Abstract
Background
This study aims to determine the feasibility of identifying circulating tumor cells (CTCs) in patients with high-risk, localized prostate cancer (HRLPC), using a modified isolation procedure on the CellSearch platform, and to assess the expression of stem cell and epithelial-mesenchymal transition (EMT) markers on the CTCs.
Methods
Thirty five patients with HRLPC who had chosen prostatectomy for definitive management were prospectively identified. After obtaining consent, four 30mL blood draws were performed, two prior to surgery and two following surgery. The CTC-containing fraction was Ficoll purified and transferred to a CellSave tube containing dilution buffer prior to standard enumeration using the CellSearch system. Loss of E-Cadherin expression, a marker of EMT, and CD133, a putative prostate cancer stem cell marker, were characterized using the open channel on the CellSearch platform. CTC fragments were also enumerated.
Results
Using the modified methodology, CTCs were detectable in 49% of patients prior to surgery. While no correlation between CTC count and biochemical recurrence (BR) was observed, the percentages of CD133 and E-Cadherin positive CTC fragments were associated with BR at one year.
Conclusion
Our results suggest that further research into the development of CTCs as prognostic biomarkers in HRLPC is warranted.
Keywords: fragment, stem cell, EMT
Introduction
An estimated 15-25% of patients with localized prostate cancer develop biochemical recurrence after definitive intervention with radical prostatectomy 1, 2. This finding suggests the presence of occult disease that exists beyond the boundaries of the prostate. Several strategies have been undertaken to detect this disease. Morgan et al evaluated bone marrow aspirates in a series of 569 patients with localized prostate cancer treated with prostatectomy 3. They found that disseminated tumor cells (DTCs), assessed through flow cytometric methods, were present in the majority of patients (72%) prior to prostatectomy, and the presence of DTCs served as an independent predictor of biochemical recurrence. Several other studies have similarly reported the prognostic value of DTCs, but the physical burden of a bone marrow biopsy represents a practical challenge that limits the utility of DTCs as a biomarker 4. Other techniques, including RT-PCR and flow-cytometry based approaches have also been used to detect disseminated disease in patients diagnosed with organ-confined disease; however, none of these approaches have yet been adopted clinically for a variety of reasons 5.
Circulating tumor cells (CTCs) are a non-invasive alternative to DTCs. The ease of acquisition of CTCs allows for serial collection and analysis, a distinct advantage over DTC acquisition. Furthermore, collection and enumeration of CTCs has been standardized and validated for several clinical conditions 6. In the setting of metastatic castration resistant prostate cancer (mCRPC), CTCs can be enumerated through the FDA approved CellSearch System (Veridex) 7. Despite the potential predictive and prognostic roles of CTC enumeration in mCPRC, the role of this entity in localized disease has not yet been clearly defined. Here, we confirm that it is feasible to identify CTCs in HRLPC patients using the CellSearch platform, as others have shown 8-10, and that these cells have phenotypes associated with aggressive disease, including expression of markers that suggest stem cell and epithelial-mesenchymal transition (EMT) phenotypes. Expression of stem cell markers on cancer cells has been proposed to define a subset of cells with increased plasticity and a better ability to adapt to selective pressures11. CD133 is a cell surface antigen that helps to define a population of prostate and prostate cancer cells with stem cell-like characteristics12, 13. Therefore, we chose to examine CD133 expression on CTCs as a marker of the stem cell phenotype. EMT, and the reverse of this process, MET, are considered to be crucial for the progression of many different cancers 14, 15. In prostate cancer, markers of EMT, including loss of E-cadherin expression and over-expression of N-cadherin, correlate with Gleason grade and disease progression following radical prostatectomy, suggesting that EMT is related to more aggressive clinical behavior 16, 17. We reasoned that if we were to detect cells with an intermediate epithelial-mesenchymal phenotype, we should use an early marker of the transition, since the CellSearch platform uses immunocapture of Epithelial Cell Adhesion Molecule, and epithelial cell marker, as a first step in CTC isolation. Therefore, we chose to examine loss of E-Cadherin expression on CTCs as a marker of EMT, since tis is occurs at the earliest stages of EMT 18.
