Abstract
Background
Dysregulation of HPA axis has been widely described in subjects with bipolar disorder (BD), including changes in cortisol levels during mood episodes and euthymia. However, most of the studies were done with medicated BD patients with variable length of illness, which was shown to interfere on peripheral cortisol levels. Therefore, the present study aims to evaluate plasma cortisol levels in drug-naïve BD subjects during the first manic episode, as well as investigate the relationship between plasma cortisol levels and manic symptomatology.
Methods
Twenty-six drug-naïve patients were enrolled meeting criteria for a first manic episode in bipolar I disorder. Severity of mania was assessed using the Young Mania Rating Scale (YMRS). The control group included 27 healthy subjects matched by age and gender. Cortisol was quantified using a direct radioimmunoassay.
Results
Plasma cortisol levels were decreased during first manic episode compared to healthy controls. Higher cortisol levels were positively associated with the presence of irritability (dysphoria), while elated mania showed lower cortisol levels compared to controls.
Limitation
Data including larger samples are lacking.
Conclusion
Higher cortisol in dysphoric mania compared to predominantly elated/euphoric mania may indicate a clinical and neurobiological polymorphic phenomenon, potentially involving a higher biological sensitivity to stress in the presence of irritable mood. The present findings highlight the importance to add a dimensional approach to the traditional categorical diagnosis for future neurobiological studies in BD.
Keywords: Cortisol, Bipolar disorder, Mania, Depression, Stress, Dimensional
1. Introduction
The relationship between stress and mood disorders is well documented (Hammen, 2005; Kessler, 1997). Changes in the Hypothalamic-Pituitary-Adrenal (HPA) axis represent one a potential link between stress and the risk for mood disorders (McEwen, 2008). Altered HPA axis activity have mostly been demonstrated in remitted and non-remitted subjects with bipolar disorder (BD) (Vieta et al., 1999; Watson et al., 2004). Most of these studies were done under pharmacological treatment and report an association between depressive episodes and higher cortisol levels or non-suppression in the dexamethasone suppression test (DST) (Rush et al., 1997; Rybakowski and Twardowska, 1999; Schmider et al., 1995). Abnormal DST results are more common during depressive episodes in the course of BD than major depressive disorder (Rush et al., 1997; Rybakowski and Twardowska, 1999; Schmider et al., 1995). However, it is unclear whether these abnormalities are related to manic symptoms (Bradley and Dinan, 2010; Walker et al., 2008). In addition, symptoms such as anxiety, insomnia and agitation are highly correlated with cortisol levels (Rybakowski and Twardowska, 1999). However, no study evaluated cortisol levels in first manic episode.
HPA axis function is compromised in BD according to the following data: HPA axis hyperactivity, higher cortisol levels, a decrease in glucocorticoid receptors and lower negative feedback (Daban et al., 2005; Watson et al., 2004). Increased cortisol levels in BD episodes were found during euthymia (Deshauer et al., 2003), depression, mania (Schmider et al., 1995; Linkowski et al., 1994), and mixed states (Cassidy et al., 1998). Abnormalities in the HPA system have been described in young offspring of subjects with BD (Ellenbogen et al., 2004), suggesting these changes precede the onset of mood episodes. In this context, some authors have hypothesized that HPA system abnormalities could be an endophenotype of mood disorders (Mello et al., 2007), while others speculate that it would be trait marker in BD and thus an indicative of a core pathophysiological process (Daban et al., 2005). In contrast, subjects with post-traumatic stress disorder (PTSD), which is highly comorbid with BD, show decreased cortisol levels, potentially associated with hypothalamic-pituitary-adrenal (HPA) axis underactivity, and/or changes in glucocorticoid receptor sensitivity (Yehuda and Seckl, in press).
Reports of HPA axis function in mania are inconsistent. Some studies found no changes on cortisol suppression on the DST (Schlesser et al. 1980; Evans & Nemeroff 1983), while others described non-suppression rates comparable to those found in depression (Godwin et al., 1984). Moreover, it has been suggested that patients with mixed episode exhibit DST non-suppression more likely than those with pure mania. Patients with pure mania exhibit normal cortisol suppression while individuals in a mixed episode patients show cortisol non-suppression (Evans & Nemeroff 1983). Other studies confirmed the abnormal cortisol suppression in BD, especially in mixed-episode patients (Krishnan et al., 1983; Swann et al., 1992).
