Abstract
Most animals sleep more early in life than in adulthood, but the function of early sleep is not known. Using Drosophila, we found that increased sleep in young flies was associated with an elevated arousal threshold and resistance to sleep deprivation. Excess sleep results from decreased inhibition of a sleep-promoting region by a specific dopaminergic circuit. Experimental hyperactivation of this circuit in young flies results in sleep loss and lasting deficits in adult courtship behaviors. These deficits are accompanied by impaired development of a single olfactory glomerulus, VA1v, which normally displays extensive sleep-dependent growth after eclosion. Our results demonstrate that sleep promotes normal brain development that gives rise to an adult behavior critical for species propagation and suggest that rapidly growing regions of the brain are most susceptible to sleep perturbations early in life.
The ontogenetic hypothesis of sleep, proposed nearly 50 years ago, postulates that early developmental sleep is important for brain patterning (1). Average daily sleep amounts are highest early in development across multiple species (1-4), and human studies have indeed demonstrated that impaired sleep during critical periods of development can have severe and longlasting consequences (5-7). Yet it remains unknown whether sleep is required for normal structural maturation of the brain, as animal studies have focused on a role for sleep in the cortical plasticity induced by sensory deprivation in early life, or relied on drugs and lesion studies with non-specific effects (8).
Sleep in Drosophila shares many characteristics with sleep in humans (4, 9), including ontogenetic changes (4, 10). We used this model organism to explore the neural circuitry governing sleep ontogeny and examined how sleep loss during development affects adult behavior. We found that sleep need is especially high in young flies. Activity of a subset of wake-promoting dopaminergic neurons increases as normal development progresses, resulting in changes in sleep patterns across stages of development. To examine whether sleep during a critical period promotes proper brain development, we focused on courtship because it is a robust, innate behavior with well-mapped circuitry. Dopaminergic hyperexcitation and consequent sleep deprivation in young but not mature flies cause behavioral courtship abnormalities and impair development of a rapidly growing brain region involved in courtship. Thus, sleep early in life is required for the proper development of a behaviorally relevant adult brain circuit.
Young Flies Have Increased Sleep Need
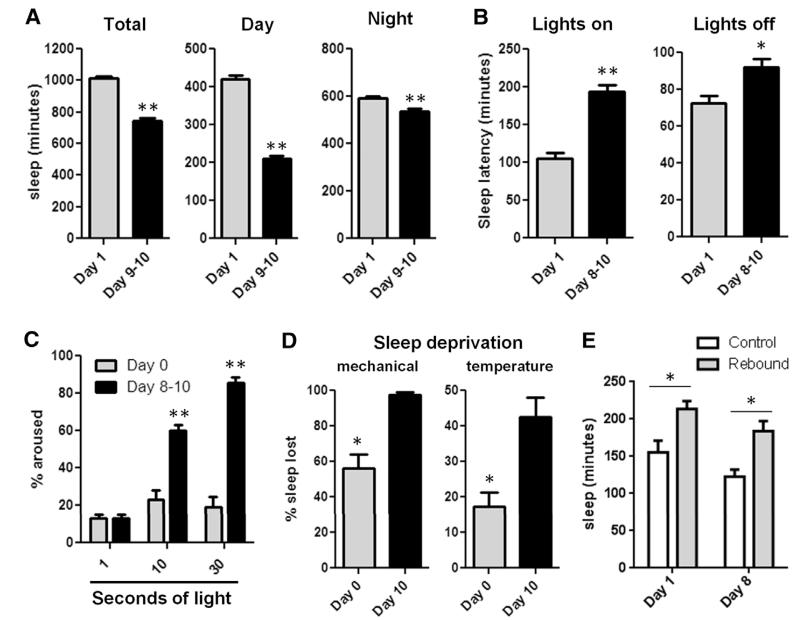
We first examined multiple aspects of sleep in young flies. Female iso31 flies were collected 2 to 3 hours after eclosion and compared to aged (day 9 to 10) flies. Consistent with previous work (4, 10), we observed increased total sleep amounts on the first day of adult life as compared to mature flies, with the majority of change resulting from increased daytime sleep in newly eclosed adult flies (Fig. 1A). Young flies initiated their first sleep bout after onset of the light period earlier than mature flies and also initiated sleep more quickly after dark onset (Fig. 1B). Sleep bout duration was lengthened during the day in young as compared to mature flies, indicating more consolidated daytime sleep, whereas bouts at night were not significantly different (fig. S1A). Developmental differences in sleep did not stem from more generalized locomotor changes, because activity during periods of wakefulness was not different between young and mature flies (fig. S1B). In addition, mutants without a functional circadian clock demonstrated ontogenetic sleep changes (fig. S1C), indicating that the sleep differences we observed were not clock-dependent.
Fig. 1. Reduced arousal and increased resistance to sleep deprivation in young flies.
Sleep measured in female iso31 flies at day 1 and day 9 to 10: (A) Sleep amounts (n = 150 flies for day 1, 97 for day 9 to 10); (B) sleep latency (n = 31 for day 1, n = 32 for day 10); and (C) arousal threshold at day 0 or day 8 to 10 (n = 5 replicates, n = 16 flies per age in each). (D) Sleep deprivation: mechanical (n = 11 flies for each condition, with multiple independent replicates); and temperature (n = 7 for day 0, n = 11 for day 10, with multiple independent replicates). (E) Rebound sleep over 6 hours after sleep deprivation (n = 15, 38, 16, and 28 flies from left to right). Bar graphs in this figure and all others are presented as means ± SEM. **P < 0.0001, *P < 0.005; unpaired two-tailed Student’s t test [(A); total sleep, (B) and (D)]; plus Welch’s correction [(A), night and day sleep]; and two-way analysis of variance (ANOVA) with post-hoc Bonferroni multiple comparisons [(C) and (E)].