Materials and Methods
Patient and Control Sample Selection
Through an IRB-approved protocol (COH IRB 11020), patients with high-risk, localized prostate cancer (defined by NCCN criteria, i.e., ≥ cT3a disease, Gleason score 8-10, and/or PSA > 20 ng/mL) who had chosen prostatectomy for their definitive management were prospectively identified. The presence of metastatic disease was ruled out as patients in the current series had negative evaluations for metastases with MRI of the abdomen and pelvis prior to surgery (or CT if contraindications existed), as well as technetium bone scan. Five volunteers without a known history of cancer were chosen as a negative control population through a separate IRB protocol (COH IRB 12311).
Processing of blood samples
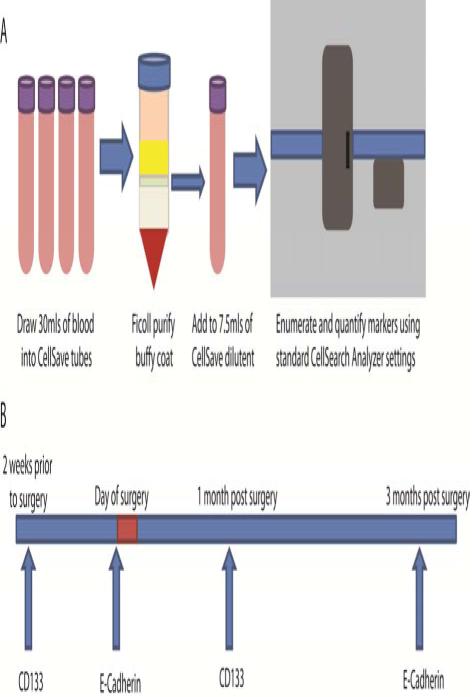
Blood was drawn into four CellSave tubes and within two hours, blood was pooled, 30 mLs were measured, and the buffy coat, which contains CTCs, was purified by Ficoll gradient (Figure A1). The buffy coat was diluted into 7.5mls of CellSave dilution buffer and transferred to a new CellSave tube, where it was processed according to standard CellSearch procedures using the CTC kit and the Celltracks Autoprep System and the Celltracks Analyzer. CTCs were enumerated by a trained operator. The blood draw schedule included sample acquisitions two weeks prior to RP, immediately prior to RP, and one and three months following RP (Figure A2).
Figure A. Blood draw and experimental design.
(1) At each blood draw, blood was drawn into four CellSave tubes and within four hours, pooled into 30 mls whereupon the buffy coat, which contains CTCs, was purified by Ficoll gradient. The buffy coat was transferred to a new CellSave tube with CellSave dilution buffer to a total of 7.5mls, which was processed according to standard CellSearch procedures using the CTC enumeration kit. (2) The blood draw schedule is shown, including which marker was evaluated in the open channel of the CellSearch system at each draw.
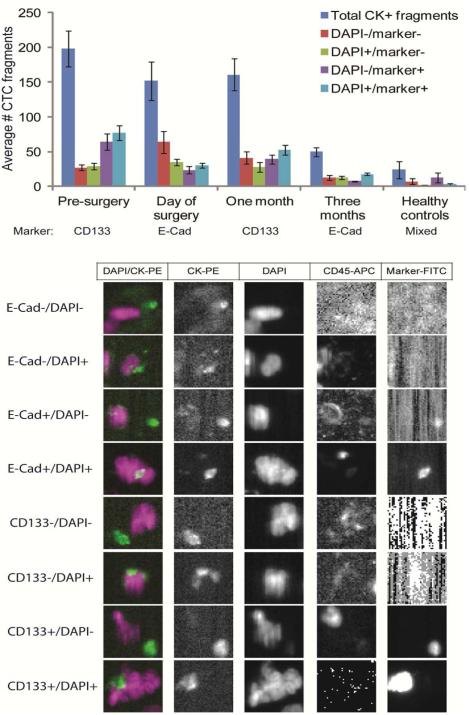
Figure B. CTC biomarker evaluation.
(Top) In all samples from which the expression of CD133 was examined, 46 CTCs were identified, of which 35 were positive for CD133 expression. In all samples from which the expression of E-cadherin was examined, 47 CTCs were identified, of which 38 were positive for E-cadherin expression. (Bottom) Representative CellSearch images of marker-positive CTCs are shown.
