Abstract
Tumor necrosis factor-alpha (TNF) is a pro-inflammatory cytokine that is implicated in the initiation of neuropathic pain. Locally administered TNF antagonist etanercept offers a promising new treatment approach to target neuropathic pain. Here we evaluate the distribution and binding specificity for TNF isoforms of locally administered etanercept into injured and uninjured rat sciatic nerve. Distribution and co-localization of etanercept and TNF in the injured and uninjured nerve was evaluated at 1, 24, 48 and 96 h after etanercept local application using immunohistochemistry. In addition, binding specificity of etanercept for TNF isoforms was analyzed using immunoblot assay system in nerve lysates. A new observation was that locally administered etanercept reached the endoneurium of the injured but not the uninjured nerve 1 h after its application and mainly co-localized with TNF-positive structures, morphologically similar to Schwann cells and macrophages. We further noticed that immunoblot analyses for etanercept demonstrated its preferential binding to transmembrane and trimer TNF isoforms. Finally, locally administered etanercept inhibited pain-related behaviors in a rat sciatic nerve crush model. We conclude that locally administered etanercept reaches the endoneurial space in the injured nerve and preferentially binds to trans-membrane and bioactive trimer TNF isoforms to modulate neuropathic pain. Locally administered etanercept has potential as a targeted immunomodulating agent to treat local pathogenesis in neuropathic pain after peripheral nerve injury.
Keywords: peripheral nerve injury, neuropathic pain, tumor necrosis factor, etanercept
Tumor necrosis factor-alpha (TNF) is a pro-inflammatory cytokine that plays crucial roles in peripheral nerve injury (Wagner and Myers, 1996b; George et al., 1999; Myers et al., 2006). Nerve injury causes local upregulation of TNF in activated Schwann cells, macrophages and other resident cells (Stoll et al., 1993; Sommer and Schroder, 1995; Wagner and Myers, 1996a). The local increase in TNF activity initiates an inflammatory cascade that produces severe persistent neuropathic pain (Redford et al., 1995; Wagner and Myers, 1996b; Schafers et al., 2003).
Several TNF inhibitors, including etanercept (Enbrel®, Amgen, Inc., Thousand Oaks, CA, USA) that is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the 75 kDa tumor necrosis factor-alpha receptor 2 (TNFR2) and the Fc portion of human immunoglobulin G (IgG), have been shown to reduce pain-related behaviors in a neuropathic pain model (Sommer et al., 2001; Schafers et al., 2003; Zanella et al., 2008). However, several adverse effects including the reemergence of latent tuberculosis, severe infections, and new and recurrent malignancies have been reported due to the chronic use of systemically delivered TNF inhibitors in patients (Scheinfeld, 2004; Bongartz et al., 2006; Hochberg et al., 2005). More recently, it has been reported that locally administered etanercept provides analgesic effects in a rat neuropathic pain model (Sommer et al., 2001; Zanella et al., 2008) and patients with sciatica and cervical radiculopathy (Tobinick and Davoodifar, 2004). Zanella et al. (2008) reported that systemic etanercept reduced thermal hyperalgesia but required a 10-fold higher dose than a locally administered compound in a rat chronic constriction injury model. These results are strongly consistent with potential efficacy for the proposed local treatment approach permitting the use of lower drug dosage, which should minimize side effects and complications of TNF inhibitor administration. However, etanercept is a macromolecule with an apparent molecular weight (MW) of approximately 150 kDa which is not expected to cross the blood–nerve barrier (Zhou, 2005; Stephen et al., 2006). After nerve injury, the blood–nerve barriers become more permeable (Weerasuriya et al., 1980), but little is known about the effects of nerve injury on drug access to the nerve. In addition, etanercept is a fusion protein consisting of TNFR2 that preferentially binds to transmembrane TNF isoforms (Grell et al., 1995), but the binding specificity for TNF isoforms of locally administered etanercept into nerve tissues has not been clarified. Binding to transmembrane TNF by TNF receptors, or even TNF antagonists, can induce reverse signaling through this membrane-anchored ligand and can trigger cell activation, cytokine suppression or apoptosis of the transmembrane TNF-bearing cell (Tracey et al., 2008). Thus, evaluation of the distribution and binding specificity for TNF isoforms of locally administered etanercept may be important for understanding the utility of etanercept for locally treating the neuropathological changes in neuropathic pain.
In the present study, we established an immunohistochemical method of etanercept detection using an antibody for human IgG, and evaluated the dynamics of etanercept uptake and distribution to injured and uninjured sciatic nerve in rats. In addition, binding specificity of etanercept for TNF isoforms was analyzed using immunoblot assay system in nerve lysates. Finally, we confirmed the pain-relieving effect of locally administered etanercept in a rat sciatic nerve crush (SNC) model using behavioral tests of mechanical sensitivity.
EXPERIMENTAL PROCEDURES
Animals and anesthesia
A total of 52 Adult female Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) weighing 200 –250 g were used. Rats were housed in pairs with a 12-h light/dark cycle with free access to food and water. The experimental protocols were approved by the VA Healthcare System Committee on Animal Research, and conform to the NIH Guidelines for Animal Use. All efforts were made to minimize animal suffering and to reduce the number of animals used. The animals were anesthetized with 4% isoflurane (IsoSol; Vedco, St. Joseph, MO, USA).