We next investigated changes in arousal threshold using dim light (~100 lux) as a stimulus (11). On the first night after eclosion, only ~20% of flies were aroused by the same stimulus that aroused >80% of mature flies (Fig. 1C). This finding was not specific to the iso31 background or the time of the light pulse in the night (fig. S1, D and E). The reduced arousal did not reflect an inability of young flies to respond to light, because young and mature flies were similarly aroused by a stronger (~1000 lux) light stimulus (fig. S1F). In addition, young flies also showed an increased arousal threshold in response to mechanical stimulation (fig. S1G). We next examined the effect of sleep-depriving stimuli by determining the percent of sleep lost during the night in young and mature flies, using two forms of deprivation (mechanical and temperature). Mature flies exhibited large amounts of sleep loss, particularly with mechanical deprivation, but young flies were resistant (Fig. 1D). Increasing the intensity of mechanical stimulus resulted in near-total sleep deprivation for 12 hours even in day-0 flies (fig. S1H), supporting the idea that young flies have an increased arousal threshold. To examine homeostatic regulation of sleep in young flies, we quantified rebound sleep during the first 6 hours of day after 12 hours of sleep deprivation using the weaker mechanical stimulus. Young and mature flies both showed significant sleep rebound, even under conditions in which young flies lost less sleep during deprivation (Fig. 1E). These results demonstrate a high sleep need during early post-eclosion development.
Reduced Dopamine Mediates Sleep Ontogeny
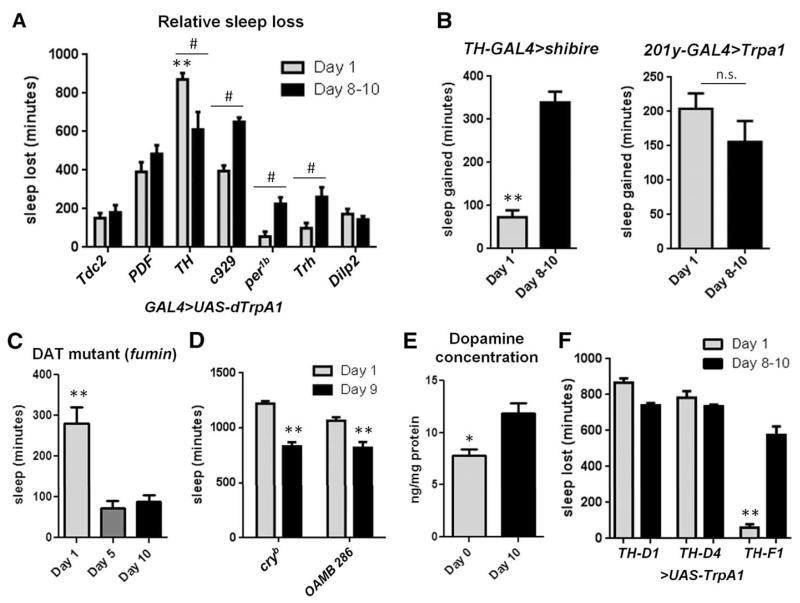
To explore the mechanism underlying ontogenetic changes in sleep, we performed a candidate-based thermogenetic screen of known wake-promoting GAL4 drivers, looking for candidates that could overcome the high sleep drive of young flies. GAL4 lines driving expression of TrpA1 [a heat-sensitive cation channel that can be used to induce neurotransmitter release (12)] were exposed to 28°C for 24 hours on the first full day after eclosion or after 8 to 10 days of aging and were compared to UAS (Fig. 2A) and GAL4 (fig. S2A) controls. In mature female adult flies, virtually all drivers increased wake as expected (fig. S2B). In young flies, most drivers also increased wake (fig. S2B). However, TH-GAL4 (tyrosine hydroxylase) was significantly more effective at driving wake in young flies than were other drivers at this age (Fig. 2A). It was also more effective in young versus mature flies, whereaas TrpA1-mediated activation by other drivers was blunted in young flies as compared to activation of the same neurons in the mature adult (Fig. 2A). The effects of TH-GAL4>UAS-dTrpA1 were similar in males and independent of day or night (fig. S2, C and D). TH-GAL4 drives expression in most dopamine neurons in the Drosophila brain (13), and dopamine is known to promote wake and arousal in the fly (14-16). We did not detect any gross changes in dopamine neuroanatomy between young and mature flies as visualized by green fluorescent protein (GFP) labeling of TH+ cell bodies and projections (fig. S3A). Thus, one interpretation of our results is that dopaminergic neurons are hypoactive in young flies, resulting in a larger relative effect of hyper-excitation as compared to other GAL4s. If this were the case, we would predict that inhibition of TH+ neurons would have a blunted effect in young as compared to mature flies, because these neurons are already less active. Using TH-GAL4 to drive the temperature-sensitive inactivator of neuronal function UAS-shibirets (17, 18), we found that silencing TH+ neurons at 29°C in mature flies caused a significantly larger increase in sleep than in young flies, as compared to UAS (Fig. 2B) and GAL4 (fig. S3B) controls. This does not simply reflect a ceiling effect of sleep in young flies, because the activation of mushroom body (MB) sleep-promoting neurons (19), using 201y-GAL4>UAS-dTrpA1, resulted in similar amounts of sleep gained in both young and mature flies (Fig. 2B and fig. S3B). Moreover, total sleep amounts were equal in young and mature TH-GAL4>UAS-shibire flies exposed to elevated temperatures, whereas 201y-GAL4>UAS-dTrpA1 flies slept significantly more when young (fig. S3C).