Analysis of CTC marker expression
The CellSearch platform maintains an open channel for assessment of an additional marker on the CTCs, which was used to assess the expression of CD133 and E-Cadherin on alternating samples from each patient (Figure A2). FITC-conjugated antibodies against CD133 (Biorbyt 3752) and E-Cadherin (Biorbyt 15536) were used to detect these proteins. Staining and exposure times were optimized by comparing immunofluorescence images of PC3 cells with CellSearch images of PC3 cells isolated from spiked blood samples. The efficiency of capture, as determined by counting the number of PC3 cells being spiked into the blood, was >85% for all samples. In the CellSearch analysis, using a 0.8 second exposure time and a 1:50 antibody dilution, a clear difference was observed between absent and present staining for both CD133 and E-Cadherin. Among 330 PC3 cells isolated by the CellSearch platform, 8% were positive and 92% were negative for CD133. Among 204 PC3 cells isolated by the CellSearch platform, 89% were positive and 21% were negative for E-Cadherin. These values were very similar to observations from immunofluorescence staining, suggesting that our method of calling positive and negative events on the CellSearch platform has high-concordance with immunofluorescence results. Similar concordance between CellSearch identification and immunofluorescence results was seen with LNCaP cells as well. Furthermore, a sampling of 10 patients demonstrated that 11/238 (5%) DAPI+/CD45+/CK− cells (leukocytes) were CD133 positive, suggesting that a small percentage of contaminating white blood cells express this stem cell marker, as would be expected. Prostate cancer cell lines have been shown to harbor ~5% of cells that are CD133+ by flow cytometry, similar to what we have observed by immunofluorescence and CellSearch identification 12, further suggesting the validity of our findings using these antibodies on the CellSearch platform. CTC cell fragments were also enumerated using methods adapted from those previously published19. Briefly, the automated algorithm in the CellTracks Analyzer software was used to identify objects that were positive for CK and/or DAPI. The algorithm identified objects at least 2 × 2 μm2 in size and of medium-to-high contrast in the DAPI and/or CK channels. We counted any epithelial cell adhesion molecule (EpCAM) purified, cytokeratin (CK)+/CD45− event meeting these criteria, and classified it by coexpression of CK with DAPI or CD133/E-Cadherin.
Statistical Analysis
A Spearman correlation coefficient or chi-square table (for continuous or categorical variables, respectively) was generated to examine the relationship between the presence of detectable CTCs prior to surgery and the following pre-defined clinicopathologic variables: (1) age, (2) clinical stage, (3) biopsy Gleason score, (4) baseline PSA, (5) pathologic stage, (6) extracapsular extension, (7) vascular invasion, and (8) seminal vesicle involvement. The association between detectable CTCs prior to or following surgery and biochemical recurrence (BR) as well as the time to biochemical recurrence (TTBR) was also explored. Notably, BR was defined as the first occurrence of a PSA ≥ 0.2. Descriptive statistics were used to characterize the extent of CD133 and E-cadherin expression in relevant subsets of patients and a Student’s t test was used to compare the frequency of BR at 1 year in biomarker-based subgroups.
Results
Patient Characteristics
A total of 35 patients were enrolled between June of 2011 and January of 2013 (Table A), with a median age of 65 (range, 48-74). Of the 35 patients enrolled, 30 patients (86%) had a Gleason score 8-10, 10 patients (29%) had a PSA > 20 ng/mL and 3 patients (9%) had ≥cT3a disease. Twenty-eight patients (80%) had 1 high-risk feature, while an additional 6 patients (17%) had two-high risk features. Only 1 patient in the series possessed all three high-risk characteristics. Amongst other clinicopathologic characteristics, the majority of patients had ≥pT3a disease identified at the time of surgery (57%), extracapsular extension (57%), vascular invasion (83%), and seminal vesicle involvement (63%). Median follow-up time in the cohort was 510 days (range, 109-894). At least one year of follow-up was available for 33/35 patients.
Table A.
