Abstract
A conformationally restricted analog of a selective cyclopropane-bearing serotonin 2C agonist was designed and synthesized. A 2,2-dimethyl-2,3-dihydrobenzofuran scaffold was investigated as a constrained variant of a biologically active isopropyl phenyl ether. Construction of the required dimethyl-2,3-dihydrobenzofuran intermediate began using a procedure that relied on a microwave-assisted alkylation reaction. The synthesis of the designed compound as its HCl salt is reported in a total of 12 steps and 17% overall yield. Biological evaluation revealed the constrained analog to be a selective serotonin 2C agonist with modest potency.
Keywords: Serotonin 2C, Agonist, Benzofuran, Cyclopropylmethanamine
Introduction
The serotonin 2C (5-HT2C) receptor has been found to represent a promising drug target in the search for new treatments for a variety of disorders, including obesity and various mental diseases, such as schizophrenia, depression, and anxiety.1,2,3 One of the many advantages of the 5-HT2C receptor as a central nervous system (CNS) drug target stems from the fact that it is almost exclusively found in the CNS,4,5 and thus compounds that selectively activate this receptor should have limited impact on peripheral tissues. However, the activation of two other closely related 5-HT2 receptor subtypes, namely the 5-HT2A and 5-HT2B receptors, has been reported to be associated with hallucinatory effects and life threatening cardiac valvulopathy, respectively.6,7,8 Therefore, the identification of ligands possessing exquisite selectivity for the 5-HT2C versus the 5-HT2A and 5-HT2B receptor subtypes is a key requirement for therapeutic advancement of 5-HT2C agonists.3 However, the achievement of such selectivity has proven to be challenging because of the high amino acid sequence homology shared by members of the 5-HT2 subfamily.
During our studies aimed at designing selective 5-HT2C agonists for possible use in CNS diseases, compound (+)-1 was found to be a relatively potent and selective 5-HT2C agonist (5-HT2C: EC50 = 71 nM, Emax = 100% (of 5-HT); 5-HT2B: EC50 = 682 nM, Emax = 57%; 5-HT2A: EC50 = 7190 nM, Emax = 17%). Based on the sum total of the structure activity relationship (SAR) data we had assembled for this lead candidate, we considered the possibility of exploring the activity of a dimethyl-2,3-dihydrobenzofuran analog 2, which represents a conformationally constrained version of the isopropyl phenyl ether 1. Conformational restriction is, of course, a well-recognized medicinal chemistry strategy for enhancing the binding affinity as well as the target selectivity of small molecules.9 This particular structure was also of interest as the heterocyclic benzofuran system is present in a number of drugs.10,11 For example, the 2,2-dimethyl-2,3-dihydrobenzofuran scaffold is found in the drug zatosetron (3),12 which is a 5-HT3 antagonist that is able to induce antinausea effects without affecting gastrointestinal motility.13 Zatosetron was also found to be an effective anxiolytic in both animal studies and human trials.14 The similarity of compound 2 with 3, and the fact that both compounds 1 and 3 act through serotonin receptors thus encouraged us to synthesize compound 2 for biological evaluation.
Result and discussion
The retro-synthetic analysis of compound (±)-2 is outlined in Figure 2. Benzaldehyde 4 was selected as a key intermediate, as the cyclopropylmethylamine side chain of 2 can be installed from this aldehyde, using methods similar to those that we have reported previously.15 This benzaldehyde could in turn be prepared from its corresponding benzoate 5.16, 17 As compound 5 has previously served as an intermediate in the synthesis of zatosetron (3), a synthetic approach to this intermediate starting from methyl 5-chloro-2-hydroxybenzoate (6) has been reported.12,18 We note that no spectroscopic data have been reported previously for the intermediates prepared in the synthesis of 5, and that this compound had previously been obtained in only very low yield.
Figure 2.
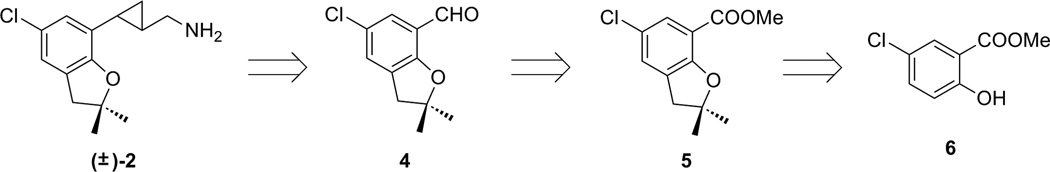
Retrosynthetic approach of compound (±)-2.
The synthesis of intermediate 5 is depicted in Scheme 1. The alkylation of the starting material methyl 5-chloro-2-hydroxybenzoate (6) was accomplished using 3-chloro-2-methylprop-1-ene under reflux conditions in acetone, however, this led to only a 29% yield of product 7.12 In order to improve the yield of this alkylation reaction, 3-bromo-2-methylprop-1-ene was used as the alkylating reagent and the reaction was carried out under microwave conditions (80 °C). This method gave an improved yield (81%) of the product 7, and the reaction time was reduced from overnight to 1.5 h. The Claisen rearrangement of intermediate 7 turned out to be sluggish. Heating of 7 in NMP at 200 °C for 8 h provided a 65% yield of product 8, with a 24% recovery of the starting material. The subsequent cyclization of compound 8 was brought about by refluxing in 95% formic acid, which gave an excellent yield of intermediate 5. With the assistance of microwave conditions, intermediate 5 was prepared in 52% yield from 6 in three steps, compared to an overall 20% yield as reported previously.12
Scheme 1.