Fig. 2. Reduced dopamine signaling underlies ontogenetic sleep changes.
(A) Wake-promoting GAL4>UAS-dTrpA1 screen examining sleep loss compared to UAS controls in day-1 and day-8 to -10 flies at 28°C (**denotes comparison to all other day-1 GAL4>UAS-dTrpA1 measures; from left to right, n = 21, 12, 23, 12, 26, 12, 24, 23, 23, 24, 22, 24, 24, and 24 flies). (B) Sleep gained as compared to UAS controls in day-1 and day-8 to -10 flies at 29°C (TH-GAL4>shi: n = 23 day-1 flies, n = 22 day-8 to -10; 201y-GAL4>TrpA1: n = 18 day-1, n = 11 day-8 to -10). (C) Total sleep time in 24 hours in fumin flies at day 1, 5, and 10 (from left to right, n = 34, 20, and 28 flies). (D) Total sleep time in 24 hours at day 1 and day 9 in cryb and OAMB 286 flies (from left to right, n = 12, 12, 10, and 10 flies). (E) HPLC detection of dopamine concentration in fly brains (n = 4 separate replicates of 20 brains each). (F) Sleep loss in TH-restricted lines as compared to UAS controls in day-1 and day-8 to -10 flies at 29°C (from left to right, n = 12, 12, 11, 12, 40, and 11 flies). **P < 0.0001, # and *P < 0.05; two-way ANOVA with post-hoc Bonferroni multiple comparisons [(A) and (D)]; one-way ANOVA with Tukey’s post-hoc test [(C) and (F)]; and unpaired two-tailed Student’s t test (E); plus Welch’s correction (B).
We next examined sleep ontogeny in fumin flies (14), which lack dopamine transporter (DAT) function. Although both young and mature fumin flies showed reduced sleep due to increased synaptic dopamine, ontogenetic changes were maintained (Fig. 2C). This supports the idea that young flies have dopaminergic hypofunction, which would limit the amount of dopamine available even in flies that lack DAT. Our hypothesis suggests that a developmental delay in dopaminergic activity underlies sleep changes during adulthood, and the inhibition of dopaminergic neurons eliminates differences in total sleep time between young and mature flies (fig. S3B). The loss of ontogenetic sleep changes is not a generalized result of abnormal arousal signaling, however, because mutations in other known wake/arousal-promoting systems [though resulting in sleep and arousal changes (20, 21)] did not disrupt sleep ontogeny (Fig. 2D). Finally, we used high-performance liquid chromatography (HPLC) to measure dopamine levels in the brains of young flies and found >30% less dopamine as compared to the brains of mature adults (Fig. 2E). Together, these results suggest that dopamine signaling is hypoactive in the brains of young flies, resulting in increased sleep and reduced arousal.
Identification of a Hypoactive Dopamine Circuit
We next sought to determine how dopaminergic hypoactivity leads to increased sleep, first by examining the temporal course of TH-GAL4 neuronal activity in young flies. We used a targetable luciferase-based reporter under the control of dCREB2 binding sites (UAS-FLP/+; Cre-F-luc/+) to achieve spatial control while monitoring CREB as a proxy for cellular activity in vivo for extended periods of time (22, 23). Expression of Cre-luciferase in dopaminergic neurons using TH-GAL4 revealed a reduction in normalized luminescence over 12 hours in day-0 as compared to day-7 to -9 flies (fig. S4A). By hour 48 of monitoring the same flies, we no longer detected a difference in signal intensity between groups. In contrast, another wake-promoting GAL4 line, c929-GAL4, showed higher reporter levels in young than in mature flies during the same period, indicating that not all wake-promoting neurons are less active by this assay in young flies (fig. S4A). Reduced TH-GAL4–driven luminescence appears to be head-specific, demonstrated by monitoring of heads and bodies independently (fig. S4B). These data confirm that a measure of cell signaling, CREB activity, is reduced in TH-GAL4 neurons of young flies.
