Abstract
Infectious diseases are the second leading cause of deaths in the world with malaria being responsible for approximately the same amount of deaths as cancer in 2012. Despite the success in malaria prevention and control measures decreasing the disease mortality rate by 45% since 2000, the development of single-dose therapeutics with radical cure potential is required to completely eradicate this deadly condition. Targeting multiple stages of the malaria parasite is becoming a primary requirement for new candidates in antimalarial drug discovery and development. Recently, 4(1H)-pyridone, 4(1H)-quinolone, 1,2,3,4-tetrahydroacridone, and phenoxyethoxy-4(1H)-quinolone chemotypes have been shown to be antimalarials with blood stage activity, liver stage activity, and transmission blocking activity. Advancements in structure-activity relationship and structure-property relationship studies, biological evaluation in vitro and in vivo, as well as pharmacokinetics of the 4(1H)-pyridone and 4(1H)-quinolone chemotypes will be discussed.
Keywords: 4(1H)-quinolone; 1,2,3,4-tetrahydroacdridone; phenoxyethoxy-4(1H)-quinolone; antimalarial activity; SAR; SPR
1 Introduction
1.1 Main biologically active derivatives
Malaria continues its devastating impact on the health of human populations in tropical regions, with 207 million cases and approximately 0.7 million deaths in 2012 [1]. Half of the world's population is at risk for malaria with the majority of deaths occurring on the African continent. Resistance to all known antimalarials and the limited availability of a large number of efficacious chemotypes are accountable for the wide spread of the disease. Even artemisinin and artemisinin combination therapies (ACT), currently considered the standard treatments, are showing signs of emerging resistance [2-5].
Over the last decade the number of deaths has decreased by approximately three times primarily due to renewed efforts to control and kill mosquito populations, as well as improved accessibility to effective medicines [6]. However, to eliminate and eradicate malaria, the discovery of novel therapeutics is required, which have potential to be used as single-dose agents targeting P. falciparum and as a radical cure of P. vivax malaria. Ideally, such a candidate possesses potent efficacy against the blood stages of multidrug resistant malaria, blocks transmission of infectious gametocytes to mosquitoes, and eradicates the liver stages infections, in particular the dormant forms (hypnozoites) of the relapsing malaria species (P. vivax and P. ovale). In the past, antimalarial drug discovery focused primarily on the erythrocytic stages of malaria, however in recent years, targeting the hypnozoites as well as the infective stages for mosquitoes has been considered to be equally important.
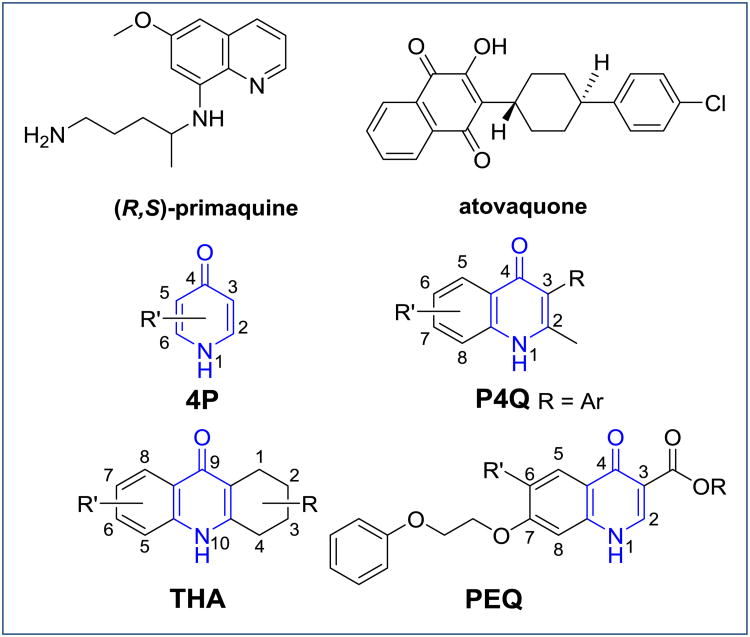
For a long period of time, 8-aminoquinolines such as primaquine were the only known antimalarials demonstrating liver stage activity, weak erythrocytic stage activity and transmission blocking activity simultaneously (Figure 1) [7]. However, toxicity in glucose-6-phosphate dehydrogenase deficient patients after administration of primaquine limits its use [8, 9]. Therefore, malaria elimination efforts continue to focus on the development of a compound that is safe and effective at killing malaria parasites at all stages of development. Fortunately, the investment into global antimalarial research and development over the last decade was expanded by non-profit organizations such as the Bill and Melinda Gates Foundation (USA), the Medicines for Malaria Venture (MMV, Switzerland), as well as by other for-profit organizations. In particular, the increased participation of academic drug discovery units in public-private partnerships with non-profit and pharmaceutical partners led to about 10 new antimalarial candidates in the early clinical phases of drug development [10]. Unfortunately, only a few of these are capable of targeting multiple stages of the malaria parasite life cycle. One of these promising preclinical candidates targeting multiple stages of the malaria parasite is ELQ-300, which is structurally related to the 4(1H)-quinolones and the 4(1H)-pyridones [11].
Figure 1. Structures of (R,S)-primaquine, atovaquone, as well as 4(1H)-quinolone and 4(1H)-pyridone-based chemotypes with antimalarial activity.
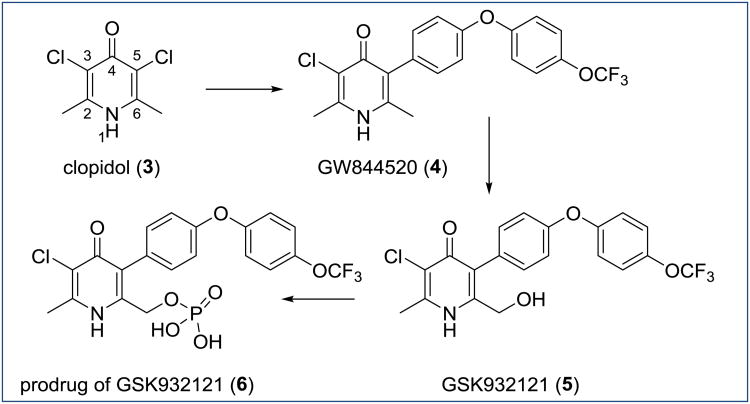
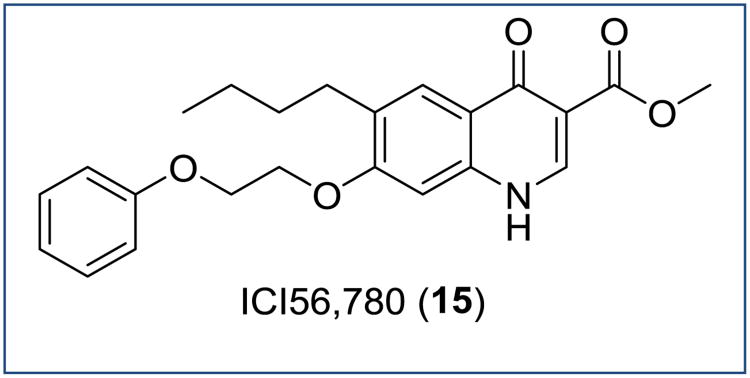
Historically, 3-alkyl- and 3-phenyl-substituted 4(1H)-quinolones (P4Qs) and 1,2,3,4-tetrahydroacridones (THAs) were reported to possess causal prophylactic activity (kill growing exoerythrocytic stage parasites) and potent erythrocytic stage inhibition in avian malaria models, but not against malaria parasites in mammals [12-14]. Similarly, compound ICI56,780, a phenoxyethoxy-substituted quinolone ester (PEQ) produced a radical cure (eradication of dormant exoerythrocytic stage parasites known as hypnozoites) in P. cynomolgi infected rhesus monkeys [15, 16]. Finally, the 4(1H)-pyridone clopidol (Figure 3) was an in vivo efficacious antimalarial against blood stages and it was also considered to be curative against the exoerythrocytic stages (P. gallinaceum and P. cynomolgi) provided it was administered at high doses over a period of seven consecutive days [17]. As these studies were conducted over 20-30 years ago without an adequate evaluation in current preclinical efficacy models and without assessing physicochemical properties of the compounds, a large number of research groups recently revisited the development of these chemotypes as antimalarials targeting multiple stages of the parasite [18-24]. Herein, we will discuss these 4(1H)pyridone and 4(1H)-quinolone chemotypes, which have potential to be unique antimalarials with blood stage activity, exoerythrocytic stage activity and transmission blocking activity.
Figure 3. Chemical structures of clopidol, GW844520, GSK932121 and its phosphate prodrug.
The 4(1H)-pyridone and 4(1H)-quinolone pharmacophores possess a common structural moiety, which is probably also responsible for their unique antimalarial activity. It has been shown that the majority of these chemotype analogues are inhibitors of the plasmodial electron transport chain targeting the parasite's bc1 complex [11]. Remarkably, atovaquone (Figure 1) is the only clinically relevant antimalarial drug inhibiting the same target [25].