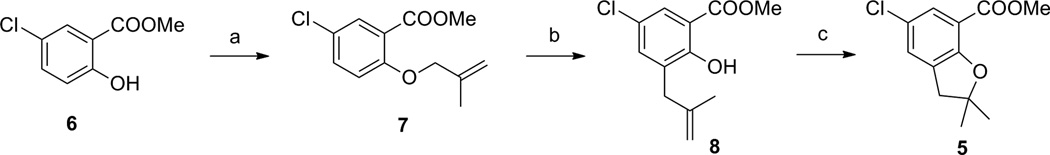
Synthesis of benzaldehyde 5. Reagents and conditions: (a) 3-bromo-2-methylprop-1-ene, K2CO3, DMF, microwave, 80 °C, 1.5h, 81%; (b) NMP, 200 °C, 8h, 65%; (c) formic acid (95%), reflux, 3h, 98%.
The ester 5 was then subjected to a sequence of lithium aluminum reduction and pyridinium dichromate (PDC) oxidation to give the benzaldehyde 4 in good yield. Wittig reaction of 4 with a commercially available reagent N-methoxy-N-methyl(triphenylphosphoranylidene)acetamide in dichloromethane at room temperature provided the Weinreb amide 10 in high yield. The double bond was obtained in solely the E form, without the observation of its Z isomer. The coupling constant of the two protons attached to the double bond is 16.0 Hz, which is consistent with the data reported for similar compounds.15 Subsequent Corey-Chaykovsky cyclopropanation of the E double bond, with the sulfur ylide which was generated from trimethylsulfoxonium iodide upon treatment with sodium hydride, gave the cyclopropane 11 as its trans isomer. The Weinreb amide was then reduced with diisobutylaluminium hydride at low temperature to give aldehyde 12, which was subsequently reduced to the corresponding alcohol 13 in excellent yield using sodium borohydride. The primary amine 2 was prepared from its alcohol 13 through a sequence of steps involving a Mitsunobu reaction employing phthalimide to afford the Gabriel imide 14, followed by de-protection with hydrazine. Finally, the hydrochloride salt of 2 was prepared using HCl in diethyl ether. This sequence of transformations provided the target compound in 9 steps from 5, with an overall yield of 33%. While preparative chiral HPLC methods were investigated for the separation of the optically pure enantiomers of the racemic Boc derivative of compound (±)-2, no separation was observed using either OD or OJ columns employing various solvent systems. Therefore, the biology activity of compound 2 was evaluated using its racemate.
Functional activity at the 5-HT2A, 5-HT2B and 5-HT2C receptors was determined using the calcium flux assay (for details of the assay, see supplementary data) for the racemic form of compound 2. Moderate potency was observed for compound 2 at the 5-HT2C receptors (EC50 = 600 nM) where it acts as a partial agonist (Emax = 66% of 5-HT). No activity was observed at either the 5-HT2A or 5-HT2B receptors. The absence of any 5-HT2B activity for compound 2 is beneficial, as this would preclude any undesirable cardiac toxicity as noted above. The decreased activity of this compound at 5-HT2C receptors may be a result of its increased steric size compared to compound 1, as previous SAR studies have shown that increasing the length of the ether chain by one carbon led to a significant decrease in 5-HT2C activity.15
Conclusion
In this letter we report the design and synthesis of a selective 5-HT2C agonist compound 2 bearing a dimethyl-2,3-dihydrobenzofuran moiety. An improved synthesis of the dimethyl-2,3-dihydrobenzofuran scaffold is reported that makes use of a microwave-assisted alkylation reaction followed by a Claisen rearrangement and a formic acid promoted cyclization. The 2-phenylcyclopropylmethylamine side chain of the target compound was synthesized using a sequence of steps involving a Wittig reaction, Corey-Chaykovsky cyclopropanation, reduction of a Weinreb amide, and a Mitsunobu reaction followed by deprotection of the thus formed Gabriel imide. The HCl salt of the target compound was prepared in 12 steps in an overall yield of 17%. The functional activity of this compound at the 5-HT2C, 5-HT2A and 5-HT2B receptors was then determined using a calcium flux assay, and compound 2 was found to be a selective 5-HT2C partial agonist of moderate potency. The design of other analogs of 2 would thus appear to be valuable to explore.
Supplementary Material
Figure 1.
