Abstract
Aims
To determine antibacterial activity of Ocimum suave essential oils against bacterial uropathogens.
Study Design
A cross sectional and experimental study.
Place and Duration of Study
Six selected hospitals in Bushenyi District, Uganda between June 2012 and July 2013.
Methodology
Clean catch midstream urine samples were collected and inoculated on Cystine Lysine Electrolyte Deficient (CLED) agar. The plates were incubated at 37°C for 24hrs to 48hrs. The O. suave essential oils were extracted by hydrodistillation of leaves for 4hrs using a Clevenger apparatus. The oil was collected and dried over anhydrous sodium sulphate (Na2SO4) and kept at 4°C till further use. The antimicrobial activity of O. suave essential oils against isolates was determined by agar well method. The MIC of O. suave essential oil extract was carried out by microbroth dilution method.
Results
Of the three hundred (300) midstream urine samples collected, 67(22.33%) had significant bacterial growth. Escherichia coli is the most common isolate (61.19%, n = 41). The essential oil from O. suave showed activity against isolates of E. coli, K. pneumoniae, S. aureus, E. feacalis, M. morganii, Citrobacter species, Enterobacter species and P. aeruginosa with mean zone of inhibition (ZI) ranging from 10–22 mm. The essential oils had no inhibitory activity on Acinetobacter species. The minimum inhibitory concentration (MIC) for O. suave essential oils ranged from 0.78 to 22 μg/ml. This study showed that O. suave essential oils had MIC value of 0.78 μg/ml against S. aureus and MIC values ranging from 3 to 22 μg/ml against the other tested isolates.
Conclusion
The most common uropathogen was E. coli (61.19% n = 41). O. suave essential oils exhibited antibacterial activity against majority of the uropathogens, except Acinetobacter species, mean ZI of 10–22 mm and MIC of 0.78 – 22 μg/ml.
Keywords: Aromatic medicinal plants, bacteriauria, E. coli, resistance
1. INTRODUCTION
Urinary tract infections (UTIs) are commonly encountered conditions, especially in developing countries, with an estimated annual global incidence of at least 250 million [1–3]. About 150 million people worldwide are diagnosed with UTIs each year costing the global economy in excess of 6 billion US dollars [4–6]. The Uganda Bureau of Statistics, National Household Survey found the national prevalence of UTIs to be 0.2%. However, its impact and frequency vary in different populations [7]. The recent studies by Andabati and Byamugisha [8] found the prevalence at 13.3% and drug resistance occurrence of 20–62% in Mulago Hospital, Uganda.
Escherichia coli and other enterobacteriacae are the most common cause of UTIs and account for approximately 75% of the isolates [3]. The relative frequencies of the pathogens vary with age, sex, catheterization, and hospitalization [3,9]. Worldwide, E. coli causes 75–90% acute uncomplicated cystitis while S. saprophyticus accounts for 5–15%, mainly in younger women [4,10–12]. Although it is not always possible to trace the mode of entry of bacteria into the urinary tract, four possible routes of entry have been suggested; ascending infection; haematogenous spread; lymphogenous spread, and direct extension from another organ [13].
The search for antimicrobials of plant origin has been mainly stimulated by the fact that they contain multiple biochemical compounds to which microbes cannot develop resistance simultaneously. Due to occurrence of drug resistant uropathogens, there is a need to search for alternative and effective antimicrobial agents. Although, the drug resistance development by microbes cannot be stopped, appropriate use of more effective antibiotics including products of plant origin may reduce the mortality and health care costs [14,15]. Essential oils from aromatic medicinal plants have been reported by various researchers to exhibit antimicrobial activity against bacteria, yeasts, filamentous fungi, viruses and cancer [16–22]. However, there are few reports on its activity against uropathogens [22,23].
Traditional remedies utilizing plant products occupy the central place among rural communities in developing countries for curing various diseases, in the absence of an efficient primary health care system [15,24,25]. Traditional people, especially in the rural areas use O. suave for treatment of discomforts associated with the urinary and reproductive tracts. However, little information on the activity of this plant against uropathogens exists. Therefore, the aim of this study is to determine antibacterial activity of O. suave essential oils against uropathogens.
2. MATERIALS AND METHODS
2.1 Study Design
A cross sectional and experimental study was carried out in six selected health facilities (i.e. Kampala International University-Teaching Hospital (KIU-TH), Ishaka Adventist Hospital, Comboni Hospital, Bushenyi Medical Centre (BMC), Bushenyi Health Centre IV and Kyabugimbi Health Centre IV) in Bushenyi District, Uganda between June 2012 and July 2013.
2.1.1 Inclusion and exclusion criteria
The study included patients aged 18 to 51 years attending out-and-in patient clinics, who were confirmed to have UTI signs and symptoms by the attending Clinician. All the patients with no history of antimicrobial drug administration for UTIs in the last two weeks and had consented to participate in the study.