1.2 Physicochemical properties of 4(1H)-pyridones and 4(1H)-quinolones
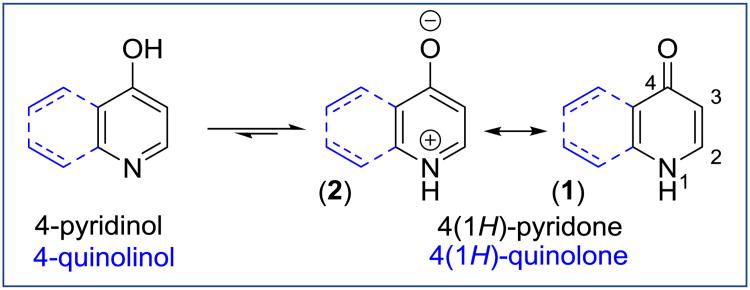
Pyridones are the carbonyl tautomeric forms of hydroxypyridines containing one oxygen at either the 2-, 3- or 4-position, while quinolones, often also called quinolinones, are pyridones to which an aromatic ring has been fused (Figure 2). Structural studies of 4(1H)-pyridones using X-ray crystallography, IRand liquid NMR suggest that the keto-form is favored in both solid and liquid states [26, 27]. Ion binding, self-association, and strong solvent effects are responsible for the domination of the keto tautomer in the liquid state, in the solid state, and in solution. In particular, the crystal structure of 4(1H)-quinolone exists as a dimer linked by intermolecular hydrogen bonding (NH1..O4 distance 1.795 Å while the carbon oxygen bond length of 1.246 Å is in the range of a C=O double bond. At the same time, the presence of a carbon C4 chemical shift at 176.8 ppm in 13C NMR as well as the splitting of the C2 proton to a triplet in 1H NMR in DMSO indicate the carbonyl nature of the 4(1H)-quinolone [28]. Both 1H NMR and 13C NMR data confirm that 4(1H)-pyridones and 4(1H)-quinolones also exist as π-electron delocalized systems with aromatic character. 4(1H)-Pyridones 1 are aromatic as long as the lone pair of the nitrogen electrons can be delocalized into the ring. However, the degree of the aromaticity depends on the contribution of the pyridinium 2 to the overall structure. 4(1H)-pyridones act like classical aromatic compounds with electrophilic aromatic substitutions (halogenation, nitration, etc.) occurring selectively at position C3, while the C4 carbonyl undergoes nucleophilic substitution by halogens when reacting with POCl3 or PCl5. Finally, the powder FT-IR of 4(1H)-quinolones displays only a stretching of the C=O bond at 1505 cm-1 without a hydroxyl peak [28]. Despite the predominance of the keto-form in solid and liquid states, structural studies of gas phase pyridones reveal an enol character which strongly suggests the existence of a tautomeric equilibrium [27].
Figure 2. Tautomer forms of 4(1H)-pyridones and 4(1H)-quinolones.
1.3 Synthesis of 4(1H)-pyridones and 4(1H)-quinolones
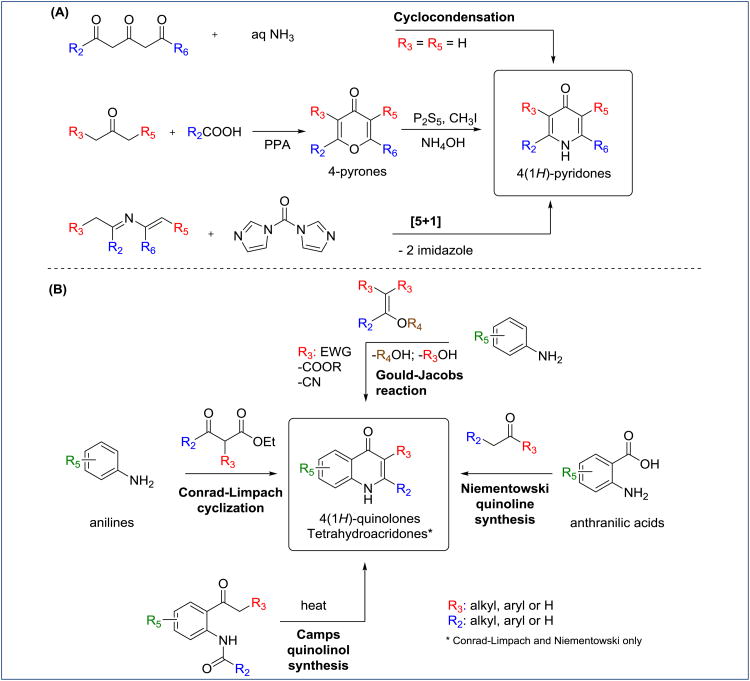
The synthesis of 4(1H)-pyridones proceeds by a cyclocondensation of 1,3,5-tricarbonyl compounds with Ammonia [29]. The formation of asymmetrical 2,6-substituted 4(1H)-pyridones is possible via a cyclocondensation of dianions of β-dicarbonyl compounds with nitriles, although the best results are obtained by the procedure described by Letsinger through pyrone intermediates [30]. Finally, 4(1H)-pyridones are also accessed via [5+1] hererocyclization of 2-azadienes with 1,1-carbonyldiimidazole (Scheme 1A) [31]. At the same time, synthesis of 4(1H)-quinolones is traditionally accomplished via Conrad-Limpach [32], Niementowski [33], Gould-Jacobs [34] or Camps [35] cyclizations (Scheme 1B). Among common disadvantages, these transformations require elevated reaction temperatures, and suffer from low reproducibility and poor-to-moderate reaction yields. Recently, several novel synthetic approaches involving transition metal catalysis with improved yields and regioselectivity have been reported [36-38].
Scheme 1. (A) Synthesis of 4(1H)-pyridones; (B) synthesis 4(1H)-quinolones and tetrahydroacridones.
2. 4(1H)-Pyridones
2.1 Historical overview
The antimalarial activities of clopidol 3, 3,5-dichloro-2,6-dimethyl-4(1H)-pyridone (Figure 3), were described by the Walter Reed Army Institute of Research (WRAIR) in the late 1960s [17]. In a concise article, compound 3 was reported to have in vivo activity against P. falciparum (chloroquine resistant), P. berghei, P. cynomolgi and P. gallinaceum. Unfortunately, clinical trials in humans at that time failed most likely because of pharmacokinetic issues (high clearance and low solubility). Simple acylations or sulfonations did not yield 3 derivatives with improved physicochemical properties [17]. Early investigations on the mechanism of action of 3 indicated that the mitochondria electron chain was inhibited [39]. This outcome was later confirmed by the Burroughs Welcome (BW) Laboratories [40]. Importantly, compound 3 maintained potent antimalarial activity against atovaquone resistant strains of malaria suggesting a different site of action for 3 than the ubiquinol oxidation site Qo of the bc1 complex for atovaquone [40].
2.2 Structure-activity relationship studies of 4(1H)-pyridones
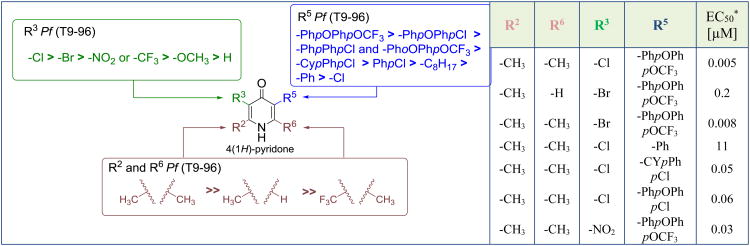
A few decades later, these results motivated scientists at GSK to renew lead optimization studies of a pyridine lead identified during the atovaquone development at BW. The C2, C3, C5, and C6 positions of 3 were optimized in a detailed structure-activity relationship study on the 4(1H)-pyridone scaffold [18]. First, the halogenation of the C3 position significantly improved the activity in vitro and in vivo over unsubstituted analogues. However, no significant differences between the 3-Br or the 3-Cl analogues were observed (EC50 (3-Cl) = 0.005 μM, EC50 (3-Br) = 0.008 μM, and EC50 (3-H) = 0.16 μM). Relative to the 3-halo-4(1H)-pyridones, other electron withdrawing groups (3-NO2 or 3-CF3) did not display any improvements, while compounds with an electron donating substituent (3-OCH3) lost all antimalarial activity.
For the C2 and C6 positions, a substantial loss in activity was observed when the 2-CH3 and 6-CH3 (EC50 3D7A = 0.2 μM) groups were replaced by CF3 groups (EC50 (3D7A) > 1 μM). This observation is in alignment with a previous report, in which electron-withdrawing groups (2-CF3) have been shown to shift the tautomeric equilibrium towards the enol isomer [26]. These results strongly suggest the importance of the keto-tautomer to maintaining antimalarial activities.
Finally, substitution at the C5 position proved to play the most important role in terms of efficacy as well as physicochemical properties. Initial improvements of 3 were achieved by changing the chloro substituent in 5-position to an alkyl chain, a phenyl, the atovaquone-like side chain 4-(4-chlorophenyl)cyclohexyl, or a diaryl ether moiety (Figure 4) [18]. Introduction of a phenyl group at the C5 position modestly improved the in vitro activity against T9-96, a chloroquine sensitive strain of P. falciparum, by a factor of two over 3, while the installation of an n-octyl chain at C5 further enhanced the potency yielding an EC50 of 4 μM. Nevertheless, both compounds lost their in vivo activity in a 4-day suppressive mouse P. yoelii assay (ED50 = 20 mg/kg and ED50 > 60 mg/kg respectively) presumably due to metabolic issues. The use of a diaryl group mimicking the side chain of atovaquone dramatically decreased the EC50 value to 0.4 μM, while the in vivo activity improved from an ED50 value of 40 mg/kg for 3 to an ED50 value of 0.6 mg/kg for the 5-diaryl-substituted 4(1H)-pyridone. Additional optimization of the distal aromatic ring and introduction of an oxygen as a spacer between the two aromatic rings of the 5-diaryl moiety provided compound 4 with promisingly low nanomolar in vitro activity and excellent in vivo activity with an ED50 value of 0.2 mg/kg in the murine P. yoelii model. This optimization leading to compound 4 corresponds to an approximate 200-fold improvement in potency over the starting point compound 3 [18].
Figure 4.
Optimization of clopidol 3 leading to 4(1H)-pyridones with improved in vitro and in vivo antimalarial activity. Note that the residues are ordered according to decreasing in vitro antimalarial activity against the T9-96 strain of P. falciparum.* T9-96 strain
In addition to excellent activity against erythrocytic stages of P. falciparum parasites, compound 4 also showed remarkable activity towards liver stages of P. falciparum (EC50 = 48.8 μM) and blood stages of P. vivax (EC50 < 4.88 μM), while no cytotoxicity against human cell lines was observed (EC50 > 6.1 μM). Furthermore, a selectivity index was confirmed in inhibition studies, in which 4 was shown to selectively inhibit plasmodial cytochrome bc1 complex over mammalian cytochrome bc1. The IC50 for plasmodial cytochrome bc1 complex III (IC50 = 0.002 μM) was shown to be approximately 250-fold lower than the corresponding human protein (IC50 = 0.51 μM) [41]. The inhibition of P. falciparum complex II and complex IV was weak (IC50 > 3 μM).