Surgical procedure
All surgical procedures were performed in an aseptic manner using microsurgical techniques. Rats were placed in a lateral position and a skin incision was made from the greater trochanter to the mid-thigh. The left sciatic nerve was exposed through a gluteal muscle splitting incision. Rats were divided into four groups: (1) the control group was injected intraneurially with 125 μg etanercept (Enbrel®, Amgen, Inc.) in 5 μl of sterile bacteriostatic water into the endoneurial space of the uninjured rat sciatic nerves using a Hamilton syringe (Hamilton Company, Reno, NV), a 30-gauge-needle (n=2). (2) The sham group was subjected to an operation in which the left sciatic nerve was exposed and 100 μg etanercept in 50 μl of sterile bacteriostatic water (Sterile Water for Injections, Hospira, Inc., Lake Forest, IL, USA) was applied into the epineurial space immediately adjacent to the nerve (n=16). Careful insertion of the needle into the epineurial space of the crushed nerve site and slow injections to avoid an overflow were ensured. (3) The SNC group was subjected to the crush operation using smooth-surface forceps once for 5 s (n=24). The muscle layer was closed using silk suture and the skin was closed with metal clips. (4) The vehicle-treated SNC group was subjected to the crush operation as described above, and then 50 μl of sterile bacteriostatic water was applied into the epineurial space immediately adjacent to the nerve 24 h after the first surgery (n=10). The sterile bacteriostatic water that we used contains 0.9% benzyl alcohol. Although benzyl alcohol in this low concentration has been widely used as a preservative in parenteral preparations, it is known to have potential neurotoxic effects at higher concentrations (Deland, 1973).
Tissue processing
For immunohistochemical experiments, SNC group rats were anesthetized with 4% isoflurane 24 h after the first surgery and the sciatic nerve was exposed and 100 μg etanercept in 50 μl of sterile bacteriostatic water was applied into the epineurial space immediately adjacent to the nerve as described above, and then the incision was closed. The dose of etanercept was based on previous studies (Sommer et al., 2001; Zanella et al., 2008). Immunohistologic examinations were performed in the control (n=2 at 1 h after the application of etanercept), the sham and SNC (n=8 each at 1, 24, 48 and 96 h after the application of etanercept) and the vehicle-treated SNC groups (n=2 at 96 h after the application of sterile bacteriostatic water). Rats were anesthetized using an i.p. injection of a cocktail containing sodium pentobarbital (Nembutal, 50 mg/ml; Abbott Labs, North Chicago, IL, USA) diazepam (5 mg/ml, Steris Laboratories, Phoenix, AZ, USA) and saline (0.9%, Steris Laboratories) in a volume proportion of 1:1:2, respectively and perfused with fresh 4% paraformaldehyde in 0.1 mol/l phosphate buffer. Bilateral sciatic nerves were removed and post-fixed briefly in 4% paraformaldehyde overnight. Tissue was processed in an Autotechnicon Cycler TP 1010 (Leica Microsystems, Inc., Bannockburn, IL, USA), and embedded in paraffin.
For immunoblotting analysis, rats (n=8) were sacrificed at 1 and 5 days after SNC by overdose of i.p. injection of the cocktail as described above followed by intracardiac Euthasol (Virbac, Fort Worth, TX, USA). Rats were decapitated rapidly under anesthesia, and bilateral sciatic nerves were removed and frozen in liquid nitrogen.
Immunohistochemistry
Sections (10 μm) of the nerves were cut from each sample and placed on slides. Sections were deparaffinized with xylenes and rehydrated in graded ethanol, and endogenous peroxidase was blocked with 3% H2O2. Non-specific binding sites were blocked with 10% normal horse or rabbit serum. Anti-human IgG (AbD Serotec, Oxford, UK; 1:500) or rat-TNF (R&D Systems, Minneapolis, MN, USA; 1:100) antibodies were incubated overnight at 4 °C in 1% horse or rabbit serum. Slides were rinsed in phosphate-buffered saline and subsequently incubated for 30 min with bio-tinylated conjugated horseradish peroxidase antimouse or goat antibodies (Vector Laboratories, Burlingame, CA, USA). Sections were incubated for 30 min with avidin– biotin–peroxidase complex (ABC Elite, Vector Laboratories), developed with 3′-3-diamino-benzidine (DAB) (Vector) and counterstained with Methyl Green. For dual immunofluorescence, sections were immersed in 0.5% sodium borohydride followed by antigen retrieval and nonspecific binding block as described above, then primary antibody incubation overnight at 4 °C, rabbit antimouse Alexa 488 (green) fluorescent antibody (Molecular Probes, 1:400) or rabbit anti-goat Alexa 594 (red) fluorescent antibody (Molecular Probes, 1:400) for 1 h, and nuclear 4,6-diamidino-2-phenylindole (DAPI) stain (Molecular Probes, 1:20,000, blue) for 5 min. Sections were mounted using Slowfade gold antifade reagent (Molecular Probes). Some sections were run without primary antibodies as controls or were preabsorbed, showing no human IgG or TNF immunoreactivity. Imaging was done on a Leica microscope using Open Laboratory Software (Improvision, Coventry, UK).
