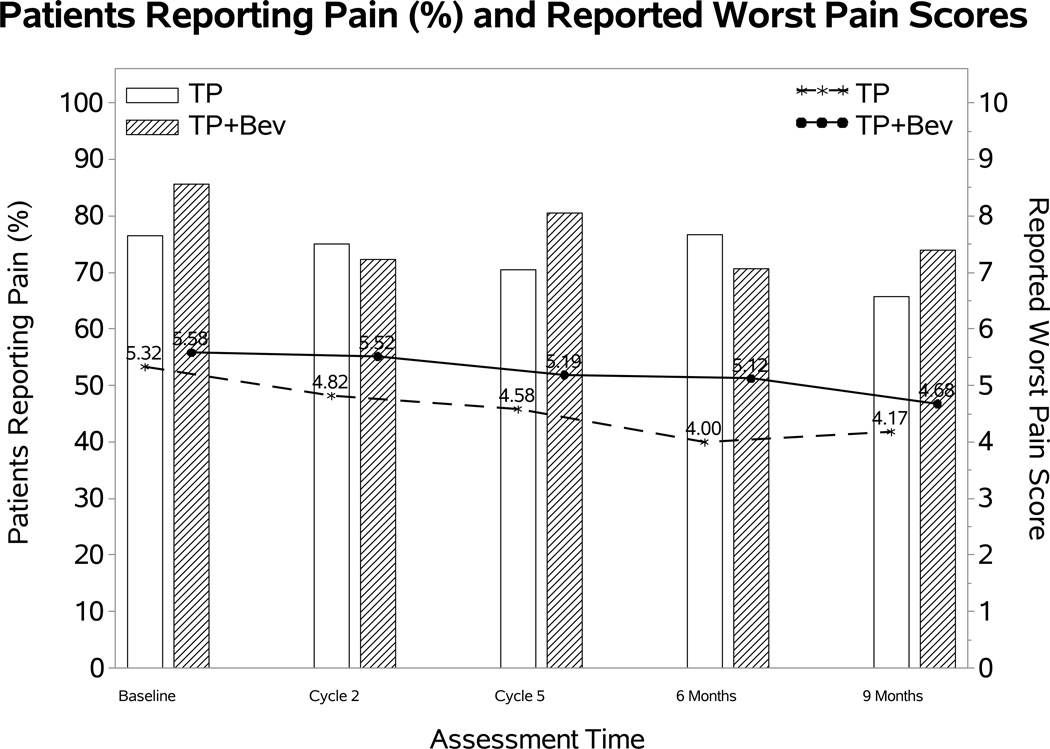
Figure3B and 3C depict the effect of bevacizumab on observed BPI worst pain score in each of chemotherapy backbones. After adjusting for baseline score, bevacizumab was not associated with the odds of having pain in either chemotherapy backbone: cisplatin-paclitaxel with and without bevacizumab (OR 1.43; 95% CI 0.03, 2.82; p=0.54); topotecan-paclitaxel with and without bevacizumab (OR 0.65; 95% CI 0.15, 1.15; p=0.16). When experiencing pain, bevacizumab was not associated with the reported BPI score in either the cisplatin-paclitaxel backbone (diff: 0.77; 95% CI: −0.13, 1.68; p=0.09) or topotecan-paclitaxel backbone (diff: 0.32; 95% CI: −0.61, 1.25; p=0.51).