To narrow down the role of distinct dopaminergic inputs in sleep ontogeny, we took advantage of TH-GAL4 drivers with expression restricted to different subsets of dopaminergic neurons (11). The dorsal fan-shaped body (dFSB) is a sleep-promoting region in the fly brain (24, 25), and dopamine neurons project to the dFSB in their role as a wake-promoting signal, presumably to inhibit dFSB function (11, 26). TH-D1 and TH-D4 both include projections to the dFSB, with TH-D4 being the most restricted; TH-F1 is also expressed in a small number of dopaminergic neurons but excludes those projecting to dFSB. At 29°C, we found that each of these lines promotes wakefulness in mature flies when driving expression of UAS-dTrpA1 (Fig. 2F, black bars), indicating the presence of dFSB-independent dopaminergic arousal circuitry (Fig. 2F is compared to UAS controls, fig. S4C is compared to GAL4 controls). However, in young flies, TH-D1 and TH-D4 result in robust sleep loss when driving TrpA1 expression, whereas TH-F1 has minimal effect (Fig. 2F, gray bars). This result is consistent with the idea that dopaminergic hypofunction in young flies is specific for the dFSB. The TH-F1 data indicate that excitation of dFSB-independent dopamine neurons cannot overcome hypoactivity of dFSB-dependent dopamine neurons to promote wakefulness in young flies. To examine the activity of these TH+ neuronal subsets during development, we again used the Cre-based reporter system. We found reduced luminescence in young as compared to mature TH-D4-GAL4>UAS-FLP/+;Cre-F-luc/+ flies (fig. S4D); in contrast, luminescence was higher in young TH-F1-GAL4>UAS-FLP/+;Cre-F-luc/+ flies (fig. S4D). These findings indicate lower CREB-dependent transcription in TH-GAL4 neurons with projections to the dFSB. Additionally, we noted no differences in TH-D4 or TH-F1 expression at different ages (fig. S5A), and found that contacts between TH+ neurons and the dFSB were indistinguishable in the different age groups (fig. S5B). Thus, a subset of dopaminergic neurons (those with projections to the dFSB) is hypoactive in young flies, whereas other dopaminergic neurons are active at or above mature adult levels.
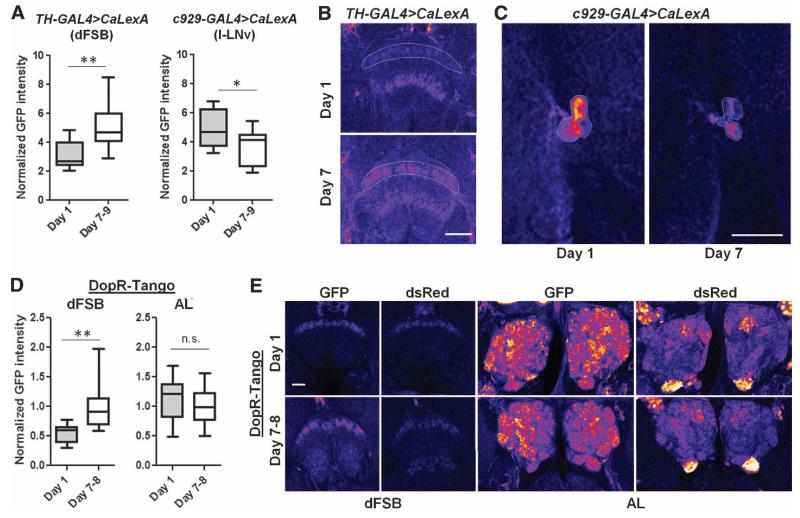
To more directly investigate developmentally regulated activity changes in specific neural circuits that might underlie sleep changes, we took advantage of the CaLexA (calcium-dependent nuclear import of LexA) system (27). This system is based on the activity-dependent nuclear import of a chimeric transcription factor, the nuclear factor of activated T cells (NFAT), which then drives GFP reporter expression (27, 28). With TH-GAL4 driving expression of CaLexA, we focused on fluorescence in dopaminergic projection to the dFSB itself, because reporter expression has been demonstrated to label all compartments of a neuron (27). We found reduced fluorescence in the dFSB on day 1 as compared to day 8, when CaLexA was expressed via the TH-GAL4 driver (Fig. 3, A and B), which is consistent with reduced dopaminergic neuronal activity in this brain region. In contrast, we noted increased fluorescence of the wake-promoting large ventral lateral neurons (l-LNvs) (29) in young flies when CaLexA was expressed via the c929-GAL4 driver (Fig. 3, A and C), which is consistent with our Cre-luciferase data. Finally, we assessed changes in postsynaptic dopaminergic input throughout development with the DopR-Tango system (30), with which transient dopamine-induced cellular activation is converted into a stable fluorescent readout. The system can be temporally restricted, because it is coupled to a pan-neuronally expressed hormone (RU486)–inducible GAL4 (31). We first demonstrated that feeding DopR-Tango flies l-dopa for 2 days after 24 hours of RU486 induced an increase in reporter expression in the dFSB (fig. S5C), similar to that found in other brain regions (30). Next, we assayed endogenous dopamine signaling in young and mature flies. We fed flies RU486 for 24 hours, followed by regular food for ~20 hours to allow time for expression of the Tango reporter (30). Young flies were dissected on day 2 after eclosion, ~3 hours after light onset, so the reporter signal should mostly reflect dopaminergic activity from day 1. With this approach, we found significantly lower reporter expression in young flies than in mature flies in the dFSB (Fig. 3, D and E), directly demonstrating reduced dopaminergic input to the dFSB. We did not detect a similar difference in dopaminergic inputs to the antennal lobe (AL; Fig. 3, D and E). Together, these results provide evidence for circuit-level specificity of dopaminergic activity changes during development.
Fig. 3. Hypoactivity of dFSB-projecting dopaminergic neurons in young flies.
(A) Normalized GFP intensity of the indicated brain region in GAL4>UAS-CaLexA flies of different ages (TH-GAL4: n = 13 at both ages; c929-GAL4: n = 14 day-1, n = 15 day-7 to -9). Representative images of (B) the dFSB and (C) large LNvs in brains immunostained for GFP. GFP is pseudocolored “fire.” Warmer colors reflect increased signal intensity. Scale bars, 37.5 μm. (D) Normalized GFP intensity in DopR-Tango flies in the dFSB and AL (dFSB: n = 22 day-1 flies, n = 25 day-7 to -8; AL: n = 34 day-1, n = 49 day-7 to -8). (E) Representative images of the dFSB and AL in DopR-Tango flies. Endogenous GFP and dsRed are pseudocolored fire. Scale bar, 20 μm. Box plots in this figure and all others represent the median value (horizontal line inside box), interquartile range (height of the box, 50% of the data within this range), and minimum and maximum value (whiskers). **P < 0.0001, *P < 0.05; unpaired two-tailed Student’s t test plus Welch’s correction [(A) and (D)].