Only one study evaluated medication-free subjects with BD (Swann et al., 1992). Medications usually used to treat mood episodes are known to alter glucocorticoids levels (Eroğlu et al., 1979; Gattaz et al., 1985; Meltzer et al., 1984; Pepin et al., 1989; Watson et al., 2007; Zobel et al., 2001). Treatment-free BD subjects in mania (not first episode) had elevated cerebrospinal fluid (CSF) and urinary free cortisol excretion compared with healthy subjects. Importantly, mixed mania had significantly higher morning plasma cortisol, post-dexamethasone plasma cortisol than pure mania (Swann et al., 1992). However, to the best of our knowledge, no study has evaluated HPA axis activity in drug-naïve BD patients during the first manic episode.
This study aimed to investigate plasma cortisol levels during the first manic episode in drug-naive BD I patients compared to matched controls, also evaluating the potential association with symptoms severity.
2. Materials and methods
Twenty-six drug-naïve patients meeting criteria for a first manic episode were enrolled in this study. Diagnosis was established by two psychiatrist using the Structured Clinical Interview for DSM-IV (SCID) (DSM-IV, 2000). Subjects with other Axis I disorders and/or severe or unstable medical illnesses were excluded from the study. All subjects were medically healthy, as determined by physical and neurological examination and laboratory tests. Severity of mania was assessed using the Young Mania Rating Scale (YMRS). The control group included twenty-seven age and gender matched healthy individuals (±3 years) evaluated with the SCID. The Hospital Espirita Ethics Committee, Porto Alegre, Brazil, approved the study. Written informed consent was obtained from all the participants in this study.
Blood samples were obtained using vacutainer tubes and kept on ice, collected at 4:00–6:00 pm. Samples were centrifuged at 3000×g for 15 min and stored at −80 °C until assay. Samples were assayed for cortisol by a sensitive radioimmunoassay using commercial kits (Human Cortisol RIA Kit from Diagnostic Systems Laboratories) Inc.
2.1. Statistical analysis
First, t-tests were used to compare the cortisol values between patients and controls (after confirming normal distribution using the Kolmogorov-Smirnov test). Cortisol levels are expressed as ug/dL and we used mead and SD to express results for the groups. YMRS 11 items (elation/euphoria, motor activity, sexual interest, sleep, irritability, speech, language/thought disorder, thought content, disruptive/aggressive behaviour, appearance and insight) and cortisol levels were tested for Pearson correlation. Group comparisons of demographic variables used ANOVAs for continuous measures and Fisher’s Exact Test for categorical variables. All analyses were conducted using Stata statistical software, version 10.0 (Statacorp, College Station, TX, USA). Results were considered significant at p<0.05, two-tailed.
3. Results
Patients and controls did not differ in age (25±3.82 vs 26.11±4.62, p=0.34). The distribution of cortisol levels for patients and controls is shown in Fig. 1.
Fig. 1.
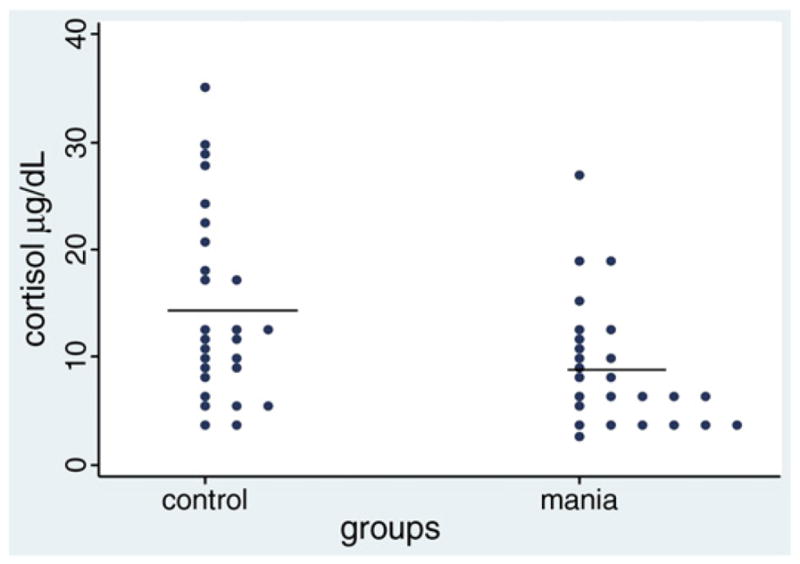
Plasma cortisol levels (μg/dl) in first episode drug-naïve mania versus healthy controls.
