Abstract
Importance
The use of a non-mydriatic camera for retinal imaging combined with the remote evaluation of images at a telemedicine reading center has been advanced as a strategy for diabetic retinopathy (DR) screening, particularly among patients with diabetes from minority populations with low eye care utilization.
Objective
To examine the rate and types of DR identified through a telemedicine screening program using a non-mydriatic camera, as well as the rate of other ocular findings.
Design
Cross-sectional.
Setting
Four urban clinic or pharmacy settings in the United States serving predominantly minority and uninsured persons with diabetes.
Participants
Persons age ≥ 18 years old who have type 1 or 2 diabetes and present to the community-based settings.
Main Outcome Measure
Percentage of DR detection including type of DR, and percentage of detection of other ocular findings.
Results
A total of 1,894 persons participated in the screening program across sites, with 21.7% having DR in at least one eye. The most common type of DR by far was background DR, which was present in 94.1% of all participants with DR. Almost half of those screened had ocular findings other than DR with 30% of other findings being cataract.
Conclusions and Relevance
In a DR telemedicine screening program in urban clinic/pharmacy settings in the US serving predominantly minority populations, 1 in 5 persons with diabetes screened positive for DR. The vast majority of DR was background indicating high public health potential for intervention in DR’s earliest phases when treatment can prevent vision loss. Other ocular conditions were detected at a high rate, a collateral benefit of DR screening programs that may be under appreciated.
There are approximately 29.1 million persons with diabetes in the United States,1 with the prevalence expected to increase dramatically in future decades.2 A common diabetes complication is diabetic retinopathy (DR),1 whose prevalence is expected to increase.3 Approximately 4.4% of Americans over 40 years old have DR.4 The personal and economic burdens of DR are noteworthy. DR is the leading cause of new blindness among working-age adults in the US,1 with an estimated economic burden of $493 million per year.5 Prevention and optimal management of DR consists of tight glycemic and blood pressure control, routine dilated comprehensive eye examination, timely treatment, and patient education.6-8 The American Academy of Ophthalmology (AAO), American Optometric Association, and American Diabetes Association recommend routine, annual dilated examination for persons with diabetes -- for type 1 diabetes, beginning 5 years after diagnosis, and for type 2, at the time of diagnosis and annually thereafter.9-11 The percentage of Americans with diabetes annually receiving dilated eye care is low. Data analysis of the Behavioral Risk Factor Surveillance System revealed a dilated examination annual rate of 63.3% in persons with self-reported diabetes.12 Among minority populations with diabetes, the annual eye exam rate is even lower, approximately 32-49% among African Americans and Hispanics.13-16 Common barriers to care for minority populations are lack of accessibility (scarcity of providers in communities; transportation challenges) and cost.17-21
The implementation of DR screening programs is associated with an increase in the percentage of people with diabetes receiving retinal screenings, a lower rate of those with sight-threatening DR detected at subsequent screenings, and a lower incidence and prevalence of blindness in the population.22-25 The use of a non-mydriatic camera for retinal imaging combined with the remote evaluation of images at a telemedicine reading center has been advanced as a strategy for DR screening and is used widely in national screening programs.26-30 Studies show that DR screening results using non-mydriatic cameras via telemedicine agree with gold-standard dilated fundus photography.31-33 This screening strategy may be particularly relevant for people with diabetes who face barriers due to transportation and cost in seeking comprehensive dilated eye care from an ophthalmologist or optometrist.34,35 Screenings are brief compared to dilated examination, less burdensome since dilation is not required, and take place in the primary care setting or in novel settings such as pharmacies. Patients express satisfaction with this screening approach.36-38 Clinic personnel can be trained to operate the camera and upload images to a reading center.33,39 There is growing evidence that DR screening programs, combined with telemedicine, are cost-effective interventions.25,40,41
Here we seek to examine the feasibility and effectiveness of non-invasive DR screening using a non-mydriatic camera combined with a telemedicine reading center. We focus on screening settings accessible to patients with diabetes in four cities in the United States, namely primary care clinics and pharmacies providing services to largely uninsured and/or minority populations.
Methods
This study was approved by the Institutional Review Boards of Johns Hopkins University (JHU), University of Alabama at Birmingham (UAB), University of Miami (UM), Wake Forest University, and Wills Eye Hospital (WEH), and followed the tenants of the Declaration of Helsinki. The protocol has been described in detail previously;42 our focus here is on the rates of DR and other ocular findings identified through the screening. Of the four study sites, three were based in outpatient clinics serving uninsured or underinsured populations with high representation of persons from ethnic/racial minorities. A fourth site was an outpatient pharmacy setting in an urban environment. Persons ages ≥ 18 years old who had been diagnosed with diabetes (Type 1 or 2) were invited to participate in a DR screening. Site-specific information is: (1) Birmingham, Alabama (UAB site): The Cooper Green Mercy Health Service’s internal medicine clinic is a county-operated, safety-net clinic serving county residents regardless of ability to pay or insurance status. English-speaking patients with diabetes were invited to participate from January to July 2012. (2) Miami, Florida (UM site): The Jessie Trice Community Health Center is a federally-qualified health center serving the uninsured or underinsured in the county. Participants were recruited via flyers and by referral from local physicians. Participants spoke English, Spanish, or Creole. Screening was from February 2012 to March 2013. (3) Winston-Salem, North Carolina (JHU site): The Downtown Health Plaza (DHP), affiliated with Wake Forest School of Medicine, serves low-income persons residing in the downtown area. Physicians and staff invited English-speaking individuals with diabetes to participate in screening, which was from May to October 2013. (4) Philadelphia, Pennsylvania (WEH site): The outpatient pharmacy at Thomas Jefferson University Hospital is located in an urban environment. English or Spanish speaking persons with diabetes were invited for screening by pharmacy personnel when picking up medications for diabetes, family practice physicians in nearby offices, flyers, or advertisements in newspapers. The screening program took place from December 2011 to March 2013. Participants provided informed consent.