Effect of replacing cisplatin with topotecan on PROs
Following adjustment for baseline score, patient age, performance status, and assignment of bevacizumab, there was no significant difference in the FACT-Cx TOI between treatment with either chemotherapy backbone (diff: 0.5; 98.75% CI: −2.4, 3.4; p=0.66) (Figure 4A). Compared to cisplatin-paclitaxel backbone, the topotecan-paclitaxel backbone was not associated with significant differences in the odds (OR: 1.05; 98.75% CI: 0.32, 1.77; p=0.87) and the severity of reported neurotoxic symptoms (diff: 0.43; 98.75% CI: −1.84, 0.99; p=0.45) (Figure 4B). No significant differences were observed in the odds of complaining of pain in either backbone (OR: 1.3; 95% CI: 0.5, 2.0; p=0.48). However, among those having pain the treatment difference in the reported BPI pain score was not constant over the assessment time (p=0.02). Compared to patients on cisplatin backbone, the patients on topotecan backbone reported 0.71 points higher (95% CI: 0.02~1.39; p=0.04) at cycle 2 but 0.86 points lower (95% CI: −1.95~0.22; p=0.12) 9 months post-cycle 1 (Figure 4C).
Figure 4. Effect of substitution of topotecan for cisplatin on the FACT-Cx TOI, FACT/GOG-Ntx, and BPI.
During the period of assessment, the mean FACT-Cx TOI (Panel 4A) and FACT/GOG-Ntx (Panel 4B) scores did not significantly differ between the cisplatin-paclitaxel and topotecan-paclitaxel chemotherapy backbones. Among patients reporting pain the treatment difference in the reported BPI pain score was not constant over the assessment time (p=0.02) (Panel 4C).