Plasma cortisol levels were lower in the first episode of mania patients (9.01ug/dL±1.15) compared to healthy controls (14.45 μg/dL±1.68) (p=0.01, df=51).
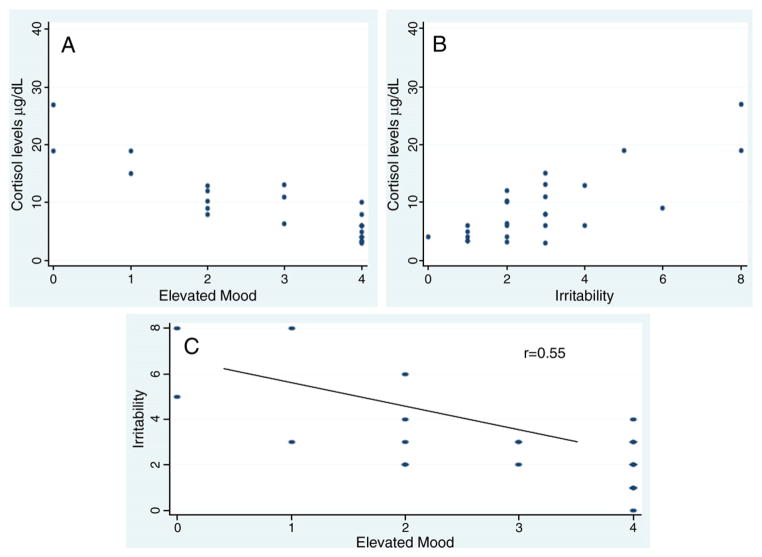
Regarding the association between YMRS items and cortisol levels we observed a positive correlation between plasma cortisol levels and irritability (p<0.001, r=0.6). Conversely, cortisol levels negatively correlated with motor activity (p<0.001, r=0.5) and elation/euphoria (p<0.001, r=0.78). There was also a negative correlation between irritability and elation/euphoria (p<0.001, r=0.55) (Fig. 2).
Fig. 2.
A) Association between cortisol (μg/dl) and elevated mood (YMRS item 1) in the first episode drug-naïve mania; B) Association between cortisol (μg/dl) and irritability (YMRS item 5) in the first episode drug-naïve mania; and C) Association between elevated mood and irritability in the first episode drug-naïve mania.
No association between cortisol levels with total YMRS (p=0.09), gender (p=0.5) or age (p=0.11) was observed in the patients group.
4. Discussion
To the best of our knowledge, this is the first study to evaluate cortisol levels in drug naïve BD subjects during the first manic episode. This study found a decrease in cortisol levels in the first manic episode compared to healthy controls. Also, lower cortisol levels in euphoric mania were observed while higher levels were associated with the presence of irritability. Importantly, an inverse association between euphoria and irritability was observed, thus reinforcing the potential dual HPA regulation in elated manic states and dysphoric mania.
These findings may reflect early neurochemical changes of mania. It was previously proposed that when mania becomes more severe and dysphoric, cortisol levels tend to increase to above the observed in euthymia (Joyce et al., 1987). In our study, lower cortisol levels were observed compared to controls in classic mania. Similarly, lower cortisol levels have been described in subjects with post-traumatic stress disorder (PTSD); this biological finding has been associated with partial primary adrenal insufficiency, hypothalamic-pituitary-adrenal (HPA) axis underactivity, increased negative feedback sensitivity and/or changes in glucocorticoid metabolism (Yehuda and Seckl, 2011). These suggested biological effects may also underlie the findings described in the present investigation.
It is important to mention that all BD subjects here evaluated were drug-naïve. Indeed, previous studies on cortisol levels in mood disorders research only investigated medicated subjects, which may represent a potential bias. For instance, anti-psychotic drugs were found to increase CSF cortisol levels (Gattaz et al., 1985). Also, lithium and other mood stabilizers showed to alter glucocorticoids response and levels (Bschor et al., 2003; Watson et al., 2007; Zobel et al., 2001). Also, the length of illness may impact on HPA activity. Thus, the present finding may represent a true biological effect.