Participants completed a questionnaire providing contact information, demographics, age when first told by a physician that they had diabetes, whether they knew their hemoglobin A1c level, when they had their most recent dilated eye examination, smoking status, and health insurance status. They were asked if they needed assistance in making an eye appointment once their DR screening results were available.
Ocular imaging was performed by trained technicians using a non-mydriatic camera with auto-focus (Model AFC-230, Nidek Inc., Fremont, CA). Dark fabric was draped over the participant’s head and/or the room was darkened. Technicians were trained by the WEH telemedicine reading center in camera use and followed the manufacturer’s standard operating instructions. Three photos were taken per eye: anterior segment, nasal fundus, temporal fundus. If images were blurry, additional images were taken to achieve satisfactory image quality. Images were generated using NavisLite software (Nidek Inc, Fremont, CA) and uploaded to a HIPPA-compliant secure website at WEH.
Trained/certified readers at WEH read the images. A HIPPA-compliant proprietary software program was used for image management and report generation. Readers evaluated images using the National Health Service’s DR grading classification system (Table 1).43 Cataracts were graded according to a protocol using anterior segment photographs. Established algorithms were used to identify other ocular disease including hypertensive retinopathy, age-related macular degeneration, and glaucoma. As described previously,42 a 10% random sample of images labeled normal by the readers were reviewed by an ophthalmologist; none were found to have signs of pathology. The intra-rater kappa coefficient for readers with respect to DR findings was 0.72 with 88.8% agreement. The inter-grader kappa coefficient for DR findings was 0.62 (95% CI 0.51 – 0.73) with agreement of 84.1%. Readers assigned preliminary grades within 48 hours of image upload. Ocular pathology other than DR was recorded. A retina specialist reviewed images showing signs of DR or other ocular findings.
Table 1.
Classifications used to grade Diabetic Retinopathy (DR) presence and severity based on the National Health Service Grading Classification System.43 The last column is the American Academy of Ophthalmology’s recommendations for diabetic patient follow-up.44
| Grade | Description | Recommendation |
|---|---|---|
| R0 | NO DIABETIC RETINOPATHY | Re-evaluate in twelve months with either eye care specialist or photographic screening |
| None | ||
| Isolated cotton wools spots (1 or more) in the absence of any microaneurysm or haemorrhage |
||
| R1 | BACKGROUND DR | Refer to eye care provider |
| 1 or more microaneurysm(s) | ||
| 1 or more retinal haemorrhage(s) | ||
| Any exudates caused by DR | ||
| R2 | PRE-PROLIFERATIVE DR | Refer to ophthalmologist promptly |
| Intraretinal microvascular abnormality (IRMA) | ||
| Venous beading | ||
| Venous loop or reduplication | ||
| Multiple deep, round or blot haemorrhages | ||
| R3 | PROLIFERATIVE DR | Refer to ophthalmologist promptly |
| New vessels on the disc (NVD) | ||
| New vessels elsewhere (NVE) | ||
| Pre-retinal or vitreous hemorrhage | ||
| Pre-retinal fibrosis with or without tractional retinal detachment due to DR |
||
| M | MACULOPATHY | Refer to ophthalmologist promptly |
| Exudate within 1 disc diameter (DD) of the center of the fovea | ||
| Circinate or group of exudates within the macula | ||
| Any microaneurysm or haemorrhage within 1 DD of the center of the fovea only if associated with a best visual acuity of 20/40 or worse |
||
| P | PHOTOCOAGULATION | Refer to eye care provider |
| Focal/grid to macula | ||
| Peripheral scatter | ||
| U | UNCLASSIFIABLE/UNGRADABLE | Refer to eye care provider |
| Due to poor photographic location, focus, or contrast |
Results from the reading center’s review of images were summarized in a screening report sent electronically to the participant’s site. The coordinator mailed a letter to participants describing the results and recommended follow-up care based on the findings; the recommendations were derived from AAO’s guidelines for DR follow-up, based on the presence and degree of DR (Table 1).44 For participants whose reports recommended normal (non-urgent) referral or follow-up (R0, R1, P), the letter encouraged him/her to seek an appointment for a dilated eye examination on an annual basis. For abnormal results for R1 or P, the letter encouraged the participant to seek an appointment for a dilated eye examination “within the next few months”. For individuals whose reports recommended prompt referral to an eye care provider due to DR or maculopathy (R2, R3, M), the coordinator telephoned the participant within 48 hours of receiving the report from the reading center advising the participant of the recommendation and offered to schedule an appointment with an ophthalmologist. Up to 5 telephone attempts were made to reach participant. A letter was also mailed to the participant with results and recommendations. Patients with images deemed ungradable due to poor quality were advised to follow-up with an appointment for a dilated examination. Results were sent to the patient’s primary care provider if he/she had requested this
Data Management and Statistical Analysis
Each site oversaw its own data entry and securely transmitted it to the data coordinating center at UAB, where a multi-site database was constructed and data analysis performed. Chi-square tests and analysis of variance was used to compare categorical and continuous variables, respectively, across groups. P-values of ≤0.05 (two-tailed) were considered statistically significant.