Patient characteristics (n=35).
| Characteristic | Number of Patients (%) |
|---|---|
| Age at surgery, median (range) | 65 (48-74) |
| Preoperative PSA PSA ≤ 10 PSA 10-20 PSA > 20 |
20 (57%) 5 (14%) 10 (29%) |
| Biopsy Gleason score 2-6 7 8-10 |
1 (3%) 4 (11%) 30 (86%) |
| Clinical stage < cT3a ≥ cT3a |
32 (91%) 3 (9%) |
| Number of high-risk features 1 2 3 |
28 (80.0%) 6 (17.1%) 1 (2.9%) |
| Pathologic stage < pT3a ≥ pT3a |
15 (42.9%) 20 (57.1%) |
| Extracapsular extension Yes No |
15 (42.9%) 20 (57.1%) |
| Vascular invasion Yes No |
6 (17.1%) 29 (82.9%) |
| Seminal Vesicle involvement Yes No |
13 (37.1%) 22 (62.9%) |
CTC Detection and Enumeration
Using the modified blood collection procedure (Figure A1), 17 of 35 patients (49%) had at least one detectable CTC in one of their two pre-surgery blood draws (Table B). No CTCs were detected in any of the five healthy control samples. Amongst patients with detectable CTCs, the median CTC count was 3 (range 1-5), 2 (range 1-7), 2 (range 1-5), and 2 (range 1-5) at the four respective blood draws (Table B). Amongst all patients, the median CTC count was 0 at each blood draw. Comparison of the two blood samples drawn pre-surgery, during which time there were no changes in health and which should be biologically similar, demonstrates that in 71% of patients, the detection of CTCs in the two pre-surgery blood draws was identical. Nine of 35 patients (26%) had at least one CTC-positive sample pre-surgery but none post-surgery, suggesting a conversion from CTC-positive to CTC-negative following surgery. However 7 of 35 patients (20%) had at least one CTC-positive sample post-surgery but none pre-surgery, suggesting the opposite conversion. Eight patients had CTCs detected in at least one pre- and post-surgery sample while eleven patients had no CTCs detected in any sample. Only two patients had detectable CTCs in all samples.
Table B.
CTC median enumeration across blood draws
| Blood draw | 1st (n=32) 2 wks pre-op |
2nd (n=32) Day of surgery |
3rd (n=34) 1 month post- surgery |
4th (n=34) 3 months post- surgery |
|---|---|---|---|---|
| Fraction with CTCs | 11/32 (34%) | 12/32 (38%) | 8/34 (24%) | 12/34 (35%) |
| Median CTC count in patients with CTCs |
3 (range 1-5, 95%CI ±0.08) |
2 (range 1-7, 95%CI ±0.13) |
2 (range 1-5, 95%CI ±0.08) |
2 (range 1-5, 95%CI ±0.07) |
| Median CTC count in all patients |
0 (range 0-5, 95%CI ±0.09) |
0 (range 0-5, 95%CI ±0.10) |
0 (range 0-5, 95%CI ±0.06) |
0 (range 0-5, 95%CI ±0.07) |
| Median total CK+ fragments |
86 (range 4-1160, 95%CI ±9.17) |
70 (range 4-1580, 95%CI ±9.90) |
37 (range 8-1030, 95%CI ±8.05) |
24 (range 2-372, 95%CI ±2.44) |
Characterization of Cell Surface Markers and CTC Fragment Analysis
The open channel on the CellSearch system was used to assess the expression of the prostate stem cell marker CD133 and the epithelial-mesenchymal transition (EMT) marker E-Cadherin on CTCs in alternating blood samples (Figure A2). In our patient samples, 38/47 (81%) CTCs were E-Cadherin positive and 35/46 (76%) CTCs were CD133 positive (Figure B). CTC cell fragments, EpCAM+/CK+/CD45− objects detectable in the CellSearch analyzer which are not intact cells and thus do not qualify as CTCs, have been shown to have similar prognostic value as intact CTCs 19. Therefore, all circulating EpCAM+/CK+/CD45− objects meeting the criteria outlined in the Methods section were enumerated and objects were segregated into DAPI +/− and marker (CD133 or E-Cadherin) +/− categories (Figure C). All evaluable samples had at least some CK+ fragments (range, 2-1580). In general, the total number of CK+ fragments was lower at the three month follow-up than the other time points (, and was lower still in healthy control patients, where the five patients averaged only 24 CK+ fragments (range 2-70). Overall, 65% and 60% of EpCAM+/CK+/CD45− events were CD133 and E-Cadherin positive, respectively. No CD133+ fragments were observed in healthy control samples, although 67% of fragments were E-Cad+.
