Abstract
Peripheral nerve regeneration begins immediately after injury. Understanding the mechanisms by which early modulators of axonal degeneration regulate neurite outgrowth may affect the development of new strategies to promote nerve repair. Tumor necrosis factor-α (TNF-α) plays a crucial role in the initiation of degenerative cascades after peripheral nerve injury. Here we demonstrate using real-time Taqman quantitative RT-PCR that, during the time course (days 1–60) of sciatic nerve crush, TNF-α mRNA expression is induced at 1 day and returned to baseline at 5 days after injury in nerve and the corresponding dorsal root ganglia (DRG). Immediate therapy with the TNF-α antagonist etanercept (fusion protein of TNFRII and human IgG), administered systemically (i.p.) and locally (epineurially) after nerve crush injury, enhanced the rate of axonal regeneration, as determined by nerve pinch test and increased number of characteristic clusters of regenerating nerve fibers distal to nerve crush segments. These fibers were immunoreactive for growth associated protein-43 (GAP-43) and etanercept, detected by anti-human IgG immunofluorescence. Increased GAP-43 expression was found in the injured nerve and in the corresponding DRG and ventral spinal cord after systemic etanercept compared with vehicle treatments. This study established that immediate therapy with TNF-α antagonist supports axonal regeneration after peripheral nerve injury.
Keywords: tumor necrosis factor, peripheral nerve injury, Wallerian degeneration, GAP-43, neurite outgrowth, Schwann cell
In contrast to the central nervous system, nerve fibers of the peripheral nervous system are able to regenerate and reinnervate distal targets (Frisen, 1997). Regenerative and repair processes of peripheral nerve begin almost immediately after injury (Fawcett and Keynes, 1990). They depend on active molecular remodeling at the nerve injury site and within the neuronal cell body of the dorsal root ganglia (DRG) that, together, provide molecular guidance cues to the growth cone and ensure efficient degeneration and permissive microenvironment for neurite outgrowth. Therefore, elucidating the mechanism by which early modulators of peripheral Wallerian degeneration regulate neuronal outgrowth may affect the development of new strategies to promote nerve repair.
Inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), function as the initiators of Wallerian degeneration. They activate resident Schwann cells and facilitate macrophage recruitment to the injury site (Stoll et al., 2002; Myers et al., 2006). Through phagocytosis of myelin and axonal debris and release of proteases and trophic factors, Schwann cells and macrophages create a permissive milieu for axonal regrowth (Luk et al., 2003). In addition, inflammatory cytokines directly regulate axonal regrowth after nerve injury (Cao et al., 2006; Myers et al., 2006; Temporin et al., 2008). For example, TNF-α inhibits neurite outgrowth of cultured DRG (Schneider-Schaulies et al., 1991; Larsson et al., 2005) and hippocampal (Neumann et al., 2002) neurons. On the other hand, TNF-α (Aoki et al., 2007) or TNF-α-containing nucleus pulposus (Hayashi et al., 2008) stimulates the expression of regeneration-related growth associated protein-43 (GAP-43) in cultured DRG neurons. However, the role of TNF-α during peripheral nerve regeneration remains unidentified.
Etanercept is a TNF-α antagonist that represents a dimeric fusion protein of the extracellular ligand-binding portion of the soluble 75-kDa TNF receptor II (p75-TNFR, or TNFRII) and the fragment-crystallizable (Fc) portion of human immunoglobulin (IgG). It has been shown to produce neuroprotective changes after brain and spinal cord injury (Genovese et al., 2006; Campbell et al., 2007) and to attenuate neuropathic pain-related behaviors associated with peripheral nerve injury (Sommer et al., 2001; Schafers et al., 2003; Zanella et al., 2008; Kato et al., 2009). But potential beneficial effects of TNF-α on neurite outgrowth raise concern for use of anti-TNF-α agents for therapy of pain and neurodegenerative conditions (Myers et al., 2006).
The present study was designed to analyze the effect of immediate systemic (i.p) or local (epineurial) etanercept therapy on functional regeneration rate of sciatic nerve using the nerve pinch test and neuropathologic and GAP-43 protein expression changes in the nerve and/or the corresponding cell bodies in the lumbar 5 (L5) DRG and ventral spinal cord.
MATERIALS AND METHODS
Animals and Surgical Procedure
Adult female Sprague-Dawley rats (N = 127; 200–225 g; Harlan Labs, Indianapolis, IN) were housed in plastic cages at room temperature on a 12-hr light-dark cycle and had free access to food and water. All surgical procedures were performed under aseptic conditions using microsurgical techniques. The animals were anesthetized with 4% Isoflurane (IsoSol; Vedco, St. Joseph, MO). About 10 mm of sciatic nerve was exposed unilaterally at the midthigh level through a gluteal muscle-splitting incision. Nerve crush injury was performed by using smooth-surface forceps once for 5 sec. For the nerve pinch test (below), the crush site was labeled using a 6–0 nylon suture to the adjacent muscle. The sham operation included unilateral sciatic nerve exposure. The muscle layer was closed with silk sutures, and the skin was closed with metal clips. Euthanasia was performed with animals under deep anesthesia from an intraperitoneal (i.p.) injection of a rodent anesthesia cocktail containing sodium pentobarbital (Nembutal; 50 mg/ml; Abbott Labs, North Chicago, IL), diazepam (5 mg/ml; Steris Labs, Phoenix, AZ), and saline (0.9%; Steris Labs) in a volume proportion of 1:1:2, followed by rapid decapitation or intracardiac injection of Euthasol (Virbac, Fort Worth, TX). Ipsilateral and contralateral to injury sciatic nerves, L5 DRG and ventral quarters of L4–6 spinal cord were removed for analyses. All experimental protocols were approved by the VA Healthcare System Committee on Animal Research and conformed to the NIH guidelines for animal use.