The study excluded female patients who were in their menstruation period, patients aged below 18 years, those with history of antimicrobial drug administration in the last two weeks, non-Bushenyi residents, patients not registered at the selected hospitals and patients who had not consented to participate.
2.1.2 Consent and counseling
A written consent was sought from the patients who satisfied the inclusion criteria. The Self-administered questionnaire and interview guide was carried out to capture demographic data, predicting factors for UTIs and counseling for specimen collection. The study subject was then sent for specimen collection and the results were kept confidential.
2.1.3 Sample size and sampling procedure
Three hundred (300) morning midstream clean catch urine samples were collected from in-and-out patients with the help of trained nursing staff. The sample size (n) was calculated using the standard formula [26].
Where: n = Sample size, Q = 100−P, Z = Level of significance (1.96) for confidence interval of 95%, P = Prevalence, I = margin of error of setting a significance level of 0.05 (i.e. 5%).
The urine samples were collected using random sampling method by taking the third patient on the waiting list of all the patients assessed for UTIs signs and symptoms by the attending Medical Officer or Clinician. Fifty samples were collected from each of the study areas with daily attendance of 150 patients with UTIs. The samples were then transported on ice to the laboratory for standard microbiological analysis within 30 minutes of collection. Baseline data such as patients’ age, sex, and clinical history were recorded at the time of sampling.
2.2 Isolation and Identification of the Uropathogens
Midstream clean catch urine samples were inoculated on CLED agar (Oxoid, UK) plates using a calibrated loop delivering 0.001ml of urine. Inoculated plates were incubated at 37°C for 24 to 48 hrs [27]. The samples were considered positive for UTI if pure culture of 105CFU/ml were obtained from uncentrifuge urine sample and ≥5 pus cells observed in urine sample per field under microscope [8,28,29]. The presumptive identification of the isolates was based on the cultural characteristics on CLED agar (Oxoid, UK) plates and identification confirmed by standard identification protocol namely; Gram staining, motility test and conventional biochemical tests (i.e. oxidase, catalase, coagulase, IMViC, TSI agar, urease, gelatinase and the ability to grow in KCN [30–33]. The isolates were preserved using 15%v/v glycerol at −70°C.
2.3 Plant Collection and Identification
The leaves of O. suave were collected from Bushenyi District, South Western Uganda. Plant identification was carried out at the Department of Botany, Makerere University using plant shoots with leaves and flowers. Voucher specimen (JT 001) was deposited at the Makerere University Herbarium.
2.3.1 Extraction of essential oils
Fresh mature leaves of O. suave were collected and thoroughly washed with distilled water twice. The excess water was drained off and the leaves cut into small pieces and hydro distilled for four (4) hours using a Clevenger apparatus. The oil was collected and dried over anhydrous sodium sulphate (Na2SO4). The extracted oil was stored at 4°C in glass bottle wrapped with aluminium foil.
A working solution of the essential oil was freshly prepared before use. Dimethyl sulfoxide (DMSO) was used to enhance oil solubility. A 100 μl of essential oil was diluted with 50 μl of dimethyl sulfoxide (DMSO) making a total volume of 150 μl. The working concentrations of essential oils were sterilized by filtering through a 0.2 μm single use filters (Sterile Acrodisc®).
2.4 Preparation of Bacterial Inoculums
The bacterial inoculum were prepared by suspending colony from a pure culture in sterile normal saline and the turbidity adjusted to match 0.5 McFarland standards; that is, about 5 × 105CFU/ml. Escherichia coli ATCC 25922 and S. aureus ATCC 12692, obtained from Department of Medical Microbiology, Makerere University, were used as reference strains.
2.4.1 Screening for antibacterial activity of essential oils
The antimicrobial activity of O. suave essential oils was screened against uropathogen isolates by the agar gel diffusion method [34], with slight modifications. Essential oil concentrations, ranging from 25 μg/ml to 50 μg/ml, were prepared with 0.5% DMSO. Ciprofloxacin and Nitrofurantoin were used as antimicrobial agent positive controls in the assay, while DMSO was the negative control.
2.4.2 Minimum inhibitory concentration (MIC) of essential oils
The MIC reference of antimicrobial drug and O. suave essential oils extract was carried out by micro-broth dilution method using Mueller-Hinton broth (Oxoid, UK) [35–37]. Two-fold serial dilutions of essential oil, ranging from 5, 10, 20: 30, 40, and 50 μg/ml, were prepared with 0.5% DMSO. Then, for both the test and reference bacterial strains, 0.01ml of the standard isolate was inoculated into each well. The test was carried out in 96-well microtitre plates; 5 μl essential oil was dispensed into the first well containing 95μl of Mueller-Hinton broth and serially diluted by transferring 5 μl and 5 μl of inoculum added to each well. The plates were then incubated at 37°C for 18–24 h. The lowest concentration showing no visible growth was considered as the MIC.