Figure C. CTC fragment evaluation.
(Top) In addition to intact CTCs, CTC fragments (CK-positive, CD45-negative events) were enumerated, including those associated with DAPI staining or not, and those with or without expression of CD133 or E-cadherin, depending on the blood draw. Fragments were also assessed in healthy control samples. The blood draw and which marker was examined are shown beneath the graph. (Bottom) Representative images of each type of fragment are shown.
Clinical correlations
The presence of CTCs prior to surgery was not associated with any of the pre-specified clinicopathologic variables noted in “Statistical Analysis”. Furthermore, no correlations were observed between the presence of intact CTCs in any given blood draw and likelihood of BR at one or two years or with TTBR. Likewise, patients with no detectable CTCs in any blood draw appear to have no lower likelihood of BR or an increased TTBR. Patients who converted from CTC positive to negative following surgery or vice versa also demonstrated no association with likelihood of BR or with TTBR. Among patients with no CTCs in either draw prior to surgery, 8/16 (50%) had BR at one year and 8/16 (50%) did not. Among patients with CTCs in either draw prior to surgery, 6/16 (37.5%) had BR at one year and 10/16 (62.5%) did not. Among patients with no CTCs in either draw post-surgery, 11/17 (65%) had BR at one year and 6/17 (35%) did not. Among patients with CTCs in either draw post-surgery, 11/15 (73%) had BR at one year and 4/15 (27%) did not.
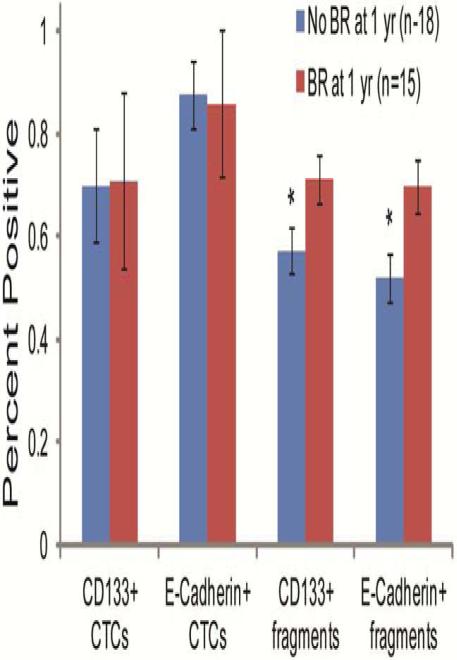
In subgroups divided by the presence or absence of BR at 1 year, there was no difference in the percentage of intact CTCs positive for CD133 or E-cadherin (Figure D). Because of the dearth of total CTCs captured in these patients, we sought to determine if CTC fragments correlated with clinical outcome. Total CK+ fragments were not different amongst the aforementioned subgroups (101 to 135, p=0.101). However, CD133 fragment positivity was significantly higher in patients with BR at one year in pre-surgery samples, post-surgery samples, and when those two blood draws were combined (p=0.028 for combined analysis). Similarly, E-Cadherin fragment positivity was significantly higher in patients with BR at one year in pre-surgery samples, post-surgery samples, and when those two blood draws were combined (p=0.006 for combined analysis) (Figure D).
Figure D. CTC fragments correlate with BR.
The percentages of intact CTCs or CTC fragments positive for CD133 or E-Cadherin in patients with or without biochemical recurrence (BR) at one year are shown. Statistically significant differences were determined using a Student’s t test (* p<0.05).
Discussion
Several studies have now attempted to identify CTCs in patients with presumed localized PC using the CellSearch platform, with detection rates from 5-21% 10, 20. Here, we detect CTCs in nearly half of patients with HRLPC prior to prostatectomy. By altering the blood collection methodology and limiting our cohort to only those men with high risk disease, we were able to increase the detection rate of CTCs compared to these similar studies.