Compound 4 was also tested against multidrug resistant strains (3D7A, FCR3, K1, Dd2, Hb3 and W2) of P. falciparum and showed no cross-resistance with any of the tested strains including the atovaquone resistant strain FCR3, suggesting a slightly different binding mode to cytochrome bc1 despite its similar mechanism of action [41]. Importantly, the FCR3 atovaquone resistant strain does not possess the clinically relevant amino acid 268 mutation in cytochrome b.
2.3 Preclinical studies of 4(1H)-pyridones
Based on the promising antimalarial profile, compound 4 entered preclinical trials in 2006 [42]. However, it was discontinued because of histopatological complications in skeletal and cardiac muscles. These results were probably caused by the high lipophilicity (log D pH7.4 = 2.79) and the long half-life (t1/2 = 143 h in a dog) of 4. Consequently, oral bioavailability of lead 4 was also extremely low at higher dosages (% F = 4 at a 2mg/kg dose in dogs and % F = 20 at a 10mg/kg dose in mice) mainly because of poor aqueous solubility (pH 2-12 < 0.1 μg/ml) [43]. The lack of dose linearity of 4 in the preclinical studies triggered the initiation of a back-up program at GSK with the goal to design 4(1H)-pyridones with higher solubilities and increased bioavailabilities [44]. Introduction of a hydroxymethyl moiety at C6 yielded compound 5 (Figure 3), whose solubility at lower pHs (simulated gastric fluid (SGF) pH 1.2 = 372 mg/ml, where the nitrogen atom of the pridone ring is protonated pKa < 1.5, fed state simulated intestinal fluid (FeSSIF) pH 5.0 = 2.34 mg/ml) was significantly increased in comparison to its solubility at pH 7.4 (PBS pH 7.4 < 0.1 mg/ml). As such, the oral bioavailability of 5 was improved to 16 % in a dog model at 2 mg/kg doses. Importantly, incorporation of the hydroxymethyl group at C6 retained the in vitro (EC50 3D7A = 0.002 μM) and in vivo efficacy (ED50 = 0.3 mg/kg in the murine P. yoelii model). The formulation approach was used to enhance the oral bioavailability and allowed 5 to progress to first-time-in-human (FTIH) studies. Despite promising early results, these studies were terminated due to unexpected acute toxicity with the water-soluble phosphate prodrug 6 (Figure 3) in rats. Prodrug approach with compound 6 progressed into preclinical trials in parallel with FTIH study as a back-up. The toxicity was attributed to the inhibition of mammalian bc1 cells by parent compound 5. Despite the difficulties associated with the toxicity of GSK compounds in humans, 4(1H)-pyridones remain an attractive chemotype with potential to be efficacious not only against the blood forms of parasite, but also against other stages such as liver and mosquito forms acting as prophylactic and transmission blocking agent.
3. 4(1H)-Quinolones
3.1 Historical overview
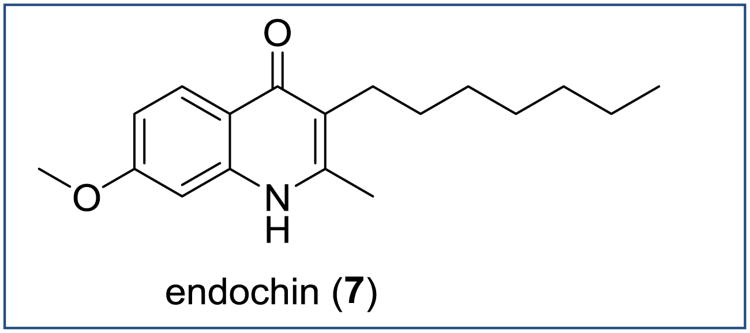
Before the beginning of World War II, German chemists from Bayer discovered prophylactic antimalarial activity with endochin 7, a 3-heptyl-7-methoxy-2-methyl-4(1H)-quinolone (Figure 5) [45]. The prefix “endo-” was chosen as this new antimalarial appeared to inhibit sporozoites and infected endothelial cells of liver stages, while the suffix “-chin” was common to many antimalarials related or unrelated to the alkaloid quinine (German: chinin) [46]. It was not until the end of the war when the first results on the antiplasmodial activity of 7 were published [14]. Remarkably, compound showed activity in an avian malaria model against all three stages of the parasite life cycle: host liver, blood, and mosquito stages [46]. Despite demonstrated efficacy of 7 towards P. gallinaceum in chicken and P. praecox in canaries, initial trials in humans failed [12], likely because of unfavorable pharmacokinetics. When better in vivo malaria models became available, 7 was tested by Walter Reed Army Institute of Research in 1974 in the gold standard P. cynomolgi infected Rhesus radical cure assay and found to be inactive [47].
Figure 5. Chemical structure of endochin.
Around the same time, Casey at the University of Bridgeport prepared 15 endochin analogues focusing on 3-alkenyl- and 3-alkyl-substituted 2-methyl-4(1H)-quinolones [12]. Only two of the tested compounds showed antimalarial activity in P. gallinaceum and P. berghei assays. The 4(1H)-quinolone with an (E)-(dodec-1-en-1-yl) side chain at the C3 position cured two out of five mice at a dose of 640 mg/kg in the Rane single dose malaria model [12]. The N-oxide of 7 was the second compound displaying in vivo activity in the same malaria model confirming the initial assumption that the poor bioavailability of compund limited its use in mammals. It is also noteworthy that in 1968 Lemke and co-workers reported a series of 3-unsubstituted 4(1H)-quinolone-2-carboxylates which were inactive against P.berghei in a murine model [48].
3.2 Structure-activity relationship studies of 4(1H)-quinolones: 3-alkyl derivatives
4(1H)-quinolones regained attention by several research teams at the beginning of this century. First, Kyle and co-workers at the Walter Reed Army Institute of Research reevaluated approximately 30 structurally related 4(1H)-quinolones for in vitro erythrocytic activity against D6 (chloroquine and atovaquone susceptible) and TM90-C2B (chloroquine, mefloquine, and atovaquone resistant) strains of P. falciparum [49]. Notably, several 4(1H)quinolones demonstrated exceptional in vitro activity with potencies in the low nanomolar ranges while the cross-resistance with atovaquone was not complete across the 4(1H)-quinolone series [49]. Moreover, the efficacy of WR193211 (3-geranyl-substituted 2-methyl-4(1H)-quinolone, one of Casey's compound) was reevaluated and proven to display modest in vivo activity in a modified Thompson Test with P. berghei (compound given daily for three days) with an ED90 of 97 mg/kg. Subsequently in 2007, Riscoe and Winter reported similar results with endochin and 8 analogues thereof [21].
3.3 Structure-activity relationship studies of 4(1H)-quinolones: optimization of the C5-C8 benzenoid ring
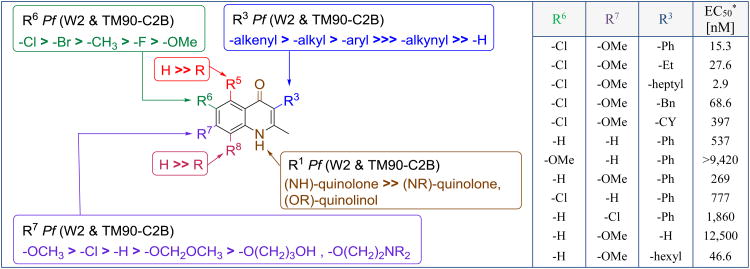
For the optimization of endochin, Manetsch and Kyle combined an extensive structure-activity relationship study with a structure-property study as compound appeared to be limited by its physicochemical properties [19]. While the n-alkyl chain at the C3 position was identified to play an important role for the antimalarial activity, it was also found to be a major liability in terms of aqueous solubility and microsomal stability (Figure 6). Nevertheless, initial optimization efforts focusing primarily on antimalarial activity yielded 3-alkenyl-substituted 4(1H)-quinolones which were approximately 2-fold more potent in comparison to 3-alkyl-4(1H)-quinolones. In contrast, 3-alkynyl-substituted compounds or 4(1H)-quinolones unsubstituted at the C3 position completely lacked in antimalarial activity. Modest results were obtained with 3-aryl-substituted 4(1H)-quinolones displaying EC50s in the submicromolar range.
Figure 6.
Structure-activity relationship (SAR) studies for the optimization of endochin.* TM90-C2B strain; In the table R5 = R8 = R1 = H
A systematic approach following the Topliss operational scheme for aromatic substituents was applied to probe the benzenoid ring for steric and electronic effects. The C5, C6, C7, and C8 positions of 3-phenyl- or 3-benzyl-2-methyl-4(1H)-quinolones were altered with a methoxy-, a chloro-, or a dichloro-substitution. Best potencies were observed with compounds substituted at the benzenoid ring with a chloro group at the C6 or with a methoxy group at the C7 position. Combination of a 6-chloro and a 7-methoxy group within the same 4(1H)-quinolone greatly synergized the substituent effects and improved the in vitro potency up to 20-fold. In addition, the 6-chloro-7-methoxy substitution combination gave rise to an array of 4(1H)-quinolones displaying equal antimalarial potency against the TM90-C2B (atovaquone resistant) and the W2 (chloroquine resistant) strains of P. falciparum which consequently lowered the atovaquone cross-resistance index (RI: EC50 ratio of TM90-C2B to W2) to an optimal value close to 1. Atovaquone resistance of TM90-C2B is caused by a specific mutation altering the wild type from Tyr268 to Ser268 in the parasite's cytochrome b gene of cytochrome bc1 [50]. Compounds with RI = 0.3 – 3.0 are considered acceptable in terms of risk to cross-resistance with atovaquone, whereas compounds with RI > 10 and RI < 0.1 are likely to have clinically relevant levels of cross-resistance with atovaquone [51, 52].