The dFSB Promotes Increased Sleep in Young Flies
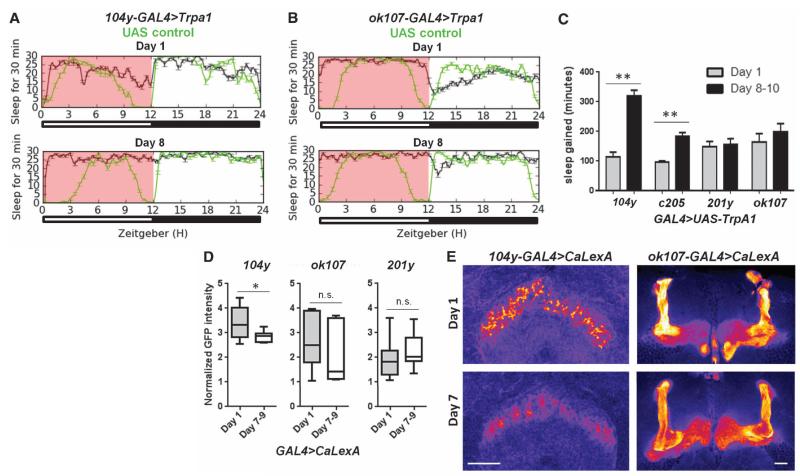
Our data suggest that a known sleep-promoting region, the dFSB, is more active in young flies because of reduced dopaminergic input. To test this idea, we used the dFSB driver 104y-GAL4, which promotes sleep at elevated temperatures when driving expression of TrpA1 (24). Inhibition of dFSB activity using 104y-GAL4 has also been shown to reduce sleep, via expression of a K+ channel that electrically silences neurons (11). We examined the consequences of dFSB activation by measuring sleep with 104y-GAL4>UAS-dTrpA1 at elevated temperatures during the first 12 hours of the day, which is a time when young and mature flies exhibit the largest difference in sleep patterns. We found that in young flies, dFSB activation had a blunted effect on sleep as compared to the large increase induced in mature flies (Fig. 4, A and C, compared to UAS controls; fig. S6A, compared to GAL4 controls); another dFSB driver, c205-GAL4, yielded similar results (Fig. 4C and fig. S6A). We did not detect any differences in 104y-GAL4–dependent expression of UAS-mCD8::GFP at day 1 as compared to day 8 (fig. S6B). The reduced effect on sleep during day 1 was specific to the dFSB, because MB activation with either ok107-GAL4>UAS-dTrpA1 or 201y-GAL4>UAS-dTrpA1 resulted in similar sleep increases in young and mature flies (Fig. 4, B and C, compared to UAS controls; fig. S6A, compared to GAL4 controls). Together these results support the idea that the dFSB in young flies is hyperactive because of reduced dopaminergic input, explaining why less sleep is induced with TrpA1 activation, whereas other sleep-promoting regions (such as the MB) function comparably in young and mature flies.
Fig. 4. The sleep-promoting dFSB is more active in young flies.
(A and B) Sleep traces over 24 hours in GAL4>UAS-dTrpA1 flies (black) or UAS controls (green) at different ages. The pink box denotes elevated temperature. The y axis denotes sleep episodes per 30 min. (C) Quantification of sleep gained during 12 hours of elevated temperature (pink box) in sleeppromoting GAL4>UAS-dTrpA1 lines at multiple developmental time points compared to UAS controls (from left to right, n = 10, 6, 12, 12, 12, 12, 10, and 8 flies, with multiple independent replicates). (D) Normalized GFP intensity of the indicated brain region in GAL4>UAS-CaLexA flies of different ages (104y-GAL4: n = 9 day-1, n = 10 day-7 to -9; ok107-GAL4: n = 15 day-1, n = 14 day-7 to -9; 201y-GAL4: n = 22 day-1, n = 16 day-7 to -9). (E) Representative images of the dFSB (left) and MB (right) in brains immunostained for GFP. GFP is pseudocolored fire. Scale bars, 37.5 μm. **P < 0.0001, *P < 0.05; one-way ANOVA with Tukey’s post-hoc test (C); and unpaired two-tailed Student’s t test plus Welch’s correction (D).
To test whether the dFSB is hyperactive in young flies, we used the CaLexA and Cre-based reporter systems described earlier. Flies with 104y-GAL4 driving expression of CaLexA showed significantly increased fluorescence in the dFSB on day 1 as compared to day 8 (Fig. 4, D and E), indicating increased activity of this sleep-promoting region in young flies. In contrast, expression of CaLexA in the MB using ok107-GAL4 or 201y-GAL4 revealed no significant differences in neural activity at different ages (Fig. 4, D and E). Likewise with Cre-luciferase, we found a higher luminescence signal during the first 12 hours of the day in day-1 compared to day-8 flies with 104y-GAL4>UAS-FLP/+; Cre-F-luc/+ (fig. S6C). Normalized luminescence over this time in 201y-GAL4>UAS-FLP/+;Cre-F-luc/+ and ok107-GAL4>UAS-FLP/+;Cre-F-luc/+ flies was the same in flies at day 1 or day 8 (fig. S6C). These results demonstrate that in young flies, a specific sleep-promoting brain area, the dFSB, is more active, leading to increased sleep and reduced arousal.