Results
A total of 1,894 persons participated in screening (Table 2), with 31.7% of the sample from Birmingham, 32.1% from Miami, 26.7% from Philadelphia and 9.5% from Winston-Salem. Mean age at each site was similar, ranging from 53 to 55 years old. There were more women (63.1%) than men (36.9%). The majority of those screened were ethnic/racial minorities (88%); however, there were site differences. In Birmingham most participants were African American (84.3%); in Philadelphia and Winston-Salem, approximately 68% of participants were African American with a larger percentage of whites than in Birmingham, whereas in Miami 63.6% were Hispanic, Haitian or Cuban American and 33.9% African American, with very few whites screened.
Table 2.
Other characteristics of sample stratified by site and overall
| Characteristic | Birmingham AL N = 600 |
Miami FL N = 608 |
Philadelphia PA N = 506 |
Winston- Salem NC N = 180 |
Total N = 1894 |
|---|---|---|---|---|---|
| Age (years), M (SD) | 53.6 (10.6) | 55.2 (9.1) | 53.8 (10.6) | 55.7 (13.0) | 54.4 (11.0) |
| Gender, n (%) | |||||
| Women | 393 (65.5) | 398 (65.6) | 282 (56.2) | 118 (66.3) | 1191 (63.1) |
| Men | 207 (34.5) | 209 (34.4) | 220 (43.8) | 60 (33.7) | 696 (36.6) |
| Race/ethnicity, n (%) | |||||
| Black | 506 (84.3) | 206 (33.9) | 345 (68.2) | 124 (68.9) | 1181 (62.4) |
| Hispanic | 2 (0.3) | 250 (41.1) | 14 (2.8) | 15 (8.3) | 281 (14.8) |
| White | 87 (14.5) | 8 (1.3) | 95 (18.8) | 38 (21.1) | 228 (12.0) |
| Haitian | 0 (0) | 70 (11.5) | 0 (0) | 0 (0) | 71 (3.7) |
| Cuban | 0 (0) | 67 (11.0) | 1 (0.2) | 0 (0) | 68 (3.6) |
| Asian | 3 (0.5) | 2 (0.3) | 23 (4.6) | 0 (0) | 28 (1.5) |
| Native Hawaiian | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) | 1 (< 0.1) |
| Native American | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) | 1 (< 0.1) |
| Other1 | 2 (0.3) | 5 (0.8) | 27 (5.3) | 1 (0.6) | 35 (1.8) |
| Age (years), M (SD) | 53.6 (10.6) | 55.2 (9.1) | 53.8 (10.6) | 55.7 (13.0) | 54.4 (11.0) |
| Age at diabetes diagnosis, years, M (SD) |
43.9 (12.6) | 46.6 (11.1) | 44.0 (15.7) | 41.2 (14.6) | 44.5 (13.3) |
| Duration of diabetes, years, M (SD) |
9.7 (9.4) | 8.6 (8.2) | 9.9 (12.5) | 14.6 (13.5) | 9.9 (10.5) |
| Currently smokes2, n (%) | 172 (28.7) | 88 (14.5) | 109 (21.6) | 61 (34.1) | 430 (22.8) |
| Knows A1C level | 81 (13.5) | 184 (30.5) | 207 (42.4) | 58 (32.4) | 530 (28.3) |
| Has any type of health insurance, n (%) |
177 (29.5) | 136 (22.6) | 370 (79.2) | 92 (51.7) | 775 (42.0) |
| Last dilated eye examination, n (%) |
|||||
| Within past year | 317 (52.8) | 155 (25.5) | 163 (32.4) | 48 (27.0) | 683 (32.2) |
| > 1 year ago but < 2 years ago |
85 (14.2) | 111 (18.3) | 116 (23.1) | 38 (21.4) | 350 (18.5) |
| ≥ 2 years | 153 (25.5) | 273 (45.0) | 168 (33.4) | 62 (34.8) | 656 (34.7) |
| Never | 23 (3.8) | 68 (11.2) | 37 (7.4) | 14 (7.9) | 142 (7.5) |
| Don’t know | 22 (3.7) | 0 (0) | 19 (3.8) | 16 (9.0) | 57 (3.0) |
Multi-racial or no data available
Refers to smoking cigarettes, pipes, cigars or any tobacco use
M = mean, SD = standard deviation
Mean age of diabetes diagnosis by self-report was in the 40s (Table 2). Mean duration of diabetes was approximately 8-10 years in Birmingham, Miami, and Philadelphia, but longer (14.6 years) in Winston-Salem. Approximately 25% of the sample reported smoking or using tobacco. The percentage of patients with health insurance was wide ranging, from 22.6% at Miami to 79.2% in Philadelphia. There was site variability for when participants reported receiving their last dilated eye examination. About half of Birmingham participants reported having a dilated eye examination within the past year, yet at other sites, those reporting eye care utilization within the past year ranged from 25.5–32.4%. At Miami almost half (45.0%) reported receiving a dilated examination 2 or more years ago and 11.2% reporting never having a dilated examination. Approximately 30–42% of participants at Miami, Philadelphia, and Winston-Salem indicated they knew their A1C level, but only 13.5% in Birmingham.