Immunoblotting assay
Samples were extracted using lysis buffer (50 mM Tris–HCl, pH 7.4, 1% Triton X-100, 150 mM NaCl, 10% glycerol, 0.1% SDS, 5 mM EDTA, 1 mM PMSF, 1 μg/ml aprotinin and leupeptin) and reduced with 10% β-mercaptoethanol (Fisher). Equal amounts of protein (60 μg, by Pierce BCA Protein Assay) per lane were run on 12.5% Tris– glycine SDS–polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, USA) for 1 h at 50 – 80 mA, and then transferred to nitrocellulose membranes using iBlot dry blotting system (Invitrogen) at 20 V for 7 min. The membranes were blocked with 5% non-fat milk (Bio-Rad), incubated with etanercept (25 mg/ml Amgen, Inc.; 1:1000) for 2 h at room temperature and anti-human IgG antibody (AbD Serotec; 1:1000) in 5% bovine serum albumin (BSA, Sigma) overnight at 4 °C, washed in TBS containing 0.1% Tween and incubated for 1 h at room temperature with HRP-conjugated antimouse or anti-goat secondary antibody (Cell Signaling; 1:5000). Anti rat-TNF (R&D Systems; 1:500) antibody was also used as primary antibody for comparison. The blots were developed using enhanced chemiluminescence (ECL, Amersham). Recombinant rat TNF-α (R&D Systems) was used for positive control (0.5 ng for anti-TNF antibody and 250 ng for etanercept and anti-human IgG antibody, as optimized in a separate experiment, using serial dilutions of recombinant rat TNF-α). As a further control for specificity, etanercept and anti rat-TNF antibody were preabsorbed with 5000 ng recombinant rat TNF-α overnight at 4 °C before the immunoblotting procedure. The blots were stripped and reprobed for gel loading controls using mouse anti β-actin antibody (Sigma; 1:10,000).
Behavioral test
Sensitivity to non-noxious mechanical stimuli was measured by the von Frey test (Chaplan et al., 1994), as we have described previously (Igarashi et al., 2000; Kato et al., 2008; Kobayashi et al., 2008). Briefly, rats were acclimated to being on a suspended 6-mm wire grid and the plantar surface of the hind paw was stimulated within the sciatic nerve innervation area using calibrated von Frey filaments (Stoelting, Wood Dale, IL, USA). Stimuli were applied through the mesh, perpendicular to the mid-paw plantar surface for 3 s with buckling forces between 0.41 and 15.2 g force sequentially with ascending filament stiffness until paw withdrawal response, determined as the lowest gram force that provoked a paw withdrawal at least twice in 10 applications. Stimuli were separated by several seconds or until the animal was calm with both hind paws placed on the grid. Testing was performed in both hind paws for 1 day before SNC or sham operation (baseline) and every other day in N of eight per group by an experimenter unaware of the experimental groups. Statistical analyses were done by one-way analyses of variance (ANOVA) and Dunnett’s post hoc test.
RESULTS
Immunohistochemistry for etanercept in uninjured nerve
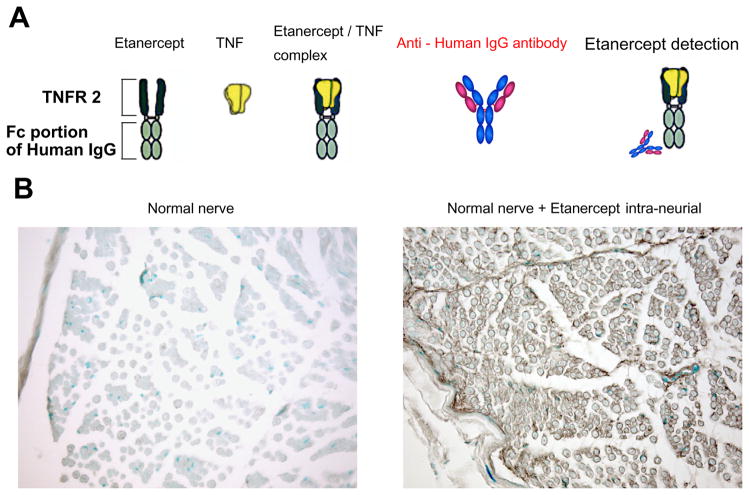
To stain for etanercept itself in the nerve tissue, we developed a method using anti-human IgG antibody that detects its Fc portion of human IgG (Fig. 1A). All the sections from the normal nerve without application of etanercept yielded negative staining with anti-human IgG antibody (Fig. 1B). At 1 h after intraneurial injection of etanercept into rat sciatic nerve, widespread distribution of the etanercept–human IgG immunoreactivity was observed in the endoneurial space of the nerve (Fig. 1B). The epineurial injections of etanercept in uninjured nerve were performed in the sham group (Fig. 2A). At 1 h after epineurial injection of etanercept, the etanercept– human IgG immunoreactivity was prominent in many epineurial cells and the perineurium. However, in the endoneurial space, no immunoreactive structures were observed (Fig. 2B; upper panels). At 24, 48 and 96 h after the epineurial injection of etanercept, the etanercept– human IgG immunoreactivity remained in the epineurial cells and the perineurium. In the endoneurial space, no or only weak immunoreactive structures were observed in the perivascular and the sub-perineurium spaces (Fig. 2B; upper panels).
Fig. 1.
Establishment of the immunohistochemical method to detect the uptake and distribution of applied etanercept in rat nerves. (A) Model diagram of the detection technique for etanercept. (B) Immunohistochemical images of rat sciatic nerve stained with an antibody for human IgG. Methyl-Green nuclear counterstain was used. Normal nerve indicating relative lack of background antibody staining. Nerve 1 h following intra-neural injection of 125 μg of etanercept indicating its widespread distribution (brown). Representative micrographs of n=2 rats/group. Magnification 200×.