2.5 Data Analysis
The data was entered in EpiData version 3.1.2701.2008, and statistical analysis was done by descriptive statistics using SPSS version 11.5. The antibacterial activity was reported in terms of diameters of the zones of inhibition (mm). The data was presented as mean ± standard deviation (SD). Comparison of means of zones of inhibition and MICs was done using student t-test and values of P < 0.05 were regarded as significant.
2.6 Ethical Approval
The ethical approval of the study was sought from Mbarara University of Science and Technology (MUST), Institutional Research and Ethics Committee (IREC) on Human Research, and Uganda National Council for Science and Technology (UNCST). All experiments were examined and approved by the appropriate ethics committees and performed in accordance with the ethical standards of the committees on human experimentation laid down in the Helsinki declaration of 1975 as revised in 2000.
3. RESULTS
Three hundred (300) morning mid-stream clean catch urine samples were collected from patients attending the selected hospitals. Sixty seven samples 67 (22.33%) had significant bacteriuria. Of the 104 male urine samples, 22(21.15%) had positive cultures, compared to the 45(22.96%) out of 196 female samples. The prevalence of UTIs was found to be high (46.27%), in the age group of 18–28 years as presented in Fig. 1 below.
Fig 1.
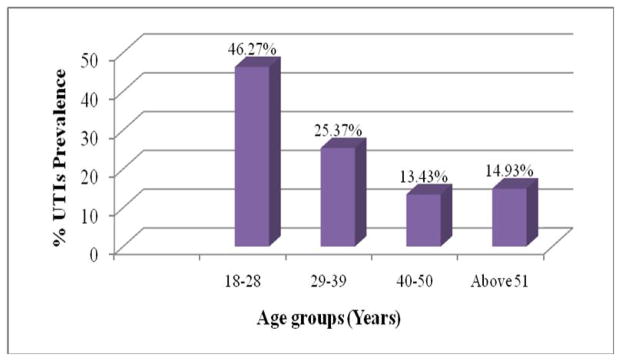
Prevalence of UTIs in different age groups
Urinary tract infections (UTIs) are mainly caused by bacteria and the findings in this study showed that nine bacterial uropathogens were isolated from 67 midstream clean catch urine samples of which E. coli was the most frequent isolate 41(61.19%), followed by S. aureus, 10(14.93%), K. pneumoniae 4(5.97%), E. feacalis 4(5.97%), M. morganii 3(4.89%), and Citrobacter species 2(2.99%). The least isolated were Acinetobacter species 1(1.49%), Enterobacter species 1(1.49%), and P. aeruginosa 1(1.49%) as shown in Fig. 2 below.
Fig 2.
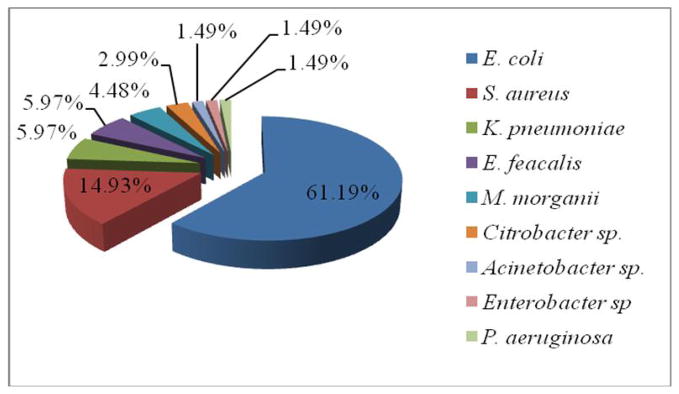
Percentage bacterial uropathogens isolated from patients
Fresh leaves of O. suave yielded 0.2%v/wt of essential oil. The O. suave essential oil exhibited antibacterial activity against isolated uropathogens. The activity of essential oil was lower compared to the drug references used. The mean zones of inhibition ranged between 16–22 mm as compared to 13–29 mm and 11–26 for Ciprofloxacin (5 μg) and Nitrofurantoin (300 μg) reference antibiotic, respectively. The highest mean zone of inhibition (ZI) of 22 mm was exhibited against E. coli, whereas it was 23mm and 11 mm for ciprofloxacin and nitrofurantoin respectively against the same organisms. The activity of the essential oil was significant against the isolated of E. coli, mean ZI of 22 mm and 18 mm at 5% confidence interval, P = .012.
However, there was no significant difference in the activity of nitrofurantoin and essential oil with (P = .786). The activity of essential oil showed no significant difference against the other uropathogens isolates when compared to ciprofloxacin (5 μg) and nitrofurantoin (300 μg) positive reference standard antibiotics. The mean ZI of essential oil were 23 mm ciprofloxacin and nitrofurantoin 15 mm against S. aureus. However, the essential oil showed no activity against Acinetobacter species and there was no inhibition of growth with the negative control (10% DMSO) as shown in Table 1 below.