Courtship Behaviors and Circuitry Require Early Sleep
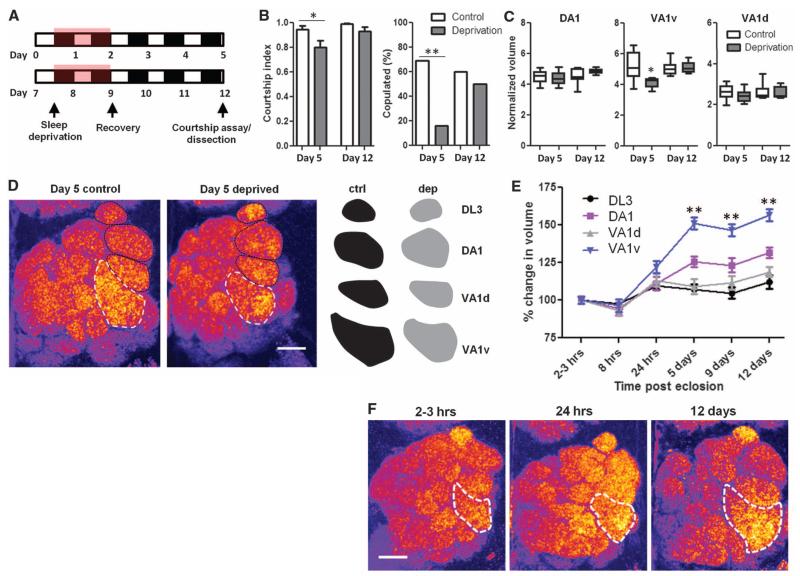
Why do young flies have mechanisms in place to maintain high levels of deep (arousal-resistant) sleep? Disruption of sleep in young flies by mechanical stimulation impairs a variety of adult behaviors, including courtship (10). We examined whether reversible hyperexcitation of TH-GAL4 neurons during a period when these cells are normally hypoactive would result in long-lasting courtship deficits. TH-GAL4>UAS-dTrpA1 males were collected within hours of eclosion and exposed to 36 hours of elevated temperature beginning either on day 0 or day 7 (Fig. 5A). Flies were then allowed to recover for 3 days before testing. On day 5 or day 12, we examined courtship behaviors (Fig. 5A). TH-GAL4>UAS-dTrpA1 males exposed to elevated temperatures beginning on day 0 but not day 7 exhibited a reduced courtship index and a severe reduction in copulation frequency (Fig. 5B). We observed no effect on courtship in GAL4 and UAS controls exposed to elevated temperatures on day 0 (fig. S7A). Thus, hyperexcitation of dopaminergic neurons during a sensitive window of development leads to long-lasting deficits in courtship.
Fig. 5. Sleep in young flies is required for courtship behaviors and circuitry development.
(A) Schematic of experimental approach. (B) Quantification of courtship index and copulation frequency (n = 14, 12, 15, and 12 flies for day-5 control, day-5 deprivation, day-12 control, and day-12 deprivation, respectively) in a 10-min assay using CS female virgin targets. (C) Quantification of olfactory glomerular volume normalized to DL3 (n = 11, 10, 10, and 9 flies for day-5 control, day-5 deprivation, day-12 control, and day-12 deprivation, respectively). (D) Representative images of the AL labeled with anti-nc82, pseudocolored “fire.” White hashed outlines demarcate VA1v; black hashed lines demarcate DL3, DA1, and VA1d (from top to bottom). Right: Solid traces of each glomerulus in black (controls) and gray (deprived). Scale bar, 20 μm. (E) Quantification of the percentage growth of glomeruli (normalized to hour-2 to -3 values) at multiple developmental time points in TH-GAL4>UAS-dTrpA1 males at 21°C (n = 11, 9, 12, 8, 11, and 10 flies for hour-2 to -3, hour-8, day-1, day-5, day-9, and day-12 respectively). (F) Representative images of the AL labeled with anti-nc82, pseudocolored “fire.” White hashed outlines demarcate VA1v. Scale bar, 20 μm. **P < 0.001, *P < 0.05; one-way ANOVA with Tukey’s posthoc test [(B); courtship index, (C)]; Fischer’s exact test [(B), copulation frequency]; and two-way ANOVA with post-hoc Bonferroni multiple comparisons (E).
Because courtship is an innate behavior, the underlying neural circuits are thought to be developmentally programmed (32). Courtship behaviors in Drosophila are sexually dimorphic and require the male-specific isoform of the fruitless gene, Fru(M) (33). fruitless expression patterns are similar in males and females, but differences have been identified in subpopulations of the ~2000 Fru+ neurons, including clusters dorsal to the antennal lobe (AL), the suboesophageal ganglion (SOG), and neurons projecting to three AL olfactory glomeruli (34-37). Olfactory glomeruli are known to be highly plastic structures (38, 39), though Fru+ glomeruli have not previously been examined. We therefore focused on this region to determine whether courtship deficits in young sleep-deprived flies might be attributed to changes in Fru+ circuitry. Total volumes were calculated for DA1 and VA1v, which are the Fru+ glomeruli known to exhibit the largest differences between males andfemales (34,37). TH-GAL4>UAS-dTrpa1 males that were sleep-deprived when young, but not those sleep-deprived when mature, showed a reduction in size of a specific Fru+ glomerulus, VA1v (Fig. 5, C and D). The volume of the other analyzed Fru+ glomerulus, DA1, was not affected, nor were two other non-Fru+ glomeruli, DL3 and VA1d, which are adjacent to DA1 and VA1v (Fig. 5, C and D and fig. S7B). These differences are apparent both in raw volumetric data (fig. S7B) and in values normalized to DL3 to control for brain-to-brain size variability (Fig. 5C) (37). Similar effects on courtship behavior and AL glomeruli were also obtained when a higher-intensity mechanical stimulus able to sleep-deprive young flies was used (fig. S7, C and D).