Etanercept Therapy
Etanercept (Enbrel; Amgen, Inc., Thousand Oaks, CA) or vehicle (Sterile Water for Injections; Hospira Inc., Lake Forest, IL) was administered at 0.3, 3.0, or 6.0 mg/kg once immediately or at 0.3 and 3.0 mg/kg twice at 1 hr and 3 days after nerve crush, intraperitoneally (i.p.) or locally, into the epineurial space using a 30-gauge needle connected to a Hamilton syringe. Careful insertion of the needle into the epineurial space of the crushed nerve site and slow injections to avoid an overflow were ensured.
Nerve Pinch Test
The rate of axonal regeneration in vivo was evaluated by nerve pinch testing (Gutmann et al., 1942; McQuarrie et al., 1977; Myers et al., 2003; Seijffers et al., 2007). Rats were anesthetized with rodent anesthesia cocktail (see above), and the sciatic and the branching tibial nerves were exposed. One-millimeter-long consecutive segments of the tibial nerve were pinched with a pair of fine forceps, starting from the distal end of the nerve and proceeding in the proximal direction, until a reflex response consisting of a contraction of the muscles of the back was observed. The distance between this pinch site and the stitch marking the original crush site was measured under a dissecting microscope and identified as the regeneration distance. The pinch test was performed in N = 5–6 per group by an experimenter unaware of the experimental groups.
Neuropathologic Evaluation
Rats were anesthetized as described above and perfused transcardially with fresh 0.5% glutaraldehyde in 0.1 M phosphate buffer. Sciatic nerve segments sectioned axially 10 mm distal to the crush site were removed, fixed in 2.5% phosphate-buffered glutaraldehyde, then postfixed in osmium tetroxide, dehydrated in graded ethanol and propylene oxide, and embedded in araldite. One-micrometer-thick sections were cut with a glass knife on an automated microtome and stained with methylene blue azure II for light microscopic analysis. Imaging was done on a Leica microscope using Openlab 4.0 software (Improvision, Coventry, United Kingdom).
Quantitative Morphometry
More than 40 sections per each sciatic nerve sample at 1, 3, and 5 days after crush were prepared. Bundles of two to six small, white, round, ultrathin structures forming clusters of regenerating axons (Young and Medawar, 1940) were detected. Individual fibers in clusters were counted in N = 6 animals in four randomly selected areas per whole transverse sciatic nerve section at ×40 objective magnification under the light microscope by an experimenter unaware of the experimental groups.
Western Blotting
Nerve, DRG, or spinal cord tissues were frozen in liquid nitrogen and stored at −80°C. Tissues were homogenized using lysis buffer (50 mM Tris-HCl, pH 7.4, 1% Triton X-100, 150 mM NaCl, 10% glycerol, 0.1% SDS, 5 mM EDTA, 1 mM PMSF, 1 μg/ml aprotinin and leupeptin). Lysates containing equal amounts (30 μg) of protein (BCA Protein Assay; Pierce, Rockford, IL) were run on 10% Trisglycine SDS-PAGE (Bio-Rad, Hercules, CA) at 50–80 mA and transferred to nitrocellulose membranes using the iBlot dry blotting system (Invitrogen) at 20 V for 7 min. The membranes were blocked with 5% nonfat milk (Bio-Rad), followed by incubation with polyclonal rabbit antibody to rat GAP-43 (Chemicon, Temcula, CA; 1:2,000) in 5% bovine serum albumin (BSA; Sigma, St. Louis, MO) overnight at 4°C, washed in TBS containing 0.1% Tween, and incubated for 1 hr at room temperature with HRP-conjugated anti-rabbit secondary antibody (Cell Signaling, Beverly, MA; 1:5,000). The blots were developed using enhanced chemiluminescence (ECL; Amersham). The membranes were stripped and reprobed with mouse anti-β-actin antibody (Sigma; 1:10,000) to control protein loading. Densitometry (optical density; OD) of the bands of interest was performed in N = 5 samples per group using Image J 1.38u (NIH, Bethesda, MD).
Dual Immunofluorescence
Rats were anesthetized as described above and perfused transcardially with fresh 4% paraformaldehyde in 0.2 M phosphate buffer. Nerve, DRG, or spinal cord tissues were removed and postfixed briefly in 4% paraformaldehyde overnight. Tissue was processed in an Autotechnicon Cycler TP 1010 (Leica Microsystems, Inc., Bannockburn, IL) and embedded in paraffin. Sections (10 μm) of the tissues were cut from each sample and placed on slides. Sections were deparaffinized with xylenes and rehydrated in graded ethanol. Sodium borohydride 0.5% in 1% dibasic sodium phosphate buffer was applied for 5 min to block endogenous aldehyde groups, followed by Dako antigen retrieval, nonspecific binding block in 5% goat serum for 1 hr, rabbit anti-rat GAP-43 (Chemicon; 1:1,000), and mouse anti-human IgG (AbD; Serotec, Oxford, United Kingdom; 1:500) antibodies incubation overnight at 4°C, then goat anti-mouse Alexa 488 (green) fluorescent antibody (Molecular Probes, Eugene, OR; 1:400) or goat anti-rabbit Alexa 594 (red) fluorescent antibody (Molecular Probes; 1:400) for 1 hr, and nuclear 4,6-diamidino-2-phenylindole (DAPI) stain (Molecular Probes; 1:20,000, blue) for 5 min. Sections were mounted with Slow-fade gold antifade reagent (Molecular Probes). Some sections were run without primary antibodies as controls, showing no human IgG or GAP-43 immunoreactivity. Imaging was done on a Leica microscope in Openlab 4.0 software (Improvision).