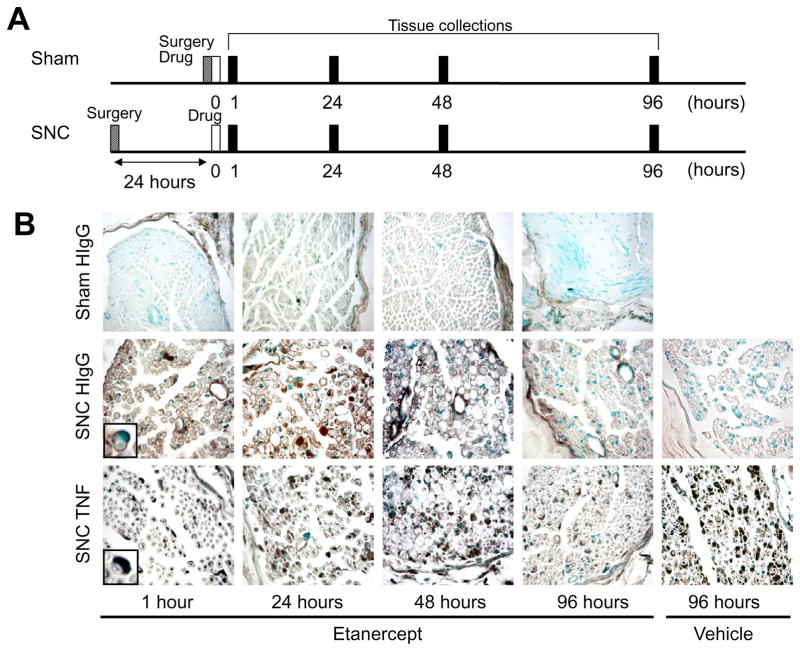
Fig. 2.
Immunohistochemistry for etanercept and TNF in nerve after the epineurial injection of etanercept. (A) Experimental schedule for surgery, therapy and tissues collection in sham and SNC groups. (B) In sham group, etanercept immunoreactivity using anti-human IgG (HIgG, brown) was observed in the epineurial space and the perineurium at 1–96 h after its injection. It was not detectable in the endoneurial space at either time point. In contrast, etanercept reached the endoneurial space after its epineurial injection to the injured rat sciatic nerve. Representative micrographs of n=8 –10 rats/group. Insets are magnified images of myelinated axons with surrounding Schwann cell cytoplasm. Magnification 200× (Sham). Magnification 400× (SNC).
Immunohistochemistry for etanercept and TNF in injured nerve
At 24 h after the nerve crush injury, the epineurial injections of etanercept were performed (Fig. 2A). At 1 h after the epineurial injection of etanercept, etanercept– human IgG immunoreactivity was observed not only in the epineurial cells and the perineurium but also in the endoneurial space. In the endoneurial space, etanercept– human IgG immunoreactivity was observed in Schwann cells (inset in Fig. 2B; middle panel), endothelial cells and fibroblasts (Fig. 2B; middle panels). TNF immunoreactivity was shown in Schwann cells (inset in Fig. 2B; lower panel), endothelial cells, fibroblasts, and intact axons at this time (Fig. 2B; lower panels). At 24 h after the epineurial injection, accumulations of etanercept– human IgG immunoreactivity were additionally observed in recruited macrophages and myelin debris (Fig. 2B; middle panels). TNF immunoreactivity was also shown in the recruited macrophages and myelin debris at this time (Fig. 2B; lower panels). At 48 h after the epineurial injection, moderate etanercept– human IgG immunoreactivity was observed in the epineurial cells, the perineurium, Schwann cells, fibroblasts, macrophages, myelin debris and endothelial cells (Fig. 2B; middle panels). TNF immunoreactivity was still seen in the epineurial cells, the perineurium, Schwann cells, endothelial cells, fibroblasts, recruited macrophages and myelin debris at this time (Fig. 2B; lower panels). At 96 h after the epineurial injection of etanercept, weak or no etanercept– human IgG immunoreactive structures were observed in the perivascular and the sub-perineurium spaces (Fig. 2B; middle panels). Weaker TNF immunoreactivity after the epineurial injection of etanercept was shown in the endoneurial space at this time compared with the vehicle-treated group (Fig. 2B; lower panels).
Etanercept– human IgG and TNF immunoreactivity mainly co-localized in the characteristic crescent or round structures in the injured rat sciatic nerve during the 96 h observation period (Fig. 3C; inset). However, in some myelinated axons, etanercept– human IgG immunoreactive structures accumulated only myelin-like structures but not TNF-positive axon-like structures, suggesting that distinct isoforms of TNF might be reactive with etanercept compared to anti-TNF antibody.
Fig. 3.
Immunofluorescence for human IgG (green), TNF (red), and DAPI nuclear stain (blue) in the injured nerve 24 h after etanercept application. (A) Anti-human IgG immunoreactivity and DAPI. (B) Anti-TNF immunoreactivity and DAPI. (C) Co-localization of human IgG and TNF immunoreactivity. Etanercept mainly co-localized in the characteristic crescent or round structures (inset), suggesting Schwann cells and macrophages. In some myelinated axons, etanercept– human IgG immunoreactive structures accumulated only myelin-like structures but not TNF-positive axon-like structures. (D) Phase-contrast light micrograph of nerve observed in A, B, and C. Representative micrographs of n=8 rats/group. Magnification 400×.