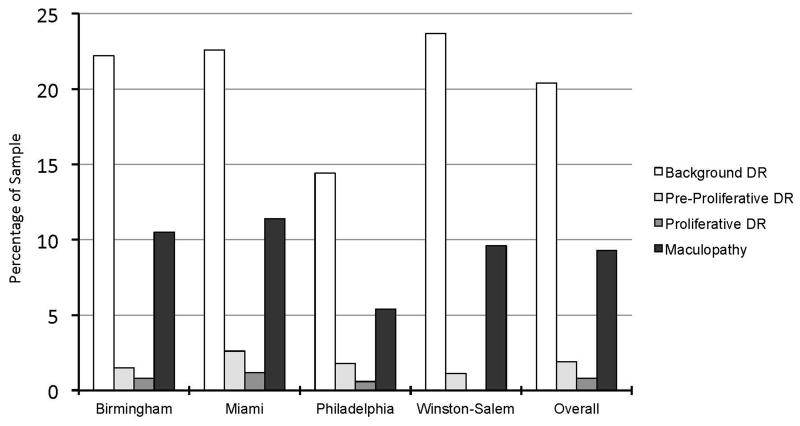
Across the sample, 21.7% of participants had DR (background, pre-proliferative, proliferative, and/or maculopathy) in either eye; by site, Birmingham 23.5%, Miami 24.1%, Philadelphia 15.8% and Winston-Salem 24.3%. Figure 1 shows the percentage of participants with specific types of DR in either eye. At Birmingham, Miami, and Winston-Salem, background DR was present in 22-23% of participants, but lower in Philadelphia (14%). Among patients with DR, the vast majority had background DR (94.1%), with rates of pre-proliferative and proliferative DR ranging from 0-11.4% depending on the site. The proportion of participants with maculopathy in the overall sample was 9.3%. The rate of maculopathy was similar in Birmingham, Miami, and Winston-Salem, ranging from 9-11%, but was approximately half that rate in Philadelphia (5.4%). Depending upon the site, no or very few participants displayed evidence of having had photocoagulation treatment. Twelve percent had at least one ungradable image in one or both eyes.
Figure 1.
Percentage of sample with various levels of diabetic retinopathy in either eye stratified by site and overall
The prevalence of DR (regardless of type) was similar for whites versus ethnic/racial minority groups taken together (22.6% vs. 21.6%, p = 0.739), and was unrelated to time since dilated eye examination (p = 0.438), smoking/tobacco use (p = 0.400), health insurance status (p = 0.211), or knowledge of hemoglobin A1C level (p = 0.819). Those with DR had a longer duration of diabetes than those without DR (mean 13.7 years (SD 9.8) vs. mean 8.8 (SD 10.4), p < 0.0001).
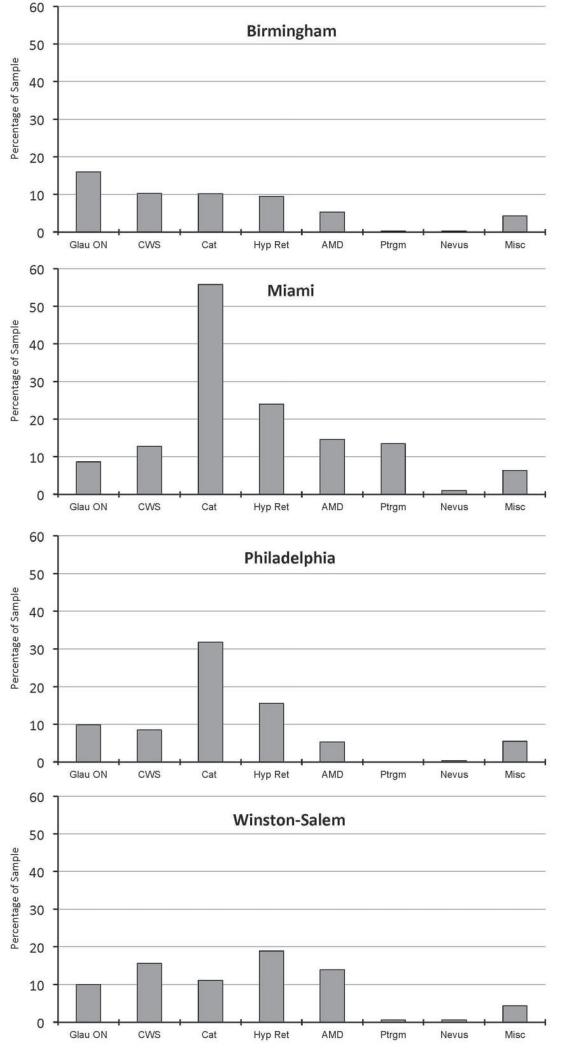
Almost half of participants (44.2%) had ocular findings besides DR, with variability across sites. Miami had double the prevalence of other ocular findings (61.1%) compared to Birmingham (29.7%), with Philadelphia and Winston-Salem between the two extremes. Table 3 lists the percentage of other ocular findings in either eye by type in the overall sample. The most common other finding was cataract, present in almost ⅓ of participants. Hypertensive retinopathy, followed by glaucomatous/optic nerve findings, cotton wool spots and age-related macular degeneration were also noted. Pterygium notations were much less common, and nevus was rare. Figure 2 displays types of other ocular findings stratified by site.