Real-Time qPCR
Primers and Taqman probes for rat TNF-α and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA) and obtained at Biosearch Technologies (Novato, CA; Table I). Their concentrations were optimized using cDNA from spleen and injured sciatic nerve to amplification efficiency of 100.3% for TNF-α and 101.6% for GAPDH. Sciatic nerve and L5 DRG samples were stored in RNA-Later (Ambion, Austin, TX) at −20°C. Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA), purified on RNeasy minicolumns (Qiagen, Valencia, CA), and treated with RNase-free DNAse I (Qiagen). The RNA purity was verified at OD260/280 absorption ratio of about 1.9–2.0. cDNA was synthesized with a SuperScript II first-strand RT-PCR kit (Invitrogen). Gene expression was measured by qPCR (MX4000; Stratagene, La Jolla, CA) with 50 ng of rat cDNA and 2x Taqman universal PCR master mix (Applied Biosystems) with a one-step program (95°C for 10 min, 95°C for 30 sec, and 60°C for 1 min for 50 cycles). Duplicate samples without cDNA (no-template control) for each gene showed no contaminating DNA. Relative TNF-α mRNA levels were normalized to GAPDH in N = 10–14 (pooled 2 N per sample) and quantified by using the comparative Ct method (Livak and Schmittgen, 2001). Fold mRNA change relative to uninjured nerve was determined using the MX4000 and based on the method described earlier (Pfaffl, 2001).
TABLE I.
Primer and Probe Sequences for Rat TNF-α and GAPDH for Real-Time Taqman qPCR*
| Gene | Accession No. | Sequences (5′–3′) | Conc. (nM) |
|---|---|---|---|
| TNF-α rat | NM_012675 | F: CCAGGAGAAAGTCCTCCT | 300 |
| R: TCATACCAGGGCTTGAGCTCA | 300 | ||
| 6-FAM d(AGAGCCCTTGCCCTAAGGACACCCCT)BHQ-l | 200 | ||
| GAPDH rat | XO2231 | F: GAACATCATCCCTGCATCCA | 100 |
| R: CCAGTGAGCTTCCCGTTCA | 100 | ||
| 6-FAM d(CTTGCCCACAGCCTTGGCAGC)BHQ-l | 100 |
Taqman probe containing 5′; reporter FAM and 3′; quencher BHQ-1 dyes.
Statistical Analysis
All data are expressed as means ± SEM. Statistical differences were assessed by the Mann-Whitney U-test or by ANOVA followed by Tukey's post hoc test, as detailed for each experiment in the Figure legends. P < 0.05 was considered significant.
RESULTS
To determine the patterns of TNF-α mRNA expression in the course of rat sciatic nerve crush, we used real-time Taqman qPCR of nerve and L5 DRG (Fig. 1). TNF-α mRNA expression in the crushed nerve (2.69 ± 0.19-fold; P < 0.001) and corresponding DRG (2.30 ± 0.17-fold; P < 0.05) was significantly elevated at 1 day and returned to baseline at 5 days after crush injury. TNF-α mRNA expression in the sham-operated tissues was not significantly different at 1 and 5 days after the surgery compared with the naïve animals (data not shown).
Fig. 1.
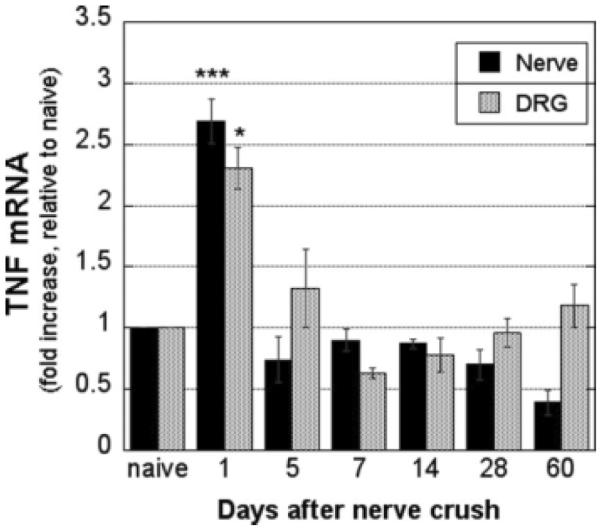
TNF-α mRNA expression in crushed sciatic nerve and ipsilateral L5 DRG. Real-time Taqman qPCR for TNF-α in nerve and DRG normalized to GAPDH. Data are expressed as the mean ± SEM -fold change relative to uninjured nerves in N = 5−7 samples. *P < 0.05, ***P < 0.001 by one-way ANOVA followed by Tukey's post hoc test.