To address the possibility that TH-GAL4>UAS-dTrpa1 in our paradigm affects courtship behaviors and circuitry independent of sleep, we sought a driver that excludes dopaminergic dFSB projections but still has widespread expression in TH+ projections near olfactory glomeruli in the AL. TH-C1 fits these criteria (11), and we found that TH-C1-GAL4>UAS-CD8::GFP is expressed in ventral projections to the AL (fig. S8A). Moreover, TH-C1 is not known to be wake-promoting when activated (11). Using TH-C1-GAL4>UAS-dTrpa1, we found that hyperexcitation of this subset of TH+ neurons in young flies failed to cause courtship deficits or glomerular volumetric changes in mature adulthood in the absence of earlier sleep loss (fig. S8, B to D). Thus, dopamine neurons excluded by TH-C1 are necessary for the observed behavioral and volumetric effects, which are not likely to be secondary to direct hyperactivation of dopaminergic inputs to the AL. Finally, we assessed TH-D4, mentioned above, which is expressed in neurons projecting only to the ellipsoid body, MB, and FSB (fig. S8A) (11). Activation of these neurons in young flies using TH-D4-GAL4>UAS-dTrpA1 resulted in sleep deprivation (fig. S8D), and after recovery days, we found a deficit in courtship measures and a specific reduction in VA1v volume (fig. S8, B and C); DA1 and VA1d volumes were unaffected. Together, these findings demonstrate that early developmental sleep deprivation affects a specific part of courtship-relevant circuitry and pinpoint a small group of TH+ neurons that are sufficient for the effect.
We wondered why VA1v in particular would be affected by sleep deprivation. The volume reduction as compared to controls could result either from volume loss or failure of normal growth. To distinguish between these two possibilities, we charted the developmental time course of growth in the same four glomeruli in TH-GAL4>UAS-dTrpa1 males. We used these flies to control for background effects on volumetric measures when comparing to preceding experiments. Flies were reared at 21°C, and dissections were performed at multiple time points after eclosion (Fig. 5E). All four glomeruli showed some degree of post-eclosion growth, although this was minimal in DL3 and VA1d (Fig. 5, E and F, and fig. S9, A and B). Both Fru+ glomeruli underwent more extensive growth after eclosion, but VA1v grew significantly more than all other glomeruli (Fig. 5, E and F, and fig. S9, A and B). Thus, sleep deprivation impairs the developmental growth of VA1v, as opposed to inducing volume loss. VA1v normally undergoes themost rapid growth beginning ~8 hours after eclosion, which is the time when sleep deprivation began in experiments showing the long-lasting effect of deprivation on volume and courtship behaviors. Our data therefore suggest that sleep deprivation might be particularly deleterious to rapidly growing regions of the brain.
Discussion
The ubiquity among animals of increased sleep early in life suggests an important role for sleep during this period. Here we have defined a specific function for sleep in structural development of the brain. In addition, we identified a neural circuit controlling sleep in young flies. Sleep ontogeny in Drosophila is controlled by a developmental program: Dopaminergic neurons projecting to the dFSB are less active in young flies, leading to increased activity of this sleep-promoting region (fig. S10). It is possible that regulation of dopamine—a conserved arousal signal—also accounts for developmental changes in sleep in other species, perhaps even through specific circuits. Sudden infant death syndrome usually occurs early in infant life, and (in addition to environmental risk factors) abnormalities in sleep structure and arousal are thought to play a significant role (40, 41). We suggest that the dysfunction of specific molecular signals or circuits could underlie pathological sleep/wake imbalances during mammalian development.
Courtship behaviors in Drosophila are innate and required for species propagation. Many studies have focused on the role of pheromone detection in this process (42). VA1v (also known as VA1lm) is the target of olfactory receptor neurons expressing Or47b (43, 44) and confers responsiveness to odors from both male and female flies (45). A specific role for VA1v in male-female courtship has remained unclear (46), although genetic disruption of Or47b suggests a role in male localization of females (47), generally consistent with our finding that reduced size of VA1v correlates with courtship deficits. We cannot rule out the possibility that other regions of the nervous system involved in courtship are affected by early sleep deprivation as well. However, antennal lobe glomeruli are known to be highly plastic structures that exhibit volume and growth changes in response to external stimuli during specific periods of development (38, 39). Post-eclosion experience-dependent glomerular plasticity could therefore be involved in encoding courtship encounters throughout life.