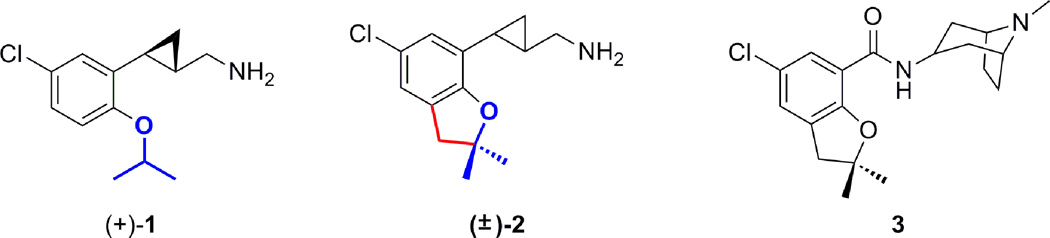
Structures of compounds (+)-1, (±)-2 and zatosetron (3).
Scheme 2.
Synthesis of target compound 2. (a) LiAlH4, THF, 0 °C to rt, 2h, 92%; (b) PDC, CH2Cl2, rt, 2h, 65%; (c) Ph3P=CHC(O)N(OMe)Me, CH2Cl2, rt, overnight, 92%; (d) Me3S+(O)I−, NaH, DMSO, rt, overnight, 98%; (e) DIBAL-H, THF, −78 °C to rt, 95%; (f) NaBH4, MeOH, 0 °C to rt, 96%; (g) Phthalimide, PPh3, DEAD, THF, rt, 85%; (h) N2H4–H2O, EtOH, reflux, 3h, 97%; (i) 2M HCl in Et2O, rt, 2h, 81%.
Table 1.
Pharmacological profiles of compound (+)-1 and (±)-2.a
| Compound | EC50, nM (Emax; % 5-HT) | ||
|---|---|---|---|
| 5-HT2C | 5-HT2B | 5-HT2A | |
| 5-HT | 0.20 (100%) | 1.3 (100%) | 2.1 (100%) |
| (+)-1 (TFA salt) | 71 (100%) | 682 (57%) | 7190 (17%) |
| (±)-2 (HCl salt) | 600 (66%) | NA | NA |
Functional data was acquired with recombinantly expressed human serotonin receptors in HEK-293 (5-HT2A and 5-HT2B) and PO1C (5-HT2C) cell lines, fluorescence imaging plate reader (FLIPR) assay; “NA”, no activity at 10 µM.
Acknowledgments
This work was financially supported by NIH grant R01MH99993.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data Supplementary data including synthetic procedures and details of the functional assay on 5-HT2 receptors associated with this article can be found, in the online version.
References and notes
- 1.Smith BM, Thomsen WJ, Grottick AJ. Expert Opin. Invest. Drugs. 2006;15:257. doi: 10.1517/13543784.15.3.257. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, Gray JA, Roth BL. Annu. Rev. Med. 2009;60:355. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meltzer HY, Roth BL. J Clin Invest. 2013;123:4986. doi: 10.1172/JCI70678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pazos A, Hoyer D, Palacios JM. Eur. J. Pharmacol. 1985;106:539. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- 5.Berg KA, Clarke WP, Cunningham KA, Spampinato U. Neuropharmacology. 2008;55:969. doi: 10.1016/j.neuropharm.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols DE. Pharmacol. Ther. 2004;101:131. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Circulation. 2000;102:2836. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 8.Huang X-P, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, Revankar C, Robers M, Doucette C, Roth BL. Mol Pharmacol. 2009;76:710. doi: 10.1124/mol.109.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann A. In: Practice of Medicinal Chemistry. Wermuth CG, editor. Elsevier; 2008. pp. 363–379. [Google Scholar]
- 10.Wu Yong-Jin. Prog. Heterocycl. Chem. 2012;24:1. [Google Scholar]
- 11.Dawood KM. Expert Opin. Ther. Pat. 2013;23:1133. doi: 10.1517/13543776.2013.801455. [DOI] [PubMed] [Google Scholar]
- 12.Robertson DW, Lacefield WB, Bloomquist W, Pfeifer W, Simon RL, Cohen ML. J. Med. Chem. 1992;35:310. doi: 10.1021/jm00080a016. [DOI] [PubMed] [Google Scholar]
- 13.Cohen ML, Bloomquist W, Gidda JS, Lacefield W. J. Pharmacol. Exp. Ther. 1990;254:350. [PubMed] [Google Scholar]
- 14.Smith WT, Londborg PD, Blomgren SL, Tollefson GD, Sayler ME. J. Clin. Psychopharmacol. 1999;19:125. doi: 10.1097/00004714-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Cho SJ, Huang X-P, Jensen NH, Svennebring A, Sassano MF, Roth BL, Kozikowski AP. ACS Med. Chem. Lett. 2011;2:929. doi: 10.1021/ml200206z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadieva MG, Oganesyan ÉT. Chem. Heterocycl. Compd. 1997;33:1245. [Google Scholar]
- 17.Abu-Hashem AA, Hussein HAR, Aly AS, Gouda MA. Synth. Commun. 2014;44:2285. [Google Scholar]
- 18.Burks JE, Jr, Espinosa L, LaBell ES, McGill JM, Ritter AR, Speakman JL, Williams MA. Org. Process Res. Dev. 1997;1:198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.