Table 1.
Antibacterial activity of O. suave essential oils (p = < .05)
| Isolates | Mean inhibition zones (mm)
|
||||
|---|---|---|---|---|---|
| ID No. | CIP (5 μg/ml) | F (300 μg/ml) | EO (50 μg/ml) | DMSO | |
| E. coli | KIUTH 010 | 22±0.58 | 18±0.58 | 16±0.00 | 0 |
| E. coli | KHC 076 | 29±0.58 | 11±0.58 | 16±0.58 | 0 |
| E. coli | KHC 053 | 13±0.58 | 18±0.58 | 21±0.58 | 0 |
| E. coli | KHC 062 | 28±0.58 | 14±0.58 | 21±0.58 | 0 |
| E. coli | IAH 148 | 23±1.00 | 11±0.58 | 22±0.58 | 0 |
| E. coli | IAH 114 | 24±1.00 | 24±0.58 | 16±0.58 | 0 |
| E. coli | IAH 112 | 25±0.58 | 21±0.58 | 16±0.58 | 0 |
| E. coli | IAH 138 | 23±1.00 | 18±0.58 | 16±0.58 | 0 |
| E. coli | CoH 122 | 26±1.00 | 26±0.58 | 17±0.58 | 0 |
| E. coli | BMC 209 | 21±0.58 | 22±0.58 | 16±0.58 | 0 |
| E. coli ATCC25922 | Control | 14±0.58 | 24±0.58 | 20±0.58 | 0 |
| S. aureus | KIUTH 001 | 26±1.00 | 24±0.58 | 17±0.58 | 0 |
| S. aureus | KIUTH 036 | 21±0.58 | 28±0.58 | 10±0.58 | 0 |
| S. aureus | KIUTH 033 | 15±0.58 | 21±0.58 | 19±0.58 | 0 |
| S. aureus | KIUTH 030 | 26±0.58 | 19±0.58 | 16±0.58 | 0 |
| S. aureus | KIUTH 038 | 29±0.58 | 20±0.58 | 14±0.58 | 0 |
| S. aureus ATCC12692 | Control | 14±0.58 | 18±0.58 | 10±0.58 | 0 |
| K. pneumoniae | CoH 135 | 10±0.58 | 18±0.58 | 16±0.58 | 0 |
| K. pneumoniae | BHC 093 | 22±0.58 | 13±0.58 | 14±0.58 | 0 |
| E. feacalis | BHC 061 | 26±0.58 | 11±0.58 | 11±0.58 | 0 |
| M. morganii | KHC 068 | 27±0.58 | 26±0.58 | 18±0.58 | 0 |
| Citrobacter species | CoH 111 | 26±0.58 | 30±0.58 | 9±0.58 | 0 |
| Acinetobacter species | IAH 129 | 24±0.58 | 20±0.58 | - | 0 |
| Enterobacter species | KIUTH 026 | 17±0.58 | 16±0.58 | 16±0.58 | 0 |
| P. aeruginosa | KHC 078 | 23±0.58 | 20±0.58 | 18±0.58 | 0 |
F – Nitrofurantoin, CIP – Ciprofloxacin, EO – Essential oil, DMSO - Dimethyl sulfoxide, - no activity
The MICs for O. suave essential oils ranged from 0.78 to 22 μg/ml. This study revealed that O. suave essential oils showed maximum activity with MIC value of 0.78 μg/ml against S. aureus and showed moderate MIC values against the other test isolates. The minimum concentration (MBC) of antimicrobial necessary to kill an organism should be equal to or greater than the MIC for that microbe. In this study eight bacterial isolates presented MBCs which were within one two-fold dilution of the MIC obtained for the isolates. The MICs and MBCs of O. suave essential oils determined by the broth microdilution method of the isolated uropathogens are shown in Table 2 below.
Table 2.