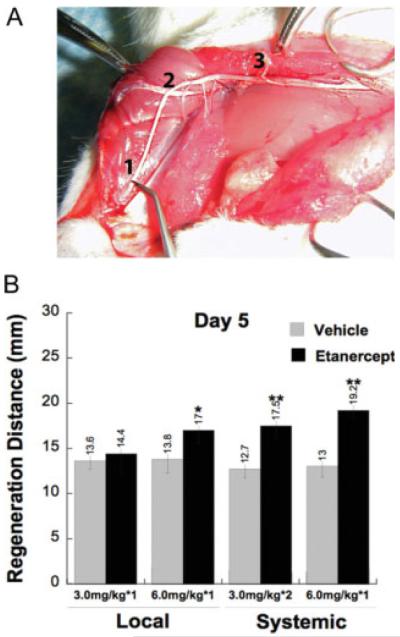
Because TNF-α mRNA expression was transient and peaked 1 day after nerve crush, we evaluated the effect of immediate anti-TNF-α (etanercept) therapy on nerve regeneration using the nerve pinch test. This test is performed between 3 and 7 days after rat sciatic nerve crush (McQuarrie et al., 1977) to determine the extent of axonal regrowth by measuring the distance between the point of crush injury and the most distal point on the nerve that produces a reflex withdrawal response when pinched with forceps (Gutmann et al., 1942; McQuarrie et al., 1977; Myers et al., 2003; Seijffers et al., 2007), as illustrated in Figure 2A. The test only measures growth of sensory axons; pinching of regenerating motor axons does not elicit withdrawal reflexes (Seijffers et al., 2007).
Fig. 2.
Etanercept therapy enhances functional regeneration of crushed sciatic nerve. A: An illustration of nerve pinch testing. Sciatic and tibial nerves are exposed in anesthetized rats, and 1-mm-long consecutive segments are pinched with a pair of forceps starting from the distal end of the tibial nerve (1), proceeding in the proximal direction until a reflex response is observed (2). The distance between this pinch site and the stitch marking the crush site (3) is measured under a dissecting microscope and defined as the regeneration distance in millimeters. B: Nerve pinch test measuring regeneration distance from the crush site (mm) at 5 days after immediate systemic, intraperitoneal (i.p.) or local epineurial administration of etanercept or vehicle after nerve crush injury. The regeneration rate was significantly enhanced after systemic and local administration of 6.0 mg/kg etanercept compared with vehicle. Single local administration of 3.0 mg/kg dose of etanercept was ineffective; however, when given twice i.p. at 1 hr and 3 days after crush, it significantly enhanced the regeneration rate compared with vehicle. Data are expressed as the mean 6 SEM regeneration distance of N = 5−6 per group. *P < 0.05, **P < 0.01, compared with vehicle-treated group by Mann-Whitney U-test.
Regeneration rates of systemic or local vehicle treatment (mean of 13.3 mm) showed close to previously reported values (13.6 mm) at 5 days after the injury for rat crushed sciatic nerve (McQuarrie et al., 1977). High-dose (6.0 mg/kg) etanercept treatment significantly improved the regeneration rate after both systemic (P < 0.01) and local (P < 0.05) administration compared with vehicle-treated animals (Fig. 2B). This difference in regeneration distance between high-dose etanercept therapy and the vehicle treatment after systemic administration (6.2 mm) was substantially higher than that of high-dose local etanercept (4.8 mm). Medium-dose (3.0 mg/kg) etanercept administered once locally immediately after injury was ineffective, but its systemic administration twice (1 hr and 3 days after crush) produced a significant increase in regeneration rate compared with vehicle (P < 0.01; Fig. 2B). Low-dose (0.3 mg/kg) etanercept demonstrated no significant change in regeneration rate after systemic or local administration (data not shown). All measurements were performed at 5 days after nerve crush. Based on its robust effect on nerve regrowth in our experimental model, etanercept was administered systemically at 6.0 mg/kg once immediately after crush injury for subsequent neuropathologic and molecular analyses.
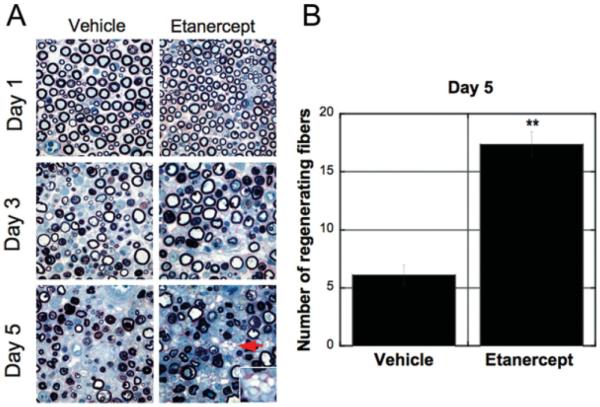
Sciatic nerve sections distal to the crush site demonstrated extensive Wallerian degeneration and inflammatory infiltrates in both vehicle- and etanercept-treated groups (Fig. 3A). Within 1 day after crush, endoneurial edema, myelin abnormalities, and hypertrophy of Schwann cell cytoplasm were observed. By day 3 after crush, myelin collapse, swelling of axons, and dark-staining axoplasm were seen as part of the early changes associated with peripheral degeneration. At 5 days after crush, etanercept therapy increased the incidence of multiple regenerative nerve clusters. Quantitative morphometry of regenerative clusters (Fig. 3B) determined that immediate systemic therapy with etanercept significantly increased the number of regenerating nerve fibers relative to vehicle treatment in the nerve segments collected 10 mm distal to nerve crush at 5 days after injury (P < 0.001).
Fig. 3.