The authors also attempted to lock a particular 4(1H)-quinolone in its tautomeric forms via N- or O-alkylation of the 4(1H)-quinolone core. Predominantly, the keto form of a 4(1H)-quinolone is favored over enol form in both solid and solution states [28]. Several N-methylated 3-benzyl-substituted 4(1H)-quinolones and their O-methylated analogues have been shown to completely lack in antimalarial activity in comparison to their corresponding 4(1H)quinolones (Figure 6). These results suggest that the hydrogen bond donors and hydrogen bond acceptors typical of a 4(1H)-quinolone scaffold are likely required for a tight interaction with the parasite target.
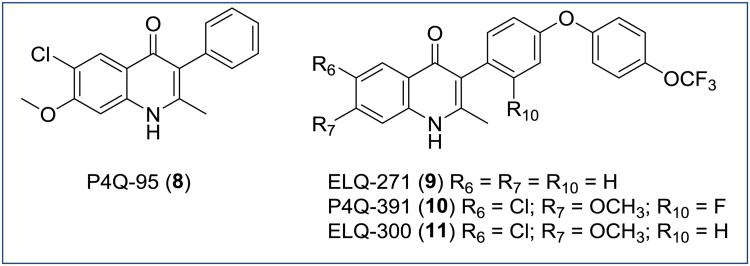
Among the most promising candidates in the initial library of approximately 70 4(1H)-quinolones was P4Q-95 (8, Figure 7) with EC50s comparable to endochin (compound 8: EC50 (W2) = 26 nM, EC50 (TM90-C2B) = 12 nM; endochin: EC50 (W2) = 8.6 nM; EC50 (TM90-C2B) = 47 nM). Importantly, 8 also displayed better microsomal stability and slightly better aqueous solubility in comparison to endochin. Based on these promising results, 3-phenyl substituted 8 was assessed in vivo in mice (Thompson test, P. berghei), but unfortunately 8 lacked in in vivo antimalarial activity due to low aqueous solubility [53].
Figure 7. Chemical structures of 3-aryl-4(1H)-quinolones P4Q-95, ELQ-271, P4Q-391, ELQ-300.
A first successful in vivo efficacy study with an optimized endochin analogue was reported by Riscoe and co-workers in 2011 [54]. For the orally administered prodrug ELQ-125, a polyethylene glycol carbonate derivative of 3-heptyl-5,7-diflouro-2-methyl-4(1H)-quinolone, an ED50 value of 11 mg/kg was obtained against P. yoelii infections in CF1 mice. ELQ-125 emerged from an optimization study comprising approximately thirty 3-alkyl-substituted 4(1H)-quinolones differing primarily at their benzenoid ring substituents. The most potent compounds had EC50 values in the picomolar range against the chloroquine resistant strain Dd2, although these compounds were not as potent against the atovaquone resistant strain TM90-C2B. For example, the resistance index for compound ELQ-125 was approximately 365 (ELQ-125: EC50 (Dd2) = 0.4 nM, EC50 (TM90-C2B) = 146 nM) suggesting strong potential for reduced efficacy against clinically relevant atovaquone resistant strains of malaria.
3.4 Structure-activity relationship studies of 3-aryl-4(1H)-quinolones and advancement of P4Q-391 and ELQ-300 into preclinical development
The experimental evidence that the orally administered prodrug ELQ-125 displayed good in vivo efficacy underscored the great promise of endochin derivatives as antimalarials. In addition to this, the potent in vitro antimalarial activity, the advantageous resistance index, the moderately improved physicochemical properties, as well as the synthetic tractability of 8 motivated the various scientists (University of South Florida, Oregon Health and Sciences University, Drexel University, Monash University, and MMV) to combine efforts in a multidisciplinary lead optimization program. Concerted optimization efforts led to the discovery of 3-diarylethers-4(1H)-quinolones ELQ-271 (9), ELQ-300 (10) and P4Q-391 (11), of which ELQ-300 was nominated as a preclinical candidate (Figure 7) [11]. The C3 substituent of 9 and 11 correspond exactly to the side chain of GSK's analogue 4, while the 6-chloro-7-methoxy benzenoid substituent in 10 and 11 is identical to the benzenoid substitution pattern of 4(1H)-quinolone 8 (Figure 7). The combination of these substitutions at C3 improved physiochemical properties whilst 6-chloro-7-methoxy proved critical for remarkable selectivity for parasite versus mammalian cytochrome bc1.
Compound 11 was potent in the in vitro erythrocytic assay against a chloroquine-sensitive strain (EC50 (Dd6) = 2.2 nM) and multidrug-resistant strains of P. falciparum (EC50 (W2) = 1.8 nM) including the atovaquone-resistant clinical clones (EC50 (TM90-C2B) = 1.7 nM). Furthermore, both 10 and 11 showed excellent in vivo efficacy against the blood stages (Thompson Test, P. berghei: compound 11 ED50 = 0.016 mg/kg; compound 10 ED50 = 0.27 mg/kg) and the liver stages (P. berghei sporozoite infection of mice followed by bioluminescence imaging: compound 11 blocking dose > 0.03 mg/kg; compound 10 blocking dose > 0.1 mg/kg). Moreover, both compounds were extremely effective in blocking transmission to the mosquito vector. Gametocytes treated with as low as 0.1 μM of 11 did not develop past stage III while a minimum concentration of 1 μM was needed for 10 to inhibit gametocyte growth past stage IV. Compounds 10 and 11 were also shown to inhibit the formation of zygote, ookinete, and oocyst forms of the mosquito life cycle. The mechanism of action for both compounds was also found to be associated to the cytochrome bc1 complex. Fortunately, although the Q0 site of plasmodial mitochondrial cytochrome bc1 complex is a common target for both drugs, the selectivity index for 11 was greatly improved (>18,000) for Plasmodium bc1 versus human bc1 due to the 6-chloro-7-methoxy substitution pattern.
One limitation of 11 is its poor aqueous solubility (fasted state simulated intestinal fluid (FaSSIF) pH 6.5 = 0.3 μM) which has direct impact on the pharmacokinetic profile of the compound [11]. The oral bioavailability of 11 in mice was excellent (∼100%) at a low dose of 0.3 mg/kg with PEG-400 vehicle in a P. yoelii efficacy model. However, increasing the dose concentration resulted in a decrease of bioavailability. The absence of dose-proportionality impedes the determination of the therapeutic index and in vivo toxicity. Alternative approaches such as prodrugs or formulation are required to overcome this solubility constraint. Nevertheless, as of today, ELQ-300 remains one of the five preclinical candidates in the MMV pipeline [10].
3.5 Structure-activity relationship studies of 4(1H)-quinolones: 2-substituted derivatives
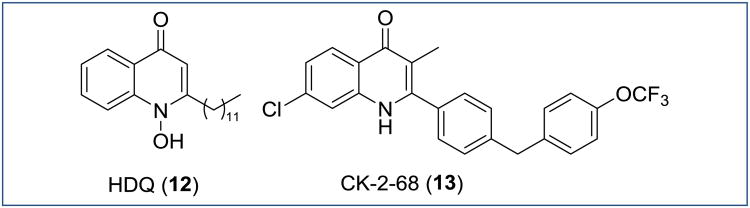
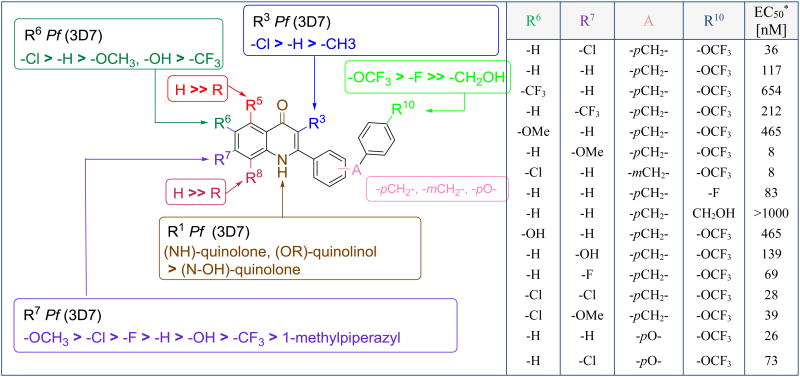
O'Neil, Ward and coworkers developed a series of 2-substituted 4(1H)quinolones with a dual mechanism of action against two enzymes of the Plasmodium mitochondrial electron transport chain: the NADH:ubiquinoneoxidoreductase (P. falciparum NDH2) and cytochrome bc1 [22]. Hydroxy-2-dodecyl-4(1H)-quinolone (12, HDQ), a known PfNDH2 inhibitor was utilized as a starting point for the selection of approximately 17,000 compounds, which were tested in a high-through-put screening against PfNDH2. Follow-up hit-to-lead optimization yielded frontrunner 4(1H)-quinolone CK-2-68 (13), in which the 2-dodecyl side chain of 12 was replaced by a bis-aryl side chain (Figure 8) [22]. Generally, the overall trends of this structure-activity relationship study (Figure 9) closely resemble the trends of the 3-substituted quinolones reported by Manetsch, Kyle and Riscoe (Figure 6).
Figure 8. Chemical structures of P. falciparum NDH2 inhibitors.
Figure 9.
SAR of 2-aryl-4(1H)-quinolone. *3D7 strain; In the table R5 = R8 = R1 = H, R3 = Cl
The benzenoid ring of the 4(1H)-quinolone core tolerated substitutions at the C6 and C7 positions, while any modification at C5 or C8 significantly decreased the antiplasmodial activities (Figure 9) [22]. Secondary, in vitro activities of C3 methyl substituted 4(1H)-quinolones versus unsubstituted analogs are overtaken by better physiochemical properties of the formers due to an increased rotational barrier along the quinolone-aryl σ bond. More importantly, the nature of the C2 and C3 substituents determined the selectivity of this 4(1H)-quinolone series with 2-diaryl substituted compounds being selective towards PfNDH2, while 3-substituted analogues preferentially target the parasite's cytochrome bc1 complex. Compound 13 has good activity against the blood stages with an EC50 value of 36 nM against 3D7 and a strong inhibition of PfNDH2 (IC50 = 16 nM). The compound administrated either as a prodrug or in conjunction with a solubilizing formulation also showed 100% suppressive activity in mice infected with P. berghei (Peter's 4 day in vivo test) [22]. Selective 4(1H)-quinolones including CK-2-68 were also tested against multidrug resistant strains of P. falciparum and while the in vitro activity against the chloroquine resistant clone W2 were similar to 3D7 (resistance index of approximately 1), compounds were not equipotent towards the atovaquone resistant strain TM90-C2B (EC50 = 178 nM).