Table 3.
Number and percentage of patients with other ocular findings
| Type of Other Ocular Findings | Total n = 1894 n (%) |
|---|---|
| Cataract | 581 (30.7) |
| Hypertensive retinopathy | 316 (16.7) |
| Cotton wool spots | 211 (11.1) |
| Glaucomatous or optic nerve findings | 197 (10.4) |
| Age-related macular degeneration (AMD) | 174 (9.2) |
| Pterygium | 90 (4.8) |
| Nevus | 11 (0.6) |
| Miscellaneous1 | 101 (5.3) |
Other ocular findings classified as miscellaneous are those that were noted on the screening reports in ≤ 5 participants at all sites. These included retinal scar, epiretinal membrane, myelinated nerve fibers, vitreous opacity, asteroid hyalosis, corneal findings, embolic material, glia, macular hole, choroidal folds, peripapillary atrophy, vein or artery occlusion, iris findings, melanocytoma, horizontal folds, retinal striae, and posterior vitreous detachment
Figure 2.
Percentage of the sample having other ocular findings in either eye stratified by site.
Discussion
One in five patients with diabetes screened positive for DR using a telemedicine screening program in four urban settings in the US serving predominantly minority populations. This rate is similar to that reported in two previous US studies also using telemedicine reading centers.45,46 Three of our sites, based at primary care clinics, had very similar rates of DR, 23-24%, but the Philadelphia site (a pharmacy) was lower (15.8%), which could result from many factors. Patients who fill prescriptions may be more medically adherent and less likely to have diabetes complications.47,48 Philadelphia had a higher percentage with health insurance (79.2%) as compared to other sites (34.6%). Patients with diabetes having health insurance are more likely to have better glycemic control and lower rates of diabetic eye disease compared to those lacking health insurance.16,49,50 Given the lower DR rate in the pharmacy cohort, it may be that screening in this setting will have lower yield than in outpatient clinics, an issue for further study.
The majority (94.1%) of persons with DR had background DR, which is similar to screening programs in primary care settings in the US and Canada.28,29,34,36,45,46 Patients with proliferative disease were rare at all sites. From a public health perspective, our finding that most patients with DR had background DR, with almost 10% of persons with diabetes screened having maculopathy, indicates high potential for intervention in DR’s earliest phases when treatment can prevent vision loss. In contrast to a United Kingdom report,51 the rate of DR detected in our program was not higher among ethnic/racial minorities compared to whites of European origin. At first glance this may seem paradoxical since the prevalence of DR among African Americans and Hispanics in the US is higher than in whites of European descent.52,53 Only 12% of participants were white; this small sample size may have made it difficult to evaluate white versus racial/ethnic minority differences in our screening program.
DR was unrelated to smoking status, health insurance status, and knowledge of one’s hemoglobin. These findings highlight the potential benefit of a DR screening program for the general population of people with diabetes, rather than a more narrow approach for only a selected subpopulation. However, DR was more likely in persons with longer durations of diabetes, a well-established risk factor. This finding underscores the importance for screening programs to target those with long-standing diabetes.
The rate of self-reported dilated eye care utilization in the past year was low for the overall sample (32.2%), suggesting that DR screening in these settings could fulfill a critical role for patients with diabetes not routinely accessing annual dilated care. There were interesting differences across sites in the reported dilated examination rates. In Birmingham over half (52.8%) reported having a dilated examination within the past year, whereas at the other sites dilated examination rate was considerably lower (25-32%). Unlike the other sites, Birmingham’s county-operated health system has an ophthalmology clinic. The other primary care sites did not have onsite eye services. This may have contributed to a higher eye care utilization rate among Birmingham patients, since care was accessible onsite.54 The situation was inverted in Miami where almost half (45%) of those screened reported not having a dilated eye examination in ≥ 2 years, with 11.2% reporting never having one. Previously the clinic had an on-site optometrist which was closed prior to study start. It remains to be determined whether these factors influenced the lower rate of eye care utilization.
Almost half of participants had other ocular findings. This is an important collateral benefit of DR screening programs since many ocular findings detected are potentially sight-threatening conditions (e.g., cataract, glaucoma, macular degeneration) yet are amenable to vision-preserving treatments. The most common other ocular finding was cataract. Glaucomatous/optic nerve findings were the most commonly noted conditions in Birmingham, not surprising given the high percentage of Blacks in the sample (84.3%), who have 4-5 times greater risk for glaucoma-associated disorders as compared to whites.55,56 Pterygium occurred in over 10% of persons screened in Miami but was rare at other sites, which may reflect the higher risk of pterygium for persons residing closer to the equator or with prolonged ultraviolet light exposure.57,58
The rate of other ocular findings differed substantially among sites. Miami had the highest other ocular finding rate at over 60%. In contrast, Birmingham had half the rate (~30%). While DR screening has the additional benefit of identifying other potentially sight threatening conditions, the particular lesion types and their frequency in the population screened depends on demographics, lifestyle, and utilization of comprehensive eye services.