Neuropathologic assessment of crushed nerve after etanercept therapy. A: Plastic-embedded 10-μm sciatic nerve sections after i.p. administration of 6.0 mg/kg of etanercept or vehicle, stained with methylene blue azure II. Nerves collected axially 10 mm distal to the crush site at 1, 3, and 5 days after injury. Endoneurial clusters of regenerative nerve fibers were identified at 5 days after crush injury (arrow). Inset is a magnified image of endoneurial clusters of regenerative nerve fibers. Representative micrographs of N = 6. ×3200. B: Quantitative morphometry of regenerating nerve fiber profiles at 5 days after nerve injury and etanercept therapy (graph) demonstrates an approximately threefold increase in the numbers of regenerating nerve fibers in the etanercept- compared with vehicle-treated group. Mean number of fibers − SEM. **P < 0.01, by Mann-Whitney U-test.
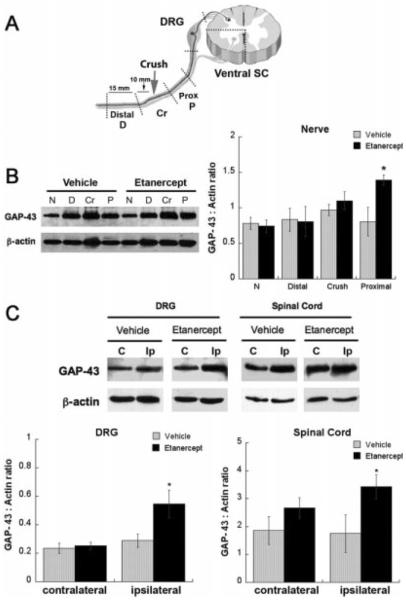
To confirm this observation, we used GAP-43, which is induced in regenerating sciatic nerve fibers and is used as a marker for axonal regeneration (Schreyer and Skene, 1991; Aigner et al., 1995; Seijffers et al., 2007). Five days after i.p. administration of high-dose (6.0 mg/kg) etanercept once immediately after crush injury (the regimen used in the above-described experiments), the following sciatic nerve segments were analyzed for GAP-43 expression: 10 mm distal to the crush site (distal), the segment encompassing the crush site (crush), and 10 mm proximal to the crush site (proximal) segment, as specified in Figure 4A. In addition, L5 DRG and ventral quarters of L4–6 spinal cords were analyzed for GAP-43 expression in sensory and motor neuronal soma, respectively. A significant increase in GAP-43 expression was found in the ipsilateral-to-injury proximal nerve segment (Fig. 4B) as well as DRG and ventral spinal cord (Fig. 4C) after etanercept therapy compared with vehicle treatment.
Fig. 4.
Etanercept increased GAP-43 expression after sciatic nerve crush. A: Graphic representation of the analyzed neuronal tissues after systemic (i.p.) etanercept (6.0 mg/kg) administration immediately after rat sciatic nerve crush. Fifteen-millimeter sciatic nerve segments were collected 10 mm distal (D) or proximal (P) to crush site (Cr); corresponding to lumbar 5 (L5) DRG and ventral quarters of L4–6 spinal cord. All tissues were collected at 5 days after crush injury and therapy. B: Western blot for GAP-43 in crushed sciatic nerve (left) and densitometry of GAP-43 to b-actin ratios (graph, right) representing the mean ± SEM of N = 5/group. Normal (N) contralateral-to-injury nerve segments were used for control. *P < 0.05, compared with vehicle-treated control group by Mann-Whitney U-test. C: Western blot for GAP-43 in DRG and ventral quarters of spinal cord, ipsilateral (Ip) or contralateral to injury. Densitometry of GAP-43 to β-actin ratios (graph, bottom) representing the mean ± SEM of N = 5/group. *P < 0.05, compared with vehicle-treated control group by Mann-Whitney U-test.
Using our previously established immunohisto-chemical method for etanercept detection in neuronal tissues (Kato et al., 2009), illustrated in Figure 5A, we analyzed the distribution of etanercept and GAP-43 in crushed nerve and the corresponding DRG and ventral spinal cord (Fig. 5B). Increased endoneurial clusters of GAP-43-positive nerve fibers were identified in the ipsi-lateral-to-injury proximal nerve segments after systemic administration of 6.0 mg/kg etanercept, consistent with the observations made by neuropathological assessment described above. Interestingly, some GAP-43-immunoreactive nerve fibers demonstrate accumulation of etanercept (Fig. 5B). Increasing numbers of GAP-43-immunoreactive DRG neurons were identified after systemic administration of 6.0 mg/kg etanercept compared with vehicle, some of which were also reactive for etanercept. Higher levels of GAP-43-immunoreactive motor neurons were identified in the ipsilateral anterior horn of spinal cord after systemic administration of 6.0 mg/kg etanercept compared with vehicle. However, no etanercept-reactive structures were detectable in these spinal cord sections.
Fig. 5.