Association between baseline QOL score and survival
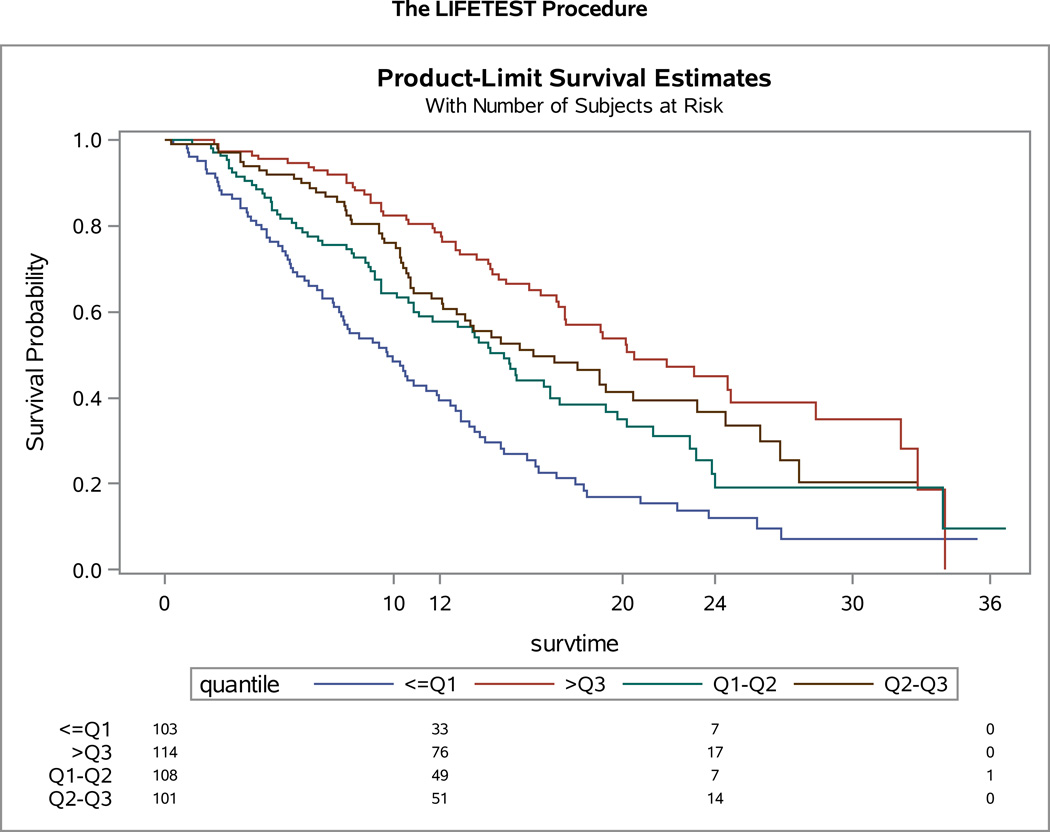
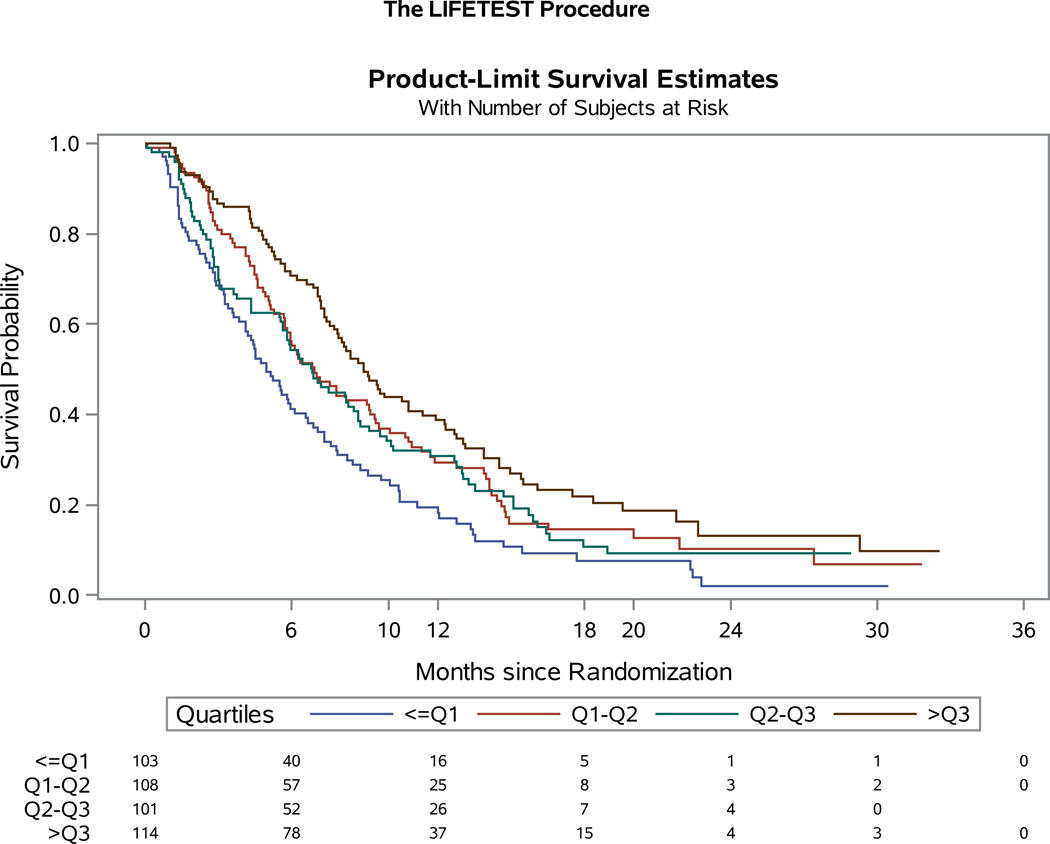
The association between baseline FACT-Cx TOI score and survival was explored using Cox proportional hazards model stratified by patients’ performance status, disease status, previous platinum radiation therapy, and treatment assignment. For the entire study population, as a continuous variable, the baseline FACT-Cx TOI was significantly associated with survival. For an increment of every 10 units of FACT-Cx TOI, the hazard ratio for death was 0.8 (95% CI: 0.74, 0.87; p<0.001), and for progression was 0.88 (95% CI: 0.83, 0.95; p<0.001). The baseline FACT-Cx TOI score was further classified into four groups with the quartiles in which the first quartile, median, and the 3rd quartile were 63, 76, and, 89, respectively. When compared to the TOI scores below the first quartile, the hazard ratios for death were 0.7 (95% CI: 0.48, 1.01, p=0.05) for TOI below median, 0.5 (95% CI: 0.35, 0.74, p<0.001) for TOI below 3rd quartile, and 0.38 (95% CI: 0.25, 0.56, p<0.001) for TOI above 3rd quartile respectively. The hazard ratios for disease progression in relative to TOI below 1st quartile were 0.69 (95% CI: 0.50, 0.97, p=0.03) for TOI below median, 0.75 (95% CI: 0.54, 1.05, p<0.10) for TOI below 3rd quartile, and 0.52 (95% CI: 0.37, 0.73, p<0.001) for TOI above 3rd quartile respectively. (Figures 5A–B).