Similar to our findings, few studies have shown differences between mixed/disphoric manic and pure manic states (Swann et al., 1994; Cassidy et al., 1998). Swann et al. (1992) reported in a sample of unmedicated classic and mixed mania, DST suppression in both groups, but higher in mixed state than pure mania (Swann et al., 1992). Furthermore, mixed episode, associated with increased irritability, had significantly higher plasma and CSF cortisol levels, as well as elevated post-dexamethasone plasma cortisol.
In our sample, elation/euphoria was negatively correlated with cortisol levels, while irritability had a positive association. That is in agreement with early studies suggesting that dysphoric mania may be a distinct affective state (McElroy et al., 1992). Dysphoric mania could be a form of atypical mania, a stage-related, a severe form, or even a transitional state between mania and depression (Himmelhoch et al., 1976; Carlson and Goodwin, 1973; Frederick K Goodwin and Jamison, 2007). Recently, the American Psychiatric Association announced that DSM-5 will include in diagnose of mania a mixed specifier, with an item defined as “proeminent dysphoria” (http://www.dsm5.org/ ).
Strengths of this research include the sample only including drug-naïve subjects during the first manic episode, thus potentially limiting biases such as time of illness and pharmacological status. Also, the exclusion of subjects with clinical and psychiatric comorbidities allowed us to better identify potential state biomarkers in mania. Limitations include the lack of cortisol response parameters and suppression tests.
In sum, the first episode of mania showed an associated with lower cortisol levels compared to healthy controls, with higher cortisol levels associated with proeminent irritability compared to elated mania. We propose that dysphoric mania may have a higher biological sensitivity to stress.
Overall, the present findings suggest the need to add a dimensional diagnosis to the traditional categorical diagnosis in neurobiological studies in BD when evaluating HPA activity and other peripheral biomarkers. Further studies are required to confirm these findings.
Acknowledgments
Role of the funding source
This study was funded by Sao Paulo Research Foundation, Fapesp (2009/ 14891-9), Brazil.
We thank Fapesp (2009/14891-9) and Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
Footnotes
Conflict of Interest
Neither the manuscript nor its data have been previously published or are currently under consideration for publication. None of the co-authors in this study have a possible conflict of interest, financial or otherwise.
References
- Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. Journal of psychopharmacology (Oxford, England) 2010;24 (4 Suppl):91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- Bschor T, et al. Lithium augmentation in treatment-resistant depression: clinical evidence, serotonergic and endocrine mechanisms. Pharmacopsychiatry. 2003;36 (Suppl 3):S230–S234. doi: 10.1055/s-2003-45135. [DOI] [PubMed] [Google Scholar]
- Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Archives of General Psychiatry. 1973;28 (2):221–228. doi: 10.1001/archpsyc.1973.01750320053009. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Murry E, Forest K, Carroll BJ. Signs and symptoms of mania in pure and mixed episodes. Journal of Affective Disorders. 1998;50 (2–3):187–201. doi: 10.1016/s0165-0327(98)00016-0. [DOI] [PubMed] [Google Scholar]
- Daban C, et al. Hypothalamic-pituitary-adrenal axis and bipolar disorder. The Psychiatric Clinics of North America. 2005;28 (2):469–480. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Deshauer D, et al. The cortisol awakening response in bipolar illness: a pilot study. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 2003;48 (7):462–466. doi: 10.1177/070674370304800706. [DOI] [PubMed] [Google Scholar]
- DSM-IVPATFO. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker CD. High levels of cortisol among adolescent offspring of parents with bipolar disorder: a pilot study. Psychoneuroendocrinology. 2004;29 (1):99–106. doi: 10.1016/s0306-4530(02)00135-x. [DOI] [PubMed] [Google Scholar]
- Eroğlu L, et al. A study of the relationship between serum lithium and plasma cortisol levels in manic depressive patients. British Journal of Clinical Pharmacology. 1979;8 (1):89–90. doi: 10.1111/j.1365-2125.1979.tb05917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Nemeroff CB. The dexamethasone suppression test in mixed bipolar disorder. American Journal of Psychiatry. 1983;140 (5):615–617. doi: 10.1176/ajp.140.5.615. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Hannak D, Beckmann H. Increased CSF cortisol levels after neuroleptic treatment in schizophrenia. Psychoneuroendocrinology. 1985;10 (3):351–354. doi: 10.1016/0306-4530(85)90012-5. [DOI] [PubMed] [Google Scholar]