Minimum bactericidal concentration (MBC) of O. suave essential oil
| Isolate | ID No. | CIP | F | EOMIC (μg/ml) | EOMBC (μg/ml) |
|---|---|---|---|---|---|
| E. coli | KIUTH 010 | 1 | 31 | 6 | 12 |
| E. coli | KHC 076 | 0.76 | 128 | 6 | 12 |
| E. coli | KHC 053 | 4.5 | 32 | 13 | 13 |
| E. coli | KHC 062 | 0.72 | 132 | 3 | 6 |
| E. coli | IAH 148 | 0.95 | 30 | 3 | 6 |
| E. coli | IAH 114 | 0.52 | 24 | 6 | 12 |
| E. coli | IAH 112 | 0.82 | 24 | 6 | 12 |
| E. coli | IAH 138 | 0.96 | 30 | 6 | 12 |
| E. coli | CoH 122 | 0.91 | 29 | 7 | 7 |
| E. coli | BMC 209 | 1 | 30 | 6 | 6 |
| E. coli ATCC25922 | Control | 4.53 | 30 | 12 | 12 |
| S. aureus | KIUTH 001 | 0.6 | 24 | 8 | 8 |
| S. aureus | KIUTH 036 | 1 | 30 | 20 | 20 |
| S. aureus | KIUTH 033 | 4.2 | 29 | 10 | 10 |
| S. aureus | KIUTH 030 | 0.45 | 28 | 0.78 | 1.5 |
| S. aureus | KIUTH 038 | 0.74 | 27 | 9 | 9 |
| S. aureus ATCC12692 | Control | 4.25 | 26 | 11 | 11 |
| K. pneumoniae | CoH 135 | 0.46 | 24 | 11 | 11 |
| K. pneumoniae | BHC093 | 4.75 | 31 | 16 | 16 |
| E. feacalis | BHC 061 | 0.46 | 24 | 9 | 9 |
| M. morganii | KHC 068 | 0.9 | 29 | 8 | 8 |
| Citrobacter species | CoH 111 | 0.8 | 27 | 6 | 12 |
| Enterobacter species | KIUTH 026 | 0.87 | 27 | 11 | 11 |
| P. aeruginosa | KHC 078 | 2 | 32 | 22 | 22 |
F – Nitrofurantoin, CIP – Ciprofloxacin, EO – Essential oil, EOMIC – Essential oil minimum inhibitory concentration, EOMBC – Essential oil minimum bactericidal concentration
4. DISCUSSION
Urinary tract infections (UTIs) are the most common infections that affects all age groups, men, women and children worldwide [13,38–40]. The results obtained were in line with the previous studies due to the fact that UTI signs and symptoms are not reliable indicators of the infection. Early diagnosis, timely and appropriate antimicrobial treatment are considered key factors for elimination of the uropathogens, prevent urosepsis and reduce the risk of renal scarring[13].
These findings are in agreement with most previous studies on UTIs [12,41]. UTIs due to E. coli are common in women because of its inherent virulence for urinary colonization particularly its adhesive abilities and the association with microorganisms ascending from the periurethral areas contaminated by fecal flora due to the close proximity to the anus and warm moist environment of the female genitalia[8]. The findings of this study are in line with the previous studies where similar results were observed by Tambekar et al. [42], reported significant bacteriuria in 39.1 of the cases, with the most common pathogens being E. coli (59%). The UTIs were found to be most frequent in female (63%) than male (37%) patients. According to Amin et al. [43], reported 68% females’ and 32% males’ urine cultures were positive for bacteria. The predominant isolate was E. coli with frequency rate of 59%. Other studies have also reported higher incidence of E. coli (47.30%) in urine samples [44]. It is interesting to note that only few have reported the presence of Citrobacter species, in UTIs [45,46].
The indiscriminate use of antimicrobial drugs has led to resistance in uropathogens globally [22]. Concurrent resistance to different antimicrobial agents has given rise to multi-drug resistance in uropathogens, which also complicates the therapeutic management of UTIs [4,22]. The spread of drug resistant uropathogens is one of the most serious threats to successful treatment of microbial diseases. Thus, the need for the discovery of new antimicrobial substances from natural sources, including plants. Essential oils from aromatic medicinal plants have been reported to exhibit antimicrobial effects against bacteria, yeasts, filamentous fungi, and viruses [21]. Essential oils are potential sources of novel antimicrobial compounds especially against bacterial pathogens [47]. In vitro studies in this study showed that O. suave essential oils inhibited bacterial growth. These findings are comparable to studies by Lima et al. [48], on in vitro antifungal activity of 13 essential oils obtained from plants against dermatophytes, O. gratissimum was found to be the most active, inhibiting 80% of the dermatophyte strains tested and producing zones of inhibition greater than 10mm in diameter. The MICs of the reference drugs used in this study were similar to those presented in [49].
The similar results were observed by Ilory et al. [50], on the antidiarrhoeal activities of leaf extracts of O. gratissimum with activity against Aeromonas sobria, E. coli, P. shigelloides, S. typhi and S. dysenteriae. The MIC for isolates ranged from 8 to 50mg/ml, while the MBC were from 8 to 62mg/ml. Other reports have shown MIC results similar to or higher [51,52]. These differences may be explained by susceptibility testing conditions, physicochemical characteristics of the oil, or even strain-to-strain differences and units of measurements.
According to Lopez et al. [53], ocimum oil has been described to be active against several species of bacteria and fungi. The chemical compositions in the essential oils are mainly of monoterpenes or sesquiterpenes with predominant features representing the terpenic chemotype group such as linalool and geraniol or the phenylpropenic chemotype groups, while the observed biological activities are attributable to either the individual components within the matrix of the oil or due to a synergistic effect of the components [54–60]. The prospect of further developing and using essential oils exhibiting broad spectrum biological activities holds promise in medicine and agriculture, owing to its low mammalian toxicity, biodegradability, non-persistence in the environment and affordability [60].