Figure 5. Quartile Analysis of patient-reported outcomes (PROs) using the Functional Assessment of Cancer Therapy – Cervix (FACT-Cx) Trial Outcome Index (TOI).
For the entire study population, the baseline FACT-Cx TOI was significantly associated with overall survival (OS) (Panel 5A) and progression-free survival (PFS) (Panel 5B).
DISCUSSION
This phase III study of the integration of anti-angiogenesis therapy for advanced cervical cancer, demonstrated that significant improvements in OS, PFS, and RR conferred by the addition of bevacizumab to chemotherapy did not come at the cost of a concomitant deterioration of HRQoL as defined by FACT TOI. Specifically, the fitted mixed model estimates for the FACT-Cx TOI scores indicated that the addition of bevacizumab did not adversely affect QoL. The effect of substitution of cisplatin with topotecan did not alter QoL, nor abrogate neurotoxic symptoms. In this population, bevacizumab extends life without a significant negative impact on patients’ QoL. Both cisplatin, paclitaxel, and bevacizumab and topotecan, paclitaxel, and bevacizumab have been granted regulatory approval by the US FDA for first line therapy for metastatic and recurrent cervical carcioma. This report confirms the tolerability of these new combinations.
This study demonstrated that baseline FACT-Cx as a continuous variable is associated with not only OS, but also PFS. Clearly baseline HRQoL is strongly predictive of survival in this population. (19, 20) Importantly, to further explore the impact of HRQoL on survival our quartiles analysis of the FACT-Cx score indicate that the estimated HR of death for patients in the highest HRQoL quartile was 62% lower than for patients in the lowest quartile (HR 0.38; 95% CI 0.25, 0.56; p<0.001) (Figure 5A). Baseline predictors of OS and PFS have clear clinical relevance, since they permit future studies to potentially pursue stratification based on baseline scores in order to further determine differential treatment responses, and importantly also provide an upfront opportunity to monitor and remediate symptoms which may be contributing to the QoL decline.
A 2.1 points lower FACT-Cx TOI measured in the cisplatin-paclitaxel-bevacizumab arm after adjustment for baseline score and patients' characteristics could be considered to be approaching a clinically significant difference of 5.8 points for the FACT-Cx TOI and can be interpreted to encourage the development of combination bevacizumab with the less toxic and equally effective carboplatin-paclitaxel combination. However, carboplatin related hematologic toxicity can be considerable when patients have previously been treated with chemoradiotherapy, and this should not yet be considered a standard.
There are a number of possible reasons for the observed trend that patients receiving bevacizumab were less likely to report neurotoxic symptoms (eg. secondary gain from increased tumor shrinkage, better health, or more activity). (13, 19, 21) Conversely, the myalgias of bevacizumab, as perhaps documented in the BPI score, may function as a counterirritant ‘distraction’ from symptoms of neurotoxicity. (12) This is consistent with the gate theory of pain proposed by Melzack and Wall in 1965. (22) Gate theory suggests that physical pain is not a direct result of activation of pain receptor neurons, but rather its perception is modulated by interaction of a network of neurons, and complex central processing. In addition to inducing angiogenesis and lymphangiogenesis, VEGF is also directly involved in neuroplasticity, nerve bundle maturation, and also exerts protective effects on neurons. VEGF protects dorsal root ganglion neurons against paclitaxel-induced neurotoxicity. (23) While factors such as disease site, histology, and intrinsic and acquired drug resistance mechanisms may ultimately determine which patients respond to anti-VEGF therapy, differential expression of the VEGF transcript must also be considered. (24, 25) In GOG 240, patients who responded and ultimately benefited from treatment on the bevacizumab-containing regimens may have comprised an enriched population expressing high levels of the target. Such patients would also be expected to experience and report less severe neurotoxicity. However, predictive factors have not yet been defined. It is worth noting that lack of demonstrated difference in neurotoxicity between cisplatin-paclitaxel and topotecan-paclitaxel suggest that we have not yet identified the appropriate tools to evaluate neurotoxicity with sufficient sensitively.