Study strengths include a focus on evaluating a DR screening program in urban settings that predominantly serve patients with diabetes from racial/ethnic minorities and uninsured or underinsured populations, an approach receiving only scant attention previously.59,60 Our target populations have among the lowest comprehensive eye care utilization rates in the US, thus being at high risk for undetected DR. Screening incorporated a non-mydriatic camera that is rapid and less burdensome and a central reading center through telemedicine. Multiple sites allowed us to implement the program in diverse geographic locations. Study limitations include selection bias during enrollment; it is unknown whether those who participated versus did not were systemically different. Information is unavailable on the percentage of persons who declined participation. Although inclusion of four different sites enhances generalizability, sites differed in many ways; factors contributing to site differences cannot be precisely determined, yet can be addressed in future research. One site had fewer participants than the others because of delayed start-up. Although here we have not focused on patient follow-up for recommended eye appointments, acuity screening, and patient satisfaction, these issues will be addressed in subsequent reports.
In a DR telemedicine screening program in urban clinic and pharmacy settings in the US serving predominantly minority populations, 1 in 5 persons with diabetes screened positive for DR. Most had background DR, suggesting high potential for intervention in DR’s earliest phases when management can prevent vision loss. Other ocular conditions were detected in almost 50% of patients screened, a potentially under-appreciated feature of DR screening programs for preventing vision loss.
Acknowledgments
A complete listing of members of the INSIGHT Research Group is in reference 42. Cynthia Owsley and Gerald McGwin had full access to all the data in the study and take responsibility for the integrity of the data at the coordinating center and the accuracy of the data analysis.
Financial Support
This research was supported through Centers for Disease Control and Prevention (CDC) Cooperative Agreements with Johns Hopkins University, University of Alabama at Birmingham, University of Miami, and Wills Eye Hospital (5U58DP002651, 5U58DP002652, 5U58DP002653, 5U58DP002655). CDC participated in the design and conduct of the study, analysis and interpretation of the data, and preparation, review and approval of the manuscript. The grantees received additional support directly from Alcon Research Institute (Johns Hopkins University), the EyeSight Foundation of Alabama (University of Alabama at Birmingham), Research to Prevent Blindness (University of Alabama at Birmingham), and the Buck Trust (University of Alabama at Birmingham); these other funding sources had no role in the design or conduct of the study, analysis and interpretation of the data, and preparation, review and approval of the manuscript. Nidek provided cameras and operator training free of charge.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of Interest Disclosure: Nidek Inc. loaned the fundus cameras used in this study at no charge. David S. Friedman has consulted to Nidek Inc about devices unrelated to this project. None of the authors have any proprietary interests or conflicts of interest related to this article.
Contributions of Authors
All authors participated in the design of the study, interpretation of the results, and preparation of the manuscript and its revision. Drs. Owsley, Lee, Lam, Friedman, Gower, Haller, and Hark oversaw implementation of data collection and management at individual sites. Dr. McGwin conducted the data analysis.
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. US Department of Health and Human Services; Atlanta GA: 2014. http://www.cdc.gov/diabetes/pubs/statsreport14.htm. [Google Scholar]
- 2.Boyle JP, Honeycutt AA, Narayan KM, et al. Projection of diabetes burden through 2050. Diabetes Care. 2001;24:1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 3.Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseaes among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126:1740–1747. doi: 10.1001/archopht.126.12.1740. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rein D, Zhang P, Wirth K, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: A systematic review. JAMA. 2007;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 7.Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. Am J Public Health. 1999;89:1878–1882. doi: 10.2105/ajph.89.12.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sloan FA, Grossman DS, Lee PP. Effects of receipt of guideline-recommended care on onset of diabetic retinopathy and its progression. Ophthalmology. 2009;116:1515–1521. doi: 10.1016/j.ophtha.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Ophthalmology Retina Panel . Preferred Practice Pattern Guidelines Diabetic Retinopathy. American Academy of Ophthalmology; San Francisco CA: 2012. http://one.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp--september-2008-4th-print. [Google Scholar]
- 10.Cavallerano J. Optometric Clinical Practice Guideline, Care of the Patient with Diabetes Mellitus, Reference Guide for Clinicians. American Optometric Association; St. Louis MO: 2009. http://www.aoa.org/documents/CPG-3.pdf. [Google Scholar]
- 11.American Diabetes Association Standards of medical care in diabetes -- 2013. Diabetes Care. 2013;36(Supplement):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thomspson TJ. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 13.MacLennan PA, McGwin G, Jr, Heckemeyer C, et al. Eye care utilization among a high-risk diabetic population seen in a public hospital’s clinics. JAMA Ophthalmol. 2014;132:162–167. doi: 10.1001/jamaophthalmol.2013.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez CM, Febo-Vázquez U, Guzmán M, Ortiz AP, Suárez E. Are adults diagnosed with diabetes achieving the American Diabetes Association clinical practice recommendations? P R Health Sci J. 2012;31:18–23. [PMC free article] [PubMed] [Google Scholar]
- 15.Paz SH, Varma R, Klein R, Wu J, Azen S. Noncompliance with vision care guidelines in Latinos with type 2 diabetes mellitus. Ophthalmology. 2006;116:1372–1377. doi: 10.1016/j.ophtha.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Baker RS, Watkins NL, Wilson MR, Bazargan M, Flowers CW., Jr. Demographic and clinical characteristics of patients with diabetes presenting to an urban public hospital ophthalmology clinic. Ophthalmology. 1998;105:1373–1379. doi: 10.1016/S0161-6420(98)98015-0. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Improving the nation’s vision health: A coordinated public health approach. http://www.cdc.gov/visionhealth/pdf/improving_nations_vision_health.pdf.