Immunoblotting for TNF using etanercept in nerve lysates
Binding specificity of etanercept for TNF isoforms was evaluated by immunoblotting with etanercept and anti-human IgG antibody in the normal and injured nerve 1 and 5 days after SNC injury. Immunoblotting for etanercept in the injured nerves (Fig. 4A) showed predominant 51, 26, and 25 kDa bands, representing a trimer of soluble tumor necrosis factor-alpha monomer (sTNF, 17 kDa), trans-membrane tumor necrosis factor-alpha (tmTNF), and lymphotoxin-alpha (LTα), respectively. Specificity for 51 and 26 kDa TNF isoforms but not 25 kDa band was confirmed by preabsorption with sTNF. Recombinant rat TNF, a 17 kDa monomer was detected as a positive control, but a large amount of proteins (250 ng) was needed for the detection in the gel loading. Immunoblotting for anti-TNF antibody in the injured nerves (Fig. 4B) showed 51, 34, 26 and 14 kDa bands, representing presumably, a trimer of sTNF, a dimmer of sTNF, tmTNF, and a trypsin- or thermolysin-cleaved sTNF is form, respectively (Shubayev and Myers, 2000; Shubayev et al., 2006). All 51, 34, 26 and 14 kDa TNF isoforms were confirmed by preabsorption with sTNF. Recombinant rat TNF (0.5 ng), a 17 kDa monomer was used for positive control. Gel loading was controlled with β-actin at 42 kDa.
Fig. 4.

Immunoblotting assay for TNF using etanercept (A) or anti-TNF antibody (B) in the normal (N) or injured nerves 1 and 5 days after SNC. Etanercept preferentially binds to 26, 25 and 51 kDa TNF isoforms in nerve lysates, presumably, corresponding to a transmembrane TNF, LTα, and a soluble TNF trimer, respectively. In contrast, anti-TNF antibody detects same levels of the 51 kDa soluble TNF trimer but very low levels of the 26 kDa transmembrane TNF compared with the etanercept detection. Recombinant rat TNF (rTNF; a 17 kDa soluble TNF monomer) was detected as a positive control, but a large amount of proteins (250 ng) was needed for the detection with etanercept compared with anti-TNF antibody (0.5 ng), as optimized in a separate experiment. Specificity of bands was confirmed by preabsorption of etanercept or anti-TNF antibody with rTNF prior to the immunoblotting procedure.
Behavioral test
After SNC, transient mechanical hypoalgesia usually develops for a brief period and then is replaced by mechanical hyperalgesia which lasts for weeks (Bester et al., 2000; George et al., 2004). We tested the effect of locally administered etanercept therapy on mechanical withdrawal thresholds using the von Frey test after SNC in line with the treatment protocols for the immunohistochemistry (Fig. 5). A robust increase in crush-induced mechanical hyperalgesia was evident 6, 7, 9, 10, 11, and 12 days after vehicle treatment in the SNC group (SNC+vehicle) relative to sham group (SHAM+vehicle). Locally administered etanercept therapy (SNC+etanercept) inhibited the decrease of the withdrawal thresholds relative to vehicle (SNC+vehicle). At 9 –12 days, the thresholds of animals receiving the locally administered etanercept therapy tended to decrease relative to sham (SHAM+vehicle), indicating the effective duration of an etanercept-induced effect might be limited.
Fig. 5.

Locally administered etanercept therapy attenuates mechanical hyperalgesia. von Frey mechanical withdrawal thresholds after SNC or sham operation and locally administered etanercept or vehicle therapy. All animals received two consecutive days of baseline testing before operation. Thereafter, the SNC+vehicle group displayed a mechanical hyperalgesia from 6 to 12 days after the crush. In contrast, the sham-operated, vehicle-treated group (SHAM+vehicle) displayed no mechanical sensitivity to von Frey stimuli. Locally administered etanercept attenuated mechanical hyperalgesia (SNC+etanercept). Data represent the mean withdrawal thresholds (gram force)±SEM of n=8/group. * P<0.05, ** P<0.01 by one-way ANOVA and Dunnett’s post hoc.
DISCUSSION
Etanercept has great promise in managing the progression of inflammatory diseases, and it has been used experimentally in painful neuropathy. Locally administered etanercept has potential as a targeted immunomodulating agent to treat local pathogenesis in neuropathic pain after peripheral nerve injury and might therefore minimize potential systemic adverse effects of etanercept administration (Tobinick and Britschgi-Davoodifar, 2003; Tobinick, 2009). This is perhaps the more appropriate method for treating neuropathic pain arising from a focal lesion. In the present study, we demonstrated three novel findings. First, locally administered etanercept reached the endoneurium of the injured but not the uninjured nerve. Its local distribution was evident as soon as 1 h after the application. Second, immunoblot analyses for etanercept demonstrated its preferential binding to transmembrane and trimer TNF isoforms. Third, locally administered etanercept inhibited pain-related behaviors in a rat SNC model.
Etanercept is a cloned and engineered fusion protein consisting of two identical chains of recombinant TNFR2 monomer fused to the Fc portion of human immunoglobulin G1 (Dower et al., 1990). We first evaluated whether the anti-human IgG antibody specifically reacted with the Fc portion of human IgG1 in etanercept using immunohistochemical methods. Results from immunohistochemistry demonstrated a relative lack of background antibody staining in normal nerve. In contrast, 1 h following intraneurial injection of etanercept, nerve showed widespread distribution of the etanercept– human IgG immunoreactive structures in the endoneurial space. Therefore, we confirmed that the human IgG antibody could specifically detect etanercept.