In successive phase III randomized trials in the advanced cervical cancer population conducted by the GOG, eligibility criteria have become stricter. (2−4) This is even more true of GOG 240 with the inclusion of an antiangiogenic agent, and undoubtedly contributed to a migration in population demographics. The study required better renal function, and excluded patients with GOG performance status 2. Given that this population for the most part is underserved with limited resources, it was not uncommon in previous studies to have very sick patients on trial. Median survival in the preceding phase III protocol (GOG 204) was 12 months for the cisplatin-paclitaxel doublet, (3) closer to the real world advanced cervix population, which is likely to experience a median survival time of seven months or less, closer to the GOG phase III experiences from the 1990s. (2, 4) Therefore, the 17 months median survival reported in GOG 240 associated with the arms in which anti-angiogenesis therapy was administered with chemotherapy represents a new benchmark that is greater than double the median survival experienced off protocol and one that reflects a degree of selection bias. (6) Furthermore, investigators were required to medically optimize their patients in order to participate on this trial, and clinicians have become pro-active with nephrostomies and ureteral stents, correcting anemia, electrolyte abnormalities, and aggressively improving nutritional status. While it cannot be quantified, the contribution of medical optimization to maintenance of QoL in GOG 240 is implicit, and the generalizability of study data requires that improvements in supportive care be taken into account when considering the historical context.
Fatigue is the dominant symptom in cancer patients. (26) Achieving antiangiogenic blockade using tyrosine kinase inhibitors is associated with more fatigue than is seen with bevacizumab. Given the favorable side-effect and QoL profile of bevacizumab it appears to be one of the better novel biologics to use to achieve clinical benefit. (27) This prompts the question as to whether fewer cycles of chemotherapy with maintenance bevacizumab is a good future strategy, and ongoing analyses are examining whether it is possible to predict which patients are at risk of fistula or gastrointestinal perforation, and whether these can be avoided.
This study has the anticipated challenge of any randomized trial in that lower completion rates in sicker patients produce non-random bias to the evaluation of the impact of therapy. Nevertheless, the reported improvement in OS attributed to bevacizumab did not come at the cost of a significant deterioration in QoL. This observation represents the crux of why the OS gain of 3.7 months is not only statistically significant in terms of the study design, but also clinically important. While patients living 3.7 months longer may be another small incremental improvement, if this survival gain is considered in context of a sustained QoL the therapeutic impact becomes clinically meaningful. This population has very limited options, and unlike many other solid tumors, the advanced cervical cancer population is not one in which multiple lines of chemotherapy and durable remissions over years are possible. By optimizing medical performance, the administration of chemotherapy with bevacizumab in this patient population has, for the first time, created a potential window of opportunity lasting nearly four months without QoL deterioration. Four months is an important, but short time. However, it may provide a window of opportunity for patients deriving benefit from anti-angiogenic therapy with other classes of anti-angiogenic drugs, targeted, or immunotherapy, in a disease that is no longer so rapidly fatal. Through the NCI and the CTEP mechanism, the NRG is currently studying other novel drugs for what has been anticipated will become a new population of advanced cervical cancer patients, namely, those who have progressed following treatment with anti-VEGF therapy.