- Godwin CD. The dexamethasone suppression test in acute mania. Journal of Affective Disorders. 1984;7 (3–4):281–286. doi: 10.1016/0165-0327(84)90049-1. [DOI] [PubMed] [Google Scholar]
- Goodwin Frederick K, Jamison KR. Manic-depressive illness. Oxford University Press; USA: 2007. [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Himmelhoch JM, et al. Incidence and signficiance of mixed affective states in a bipolar population. Archives of General Psychiatry. 1976;33 (9):1062–1066. doi: 10.1001/archpsyc.1976.01770090052004. [DOI] [PubMed] [Google Scholar]
- Joyce PR, Donald RA, Elder PA. Individual differences in plasma cortisol changes during mania and depression. Journal of Affective Disorders. 1987;12 (1):1–5. doi: 10.1016/0165-0327(87)90054-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Krishnan RR, Maltbie AA, Davidson JR. Abnormal cortisol suppression in bipolar patients with simultaneous manic and depressive symptoms. The American Journal of Psychiatry. 1983;140 (2):203–205. doi: 10.1176/ajp.140.2.203. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L’Hermite-Baleriaux M, et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Archives of General Psychiatry. 1994;51 (8):616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- McElroy SL, et al. Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. The American Journal of Psychiatry. 1992;149 (12):1633–1644. doi: 10.1176/ajp.149.12.1633. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583 (2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello AF, et al. Depression and stress: is there an endophenotype? Revista Brasileira de Psiquiatria (São Paulo, Brazil : 1999) 2007;29 (Suppl 1):S13–8. doi: 10.1590/s1516-44462007000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, et al. Effect of 5-hydroxytryptophan on serum cortisol levels in major affective disorders. II. Relation to suicide, psychosis, and depressive symptoms. Archives of General Psychiatry. 1984;41 (4):379–387. doi: 10.1001/archpsyc.1984.01790150069010. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Beaulieu S, Barden N. Antidepressants regulate glucocorticoid receptor messenger RNA concentrations in primary neuronal cultures. Brain Research Molecular Brain Research. 1989;6 (1):77–83. doi: 10.1016/0169-328x(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Rush AJ, et al. Dexamethasone response, thyrotropin-releasing hormone stimulation, rapid eye movement latency, and subtypes of depression. Biological Psychiatry. 1997;41 (9):915–928. doi: 10.1016/S0006-3223(97)00148-0. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. Journal of Psychiatric Research. 1999;33 (5):363–370. doi: 10.1016/s0022-3956(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Schlesser MA, Winokur G, Sherman BM. Hypothalamic-pituitary-adrenal axis activity in depressive illness. Its relationship to classification. Archives of General Psychiatry. 1980;37 (7):737–743. doi: 10.1001/archpsyc.1980.01780200015001. [DOI] [PubMed] [Google Scholar]
- Schmider J, et al. Combined dexamethasone/corticotropin-releasing hormone test in acute and remitted manic patients, in acute depression, and in normal controls: I. Biological Psychiatry. 1995;38 (12):797–802. doi: 10.1016/0006-3223(95)00064-X. [DOI] [PubMed] [Google Scholar]
- Swann AC, et al. Hypothalamic-pituitary-adrenocortical function in mixed and pure mania. Acta Psychiatrica Scandinavica. 1992;85 (4):270–274. doi: 10.1111/j.1600-0447.1992.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Swann AC, et al. Depressive mania versus agitated depression: biogenic amine and hypothalamic-pituitary-adrenocortical function. Biological Psychiatry. 1994;35 (10):803–813. doi: 10.1016/0006-3223(94)91143-6. [DOI] [PubMed] [Google Scholar]
- Vieta E, et al. Enhanced corticotropin response to corticotropin-releasing hormone as a predictor of mania in euthymic bipolar patients. Psychological Medicine. 1999;29 (4):971–978. doi: 10.1017/s0033291799008727. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Watson S, et al. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. The British Journal of Psychiatry : The Journal of Mental Science. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- Watson S, et al. Lithium, arginine vasopressin and the dex/CRH test in mood disordered patients. Psychoneuroendocrinology. 2007;32 (5):464–469. doi: 10.1016/j.psyneuen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Seckl J. Minireview: stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152 (12):4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- Zobel AW, et al. Cortisol response in the combined dexamethasone/ CRH test as predictor of relapse in patients with remitted depression. a prospective study. Journal of Psychiatric Research. 2001;35 (2):83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]