After its epineurial injection in uninjured nerve, etanercept uptake was not evident in the endoneurial space during the 96 h observation period. This lack of entry into the endoneurial space after the epineurial injection may be explained by the integrity of perineurial and blood–nerve barriers, which has tight junctions between adjacent cells along with tight endothelial junctions of vessels in the endoneurium (Olsson et al., 1966; Weerasuriya et al., 1980). Several studies have shown that there was minimal transfer of relatively small molecules (sodium fluorescein; MW 376 Da and horseradish peroxidase; MW 40,000 Da) into the normal sciatic nerve after both i.v. and perineurial injection of the sciatic nerve in intact animals (Malmgren et al., 1980; Myers et al., 1986; Stephen et al., 2006). Etanercept is a macromolecule with an apparent MW of approximately 150 kDa; its extravascular distribution in nervous system tissue is expected to be very small to nonexistent (Zhou, 2005). Thus, we speculate that etanercept does not reach the endoneurium of normal nerve in significant volume due to integrity of its perineurial and blood–nerve barriers. In support of this conclusion, our immunoblotting results demonstrated the ability of etanercept to bind to transmembrane TNF in normal nerves.
Interestingly, at 24 h after the nerve crush injury, widespread distribution of etanercept can be observed in the endoneurial space 1 h after its epineurial injection. SNC injury increases perineurial permeability within the first day after the crush at the site of the trauma (Olsson and Kristensson, 1973) and endoneurial permeability to morphological tracers within a few days to weeks after nerve crush (Myers et al., 1980; Weerasuriya et al., 1992). In the present study, when etanercept was administrated after the nerve injury, Schwann cells, fibroblasts, and endothelial cells already expressed local TNF. Local TNF alters the blood–nerve barrier causing upregulation of vascular adhesion molecules and attraction of hematogenous macrophages that enter the nerve during matrix metalloproteinase-related breakdown of the barrier (Bereta et al., 1993; Shubayev et al., 2006). Thus, we believe that cytokine-induced permeability of the blood–nerve barriers facilitates etanercept entry into the endoneurium after the nerve injury.
Fast local intraneurial delivery of etanercept 1 h after the application is consistent with the efficacy of locally administered soluble TNF receptors on the dorsal root ganglion (DRG) in reducing mechanical allodynia in the chronic DRG compression model (Homma et al., 2002). Pretreatment starting 2 days before spinal nerve ligation with systemically administered etanercept attenuated mechanical allodynia; however, treatment starting 1 or 7 days after spinal nerve ligation was not effective (Schäfers et al., 2003). These findings suggest that TNF plays an important role in the initiation of neuropathic pain. However, etanercept is administered most commonly by s.c. injection and its peak plasma concentrations are achieved 48 – 60 h after the injection, indicating its slow absorption (Zhou, 2005). Because locally administered etanercept reaches the endoneurial TNF in the injured nerve as soon as 1 h after the application, early administration of etanercept using interventional procedures such as nerve block may be effective in mitigating neuropathic pain after the nerve injury. In line with this speculation, our behavioral testing results demonstrated that etanercept that was locally administered 1 day after the crush injury attenuated crush-induced mechanical hyperalgesia.
Etanercept mainly co-localized with TNF-positive structures in dual immunofluorescence studies. We suggest that these structures are Schwann cells and macrophages, based on our immunohistochemical results and previous morphological studies of TNF co-localization (Wagner and Myers, 1996a; George et al., 2004), although we cannot exclude the possibility of co-localization with other cell types having similar morphological appearance in paraffin sections at the light microscopic level, such as mast cells. In addition, immunoblot analyses for etanercept demonstrated its preferential binding to a 26 kDa trans-membrane and the 51 kDa trimer TNF isoforms compared to soluble 17 kDa monomer. Accordingly, 125I-labeled TNF binding assays showed that etanercept preferentially binds to the 51 kDa trimer isoforms of soluble TNF not the 17 kDa monomer (Scallon et al., 2002) and transmembrane TNF preferentially binds to TNFR2 (Grell et al., 1995), a component of etanercept. In fact, etanercept can bind transfected transmembrane TNF expressed in human cell lines at a 1:1 ratio (Scallon et al., 2002). Current evidence suggests that TNF antagonists have dual functions and can act as antagonists by blocking tmTNF interactions with TNFRs, or as agonists by initiating reverse (receptor-transmembrane TNF ligand) signaling, leading to apoptosis or cytokine suppression (Tracey et al., 2008). For example, etanercept suppresses the secretion of lipopolysaccharide-induced endothelial cell apoptotic factor via reverse-signaling mechanism (Kirchner et al., 2004). In addition, an interesting new fusion protein of elastin-like polypeptide and TNFR2 designed for local anti-TNF therapy demonstrated attenuating levels of inflammatory cytokines (interleukin-1β, interleukin-6) after TNF stimulation of DRG explants (Shamji et al., 2008). Because TNF immunoreactive structures were decreased 96 h after the epineurial injection of etanercept compared with the injection of vehicle, locally administered etanercept might affect the level of TNF by reverse signaling. While the beneficial action of etanercept to antagonize bio-active TNF trimer and transmembrane isoforms in nerve are clear (Myers et al., 2006), clinical significance of reverse signaling remain to be elucidated.