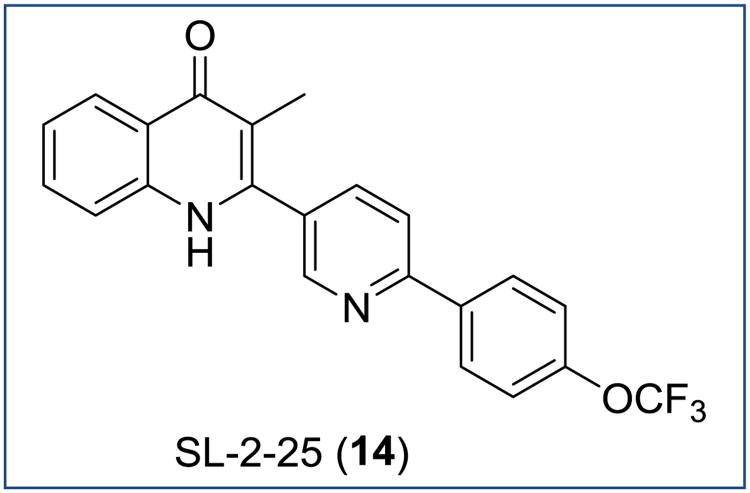
The low aqueous solubility and the necessity of approaches utilizing a prodrug moiety or formulations led to a design of compounds containing a heterocyclic sidechain [55]. In comparison to 13, compound SL-2-25 (14, Figure 10) conserved the promising antimalarial activity against the 3D7 strain of P. falciparum (EC50 = 54 nM) and the target PfNDH2 (IC50 = 14 nM), while an oral administration showed activity with an ED50 value of 1.9 mg/kg in a P. berghei model of malaria when given as a phosphate salt. At the same time 14 is also equipotent against the parasite bc1 complex (IC50 = 15 nM). However, it shows reduced activity towards the atovaquone-resistant strain TM90-C2B (EC50 = 156 nM), yielding a resistance index of approximately 3. Generally, 2-pyridyl-4(1H)-quinolones are considered to be attractive templates for further lead optimization of 4(1H)-quinolones with dual mechanisms of action.
Figure 10. Chemical structure of SL-2-25.
4. 3-Ester-4(1H)-Quinolones
4.1 Historical overview
At Imperial Chemical Industries (ICI), Ryley and Peters reported a study on the development of an anti-coccidial agent as a potent antimalarial [15]. Compound ICI56,780, a 7-(2-phenoxyethoxy)-4(1H)-quinolone (PEQ) (15, Figure 11), was shown to possess causal prophylactic (single dose of 30 mg/kg subcutaneous) and blood schizonticidal activity (ED50 = 0.05 mg/kg) in rodent malaria models. Unfortunately, a high degree of resistance to 15 was obtained after one passage in P. berghei infected mice and the lack of oral bioavailability led to abandonment of this series of compounds. Approximately twenty years later, Puri and Dutta retested 15 and produced radical cures (eradicate dormant hypnozoites) in P. cynomolgi infected Rhesus monkeys [16]. Antimalarials which have proven in vivo activity at eradicating hypnozoites of P. cynomolgi have potential to be used as radical cures representing targets of highest priority for the worldwide malaria eradication initiative.
Figure 11. Chemical structure of ICI56,780.
4.2 Structure-activity relationship studies of phenoxyethoxy ester quinolones (PEQs)
To overcome the resistance issues related to the PEQs, the Kyle and Manetsch laboratories conducted a structure-activity relationship study on a series of 29 novel PEQs in 2011 [23]. The most promising compound of this PEQ series was 6-butyl-3-(2-fluoro-4-(trifluoromethyl)phenyl)-7-(2-phenoxyethoxy)-4(1H)-quinolone which was reported to have a more than 200 fold improvement in RI (EC50 (TM90-C2B) = 31 nM, EC50 (W2) = 28 nM; RI = 1.1) over the parent compound 15 (EC50 (TM90-C2B) = 0.05 nM, EC50 (W2) = 11.2 nM; RI = 224). Superior blood stage activity and improved RI values were obtained with the 7-(2-phenoxyethoxy) substituted 4(1H)-quinolone, while the presence of the hydrophobic butyl group at the C6 position was identified to be important in maintaining potent antimalarial activities.
Similarly, an antimalarial series of twenty 4(1H)-quinolone esters that were mono-substituted at the C6 or C7 positions with one aryloxy or benzyloxy moiety was reported by Cowley et al in 2012 [56]. Each of the compounds was tested only in vitro against the chloroquine-sensitive strain 3D7 of P. falciparum. The structure-activity relationship data suggests that a C7 aryloxy or benzyloxy substitent was preferred over the same analogues substituted at C6 by an approximate factor of 300. Furthermore, the importance of the ester group at C3 was also established. The most promising quinolone ester from this series was confirmed to be active against the plasmodial bc1 complex (IC50 = 1.3 nM). Docking studies with the best compound binding to the Qo site of yeast bc1 complex suggested the existence of strong hydrogen bonding between the quinolone's nitrogen and oxygen with the His182 and Glu272 residues [56].
4.3 Structure-activity relationship studies of 2-aryl-3-ester-4(1H)-quinolones
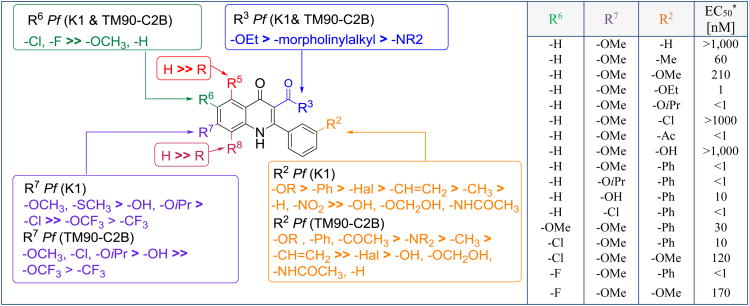
An extensive work on lead optimization of 2-aryl-3-ester-4(1H)-quinolones was reported by Guy and coworkers [24]. Approximately 100 derivatives of 4(1H)-quinolones were synthesized and tested against an array of P. falciparum strains (chloroquine and pyrimethamine resistant strain K1; chloroquine, mefloquine, pyrimethamine, and atovaquone resistant strain TM90-C2B; chloroquine sensitive strain D10; and DHODH inhibitor resistant strain D10_yDHOD). A systematic approach including a methodical variation of sterics, electrostatics and hydrophobics following Topliss and Hansch analysis was applied to conduct the optimization. A meta-substituted aryl group at the C2 position was established to be extremely important for antimalarial activity [57]. In order to investigate this trend more precisely, more than 20 additional analogues were synthesized and tested in vitro (Figure 12). Hydrophobic and electron-donating groups at the meta-position were determined to be the preferred C2 aryl substituents. Interestingly, a linear correlation between the pEC50 (K1) values and the hydrophobicity (R2 = 0.74) was identified while performing Hansch analysis. In contrast, no correlation between antimalarial activity and electronic effects were observed, which highlights a direct relationship between hydrophobicity and potency for these 2-aryl-3-ester-4(1H)-quinolones. For these 4(1H)-quinolone esters, it was therefore concluded that during the optimization process the antimalarial activity and key physicochemical properties needed to be carefully counterbalanced.
Figure 12.
SAR of 2-aryl-3-ester-4(1H)-quinolones. * TM90-C2B strain; In the table R5 = R8 = H, R3 = OEt
Introduction of an electron-donating group at the C7 position of the 4(1H)-quinolone benzenoid ring greatly improved the antimalarial activity, while an electron-withdrawing group caused a significant drop in potency (Figure 12). At the same time, the addition of a halogen substituent at the C6 position improved the antimalarial activity primarily against the TM90-C2B strain and consequently the RI value to approximately 1. Lastly, incorporation of different solubilizing moieties at the C3 ester position substantially reduced the potency in comparison to the ethyl ester 4(1H)-quinolone starting point.
Parallel assessment of the in vitro activity and physicochemical property data led to the decision to test nine of these 4(1H)-quinolone esters in a Thompson test for in vivo activity. Of all the tested compounds, only two showed suppressive activities. For instance, ethyl 2-(3-chlorophenyl)-6-fluoro-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate displayed low nanomolar EC50s against K1 (EC50 = 80 nM) and TM90-C2B (EC50 = 200 nM) strains of P. falciparum, but it was highly soluble at pH 7.4 even at concentrations of 20 μM and possessed good permeability (PAMPA pH 7.4 = 410 cm/s). Not surprisingly, it was efficacious in vivo, demonstrating a 57% suppression of parasitemia on day 6 postinfection at an oral dose of 30 mg/kg.
Despite the excellent activities in vitro for the best 2-aryl-3-ester-4(1H)-quinolones, they are approximately 50-fold less potent in vivo in comparison to the 4(1H)-quinolones such as ELQ-300 or P4Q-391 (Figure 7) developed by the Manetsch, Kyle and Riscoe groups. These compounds also appear to target the plasmodial cytochrome bc1 complex as increased toxicity was observed with repeated dosing suggesting partial inhibition of mammalian bc1 complex. Nonetheless, 2-aryl-3-ester-4(1H)-quinolones possess great potential for future development as there is high potential for additional bioavailable improvements.