5. CONCLUSION
E. coli was the most common organism detected in this study. The O. suave essential oils showed activity against E. coli, K. pneumoniae, S. aureus, E. feacalis, M. morganii, Citrobacter species, Enterobacter species and P. aeruginosa; but had no activity against Acinetobacter species. The essential oils showed antibacterial activity against the isolated of E. coli, mean ZI of 22 mm and MIC of 0.78 μg/ml against S. aureus.
Acknowledgments
We thank Kampala International University – Western Campus for the support. This study was supported by MEPI-MESAU Grant Number 5R24TW008886 supported by OGAC, NIH and HRSA.
Footnotes
Authors’ contributions
This work was carried out in collaboration between all authors. Author JT designed the study, participated in site recruitment, laboratory analysis, data entry and wrote the first draft of the manuscript. Authors MAO and JMN participated in the study design, laboratory quality assurance, supervised and reviewed the manuscript. Author JLN participated in study design, drafted and critically reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
Authors have declared that no competing interests exist.
References
- 1.Ronald AR, Nicolle LE, Stamm E. Urinary tract infection in adults: Research priorities and strategies. Int J Antimicrob Agents. 2001;17:343–348. doi: 10.1016/s0924-8579(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 2.Barišić Z, Babić-Erceg A, Borzić El. Urinary tract infections in South Croatia: Aetiology and antimicrobial. Intl J Antimicrob Agents. 2003;22:S61–S64. doi: 10.1016/s0924-8579(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 3.Getenet B, Wondewosen T. Bacterial Uropathogens In Urinary Tract Infection And Antibiotic Susceptibility Pattern In Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21(2) doi: 10.4314/ejhs.v21i2.69055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 5.Ava B, Mohammad R, Jalil VY. A survey on epidemiology of urinary tract infections and resistance pattern of uropathogens in an Iranian-1000-bed tertiary care hospital. African Journal of Microbiology Research. 2010;4(9):753–756. [Google Scholar]
- 6.Manikandan S, Ganesapandian S, Singh M, Kumaraguru AK. Antimicrobial Susceptibility Pattern of Urinary Tract Infection Causing Human Pathogenic Bacteria. Asian Journal of Medical Sciences. 2011;3(2):56–60. [Google Scholar]
- 7.UBoS. Uganda National Household Survey Report. Uganda Bureau of Statistics; 2010. Copyright ©. [Google Scholar]
- 8.Andabati G, Byamugisha J. Microbial aetiology and sensitivity of asymptomatic bacteriuriaamong ante-natal mothers in Mulago hospital, Uganda. African Health Sciences. 2010;10(4):349–352. [PMC free article] [PubMed] [Google Scholar]
- 9.Sefton AM. The impact of resistance on the management of urinary tract infections. Int J Antimicrob Agents. 2000;16:489–491. doi: 10.1016/s0924-8579(00)00282-x. [DOI] [PubMed] [Google Scholar]
- 10.Ronald A. The aetiology of urinary tract infection; traditional and emerging pathogens. Am J Med. 2002;113(Suppl 1A):14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 11.Fihn SD. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259–266. doi: 10.1056/NEJMcp030027. [DOI] [PubMed] [Google Scholar]
- 12.Mwaka AD, Mayanja KH, Kigonya E, Kaddu MD. Bacteriuria among adult non-pregnant women attending Mulago hospital assessment centre in Uganda. African Health Sciences. 2011;11(2):182–189. [PMC free article] [PubMed] [Google Scholar]
- 13.Maripandi A, Ali AA, Amuthan M. Prevalence and antibiotics susceptibility of Uropathogens in patients from a rural environment, Tamilnadu. Am J Infect Dis. 2010;6(2):29–33. [Google Scholar]
- 14.Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 15.Pandey RR, Dubey RC, Saini S. Phytochemical and antimicrobial studies on essential oils of some aromatic plants. African Journal of Biotechnology. 2010;9(28):4364–4368. [Google Scholar]
- 16.Deans SG, Svoboda KP, Gundidza M, Brechany EY. Essential oil profiles of several temperate and tropical aromatic plants: their antimicrobial and antioxidant activities. Acta Hortic. 1992;306:229–232. [Google Scholar]
- 17.Piccaglia R, Marotti M, Giovanelli E, Deans SG, Eaglesham E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Ind Crops and Prod. 1993;2:47–50. [Google Scholar]