Our immunoblot analyses using etanercept also demonstrated its strong binding to 25 kDa, presumably, a lymphotoxin α monomer (Nedwin et al., 1985). Etanercept is the only approved TNF antagonist that is known to bind and neutralize members of lymphotoxin family. There are no published values for binding affinities of etanercept to LT ligands, but reports indicate that etanercept binds to LTα3 with affinity comparable to or greater than that of soluble TNF (Tracey et al., 2008), consistent with our data. Further investigations are needed to fully elucidate the functional consequences of LT families in neuropathic pain.
In the present study, the elimination of etanercept from the endoneurial spaces was shown by 96 h after the epineurial injection. The half-life of etanercept is 102±30 h in human patients following a single s.c. injection (Enbrel Prescribing Information, 1998). Although the pattern of biodistribution, metabolism, and clearance may differ between rats and humans, the elimination of etanercept from the endoneurial spaces might be accelerated by fluid bulk flow dynamics such as the proximodistal endoneurial fluid flow we have previously characterized in the peripheral nervous system (Myers et al., 1988). Although TNF antagonist–TNF complexes are not static but constantly bind and release bioactive TNF based on the relative concentrations of drug and TNF, and the stoichiometry of the complexes (Tracey et al., 2008), after the elimination of etanercept, TNF immunoreactivity in nerve declined, compared with the vehicle (a 96 h time-point). It is possible, that the TNF-carrier effect of etanercept leads to redistribution of TNF from sites of inflammation to other tissues, followed by clearance by reticuloendothelial system in spleen and liver, Fc receptor-dependent intracellular degradation or filtration through the kidney (Lobo et al., 2004). However, our behavioral tests demonstrated that the mechanical thresholds of animals in the locally administered etanercept therapy group tended to decrease relative to sham group at 8 –11 days after the application, indicating the effective duration of etanercept might be limited. Thus, repeated injections or continuous delivery of etanercept (Zanella et al., 2008) might be a more effective protocol compared with a single acute injection.
CONCLUSION
In conclusion, locally administered etanercept reaches the endoneurial space in the injured nerve and preferentially binds to transmembrane and bioactive trimer TNF isoforms. We speculate that locally administered etanercept after nerve injury may exert more specific therapeutic effects in local lesions and minimize side effects compared with systemically administered etanercept.
Acknowledgments
The authors gratefully acknowledge Jennifer Dolkas for expert technical assistance. This work was supported by the Veteran Affairs Rehabilitation R&D Program. Disclosure statements: The devices and drugs that are the subject of this manuscript are not FDA approved for this indication. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Abbreviations
- ANOVA
analyses of variance
- BSA
bovine serum albumin
- DAB
3′-3-diamino-benzidine
- DAPI
4,6-diamidino-2-phe-nylindole
- DRG
dorsal root ganglia
- ECL
enhanced chemiluminescence
- Fc
fragment crystallizable
- IgG
immunoglobulin G
- LTα
lymphotoxin-alpha
- MW
molecular weight
- SNC
sciatic nerve crush
- sTNF
soluble tumor necrosis factor-alpha
- tmTNF
transmembrane tumor necrosis factor-alpha
- TNF
tumor necrosis factor-alpha
- TNFR2
tumor necrosis factor alpha receptor 2
References
- Bereta J, Bereta M, Cohen S, Cohen MC. Regulation of VCAM-1 expression and involvement in cell adhesion to murine microvascular endothelium. Cell Immunol. 1993;147:313–330. doi: 10.1006/cimm.1993.1072. [DOI] [PubMed] [Google Scholar]
- Bester H, Beggs S, Woolf CJ. Changes in tactile stimuli-induced behavior and c-Fos expression in the superficial dorsal horn and in parabrachial nuclei after sciatic nerve crush. J Comp Neurol. 2000;428:45–61. doi: 10.1002/1096-9861(20001204)428:1<45::aid-cne5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and metal-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55– 63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Deland FH. Intrathecal toxicity studies with benzyl alcohol. Toxicol Appl Pharmacol. 1973;25:153–156. doi: 10.1016/s0041-008x(73)80001-8. [DOI] [PubMed] [Google Scholar]
- Dower SK, Smith CA, Park LS. Human cytokine receptors. J Clin Immunol. 1990;10:289–299. doi: 10.1007/BF00917473. [DOI] [PubMed] [Google Scholar]
- Enbrel Prescribing Information. Immunex Corporation; Seattle, WA, USA: 1998. [Google Scholar]
- George A, Schmidt C, Weishaupt A, Toyka KV, Sommer C. Serial determination of tumor necrosis factor-a content in rat sciatic nerve after chronic constriction injury. Exp Neurol. 1999;160:124–132. doi: 10.1006/exnr.1999.7193. [DOI] [PubMed] [Google Scholar]
- George A, Buehl A, Sommer C. Wallerian degeneration after crush injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci Lett. 2004;372:215–219. doi: 10.1016/j.neulet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Grell M, Douni E, Wajant H, Löhden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Hochberg MC, Lebwohl MG, Plevy SE, Hobbs KF, Yocum DE. The benefit/risk profile of TNF-blocking agents: findings of a consensus panel. Semin Arthritis Rheum. 2005;34:819–836. doi: 10.1016/j.semarthrit.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor alpha to the normal and mechanically compressed lumbar ganglia in the rat. Pain. 2002;95:239–246. doi: 10.1016/S0304-3959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- Kato K, Kikuchi S, Konno S, Sekiguchi M. Participation of 5-hydroxytryptamine in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Spine. 2008;33:1330–1336. doi: 10.1097/BRS.0b013e318173298b. [DOI] [PubMed] [Google Scholar]