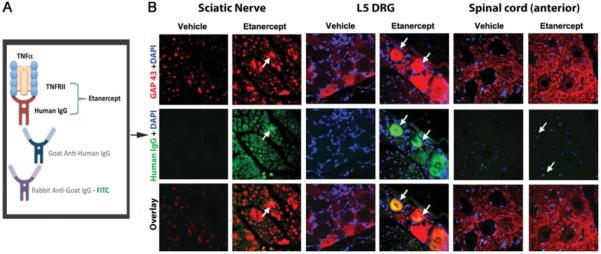
Colocalization of etanercept and GAP-43 in crushed nerve and associated DRG. A: Diagram for etanercept immunodetection method. Etanercept, a TNF-α-neutralizing agent that represents a fusion protein of TNFRII with the Fc portion of human IgG, is detected using goat anti-human IgG antibody and FITC-tagged rabbit anti-goat antibody. B: Dual immunofluorescence for GAP-43 (red) and etanercept (green) in the ipsilateral-to-injury proximal nerve segment, L5 DRG, and anterior horn of spinal cord after systemic administration of 6.0 mg/kg etanercept and vehicle treatments. Increased levels of GAP-43-positive fibers were observed in nerves, DRG, and spinal cord after etanercept therapy. In some GAP-43-immunoreactive nerve fibers, accumulation of etanercept was evident (arrows). Representative micrographs of N = 3 per group. ×400.
DISCUSSION
Multiple regenerating sprouts originate from the terminal nodes of Ranvier immediately after injury to peripheral nerve (Fawcett and Keynes, 1990). Evidence of the early events of nerve regeneration occurring parallel and often independently of Wallerian degeneration is growing (McDonald et al., 2006). Thus, identifying the role of early modulators of peripheral nerve injury in axonal regrowth may affect the development of new therapeutic strategies to promote nerve repair. The present study provides the first evidence that immediate anti-TNF-α (etanercept) therapy enhanced the rate of axonal regeneration after nerve injury. Elevated GAP-43 expression, a marker for axonal regeneration, was observed after systemic etanercept therapy in crushed nerve ipsilateral DRG and ventral spinal cord, the sites of sensory and motor neuronal soma, respectively.
TNF-α mRNA expression in nerve and corresponding DRG was elevated at 1 day and returned to baseline at 5 days after nerve crush. This early and transient increase in TNF-α mRNA expression is characteristic of sciatic nerve crush injury (George et al., 2004; Kleinschnitz et al., 2004). Considering this pattern of TNF-α expression and the half-life of etanercept of 3–5 days, its single administration immediately after crush injury and 5 days after its administration were utilized for functional and molecular assessments. Therapeutic efficacy of immediate, single etanercept administration may be valuable in minimizing potential adverse effects of prolonged anti-TNF-α therapy (Shin et al., 2006).
The nerve pinch test established enhanced rates of functional regeneration after etanercept therapy. Based on the rates established by McQuarrie et al. (1977) of approximately 4 mm/day × 3.4 days (i.e., 5 days of crush less the initial 1.6 days delay of axonal outgrowth after nerve crush), the anticipated regeneration distance at 5 days after crush equals 13.6 mm, which is close to that observed here after vehicle treatment, averaging 13.3 mm. Although it was based on the anticipated speed of nerve regrowth, day 5 after sciatic nerve crush presented an optimal time point for nerve pinch testing and targeting transient TNF-α induction; future studies should assess the effect of immediate etanercept therapy on the late stages of functional regeneration. Several interesting observations on the effects of etanercept therapy on the rate of regeneration were made. Although effective after both systemic and local administration, high-dose (6.0 mg/kg) etanercept therapy was more potent in promoting axonal regrowth after its systemic administration, consistent with its ability to neutralize not only endoneurial but also circulating and DRG neuronal TNF-α (Schneider-Schaulies et al., 1991; Larsson et al., 2005). Although, as a 150 kDa macromolecule, etanercept is not expected to cross neurovascular barriers (Zhou, 2005), DRG represent a unique structure lacking a blood–nerve barrier (Devor, 1999). In support of this conclusion, our immunofluorescence for etanercept confirmed its reactivity in DRG neurons in spinal cord. Furthermore, nerve injury-induced breakdown of neurovascular and perineurial barriers facilitates etanercept uptake by endoneurial structures compared with uninjured nerve (Kato et al., 2009).
The etanercept doses used here relate to its other reported therapeutic applications in experimental peripheral and central neuronal degeneration. For example, local and systemic administration of etanercept (2.5–5.0 mg/kg) produced a dose-dependent attenuation of thermal hyperalgesia and mechanical allodynia after sciatic nerve chronic constriction injury (CCI; Sommer et al., 2001). Systemic 3.0 mg/kg but not 0.3 mg/kg etanercept reduced thermal hyperalgesia after CCI (Zanella et al., 2008). After spinal nerve ligation (a peripheral nerve lesion closest to DRG neuronal soma), pretreatment with systemic (6.7–8.3 mg/kg) etanercept for 2 days was required to attenuate mechanical allodynia, but therapy starting at 1 and 7 days after injury was ineffective (Schafers et al., 2003), emphasizing the special importance of TNF-α in the initiation of nerve injury located close to neuronal soma. Furthermore, endoneurial administration of etanercept once immediately after injury at 6.0 mg/kg produced sustained attenuation of mechanical allodynia after sciatic nerve crush (Kato et al., 2009). Although these reports assess pain-related behaviors, they support our finding of the dose-dependent effect of etanercept and the value of its immediate administration after peripheral nerve injury. In addition, systemic (5.0 mg/kg) etanercept administration 1 hr before and 6 hr after spinal cord injury significantly ameliorated the recovery of limb function in mice (Genovese et al., 2006). Because in the clinical trials for local (perispinal) etanercept therapy for radiculopathy the estimated therapeutic dose is less than 1.0 mg/kg (Tobinick and Britschgi-Davoodifar, 2003; Tobinick and Davoodifar, 2004; Tobinick, 2009), we caution against direct extrapolation of the etanercept doses established as efficacious in rodents into its clinical use.