- 18.Owsley C, McGwin G, Scilley K, Girkin CA, Phillips JM, Searcey K. Perceived barriers to care and attitudes about vision and eye care: Focus groups with older African Americans and eye care providers. Invest Ophthalmol Vis Sci. 2006;47:2797–2802. doi: 10.1167/iovs.06-0107. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan PA, Owsley C, Searcey K, McGwin G., Jr. A survey of Alabama eye care providers in 2010. BMC Ophthalmol. 2014;3:44. doi: 10.1186/1471-2415-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rask KJ, Williams MV, Parker RM, McNagny SE. Obstacles predicting lack of a regular provider and delays in seeking care for patients at an urban public hospital. JAMA. 1994;271:1931–1933. [PubMed] [Google Scholar]
- 21.Chou C-F, Sherrod CE, Zhang X, et al. Barriers to eye care among people aged 40 years and older with diagnosed diabetes, 2006-2010. Diabetes Care. 2014;37:180–188. doi: 10.2337/dc13-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefánsson E, Bek T, Porta M, Larsen N, Kristinsson JK, Agardh E. Screening and prevention of diabetic blindness. Acta Ophthalmol. 2000;78:374–385. doi: 10.1034/j.1600-0420.2000.078004374.x. [DOI] [PubMed] [Google Scholar]
- 23.Forster AS, Forbes A, Dodhia H, et al. Changes in detection of retinopathy in type 2 diabetes in the first 4 years of a population-based diabetic eye screening program: retrospective cohort study. Diabetes Care. 2013;36:2663–2669. doi: 10.2337/dc13-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg S, Jani PD, Kshirsagar AV, King B, Chaum E. Telemedicine and retinal imaging for improving diabetic reitnopathy evaluation. Arch Intern Med. 2012;172:1677–1678. doi: 10.1001/archinternmed.2012.4372. [DOI] [PubMed] [Google Scholar]
- 25.Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604–2610. doi: 10.1016/j.ophtha.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Zimmer-Galler IE, Zeimer R. Telemedicine in diabetic retinopathy screening. Int Ophthalmol Clin. 2009;49:75–86. doi: 10.1097/IIO.0b013e31819fd60f. [DOI] [PubMed] [Google Scholar]
- 27.Silva PS, Cavallerano JD, Aiello LM, Aiello LP. Telemedicine and diabetic retinopathy. Arch Ophthalmol. 2011;129:236–242. doi: 10.1001/archophthalmol.2010.365. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RR, Silowash R, Anthony L, Cecil RA, Eller A. Telemedicine process used to implement an effective and functional screening program for diabetic retinopathy. J Diabetes Sci Technol. 2008;2:785–791. doi: 10.1177/193229680800200506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansberger SL, Gleitsmann K, Gardiner S, et al. Comparing the effectiveness of telemedicine and traditional surveillance in providing diabetic retinopathy screening examinations: A randomized controlled trial. Telemed J E Health. 2013;19:942–948. doi: 10.1089/tmj.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li HK, Horton M, Bursell S-E, et al. Telehealth practice recommendations for diabetic retinopathy, second edition. Telemed J E Health. 2011;17:1–24. doi: 10.1089/tmj.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field monmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol. 2002;134:204–213. doi: 10.1016/s0002-9394(02)01522-2. [DOI] [PubMed] [Google Scholar]
- 32.Massin P, Erginay A, Mehidi AB, et al. Evaluation of a new non-mydriatic digital camera for detection of diabetic retinopathy. Diabetic Medicine. 2003;20:635–641. doi: 10.1046/j.1464-5491.2003.01002.x. [DOI] [PubMed] [Google Scholar]
- 33.Bragge P, Gruen RL, Chau M, Forbes A, Taylor HR. Screening for presence or absence of diabetic retinopathy: a meta-analysis. Arch Ophthalmol. 2011;129:435–444. doi: 10.1001/archophthalmol.2010.319. [DOI] [PubMed] [Google Scholar]
- 34.Ogunyemi O, Terrien E, Eccles A, et al. Teleretinal screening for diabetic retinopathy in six Los Angeles urban safety-net clinics: initial findings. AMIA Annu Symp Proc. 2011;2011:1027–1035. [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson DM. Eye care availability and access among individuals with diabetes, diabetic retinopathy, or age-related macular degeneration. JAMA Ophthalmol. 2014;132:471–477. doi: 10.1001/jamaophthalmol.2013.7682. [DOI] [PubMed] [Google Scholar]
- 36.Nathoo N, Ng M, Rudnisky CJ, Tennant MTS. The prevalence of diabetic retinopathy as identified by teleophthalmology in rural Alberta. Can J Ophthalmol. 2010;45:28–32. doi: 10.3129/i09-220. [DOI] [PubMed] [Google Scholar]
- 37.Arora S, Kurji AK, Tennant MTS. Dismantling sociocultural barriers to eye care with tele-ophthalmology: Lessons from an Alberta Cree community. Clin Invest Med. 2013;36:E57–E63. doi: 10.25011/cim.v36i2.19567. [DOI] [PubMed] [Google Scholar]