- Kirchner S, Holler E, Haffner S, Andreesen R, Eissner G. Effect of different tumor necrosis factor (TNF) reactive agents on reverse signaling of membrane integrated TNF in monocytes. Cytokine. 2004;28:67–74. doi: 10.1016/j.cyto.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Chattopadhyay S, Kato K, Dolkas J, Kikuchi SI, Myers RR, Shubayev VI. MMPs initiate Schwann cell-mediated MBP degradation and mechanical nociception after nerve damage. Mol Cell Neurosci. 2008;39:619–627. doi: 10.1016/j.mcn.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharmacol Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- Malmgren LT, Olsson Y. Differences between the peripheral and the central nervous system in permeability to sodium fluorescein. J Comp Neurol. 1980;191:103–117. doi: 10.1002/cne.901910106. [DOI] [PubMed] [Google Scholar]
- Myers RR, Powell HC, Shapiro HM, Costello ML, Lampert PW. Changes in endoneurial fluid pressure, permeability, and peripheral nerve ultrastructure in experimental lead neuropathy. Ann Neurol. 1980;8:392–401. doi: 10.1002/ana.410080410. [DOI] [PubMed] [Google Scholar]
- Myers RR, Kalichman MW, Reisner LS, Powell HC. Neurotoxicity of local anesthetics: altered perineurial permeability, edema, and nerve fiber injury. Anesthesiology. 1986;64:29–35. [PubMed] [Google Scholar]
- Myers RR, Rydevik BL, Heckman HM, Powell HC. Proximodistal gradient in endoneurial fluid pressure. Exp Neurol. 1988;102:368–370. doi: 10.1016/0014-4886(88)90233-6. [DOI] [PubMed] [Google Scholar]
- Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006;11:8–20. doi: 10.1016/S1359-6446(05)03637-8. [DOI] [PubMed] [Google Scholar]
- Nedwin GE, Naylor SL, Sakaguchi AY, Smith D, Jarrett-Nedwin J, Pennica D, Goeddel DV, Gray PW. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985;13:6361– 6373. doi: 10.1093/nar/13.17.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol (Berl) 1966;7:1–15. doi: 10.1007/BF00686605. [DOI] [PubMed] [Google Scholar]
- Olsson Y, Kristensson K. The perineurium as a diffusion barrier to protein tracers following trauma to nerves. Acta Neuropathol (Berl) 1973;23:105–111. doi: 10.1007/BF00685764. [DOI] [PubMed] [Google Scholar]
- Redford EJ, Hael SM, Smith KJ. Vascular changes and demyelination induced by the intraneural injection of tumor necrosis factor. Brain. 1995;118:869– 878. doi: 10.1093/brain/118.4.869. [DOI] [PubMed] [Google Scholar]
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418– 426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Derm. 2004;15:280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- Shamji MF, Jing L, Chen J, Hwang P, Ghodsizadeh O, Friedman AH, Richardson WJ, Setton LA. Treatment of neuroinflammation by soluble tumor necrosis factor receptor type II fused to a thermally responsive carrier. J Neurosurg Spine. 2008;9:221–228. doi: 10.3171/SPI/2008/9/8/221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Upregulation and interaction of TN-Falpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83– 89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNF alpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31:407– 415. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Schroder JM. HLA-DR expression in peripheral neuropathies: the role of Schwann cells, resident and hematogenous macrophages, and endoneurial fibroblasts. Acta Neuropathol (Berl) 1995;89:63–71. doi: 10.1007/BF00294261. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schäfers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- Stephen EA, Johnny YBA, Andreas F, Quinn HH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105:146–153. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993;45:175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133:170–177. doi: 10.4414/smw.2003.10163. [DOI] [PubMed] [Google Scholar]
- Tobinick E, Davoodifar S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc-related pain: a study of clinical observations in 143 patients. Curr Med Res Opin. 2004;20:1075–1085. doi: 10.1185/030079903125004286. [DOI] [PubMed] [Google Scholar]
- Tobinick E. Perispinal etanercept for neuroinflammatory disorders. Drug Discov Today. 2009;14:168–177. doi: 10.1016/j.drudis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Schwann cells produce TNF-alpha: expression in injured and non-injured nerves. Neuroscience. 1996a;73:625– 629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Endoneurial injection of TNF-a produces neuropathic pain behaviors. Neuroreport. 1996b;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A, Rapoport SI, Taylor RE. Perineural permeability increases during wallerian degeneration. Brain Res. 1980;192:581–585. doi: 10.1016/0006-8993(80)90911-7. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A, Hockman CH. Perineurial permeability to sodium during wallerian degeneration in rat sciatic nerve. Brain Res. 1992;581:327–333. doi: 10.1016/0006-8993(92)90727-q. [DOI] [PubMed] [Google Scholar]
- Zanella JM, Burright EN, Hildebrand K, Hobot C, Cox M, Christoferson L, McKay WF. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine. 2008;33:227–234. doi: 10.1097/BRS.0b013e318162340a. [DOI] [PubMed] [Google Scholar]
- Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490– 497. doi: 10.1177/0091270004273321. [DOI] [PubMed] [Google Scholar]