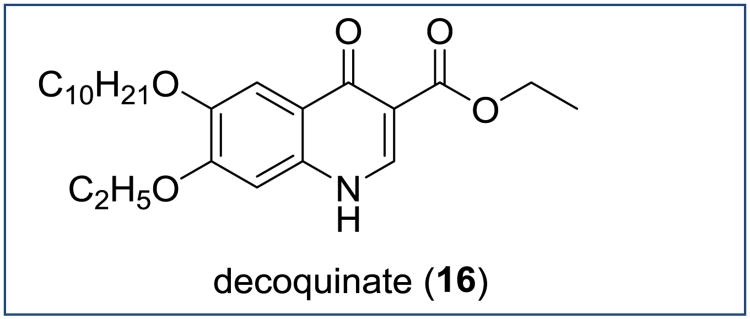
Recent screening of 1037 existing drugs targeted against P. berghei liver stages identified decoquinate 16 as the most potent inhibitor (EC50 = 2.6 nM) (Figure 13) [58]. This result is not unexpected considering that antimalarial activity of decoquinate against P. berghei was first reported by Ryley and Peters in their anti-coccidial study [16]. In addition, Puri and Dutta also showed that compound 16 possessed anti-hypnozoite activity in a P. cynomolgi infected Rhesus model when administrated as the ester prodrug WR 194905. Further investigation of this quinolone ester confirmed selective inhibition of plasmodial cytochrome bc1 complex [58].
Figure 13. Chemical structure of decoquinate.
5. 1,2,3,4-Tetrahydroacridones
5.1 Historical overview
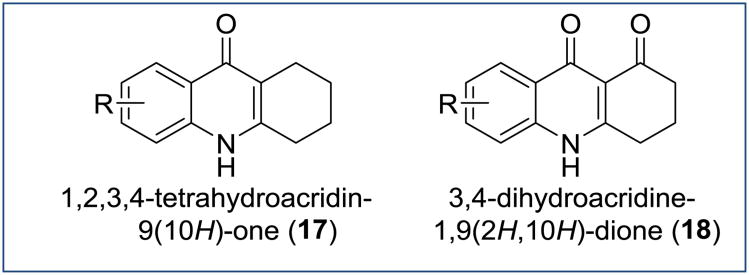
Antimalarial activity of 1,2,3,4-tetrahydroacridin-9(10H)-ones (THAs) was reported initially by Stephen and co-workers in 1947 [13]. More than a dozen THA analogues were tested using an in vivo assay against P. gallinaceum infections in chicks with a few compounds having minimum effective doses as low as 12.5 mg per 100 g (Figure 14, compound 17). At approximately the same time, endochin (Figure 5) was discovered to have prophylactic antiplasmodial activity, which surpassed the prophylactic activity of most of the THAs. Because of this, the next 50 years of optimization efforts focused primarily on 4(1H)quinolones and 4(1H)-quinolone esters instead of the THAs. Nevertheless, interesting reports on the antimalarial properties of THAs were published in the past decades.
Figure 14. Chemical structure of 1,2,3,4-tetrahydroacridin-9(10H)-ones and 3,4-dihydroacridine-1,9(2H,10H)-diones.
5.2 Floxacrine and other dihydroacridinediones
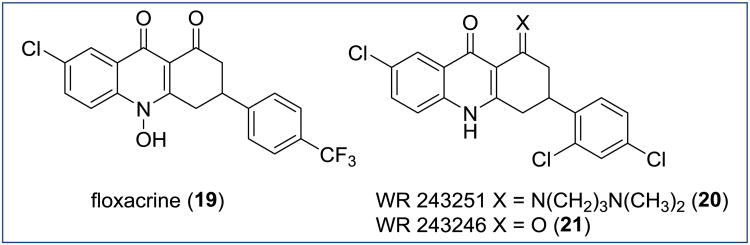
The dihydroacridinediones, which are structurally related to the THAs (Figure 14, compound 18), were studied in great detail over the course of last 30 years. Floxacrine (Figure 15, compound 19), first reported by Durckheimer in 1975, was discovered to have promising antimalarial activity including causal prophylactic activity [59]. Extensive optimization of floxacrine was conducted by several research groups with the hope to address the poor aqueous solubility, atovaquone cross resistance, and the dose-dependent chronic periarteritis caused by 19 [60]. Compound WR243251 20, a prodrug of WR243246 21, was developed and shown to generate cures in Aotus monkeys infected with the Smith strain (chloroquine, quinine, and pyrimethamine resistant) of P. falciparum at a dose of 16 mg/kg. Unfortunately, prodrug 20 was abandoned in the late preclinical development stage because of cross-resistance to atovaquone [61] and compound instability possibly related to a spontaneous decomposition of the prodrug 20 into 21 . Noteworthy, despite a substantial similarity between 19 and 21, both compounds appear to have different mechanisms of action with 19 inhibiting parasites via heme-mediated processes whereas the single (S)-enantiomer of 21 was shown to be a highly selective inhibitor of the ubiquinol oxidation site Qo of the P. falciparum bc1 complex [62].
Figure 15. Chemical structures of floxacrine, WR 243251, and WR 243246.
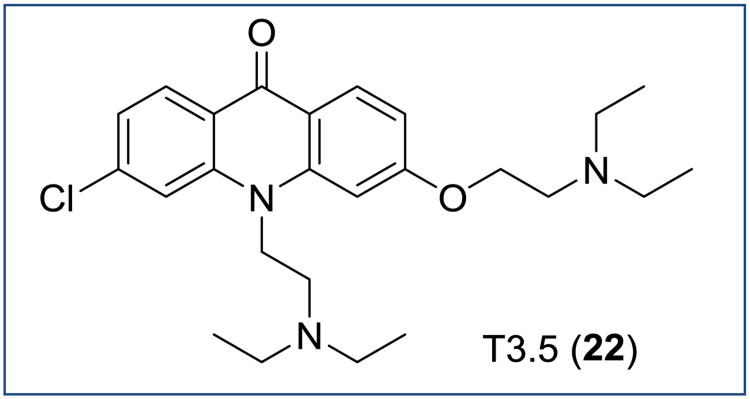
Recently, Riscoe and co-workers developed acridone T3.5 22, a fully aromatized THA analogue, which possesses excellent blood stage antimalarial activity via a dual mode of action (Figure 16) [63]. Compound 22 was shown to be equipotent (RI ∼ 1) in in vitro erythrocytic assays against the chloroquine-sensitive D6 (EC50 = 45 nM) and the multidrug-resistant P. falciparum strains Dd2 (EC50 = 77 nM) and also the atovaquone-resistant clinical clone TM90-C2B (EC50 = 71 nM). Compound 22 also showed suppressive in vivo efficacy in a P. berghei murine model decreasing parasitemia by 95% at a dose of 100 mg/kg per day. The curative effect was recorded at higher doses (256 mg/kg per day). The dual mode of action of compound 20 was attributed to the ability of disrupting hemozoin formation (tricyclic aromatic acridone core) as well as chemosensitizing via acid trapping. While no in vivo pharmacokinetic data has been reported so far, the T3.5 acridone chemotype is a promising template for further development of novel antimalarials.
Figure 16. Chemical structure of acridone T3.5.
5.3 Structure-activity relationship studies of 1,2,3,4-tetrahydroacridones
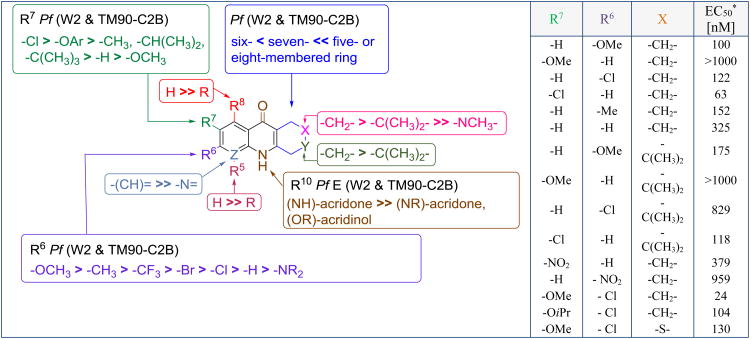
Finally, an extensive structure-activity relationship and structure-property relationship study on more than 100 THAs was reported by Kyle and Manetsch [20]. A systematic approach following the Topliss operational scheme for aromatic substituents was applied to investigate the benzenoid ring while also looking at the saturated ring using the findings of Stephen and Kesten [60]. In general, the results (Figure 17) were similar to the previously observed structure-activity relationship data for the 4(1H)-quinolones (Figure 6). Despite the initial report from Kesten on the importance of the mono-chloro substitution of the THA core, best antimalarial activity was obtained when the C6 position was substituted with an electron-donating substituent and C7 was substituted with a weakly electron-withdrawing group such as a halogen. Substitutions at C5 and C8 were not well tolerated perhaps because of the disruption of intermolecular hydrogen bonding between amine and carbonyl oxygen to the biological target. The THA core containing a six member unsubstituted aliphatic ring was reported to be most active. 7-Chloro-6-methoxy-1,2,3,4-tetrahydroacridin-9(10H)-one was reported with EC50 values lower than 30 nM for the W2 and TM90-C2B strains. Although the THAs are less potent than the 4(1H)-quinolones, the best THAs had an excellent in vitro therapeutic index and were equipotent for the atovaquone resistant and susceptible strains. Unfortunately, the THAs suffered from poor solubility and unfavorable microsomal stability yielding poor performance of the THA analogues in vivo [rodent models, unpublished data]. Additional functionalization of acridin-9(10H)-ones via alternative approaches such as prodrugs or formulations are required to obtain good oral efficacy.
Figure 17.
SAR of 1,2,3,4 -tetrahydroacridones.* TM90-C2B strain; In the table R5 = R8 = H, Y = -CH2-
6. Conclusions
4(1H)-pyridone and 4(1H)-quinolone chemotypes display potent antimalarial activities against Plasmodium species in vitro and in vivo. Some of these chemotypes inhibit the electron-transport chain in P. falciparum and hence possess a mechanism of action similar to the one of atovaquone. The progression of 4(1H)-pyridone 5 into first-time-in-human studies featured the full potential that these chemotypes have, although the program had to be stopped because of potential toxicity issues. Not surprisingly, several research groups focus their resources in developing and optimizing 4(1H)-quinolone-based antimalarials. Recently, compound 11 and structurally related 4(1H)-quinolone analogues have been shown to be highly active against P. falciparum and P. vivax at all life cycle stages. Due to this antimalarial activity profile in conjunction with favorable pharmacokinetics, 11 has been selected as a preclinical candidate in the MMV drug discovery program. At the same time, in vitro and in vivo efficacious 2-diheteroaryl 4(1H)quinolones with dual mechanism of action have been developed, which inhibit the NADH:ubiquinone oxidoreductase P. falciparum NDH2 and the cytochrome bc1 complex simultaneously. These 2-substituted 4(1H)-quinolones are currently undergoing further development in the MMV pipeline.