With National Comprehensive Cancer Network (NCCN) listing of the cisplatin-paclitaxel-bevacizumab-containing triplet regimen in July 2013 (one month following presentation of the data at ASCO 2013), despite the absence of a label, there was an approximately 45% uptake of bevacizumab nationally for this condition over the preceding eight months in the United States [Genentech Roche, Personal Communication through market research]. Upon publication of the primary manuscript in February 2014, the Cancer Drug Fund approved bevacizumab for advanced cervical cancer in England, (6) and, four months after filing with the U.S. FDA, bevacizumab was granted a label as indicated for the treatment of persistent, recurrent, or metastatic carcinoma of the cervix on August 14, 2014. As drug uptake will continue to increase, being proactive with physician and patient education is necessary to ensure that GOG 240 eligibility criteria are adhered to in order to mitigate severe treatment-related toxicity and sustain HRQoL in order to allow patients to be treated with other novel agents when they ultimately progress.
Cervical cancer patients frequently experience quality of life disruptions from physical symptoms including pain, bowel, bladder, and sexual dysfunction and lymphedema. (21) Additional key psychological and physical health factors have been identified, which contribute significantly to poor quality of life subsequent to definitive cancer treatment. (28) The majority of these factors are amenable to supportive care interventions and could be evaluated at the time of primary treatment.
Research in context
Systematic review
No formal systematic review was performed in planning for this trial, which built on the preceding studies. Awareness of planned international and cooperative trials though the Gynecologic Cancer Intergroup (GCIG) factored into the design which was ratified by the Cervix Committee of Gynecologic Oncology Group (GOG), Protocol Development Committee (GOG), Cervical Cancer Task Force, Gynecologic Cancer Steering Committee, GEICO, Genentech/Roche, Central IRB, and CTEP between 2006 and 2008 prior to study activation April 9, 2009.
Interpretation
The study established a new standard of care with a substantial, statistically significant, and clinically beneficial improvement in overall survival, progression free survival and response rate. This publication adds to the literature data on the impact of bevacizumab on health-related quality of life (HRQoL) of women with advanced cervical cancer, confirming that it can be administered without a significant deterioration in HRQoL. There has already been a significant uptake of this as the preferred regimen, with endorsements in guidelines (National Comprehensive Cancer Network (NCCN)) and addition of this indication to the label by the FDA (August 14, 2014). Regulatory approval has also been granted in England, and some Latin American countries (eg., Ecuador).
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest statement
Dr. Richard T. Penson served on Advisory Boards and received research funding from Genentech Inc. Dr. Bradley Monk discloses his institution has received grants/contracts from Genentech. Dr. Monk has received honorarium from speakers bureaus from Roche/Genentech and has been a consultant for Roche/Genentech.
Dr. Krishnansu Tewari served as a consultant for Genentech/Roche (travel/accommodations) to advisory board.
All other co-authors have no conflicts of interest to report.