All patients in the current series had negative evaluations for metastases with MRI of the abdomen and pelvis prior to surgery (or CT if contraindications existed), as well as technetium bone scan. The presence of CTCs may reflect the presence of occult micrometastases, or perhaps a subset of patients with a greater predisposition for future metastases. It is very likely that not all CTCs are precursors to metastases and that subsets of CTCs, defined by surface markers, have a greater propensity to form metastases. We hypothesized that CTCs with a stem cell phenotype or with an intermediate epithelial-mesenchymal phenotype would be more malignant, thus, we used the open channel in the CellSearch system to quantify the expression of CD133, a prostate stem cell antigen, and of E-Cadherin, the loss of which is one of the earliest events in EMT. Our findings of high levels of expression of CD133 and non-universal expression of E-Cadherin on prostate cancer CTCs lends support to the hypothesis that prostate CTCs exhibit high plasticity 11. This hypothesis predicts that CTCs with stem cell characteristics are transitional in nature and display an intermediate epithelial-mesenchymal phenotype, which leads them to be highly malignant. We found that 76% of CTCs examined in our study expressed CD133. Using the CellSearch system to enrich CTCs and immunocytochemistry to detect marker expression on fixed cells, Armstrong et al found that 11/11 men with mCRPC had CTCs positive for CD133, with 127/153 (83%) CTCs total being CD133 positive 11. Our findings in HRLPC patient samples, although using slightly different methodology and a different antibody, are very similar to these results and suggest that CD133 expression is fairly common on prostate cancer CTCs, regardless of disease stage. Additionally, OS and DFS of colorectal cancer patients who had CTCs positive for CD133 were significantly worse than those of patients who were negative for CD133 21.
Using IHC, Armstrong et al also looked for co-expression of E-Cadherin and N-Cadherin on 361 EpCAM-captured, CD45-negative cells from CRPC patients. They found that 45% were double positive and 31% were double negative. Furthermore, 7% were E-cadherin positive/N-cadherin negative- and 16% were E-cadherin negative/N-cadherin positive. These results suggest that CTCs from CRPC patients have an intermediate phenotype, even within an individual. Furthermore, as 47% of cells were E-Cadherin negative, it demonstrates quite clearly that E-Cadherin negative cells can be captured using the EpCAM-based CellSearch system, which has been a highly debated question. Using the open channel on the CellSearch system as opposed to downstream immunohistochemical analysis, we show that in our CTCs from local prostate cancer patients, E-Cadherin expression is 81%. While the lower rate of E-Caherin negative cells may be a result of technique or disease stage, it quite clearly demonstrates that E-Cadherin negative cells can be captured by the CellSearch system.
We observed no correlations between the presence of CTCs prior to surgery and a number of clinicopathological features that are typically used to predict disease outcome. Furthermore, we found no correlation between the presence of CTCs in any blood draw and BR or TTBR, nor did we find a correlation between CD133 or E-Cadherin positive CTCs and BR or TTBR. The lack of correlation may be explained by the low number of intact CTCs detected in our patient population, which made statistical correlations difficult. To overcome the limited number of events, we enumerated all EpCAM+/CK+/CD45− events in our patient samples, as these events have also been shown to be prognostic in mCRPC.19 The CellSearch methodology is thought to underestimate the actual number of CTCs in patient samples, perhaps by fragmentation of sensitive CTCs. Thus, CTC fragments likely reflect the presence of additional CTCs in patients, which lends them their prognostic value. We identified CK+ events in every sample, even controls, which is to be expected since epithelial components are occasionally shed into the bloodstream, particularly following blood draw. Importantly, we found that men with BR at one year had an increased percentage of CTC fragments that were positive for CD133 or E-Cadherin. An increased amount of CD133 expression on CTCs fits well with the hypothesis that CTCs with a stem-like phenotype are more aggressive. E-Cadherin expression is present in cancer cells both before they metastasize and following the establishment of a metastasis after having undergone MET, the reverse of EMT. Therefore, E-Cadherin expression can reflect the presence of local or established metastatic cancer, so it is possible that the increased amount of E-Cadherin expression on CTC fragments arose from metastatic sites, which is why it was positively correlated with BR. It is also possible that these cells express both epithelial markers and mesenchymal markers, and that dual expression portends the most aggressive disease. A validated CTC detection methodology that allows detection of multiple markers in the EMT pathway is needed to answer this question.