Nevertheless, the development of 4(1H)-pyridone and 4(1H)-quinolone antimalarials are also associated with risks. 4(1H)-Pyridones and 4(1H)-quinolones are known to be poorly soluble and thus represent challenging drug candidates for pharmacokinetic and bioavailability reasons. In addition, as the parasite electron-transport chain is quite often the biological target, 4(1H)-pyridone or 4(1H)-quinolone inhibitor design requires structural features inheriting a high selectivity over the corresponding mammalian targets. Furthermore, the potential to induce cross-resistance with atovaquone has to be carefully monitored during the optimization process. In particular compounds with greatly reduced potential for inducing resistance (e.g., ELQ-300) are required due to the high frequency of resistance selection with atovaquone. Also an ideal antimalarial compound should have a rapid onset of action, which is a property none of the compounds of this series has yet overcome. Despite these hurdles, the potent blood stage activity and the demonstrated potential to kill hypnozoites in the gold standard P. cynomolgi infected Rhesus model makes the herein discussed 4(1H)-pyridones, the 4(1H)-quinolones, the THAs and the PEQs attractive chemotypes for the development of novel drugs to treat multidrug resistant malaria and to aid the malaria elimination campaign.
Acknowledgments
We thank the National Institutes of Health (NIH 1R01GM097118-01) for financial support. We also thank Dr. David L. Flanigan and Dr. Kurt Van Horn for a fruitful discussion and the critical proofreading of the manuscript.
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflict of interest.
References
- 1.World Malaria Report 2012. World Health Organization; 2013. [Google Scholar]
- 2.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimde AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, Oyola SO, Rayner JC, Newbold CI, Berriman M, Spencer CC, McVean G, Day NP, White NJ, Bethell D, Dondorp AM, Plowe CV, Fairhurst RM, Kwiatkowski DP. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45(6):648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plowe CV. Malaria: Resistance nailed. Nature. 2014;505(7481):30–31. doi: 10.1038/nature12845. [DOI] [PubMed] [Google Scholar]
- 6.Burrows JN. Antimalarial drug discovery: where next? Future Med Chem. 2012;4(18):2233–2235. doi: 10.4155/fmc.12.189. [DOI] [PubMed] [Google Scholar]
- 7.White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis. 2013;13(2):175–181. doi: 10.1016/S1473-3099(12)70198-6. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S, Gupta UR, Daniel CS, Gupta RC, Anand N, Agarwal SS. Susceptibility of glucose-6-phosphate dehydrogenase deficient red cells to primaquine, primaquine enantiomers, and its two putative metabolites. II. Effect on red blood cell membrane, lipid peroxidation, MC-540 staining, and scanning electron microscopic studies. Biochem Pharmacol. 1991;41(1):17–21. doi: 10.1016/0006-2952(91)90005-p. [DOI] [PubMed] [Google Scholar]
- 9.Ziai M, Amirhakimi GH, Reinhold JG, Tabatabee M, Gettner ME, Bowman JE. Malaria prophylaxis and treatment in G-6-PD deficiency. An observation on the toxicity of primaquine and chloroquine. Clin Pediatr (Phila) 1967;6(4):242–243. doi: 10.1177/000992286700600416. [DOI] [PubMed] [Google Scholar]
- 10.MMV R&D portfolio. [Accessed December 1, 2013]; http://www.mmv.org/research-development/rd-portfolio.
- 11.Nilsen A, LaCrue AN, White KL, Forquer IP, Cross RM, Marfurt J, Mather MW, Delves MJ, Shackleford DM, Saenz FE, Morrisey JM, Steuten J, Mutka T, Li Y, Wirjanata G, Ryan E, Duffy S, Kelly JX, Sebayang BF, Zeeman AM, Noviyanti R, Sinden RE, Kocken CH, Price RN, Avery VM, Angulo-Barturen I, Jimenez-Diaz MB, Ferrer S, Herreros E, Sanz LM, Gamo FJ, Bathurst I, Burrows JN, Siegl P, Guy RK, Winter RW, Vaidya AB, Charman SA, Kyle DE, Manetsch R, Riscoe MK. Quinolone-3-diarylethers: a new class of antimalarial drug. Sci Transl Med. 2013;5(177):177ra137. doi: 10.1126/scitranslmed.3005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey AC. 4(1H)-quinolones. 2. Antimalarial effect of some 2-methyl-3-(1′-alkenyl)-or-3-alkyl-4(1H)-quinolones. J Med Chem. 1974;17(2):255–256. doi: 10.1021/jm00248a030. [DOI] [PubMed] [Google Scholar]
- 13.Stephen JM, Tonkin IM, Walker J. Tetrahydroacridones and related compounds as antimalarials. J Chem Soc. 1947:1034–1039. doi: 10.1039/jr9470001034. [DOI] [PubMed] [Google Scholar]
- 14.Salzer W, Timmler H, Andersag H. Über einen neuen, gegen Vogelmalaria wirksamen Verbindungstypus. Chem Ber. 1948;81(1):12–19. [Google Scholar]
- 15.Ryley JF, Peters W. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 1970;64(2):209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 16.Puri SK, Dutta GP. Quinoline esters as potential antimalarial drugs: effect on relapses of Plasmodium cynomolgi infections in monkeys. Trans R Soc Trop Med Hyg. 1990;84(6):759–760. doi: 10.1016/0035-9203(90)90066-n. [DOI] [PubMed] [Google Scholar]
- 17.Markley LD, Van Heertum JC, Doorenbos HE. Antimalarial activity of clopidol, 3,5-dichloro-2,6-dimethyl-4-pyridinol, and its esters, carbonates, and sulfonates. J Med Chem. 1972;15(11):1188–1189. doi: 10.1021/jm00281a029. [DOI] [PubMed] [Google Scholar]
- 18.Yeates CL, Batchelor JF, Capon EC, Cheesman NJ, Fry M, Hudson AT, Pudney M, Trimming H, Woolven J, Bueno JM, Chicharro J, Fernandez E, Fiandor JM, Gargallo-Viola D, Gomez de las Heras F, Herreros E, Leon ML. Synthesis and structure-activity relationships of 4-pyridones as potential antimalarials. J Med Chem. 2008;51(9):2845–2852. doi: 10.1021/jm0705760. [DOI] [PubMed] [Google Scholar]
- 19.Cross RM, Monastyrskyi A, Mutka TS, Burrows JN, Kyle DE, Manetsch R. Endochin optimization: structure-activity and structure-property relationship studies of 3-substituted 2-methyl-4(1H)-quinolones with antimalarial activity. J Med Chem. 2010;53(19):7076–7094. doi: 10.1021/jm1007903. [DOI] [PubMed] [Google Scholar]
- 20.Cross RM, Maignan JR, Mutka TS, Luong L, Sargent J, Kyle DE, Manetsch R. Optimization of 1,2,3,4-tetrahydroacridin-9(10H)-ones as antimalarials utilizing structure-activity and structure-property relationships. J Med Chem. 2011;54(13):4399–4426. doi: 10.1021/jm200015a. [DOI] [PubMed] [Google Scholar]
- 21.Winter RW, Kelly JX, Smilkstein MJ, Dodean R, Hinrichs D, Riscoe MK. Antimalarial quinolones: synthesis, potency, and mechanistic studies. Exp Parasitol. 2008;118(4):487–497. doi: 10.1016/j.exppara.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pidathala C, Amewu R, Pacorel B, Nixon GL, Gibbons P, Hong WD, Leung SC, Berry NG, Sharma R, Stocks PA, Srivastava A, Shone AE, Charoensutthivarakul S, Taylor L, Berger O, Mbekeani A, Hill A, Fisher NE, Warman AJ, Biagini GA, Ward SA, O'Neill PM. Identification, design and biological evaluation of bisaryl quinolones targeting Plasmodium falciparum type II NADH:quinone oxidoreductase (PfNDH2) J Med Chem. 2012;55(5):1831–1843. doi: 10.1021/jm201179h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross RM, Namelikonda NK, Mutka TS, Luong L, Kyle DE, Manetsch R. Synthesis, antimalarial activity, and structure-activity relationship of 7-(2-phenoxyethoxy)-4(1H)-quinolones. J Med Chem. 2011;54(24):8321–8327. doi: 10.1021/jm200718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Clark JA, Connelly MC, Zhu F, Min J, Guiguemde WA, Pradhan A, Iyer L, Furimsky A, Gow J, Parman T, El Mazouni F, Phillips MA, Kyle DE, Mirsalis J, Guy RK. Lead optimization of 3-carboxyl-4(1H)-quinolones to deliver orally bioavailable antimalarials. J Med Chem. 2012;55(9):4205–4219. doi: 10.1021/jm201642z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessl JJ, Lange BB, Merbitz-Zahradnik T, Zwicker K, Hill P, Meunier B, Palsdottir H, Hunte C, Meshnick S, Trumpower BL. Molecular basis for atovaquone binding to the cytochrome bc1 complex. J Biol Chem. 2003;278(33):31312–31318. doi: 10.1074/jbc.M304042200. [DOI] [PubMed] [Google Scholar]
- 26.Katritzky AR, Karelson M, Harris PA. Prototropic Tautomerism of Heteroaromatic-Compounds. Heterocycles. 1991;32(2):329–369. [Google Scholar]