| RTP | Study design; data analysis; data interpretation; manuscript writing |
| HQH | Data analysis and data interpretation |
| LW | Study design: feedback on PRO’s; missing PRO data monitoring; interpretation; manuscript writing |
| BJM | Conception and design; collection and assembly of data, data analysis and interpretation; manuscript writing; final approval of manuscript; provision of study material or patients |
| SS | Assisted in improving compliance of completing QoL assessments at all time points |
| HJL | Study design |
| LMR | Data interpretation/writing and study design (GOG Cervix Committee) |
| LML | Final approval of manuscript |
| AO | Final approval of manuscript |
| TJAR | Data collection |
| MML | Final approval of manuscript |
| MM | Final approval of manuscript |
| HM | Study design; data collection; writing |
| KST | Final approval of manuscript |
REFERENCES
- 1.Tewari K, Monk B. Invasive Cervical Cancer. In: DiSaia P, Creasman W, editors. Clinical Gynecologic Oncology. 8th ed. Mosby: 2012. [Google Scholar]
- 2.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005;23(21):4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(28):4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004;22(15):3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 5.Moreira IS, Fernandes PA, Ramos MJ. Vascular endothelial growth factor (VEGF) inhibition--a critical review. Anti-cancer agents in medicinal chemistry. 2007;7(2):223–245. doi: 10.2174/187152007780058687. [DOI] [PubMed] [Google Scholar]
- 6.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella D, Huang HQ, Monk BJ, Wenzel L, Benda J, McMeekin DS, et al. Health-related quality of life outcomes associated with four cisplatin-based doublet chemotherapy regimens for stage IVB recurrent or persistent cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;119(3):531–537. doi: 10.1016/j.ygyno.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long HJ, 3rd, Monk BJ, Huang HQ, Grendys EC, Jr, McMeekin DS, Sorosky J, et al. Clinical results and quality of life analysis for the MVAC combination (methotrexate, vinblastine, doxorubicin, and cisplatin) in carcinoma of the uterine cervix: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;100(3):537–543. doi: 10.1016/j.ygyno.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 9.McQuellon RP, Thaler HT, Cella D, Moore DH. Quality of life (QOL) outcomes from a randomized trial of cisplatin versus cisplatin plus paclitaxel in advanced cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101(2):296–304. doi: 10.1016/j.ygyno.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Monk BJ, Huang HQ, Cella D, Long HJ., 3rd Quality of life outcomes from a randomized phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005;23(21):4617–4625. doi: 10.1200/JCO.2005.10.522. [DOI] [PubMed] [Google Scholar]
- 11.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 12.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17(2):387–393. doi: 10.1111/j.1525-1438.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 13.Cleeland C, editor. Pain assessment in cancer. Boston: CRC Press; 1991. [Google Scholar]
- 14.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Evaluation & the health professions. 2005;28(2):172–191. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 15.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116(1):44–49. doi: 10.1016/j.ygyno.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berk K, Lachenbruch P. Repeated measures with zero. Stat Methods in Medical Research. 2003;11(4):303–316. doi: 10.1191/0962280202sm293ra. [DOI] [PubMed] [Google Scholar]
- 17.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. [PubMed] [Google Scholar]
- 18.Yost K, Eton D, editors. 2005. Combining distribution- and anchor-based approaches to determine minimally important difference, the FACIT experience. [DOI] [PubMed] [Google Scholar]
- 19.Bottomley A, Jones D, Claassens L. Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. Eur J Cancer. 2009;45(3):347–353. doi: 10.1016/j.ejca.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Slevin ML, Stubbs L, Plant HJ, Wilson P, Gregory WM, Armes PJ, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ. 1990;300(6737):1458–1460. doi: 10.1136/bmj.300.6737.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chase DM, Huang HQ, Wenzel L, Cella D, McQuellon R, Long HJ, et al. Quality of life and survival in advanced cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125(2):315–319. doi: 10.1016/j.ygyno.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 23.Verheyen A, Peeraer E, Lambrechts D, Poesen K, Carmeliet P, Shibuya M, et al. Therapeutic potential of VEGF and VEGF-derived peptide in peripheral neuropathies. Neuroscience. 2013;244:77–89. doi: 10.1016/j.neuroscience.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 24.Efficace F, Bottomley A, Smit EF, Lianes P, Legrand C, Debruyne C, et al. Is a patient's self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2006;17(11):1698–1704. doi: 10.1093/annonc/mdl183. [DOI] [PubMed] [Google Scholar]
- 25.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 26.Butt Z, Rosenbloom SK, Abernethy AP, Beaumont JL, Paul D, Hampton D, et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6(5):448–455. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monk BJ, Willmott LJ, Sumner DA. Anti-angiogenesis agents in metastatic or recurrent cervical cancer. Gynecol Oncol. 2009;116(2):181–186. doi: 10.1016/j.ygyno.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Osann K, Hsieh S, Nelson EL, Monk BJ, Chase D, Cella D, et al. Factors associated with poor quality of life among cervical cancer survivors: Implications for clinical care and clinical trials. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.