Sleep is involved in alterations of synaptic strength (48-50). Work in adolescent mice also suggests changes in dendritic spine number depending on the wake or sleep state, but only within a developmental period of heightened spine turnover (51). Whether ontogenetic sleep changes are required for synapse formation is not known. The antibody we used for volumetric glomerular measurements, anti-nc82, specifically recognizes the presynaptic active zone protein Bruchpilot, which exhibits homology to human presynaptic protein ELKS/CAST/ERC (52), and previous work has shown that glomerular volume correlates with synapse number (53). We propose a model whereby VA1v undergoes a period of rapid synapse addition in young flies, and we suggest that sleep is required for this process. Sleep disruption during this time affects VA1v and probably other regions with heightened rates of synaptogenesis. Many neuropsychiatric diseases are increasingly viewed as synaptic and developmental in origin (54), and sleep abnormalities are pervasive in psychiatric illness (55). Sleep during critical periods of development may therefore play an important role in diseases that manifest later in life.
Supplementary Material
Acknowledgments
We thank D. Raizen, T. Abel, M. Frank, and members of the Sehgal lab for helpful input and advice. This work was supported by grants T32HL07713 and R25MH060490 from the National Institutes of Health (M.S.K.) and the Howard Hughes Medical Institute (A.S.). M.S.K. and A.S. designed the experiments. M.S.K. and Z.Y. performed the research. M.S.K., Z.Y., and A.S. collected, analyzed, and interpreted the data. M.S.K., Z.Y., and A.S. wrote and edited the paper.
Footnotes
www.sciencemag.org/content/344/6181/269/suppl/DC1
Materials and Methods
Figs. S1 to S10
References (56-61)
The authors have no patents pending or financial conflicts to disclose.
References and Notes
- 1.Roffwarg HP, Muzio JN, Dement WC. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 2.Jouvet-Mounier D, Astic L, Lacote D. Dev. Psychobiol. 1969;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 3.Frank MG, Heller HC. Am. J. Physiol. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- 4.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien LM, et al. Pediatrics. 2004;114:44–49. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Halbower AC, et al. PLOS Med. 2006;3:e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ednick M, et al. Sleep. 2009;32:1449–1458. doi: 10.1093/sleep/32.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank MG. Prog. Brain Res. 2011;193:221–232. doi: 10.1016/B978-0-444-53839-0.00014-4. [DOI] [PubMed] [Google Scholar]
- 9.Hendricks JC, et al. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 10.Seugnet L, Suzuki Y, Donlea JM, Gottschalk L, Shaw PJ. Sleep. 2011;34:137–146. doi: 10.1093/sleep/34.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu MN, Kodama L, Liu Q, Liu S, Driscoll MR. Curr. Biol. 2012 doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada FN, et al. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friggi-Grelin F, et al. J. Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 14.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. J. Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andretic R, van Swinderen B, Greenspan RJ. Curr. Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. Sleep. 2008;31:465–472. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeiffer BD, Truman JW, Rubin GM. Proc. Natl. Acad. Sci. U.S.A. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamoto T. J. Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 19.Joiner WJ, Crocker A, White BH, Sehgal A. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 20.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Chen D, Sehgal A. Genes Dev. 2012;26:1224–1234. doi: 10.1101/gad.186338.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanenhaus AK, Zhang J, Yin JCP. PLOS ONE. 2012;7:e45130. doi: 10.1371/journal.pone.0045130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheng M, Thompson MA, Greenberg ME. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 24.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donlea JM, Pimentel D, Miesenböck G. Neuron. 2014;81:860–872. doi: 10.1016/j.neuron.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno T, et al. Nat. Neurosci. 2012;15:1516–1523. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- 27.Masuyama K, Zhang Y, Rao Y, Wang JW. J. Neurogenet. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim WJ, Jan LY, Jan YN. Neuron. 2013;80:1190–1205. doi: 10.1016/j.neuron.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang Y, Griffith LC, Rosbash M. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagaki HK, et al. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osterwalder T, Yoon KS, White BH, Keshishian H. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall JC. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto D, Koganezawa M. Nat. Rev. Neurosci. 2013;14:681–692. doi: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- 34.Kondoh Y, Kaneshiro KY, Kimura K, Yamamoto D. Proc. Biol. Sci. 2003;270:1005–1013. doi: 10.1098/rspb.2003.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura K-I, Ote M, Tazawa T, Yamamoto D. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 36.Manoli DS, et al. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 37.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Sachse S, et al. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Devaud J-M, Acebes A, Ramaswami M, Ferrús A. J. Neurobiol. 2003;56:13–23. doi: 10.1002/neu.10215. [DOI] [PubMed] [Google Scholar]
- 40.Schechtman VL, Harper RM, Wilson AJ, Southall DP. Pediatrics. 1992;89:865–870. [PubMed] [Google Scholar]
- 41.Harper RM, et al. Science. 1981;213:1030–1032. doi: 10.1126/science.7268406. [DOI] [PubMed] [Google Scholar]
- 42.Dickson BJ. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 43.Couto A, Alenius M, Dickson BJ. Curr. Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Fishilevich E, Vosshall LB. Curr. Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 45.van der Goes van Naters W, Carlson JR. Curr. Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, et al. Nat. Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Root CM, et al. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abel T, Havekes R, Saletin JM, Walker MP. Curr. Biol. 2013;23:R774–R788. doi: 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bushey D, Tononi G, Cirelli C. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank MG, Issa NP, Stryker MP. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 51.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Nat. Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagh DA, et al. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Devaud J-M, Acebes A, Ferrús A. J. Neurosci. 2001;21:6274–6282. doi: 10.1523/JNEUROSCI.21-16-06274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoghbi HY. Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 55.Kupfer DJ. Biol. Psychiatry. 1995;38:391–403. doi: 10.1016/0006-3223(94)00295-E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.