- 38.Cavallerano AA, Conlin PR. Teleretinal imaging to screen for diabetic retinopathy in the Veterans Health Administration. J Diabetes Sci Technol. 2008;2:33–39. doi: 10.1177/193229680800200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maberley D, Morris A, Hay D, Chang A, Hall L, Mandava N. A comparison of digital retinal image quality among photographers with different levels of training using a non-mydriatic fundus camera. Ophthalmic Epidemiol. 2004;11:191–197. doi: 10.1080/09286580490514496. [DOI] [PubMed] [Google Scholar]
- 40.Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabetic Medicine. 2010;27:249–256. doi: 10.1111/j.1464-5491.2009.02870.x. [DOI] [PubMed] [Google Scholar]
- 41.Rein DB, Wittenborn JS, Zhang X, et al. The cost-effectiveness of three screening alternatives for people with diabetes with no or early diabetic retinopathy. Health Serv Res. 2011;46:1534–1561. doi: 10.1111/j.1475-6773.2011.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murchison A, Friedman D, Gower EW, et al. A multi-center diabetes eye screening study in community settings: Study design and methodology. doi: 10.3109/09286586.2015.1099682. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding SP, Greenwood R, Adlington S, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabetic Medicine. 2003;20:965–971. doi: 10.1111/j.1464-5491.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- 44.American Academy of Ophthalmology Preferred Practice Patterns Committee . Preferred Practice Pattern® Guidelines. Comprehensive Adult Medical Eye Evaluation. American Academy of Ophthalmology; San Francisco CA: 2010. Available at: http://www.aao.org/ppp. [Google Scholar]
- 45.Zimmer-Galler I, Zeimer R. Results of implementation of the DigiScope for diabetic retinopathy assessment in the primary care environment. Telemed J E-Health. 2006;12:89–98. doi: 10.1089/tmj.2006.12.89. [DOI] [PubMed] [Google Scholar]
- 46.Cavallerano AA, Cavallerano JD, Katalinic P, et al. A telemedicine program for diabetic retinopathy in a Veterans Affairs Medical Center - The Joslin Vision Network Eye Health Care Model. Am J Ophthalmol. 2005;139:597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 47.An J, Nichol MB. Multiple medication adherence and its effect on clinical outcomes among patients with comorbid type 2 diabetes and hypertension. Med Care. 2013;51:879–887. doi: 10.1097/MLR.0b013e31829fa8ed. [DOI] [PubMed] [Google Scholar]
- 48.Krapek K, King K, Warren S, et al. Medication adherence and association hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004;38:1357–1362. doi: 10.1345/aph.1D612. [DOI] [PubMed] [Google Scholar]
- 49.Gregg EW, Geiss LS, Saaddine J, et al. Use of diabetes preventive care and complications risk in two African American communities. Am J Prev Med. 2001;21:197–202. doi: 10.1016/s0749-3797(01)00351-8. [DOI] [PubMed] [Google Scholar]
- 50.Flavin NE, Mulla ZD, Bonilla-Navarrete A, et al. Health insurance and the development of diabetic complications. South Med J. 2009;102:805–809. doi: 10.1097/SMJ.0b013e3181aa5f5b. [DOI] [PubMed] [Google Scholar]
- 51.Sivaprasad S, Gupta B, Gulliford MC, et al. Ethnic variations in the prevalence of diabetic retinopathy in people with diabetes attending screening in the United Kingdon (DRIVE UK) PLoS ONE. 2012;7:e32182. doi: 10.1371/journal.pone.0032182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 53.Wong TY, R K, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin P, Finnegan B. Assessing the need for on-site care professionals in community health centers. Policy Brief (George Washington University Center for Health Services Research and Policy) 2009 Feb 1-23; [PubMed] [Google Scholar]
- 55.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt JC. Racial variations in the prevalence of primary open-angle glaucoma. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 56.Javitt JC, Bean AM, Nicolson GA, Babish JD, Warren JL, Krakauer H. Undertreatment of glaucoma among black Americans. NEJM. 1991;325:1418–1422. doi: 10.1056/NEJM199111143252005. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk for pterygium: a systematic review and meta-analysis. BMJ Open. 2013;3:e003787. doi: 10.1136/bmjopen-2013-003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999;6:219–228. doi: 10.1076/opep.6.3.219.1504. [DOI] [PubMed] [Google Scholar]
- 59.Olayiwola JN, Sobieraj DM, Kulowski K, St. Hilaire D, Huang JJ. Improving diabetic retinopathy screening through a statewide telemedicine program at a large federally qualified health center. J Health Care Poor Underserved. 2011;22:804–816. doi: 10.1353/hpu.2011.0066. [DOI] [PubMed] [Google Scholar]
- 60.Jiménez-Ramirez J, Pérez R. Diabetic retinopathy education and screening at the community pharmacy in Puerto Rico. P R Health Sci J. 2011;30:139–144. [PubMed] [Google Scholar]