- 27.Johnson CD. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, Rees CW, editors. Pergamon; Oxford: 1984. pp. 99–164. [Google Scholar]
- 28.Nasiri HR, Bolte M, Schwalbe H. Tautomerism of 4-Hydroxy-4(1H) quinolon. Heterocycl Commun. 2006;12(5):319–322. [Google Scholar]
- 29.Tawara JN, Lorenz P, Stermitz FR. 4-hydroxylated piperidines and N-methyleuphococcinine (1-methyl-3-granatanone) from Picea (spruce) species. Identification and synthesis. J Nat Prod. 1999;62(2):321–323. doi: 10.1021/np9802769. [DOI] [PubMed] [Google Scholar]
- 30.Letsinger R, Jamison JD. Synthesis of Pyranones and Benzofluorenones from Ketones and Carboxylic Acids. J Am Chem Soc. 1961;83(1):193–&. [Google Scholar]
- 31.Barluenga J, Carlon RP, Gonzalez FJ, Fustero S. Synthesis of 4(1h)-Pyridones by Carbonylation of 2-Aza-1,3-Dienes. Tetrahedron Lett. 1990;31(26):3793–3796. [Google Scholar]
- 32.Conrad M, Limpach L. synthesen von Chinolinderivaten mittelst Acetessigester. Chem Ber. 1887;20(1):944–948. [Google Scholar]
- 33.Niementowski S, Orzechowski B. Synthesen der Chinolinderivate aus Anthranilsäure und Aldehyden. Chem Ber. 1895;28(3):2809–2822. [Google Scholar]
- 34.Gould RG, Jacobs WA. The Synthesis of Certain Substituted Quinolines and 5,6-Benzoquinolines. J Am Chem Soc. 1939;61(10):2890–2895. [Google Scholar]
- 35.Camps R. Synthese von α- und γ-Oxychinolinen. Chem Ber. 1899;32(3):3228–3234. [Google Scholar]
- 36.Cross RM, Manetsch R. Divergent route to access structurally diverse 4-quinolones via mono or sequential cross-couplings. J Org Chem. 2010;75(24):8654–8657. doi: 10.1021/jo1014504. [DOI] [PubMed] [Google Scholar]
- 37.Zhao T, Xu B. Palladium-catalyzed tandem amination reaction for the synthesis of 4-quinolones. Org Lett. 2010;12(2):212–215. doi: 10.1021/ol902626d. [DOI] [PubMed] [Google Scholar]
- 38.Jones CP, Anderson KW, Buchwald SL. Sequential Cu-catalyzed amidation-base-mediated camps cyclization: a two-step synthesis of 2-Aryl-4-quinolones from o-halophenones. J Org Chem. 2007;72(21):7968–7973. doi: 10.1021/jo701384n. [DOI] [PubMed] [Google Scholar]
- 39.Fry M, Williams RB. Effects of decoquinate and clopidol on electron transport in mitochondria of Eimeria tenella (Apicomplexa: Coccidia) Biochem Pharmacol. 1984;33(2):229–240. doi: 10.1016/0006-2952(84)90480-5. [DOI] [PubMed] [Google Scholar]
- 40.Hudson AT, Latter VS, Randall AW, Richards WHG. U.S. Patent 505,341,8. Antiprotozoal agents. 1991 Oct 1;
- 41.Gargallo D. 4(1H)-Pyridones as putative antimalarials. GSK: MMV; 2007. [Google Scholar]
- 42.Xiang H, McSurdy-Freed J, Moorthy GS, Hugger E, Bambal R, Han C, Ferrer S, Gargallo D, Davis CB. Preclinical drug metabolism and pharmacokinetic evaluation of GW844520, a novel anti-malarial mitochondrial electron transport inhibitor. J Pharm Sci. 2006;95(12):2657–2672. doi: 10.1002/jps.20681. [DOI] [PubMed] [Google Scholar]
- 43.Bueno JM, Herreros E, Angulo-Barturen I, Ferrer S, Fiandor JM, Gamo FJ, Gargallo-Viola D, Derimanov G. Exploration of 4(IH)-pyridones as a novel family of potent antimalarial inhibitors of the plasmodial cytochrome bcl. Future Med Chem. 2012;4(18):2311–2323. doi: 10.4155/fmc.12.177. [DOI] [PubMed] [Google Scholar]
- 44.Bueno JM, Manzano P, Garcia MC, Chicharro J, Puente M, Lorenzo M, Garcia A, Ferrer S, Gomez RM, Fraile MT, Lavandera JL, Fiandor JM, Vidal J, Herreros E, Gargallo-Viola D. Potent antimalarial 4-pyridones with improved physico-chemical properties. Bioorg Med Chem Lett. 2011;21(18):5214–5218. doi: 10.1016/j.bmcl.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 45.Fitch WK. German pharmaceutical factories. Pharm J. 1945;155:182–183. [PubMed] [Google Scholar]
- 46.Kikuth W, Mudrow-Reichenow L. Ueber kausalprophylaktisch bei Vogelmalaria wirksame Substanzen. Z Hyg Infektionskr. 1947;127(1-2):151–165. [PubMed] [Google Scholar]
- 47.Casey AC. Synthesis of some 4-quinolones and related structures for evaluation as potential antimalarial agents. University of Bridgeport; Bridgeport, CT: 1974. [Google Scholar]
- 48.Heindel ND, Bechara IS, Kennewell PD, Molnar J, Ohnmacht CJ, Lemke SM, Lemke TF. Cyclization of aniline-acetylenedicarboxylate adducts. A modified Conrad-Limpach method for the synthesis of potential antimalarials. J Med Chem. 1968;11(6):1218–1221. doi: 10.1021/jm00312a025. [DOI] [PubMed] [Google Scholar]
- 49.Kyle DE, Gerena L, Pitzer K, Gettyacamin M. American Society of Tropical Medicine and Hygiene 54th Annual Meeting; Washington, DC. 2005. [Google Scholar]
- 50.Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol Microbiol. 1999;33(4):704–711. doi: 10.1046/j.1365-2958.1999.01515.x. [DOI] [PubMed] [Google Scholar]
- 51.Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54(1):62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 52.Milhous WK, Gerena L, Kyle DE, Oduola AM. In vitro strategies for circumventing antimalarial drug resistance. Prog. Clin. Biol. Res. 1989;313:61–72. [PubMed] [Google Scholar]
- 53.Monastyrskyi A, Flanigan D, Cross RM, LaCrue AN, Mutka TS, Fronczek FR, Guida WC, Kyle DE, Manetsch R. 3-Aryl-4(1H)-quinolones with broad range in vivo antimalarial activity. Abstr Pap Am Chem Soc. 2013;245 [Google Scholar]
- 54.Winter R, Kelly JX, Smilkstein MJ, Hinrichs D, Koop DR, Riscoe MK. Optimization of endochin-like quinolones for antimalarial activity. Exp Parasitol. 2011;127(2):545–551. doi: 10.1016/j.exppara.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biagini GA, Fisher N, Shone AE, Mubaraki MA, Srivastava A, Hill A, Antoine T, Warman AJ, Davies J, Pidathala C, Amewu RK, Leung SC, Sharma R, Gibbons P, Hong DW, Pacorel B, Lawrenson AS, Charoensutthivarakul S, Taylor L, Berger O, Mbekeani A, Stocks PA, Nixon GL, Chadwick J, Hemingway J, Delves MJ, Sinden RE, Zeeman AM, Kocken CH, Berry NG, O'Neill PM, Ward SA. Generation of quinolone antimalarials targeting the Plasmodium falciparum mitochondrial respiratory chain for the treatment and prophylaxis of malaria. Proc Natl Acad Sci U S A. 2012;109(21):8298–8303. doi: 10.1073/pnas.1205651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cowley R, Leung S, Fisher N, Al-Helal M, Berry NG, Lawrenson AS, Sharma R, Shone AE, Ward SA, Biagini GA, O'Neill PM. The development of quinolone esters as novel antimalarial agents targeting the Plasmodium falciparum bc(1) protein complex. Medchemcomm. 2012;3(1):39–44. [Google Scholar]
- 57.Zhang Y, Guiguemde WA, Sigal M, Zhu F, Connelly MC, Nwaka S, Guy RK. Synthesis and structure-activity relationships of antimalarial 4-oxo-3-carboxyl quinolones. Bioorg Med Chem. 2010;18(7):2756–2766. doi: 10.1016/j.bmc.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Cruz FP, Martin C, Buchholz K, Lafuente-Monasterio MJ, Rodrigues T, Sonnichsen B, Moreira R, Gamo FJ, Marti M, Mota MM, Hannus M, Prudencio M. Drug screen targeted at Plasmodium liver stages identifies a potent multistage antimalarial drug. J Infect Dis. 2012;205(8):1278–1286. doi: 10.1093/infdis/jis184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durckheimer W, Raether W, Seliger HG. US3947449. Tetrahydroacridones having chemotherapeutic action and process for preparing them. 1976
- 60.Kesten SJ, Degnan MJ, Hung J, McNamara DJ, Ortwine DF, Uhlendorf SE, Werbel LM. Synthesis and antimalarial properties of 1-imino derivatives of 7-chloro-3-substituted-3,4-dihydro-1,9(2H,10H)-acridinediones and related structures. J Med Chem. 1992;35(19):3429–3447. doi: 10.1021/jm00097a001. [DOI] [PubMed] [Google Scholar]
- 61.Suswam E, Kyle D, Lang-Unnasch N. Plasmodium falciparum: the effects of atovaquone resistance on respiration. Exp Parasitol. 2001;98(4):180–187. doi: 10.1006/expr.2001.4639. [DOI] [PubMed] [Google Scholar]
- 62.Biagini GA, Fisher N, Berry N, Stocks PA, Meunier B, Williams DP, Bonar-Law R, Bray PG, Owen A, O'Neill PM, Ward SA. Acridinediones: selective and potent inhibitors of the malaria parasite mitochondrial bc1 complex. Mol Pharmacol. 2008;73(5):1347–1355. doi: 10.1124/mol.108.045120. [DOI] [PubMed] [Google Scholar]
- 63.Kelly JX, Smilkstein MJ, Brun R, Wittlin S, Cooper RA, Lane KD, Janowsky A, Johnson RA, Dodean RA, Winter R, Hinrichs DJ, Riscoe MK. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459(7244):270–273. doi: 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]