- 18.Svoboda KP, Hampson JB. Bioactivity of essential oils of selected temperate aromatic plants: antibacterial, antioxidant, antiinflammatory and other related pharmacological activities. Proceedings of the Specialty Chemicals for the 21st Century ADEME/IENICA Seminar; Sept. 16–17; Paris: ADEME; 1999. pp. 43–49. [Google Scholar]
- 19.Jirovetz L, Buchbauer G, Denkova Z, Slavchev A, Stoyanova A, Schmidt E. Chemical composition, antimicrobial activities and odor descriptions of various Salvia sp. and Thuja sp. essential oils. Nutrition. 2006;30:152–158. [Google Scholar]
- 20.Silva CB, Gueterres SS, Weisheimer V, Schapoval ES. Antifungal activity of lemongrass oil and citral against Candida spp. Braz J Infect Dis. 2008;12:63–66. doi: 10.1590/s1413-86702008000100014. [DOI] [PubMed] [Google Scholar]
- 21.Reichling J, Schnitzler P, Suschke U, Saller R. Essential Oils of Aromatic Plants with Antibacterial, Antifungal, Antiviral and Cytotoxic Properties – an Overview. Forsch Komplementmed. 2009;16:79–90. doi: 10.1159/000207196. [DOI] [PubMed] [Google Scholar]
- 22.Tripti M, Sigh P. Antimicrobial effects of essential oils against Uropathogens with varying sensitivity to antibiotics. Asian Journal of Biological Sciences. 2010;3:92–98. [Google Scholar]
- 23.Pereira RS, Sumita TC, Furlan MR, Jorge AO, Ueno M. Antibacterial activity of essential oils on microorganisms isolated from urinary tract infection. Rev Saude Publ. 2004;38:326–328. doi: 10.1590/s0034-89102004000200025. [DOI] [PubMed] [Google Scholar]
- 24.Ali ANA, Julich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol. 2001;74:173–179. doi: 10.1016/s0378-8741(00)00364-0. [DOI] [PubMed] [Google Scholar]
- 25.Pandey AK. Composition and in vitro antifungal activity of the essential oil of menthol mint (Mentha arvensis L.) growing in central India. Ind Drugs. 2003;40(2):126–128. [Google Scholar]
- 26.Martin SW, Meek AH, Willeberg P. Principles and Methods. Iowa State University Press; Ames, Iowa: 1987. Veterinary Epidemiology; p. 343. [Google Scholar]
- 27.Pezzlo M, York MK. Aerobic Bacteriology. In: Isenberg HD, editor. Clinical Microbiology procedure manual. Washington DC: American society of microbiology Press; 2004. pp. 3–12.pp. 1–19. [Google Scholar]
- 28.Lucas MJ, Cunningharm FG. Urinary tract infection in pregnancy. Clinical Obstetrics And Gynecology. 1993;36:855–68. doi: 10.1097/00003081-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Momoh ARM, Orhue PO, Idonije OB, Oaikhena AG, Nwoke EO, Momoh AA. The antibiogram types of Escherichia coli isolated from suspected urinary tract infection samples. J Microbiol Biotech Res. 2011;1(3):57–65. [Google Scholar]
- 30.Collee JG, Miles RS, Watt B. Test for the identification of bacteria. In: Cruickshank R, Collee JG, Marmion BP, Simmons A, editors. Macki and McCartney Practical Medical Microbiology. 14. Vol. 1. Churchill Livingstone; New York: 1996. pp. 133–49. [Google Scholar]
- 31.Foxman R, D’Arcy BH, Gillespie B. Urinary tract infection: Self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 32.Foxman B, Brown P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence and costs. Infect Dis Clin North Am. 2003;49:53–70. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 33.Sohely S, Alamgir F, Begum F, Jaigirdar QH. Use of chromogenic agar media for identification of uropathogens. Bangladesh J Med Microbiol. 2010;04(01):18–23. [Google Scholar]
- 34.Kirimuhuzya C, Waako P, Joloba M, Olwa O. The anti-mycobacterial activity of Lantana camara a plant traditionally used to treat symptoms of tuberculosis in South-western Uganda. African Health Sciences. 2009;9(1):40–45. [PMC free article] [PubMed] [Google Scholar]
- 35.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Applied Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 36.Chander J. Routine Mycological Techniques (appendix C) 2. Mehta Publishers; New Delhi: 2002. Textbook of Mycology. [Google Scholar]
- 37.Tripti M, Padma S, Shailja P, Nirpendra C, Hema L. Potentiation of antimicrobial activity of ciprofloxacin by Pelargonium graveolens essential oil against selected uropathogens. Phytother Res. 2011;25:1225–1228. doi: 10.1002/ptr.3479. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin SP, Carson CC. Urinary tract infections in women. Med Clin North Am. 2004;88:417–429. doi: 10.1016/S0025-7125(03)00148-2. [DOI] [PubMed] [Google Scholar]
- 39.Llenerrozos HJ. Evidence-based management of urinary tract infections across the lifespan: Management. Clin Fam Pract. 2004;6:157–173. [Google Scholar]