Earlier studies analyzed the effect of TNF-α inhibition on peripheral nerve regeneration. Inhibition of p38 MAPK signaling enhanced the rate of axonal regeneration after sciatic nerve crush, as it reduced TNF-α levels (Myers et al., 2003). However, a single dose of locally administered polyclonal anti-TNF-α antibody showed no effect on nerve regeneration after CCI (Lindenlaub et al., 2000). This is not surprising given the sustained TNF-α mRNA expression for over 28 days after CCI, in contrast to its transient expression after crush (Okamoto et al., 2001; Kleinschnitz et al., 2004). Lindenlaub and coauthors concluded that the single dose of anti-TNF-α antibody was not sufficient for neutralizing the sustained TNF-α levels after CCI. In addition, the mechanisms of action of anti-TNF-α antibody in nerve and their binding affinity to specific TNF-α isoforms are anticipated to differ (Kato et al., 2009). As a fusion protein containing a soluble TNFRII, etanercept preferentially binds to a cell surface-bound over a soluble isoform of TNF-α (Tracey et al., 2008). Actions of etanercept may also be influenced by the endogenously expressed TNFRII after sciatic nerve crush (George et al., 2005).
Neuropathological consequences of etanercept therapy demonstrated an increased number of regenerating clusters in the distal nerve segments. In line with this evaluation, etanercept therapy increased GAP-43-immunoreactive regenerative fibers in nerve. These data do not necessarily imply that TNF-α regulates the formation of new regenerating sprouts but correlate with the enhanced distance of distal fiber regrowth evidenced by pinch test. It is important to note that treatment with etanercept did not significantly influence the neuropathology of Wallerian degeneration. Similarly, the extent of axonal degeneration and macrophage content after CCI was not changed after systemic or local etanercept therapy despite its antinociceptive effect (Sommer et al., 2001). Thus, we suggest that immediate etanercept therapy promotes axonal regrowth without delay in myelin debris clearance and may present an alternative to acute treatment with glucocorticoids that cause significant delays in myelin debris removal and functional recovery after sciatic nerve injury (Boivin et al., 2007). Future studies are imperative to elucidate exactly how TNF-α actions during axonal degeneration relate to its role during axonal regrowth. However, the direct role of TNF-α on axonal outgrowth must be considered.
GAP-43 is produced in neuronal soma and transported axonally into the growth cone of regenerating axons (Fawcett and Keynes, 1990), suggesting that systemic etanercept therapy increased GAP-43 levels in the proximal nerve stump and the corresponding (sensory) DRG and ventral spinal cord (motor) by its effect on neuronal soma. Indeed, in cultured DRG and hippocampal neurons, TNF-α directly inhibits neurite outgrowth by activation of the RhoA/Rho-kinase pathway (Schneider-Schaulies et al., 1991; Neumann et al., 2002; Larsson et al., 2005), which is inhibitory of axonal regeneration (Hiraga et al., 2006). Interestingly, another proinflammatory cytokine, interleukin-1β (IL-1β), deactivates RhoA and promotes sensory nerve regeneration after sciatic nerve injury (Cao et al., 2006; Myers et al., 2006; Temporin et al., 2008). Thus, activation and deactivation of RhoA pathway represent a mechanism of opposing TNF-α and IL-1β effects in axonal regeneration. It will be important to investigate in future studies the effects of extended anti-TNF-α therapy on the cytokine network during neurite outgrowth.
Etanercept colocalized with GAP-43 positive structures in the crushed nerve and the corresponding DRG neurons. Current evidence suggests that TNF-α antagonists have dual functions and act as antagonists by blocking transmembrane TNF-α interactions with TNFRs or as agonists by initiating reverse (receptor-transmembrane TNF-α ligand) signaling (Tracey et al., 2008). Insofar as etanercept was not immunoreactive in the spinal cord, the mechanism by which its systemic therapy produces a GAP-43 increase in motor neurons of the spinal cord and its effect on the functional locomotive recovery that is not assessed by nerve pinch testing (Seijffers et al., 2007) remain to be elucidated. In summary, this study provides the first evidence that immediate therapy with TNF-α antagonist supports peripheral nerve regeneration.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Jennifer Dolkas and Mila Angert for expert technical assistance and Amber Millen for help in editing the manuscript. The devices and drugs that are the subject of this paper are not FDA approved for this indication. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this paper.
Contract grant sponsor: Veterans Affairs Rehabilitation Research and Development Program.