Conclusions
In summary, we have demonstrated that through enrichment of clinicopathologic features (i.e., selecting high-risk patients) and sample features (i.e., increased volume), we can detect CTCs in nearly half of patients with localized prostate cancer using the already established CellSearch System. Importantly, we demonstrate the feasibility of interrogating the phenotype of CTCs in HRLPC patients and show that many of these CTCs, in the absence of frank metastatic disease, already possess hallmarks of advanced disease, including loss of E-Cadherin and expression of CD133, and that expression of these markers on CTC fragments is associated with BR. However, larger prospective trials will be necessary to determine the predictive or prognostic value of CTCs or CTC fragments in the setting of localized prostate cancer. Ultimately, it is hoped that CTCs will evolve into a personalized prognostic tool for patients with high-risk localized prostate cancer – a tool that reflects the biology of the disease to a greater extent than PSA.
Clinical Practice Points.
The prognostic value of circulating tumor cells (CTCs) in metastatic castration-resistant prostate cancer (mCRPC) is well established
Previous studies have suggested that CTCs can be detected in patients with localized prostate cancer, albeit with low yield
Using a modified methodology, we have detected CTCs in 49% of patients with high-risk localized prostate cancer prior to surgery
CTC fragments were obtained in a even higher proportion of patients, and several markers (E-cadherin and CD133) were associated with biochemical recurrence at 1 year
MicroAbstract.
Circulating tumor cells (CTCs) have established prognostic value in the setting of metastatic castration resistant prostate cancer. However, their utility in the setting of localized prostate cancer is largely unknown. In the current study, a novel method is used to quantify and characterize CTCs in patients with high-risk localized prostate cancer.
Acknowledgements
This work was supported by NIH K12 2K12CA001727-16A1 and Tower foundation grant (S.K.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have declared no conflicts of interest.
References
- 1.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. The Journal of Urology. 2000;164:101–105. [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Morgan TM, Lange PH, Porter MP, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci. 2007;12:3000–9. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 6.Miller MC, Doyle GV, Terstappen LW. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 8.Helo P, Cronin AM, Danila DC, et al. Circulating Prostate Tumor Cells Detected by Reverse Transcription-PCR in Men with Localized or Castration-Refractory Prostate Cancer: Concordance with CellSearch Assay and Association with Bone Metastases and with Survival. Clinical Chemistry. 2009;55:765–773. doi: 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stott SL, Lee RJ, Nagrath S, et al. Isolation and Characterization of Circulating Tumor Cells from Patients with Localized and Metastatic Prostate Cancer. Science Translational Medicine. 2010;2:25ra23–25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thalgott M, Rack B, Maurer T, et al. Detection of circulating tumor cells in different stages of prostate cancer. J Cancer Res Clin Oncol. 2013;139:755–63. doi: 10.1007/s00432-013-1377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Marengo MS, Oltean S, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–11. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson SC, Hepburn AC, Wilson L, et al. Human alpha(2)beta(1)(HI) CD133(+VE) epithelial prostate stem cells express low levels of active androgen receptor. PLoS One. 2012;7:e48944. doi: 10.1371/journal.pone.0048944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras HR, Ledezma RA, Vergara J, et al. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial-mesenchymal transition markers, E-cadherin and beta-catenin, in prostate cancer. Urol Oncol. 2010;28:534–40. doi: 10.1016/j.urolonc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 18.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–36. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 19.Coumans FA, Doggen CJ, Attard G, de Bono JS, Terstappen LW. All circulating EpCAM+CK+CD45-objects predict overall survival in castration-resistant prostate cancer. Ann Oncol. 2010;21:1851–7. doi: 10.1093/annonc/mdq030. [DOI] [PubMed] [Google Scholar]
- 20.Davis JW, Nakanishi H, Kumar VS, et al. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. J Urol. 2008;179:2187–91. doi: 10.1016/j.juro.2008.01.102. discussion 2191. [DOI] [PubMed] [Google Scholar]
- 21.Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J Clin Oncol. 2011;29:1547–55. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]