- 40.Blair KA. Evidence based urinary tract infection across the life span: Current updates. J Nurse Pract. 2007;3:629–632. [Google Scholar]
- 41.Allan RR. Urologic symptoms. In: Guerrant RL, Walker HD, Weller FP, editors. Essentials of tropical infectious diseases. Churchill Livingstone; 2001. pp. 98–100. [Google Scholar]
- 42.Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotech. 2009;5:1562–1565. [Google Scholar]
- 43.Amin M, Manijeh M, Zohreh P. Study of bacteria isolated from urinary tract infections and determination of their susceptibility to antibiotics. Jundishapur J Microbiol. 2009;2:118–123. [Google Scholar]
- 44.Wazait HD, Patel HRH, Veer V, Kelsey M, Van der Meulen JHP. Catheter associated urinary tract infections: Prevalence of uropathogens and pattern of antimicrobial resistance in a UK hospital. Brit J Urol. 2003;91:806–809. doi: 10.1046/j.1464-410x.2003.04239.x. [DOI] [PubMed] [Google Scholar]
- 45.Chawla JC, Clayton CL, Stickler DJ. Antiseptics in the long-term urological management of patients by intermittent catheterization. Br J Urol. 1998;62:289–294. doi: 10.1111/j.1464-410x.1988.tb04350.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim BN, Woo JH, Ryu J, Kim YS. Resistance to extended-spectrum cephalosporins and mortality in patients with Citrobacter Freundii bacteremia. Infection. 2003;19:202–207. doi: 10.1007/s15010-003-2176-8. [DOI] [PubMed] [Google Scholar]
- 47.Mitscher LA, Drake S, Gollapudi SR, Okwute SK. A modern look at folkloric use of anti-infective agents. J Nat Prod. 1987;50:1025–1040. doi: 10.1021/np50054a003. [DOI] [PubMed] [Google Scholar]
- 48.Lima EO, Gompetz OF, Giesbrecht AM, Paulo MQ. In vitro antifungal activity of essential oils obtained from officinal plants against dermatophytes. Mycoses. 1993;36:333–336. doi: 10.1111/j.1439-0507.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 49.CLSI. Performance Standard for Antimicrobial Disk Susceptibility Tests; Approved standard. (9) 2007;26(Supplement M2 –A9)(1) [Google Scholar]
- 50.Ilory M, Sheteolu AO, Omonibgehin EA, Adeneye AA. Antibacterial activity of Ocimum gratissimum (Lamiaceae) J Diarhoeal Dis Res. 1996;14:283–285. [PubMed] [Google Scholar]
- 51.Ramanoelina AR, Terrom GP, Bianchini JP, Coulanges P. Antibacterial action of essential oils extratted from Madagascar plants. Arch Inst Pasteur Madagascar. 1987;53:217–226. [PubMed] [Google Scholar]
- 52.Janssen AM, Scheffer JJ, Ntezurubanza L, Baerheim S. Antimicrobial acitivities of some Ocimum species grown in Rwanda. J Ethnopharmacol. 1989;26:57–63. doi: 10.1016/0378-8741(89)90113-x. [DOI] [PubMed] [Google Scholar]
- 53.Lopez P, Sanchez K, Batlle R, Nerin C. Solid and vapour phase anti-microbial activities of six essential oils susceptibility of selected food borne bacterial and fungal strains. J Agric Food Chem. 2005;53(17):6939–6946. doi: 10.1021/jf050709v. [DOI] [PubMed] [Google Scholar]
- 54.Carlton RR, Waterman PG, Gray AI, Deans SG. The antifungal activity of the leaf gland volatile oil of sweet gale (Myrica gale) (Myricaceae) Chemoecology. 1992;3:55–59. [Google Scholar]
- 55.Lachowicz KJ, Jones GP, Briggs DR. The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) against acid-tolerant food microflora. Letters in Applied Microbiology. 1998;26:209–214. doi: 10.1046/j.1472-765x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 56.Sinha GK, Gulati BC. Antibacterial and antifungal study of some essential oils and some of their constituents. Indian Perfumer. 1990;34:126–129. [Google Scholar]
- 57.Holm Y. Medicinal and Aromatic Plants – Industrial Profiles. Vol. 10. UK: Harwood Academic Publishers; 1999. Bioactivity of basil. Basil – the genus Ocimum; pp. 113–135. [Google Scholar]
- 58.Vasudaran P, Kashyap S, Sharma S. Bioactive botanicals from basil (Ocimum spp.) Journal of Science and Industrial Research. 1999;58:332–338. [Google Scholar]
- 59.Svoboda KP, Kyle SK, Hampson JB, Ruzickova G, Brocklehurst S. Antimycotic activity of essential oils: The possibility of using new bioactive products derived from plants. In: Rai MK, editor. Plant-derived antimycotics: Current trends and future prospects. Binghampton, New York, USA: The Hawthorn Press Inc; 2003. pp. 198–224. [Google Scholar]
- 60.Wossa WS, Rali T, Leach ND. Volatile Chemical Constituents of three Ocimum species (Lamiaceae) from Papua New Guinea. The South Pacific Journal of Natural Science. 2008:26. [Google Scholar]