REFERENCES
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Aoki Y, An HS, Takahashi K, Miyamoto K, Lenz ME, Moriya H, Masuda K. Axonal growth potential of lumbar dorsal root ganglion neurons in an organ culture system: response of nerve growth factor-sensitive neurons to neuronal injury and an inflammatory cytokine. Spine. 2007;32:857–863. doi: 10.1097/01.brs.0000259810.48681.90. [DOI] [PubMed] [Google Scholar]
- Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SJ, Jiang Y, Davis AE, Farrands R, Holbrook J, Leppert D, Anthony DC. Immunomodulatory effects of etanercept in a model of brain injury act through attenuation of the acute-phase response. J Neurochem. 2007;103:2245–2255. doi: 10.1111/j.1471-4159.2007.04928.x. [DOI] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine inter-leukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain Suppl. 1999;6:S27–S35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Frisen J. Determinants of axonal regeneration. Histol Histopathol. 1997;12:857–868. [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P, Cuzzocrea S. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther. 2006;316:1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- George A, Buehl A, Sommer C. Wallerian degeneration after crush injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci Lett. 2004;372:215–219. doi: 10.1016/j.neulet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- George A, Buehl A, Sommer C. Tumor necrosis factor receptor 1 and 2 proteins are differentially regulated during Wallerian degeneration of mouse sciatic nerve. Exp Neurol. 2005;192:163–166. doi: 10.1016/j.expneurol.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Guttmann L, Medawar P, Young J. The rate of regeneration of nerve. J Exp Biol. 1942;19:14–44. [Google Scholar]
- Hayashi S, Taira A, Inoue G, Koshi T, Ito T, Yamashita M, Yamauchi K, Suzuki M, Takahashi K, Ohtori S. TNF-alpha in nucleus pulposus induces sensory nerve growth: a study of the mechanism of discogenic low back pain using TNF-alpha-deficient mice. Spine. 2008;33:1542–1546. doi: 10.1097/BRS.0b013e318178e5ea. [DOI] [PubMed] [Google Scholar]
- Hiraga A, Kuwabara S, Doya H, Kanai K, Fujitani M, Taniguchi J, Arai K, Mori M, Hattori T, Yamashita T. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst. 2006;11:217–224. doi: 10.1111/j.1529-8027.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Kato K, Kikuchi S, Shubayev VI, Myers RR. Distribution and tumor necrosis factor-alpha isoform binding specificity of locally administered etanercept into injured and uninjured rat sciatic nerve. Neuroscience. 2009;160:492–500. doi: 10.1016/j.neuroscience.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C, Brinkhoff J, Zelenka M, Sommer C, Stoll G. The extent of cytokine induction in peripheral nerve lesions depends on the mode of injury and NMDA receptor signaling. J Neuroimmunol. 2004;149:77–83. doi: 10.1016/j.jneuroim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Larsson K, Rydevik B, Olmarker K. Disc related cytokines inhibit axonal outgrowth from dorsal root ganglion cells in vitro. Spine. 2005;30:621–624. doi: 10.1097/01.brs.0000155410.48700.9e. [DOI] [PubMed] [Google Scholar]
- Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 2000;866:15–22. doi: 10.1016/s0006-8993(00)02190-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta CT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luk HW, Noble LJ, Werb Z. Macrophages contribute to the maintenance of stable regenerating neurites following peripheral nerve injury. J Neurosci Res. 2003;73:644–658. doi: 10.1002/jnr.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Cheng C, Chen Y, Zochodne D. Early events of peripheral nerve regeneration. Neuron Glia Biol. 2006;2:139–147. doi: 10.1017/S1740925X05000347. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B, Gershon MD. Axonal regeneration in the rat sciatic nerve: effect of a conditioning lesion and of dbcAMP. Brain Res. 1977;132:443–453. doi: 10.1016/0006-8993(77)90193-7. [DOI] [PubMed] [Google Scholar]
- Myers RR, Sekiguchi Y, Kikuchi S, Scott B, Medicherla S, Protter A, Campana WM. Inhibition of p38 MAP kinase activity enhances axonal regeneration. Exp Neurol. 2003;184:606–614. doi: 10.1016/S0014-4886(03)00297-8. [DOI] [PubMed] [Google Scholar]
- Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006;11:8–20. doi: 10.1016/S1359-6446(05)03637-8. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Proand anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies J, Kirchhoff F, Archelos J, Schachner M. Down-regulation of myelin-associated glycoprotein on Schwann cells by interferon-gamma and tumor necrosis factor-alpha affects neurite outgrowth. Neuron. 1991;7:995–1005. doi: 10.1016/0896-6273(91)90344-y. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J Neurosci. 1991;11:3738–3751. doi: 10.1523/JNEUROSCI.11-12-03738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin IS, Baer AN, Kwon HJ, Papadopoulos EJ, Siegel JN. Guillain-Barre and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429–1434. doi: 10.1002/art.21814. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Temporin K, Tanaka H, Kuroda Y, Okada K, Yachi K, Moritomo H, Murase T, Yoshikawa H. Interleukin-1 beta promotes sensory nerve regeneration after sciatic nerve injury. Neurosci Lett. 2008;440:130–133. doi: 10.1016/j.neulet.2008.05.081. [DOI] [PubMed] [Google Scholar]
- Tobinick E. Perispinal etanercept for neuroinflammatory disorders. Drug Discov Today. 2009;14:168–177. doi: 10.1016/j.drudis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133:170–177. doi: 10.4414/smw.2003.10163. [DOI] [PubMed] [Google Scholar]
- Tobinick E, Davoodifar S. Efficacy of etanercept delivered by perispinal administration for chronic back and/or neck disc-related pain: a study of clinical observations in 143 patients. Curr Med Res Opin. 2004;20:1075–1085. doi: 10.1185/030079903125004286. [DOI] [PubMed] [Google Scholar]
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Young JZ, Medawar PB. Fibrin suture of peripheral nerves: measurement of the rate of regeneration. Lancet. 1940;236:126–128. [Google Scholar]
- Zanella JM, Burright EN, Hildebrand K, Hobot C, Cox M, Christoferson L, McKay WF. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine. 2008;33:227–234. doi: 10.1097/BRS.0b013e318162340a. [DOI] [PubMed] [Google Scholar]
- Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490–497. doi: 10.1177/0091270004273321. [DOI] [PubMed] [Google Scholar]