Abstract
It is well established that chronic exposure to excess nutrients leads to insulin resistance (IR) in skeletal muscle. Since skeletal muscle is responsible for 70-80% of insulin-stimulated glucose uptake, skeletal muscle IR is a key pathological component of type 2 diabetes (T2D). Recent evidence suggests that inhibition of the nutrient-sensing enzyme AMP-activated protein kinase (AMPK) is an early event in the development of IR in response to high glucose, branched chain amino acids (BCAA), or fatty acids (FA). Whether the decrease in AMPK activity is causal to the events leading to insulin resistance (increased mTOR/p70S6K signaling) remains to be determined. Interestingly, pharmacological activation of AMPK can prevent activation of mTOR/p70S6K and insulin resistance, while inhibition of mTOR with rapamycin prevents insulin resistance, but not AMPK downregulation. AMPK can be inhibited by increased energy state (reduced AMP/ATP ratio), decreased phosphorylation of its activation site (αThr172) (by decreased upstream kinase activity or increased phosphatase activity), increased inhibitory phosphorylation at αSer485/491, changes in redox state or hormone levels, or other yet to be identified mechanisms. Excess nutrients also lead to an accumulation of the toxic lipid intermediates diacylglycerol (DAG) and ceramides, both of which can activate various protein kinase C (PKC) isoforms, and contribute to IR. The mechanism responsible for the initial downregulation of AMPK in response to excess nutrients, and whether glucose, BCAA, and FA act through similar or different pathways requires further study. Identification of this mechanism and the relative importance of other events would be beneficial for designing novel pharmacological interventions to prevent and/or reverse IR. This review will focus on the some of the mechanisms responsible for AMPK downregulation and the relative sequence and importance of these events.
Keywords: mTOR/p70S6K, Hyperglycemia; Protein Kinase C (PKC); Branched Chain Amino Acids (BCAA); Fatty Acid (FA)
INTRODUCTION
The prevalence of worldwide obesity, type 2 diabetes (T2D), and the metabolic syndrome are increasing at an alarming rate. Insulin resistance (IR) in peripheral tissues (muscle, liver, and adipose) is a key pathological event in the development of T2D. Since skeletal muscle is responsible for 70-80% of insulin-stimulated glucose uptake [1], skeletal muscle IR is considered a critical pathological component of the metabolic syndrome and T2D [2]. Acute IR may be a normal or protective response of the cell to excess nutrients under physiological conditions, possibly a mechanism to reroute unneeded fuel to other parts of the body, or to prevent oxidative stress and glucotoxicity [3]. In this setting, normal insulin signaling resumes when nutrient levels return to normal. In contrast, chronic nutrient excess seems to cause less easily reversible changes that prevent normal glucose uptake, causing hypergylcemia and hyperinsulinemia in the plasma, but glucose deprivation in the tissue. Additionally, more damaging secondary changes such as pancreatic beta cell apoptosis, inflammation, oxidative stress, and vascular/endothelial dysfunction result [4]. The molecular sequence of events by which chronic exposure to excess nutrients (high glucose, lipids) impairs insulin signaling has been studied in detail, but remains incompletely understood. Inflammation, oxidative stress, ER stress, and the accumulation of toxic lipid derivatives, such as diacylglycerol (DAG) and ceramides, have all been implicated to contribute to the development of IR [1,5]. Which of these factors is the primary cause or whether it is a combination of them remains under debate? AMP-activated protein kinase (AMPK) is an energy sensing enzyme that plays a central role in nutrient sensing/insulin sensitivity. It is a heterotrimeric protein that is activated when energy levels are low (i.e., exercise or starvation) and signals to increase ATP generating processes and decrease ATP consuming processes. The consequences of its activation (mediated through a high AMP/ATP or ADP/ATP ratio and phosphorylation of αAMPK Thr172) have been studied in detail. Pharmacologic agents that activate AMPK are currently used in the clinic (i.e., Metformin, TZD’s) and are of interest in drug development [6], as they prevent and/or reverse some of the pathologies of T2D. In contrast, the mechanism and consequences of AMPK downregulation below basal levels, which our lab has shown to occur early on in the setting of high nutrient induced IR, are less understood [7]. Notably, multiple animal models with a metabolic syndrome phenotype have decreased AMPK activity in muscle and liver [6,8-11], and loss of AMPK is detrimental in a number of metabolic challenges [9,12-16], such as diet-induced IR and obesity [17], calorie restriction [18], and exercise [19]. In addition, decreased AMPK activity in skeletal muscle [20] and adipose tissue [21,22] of humans with T2D or obesity has been reported. Although no causal inferences can be established in humans, dysfunction of AMPK may predispose obese individuals to a variety of metabolic complications, including IR and T2D.
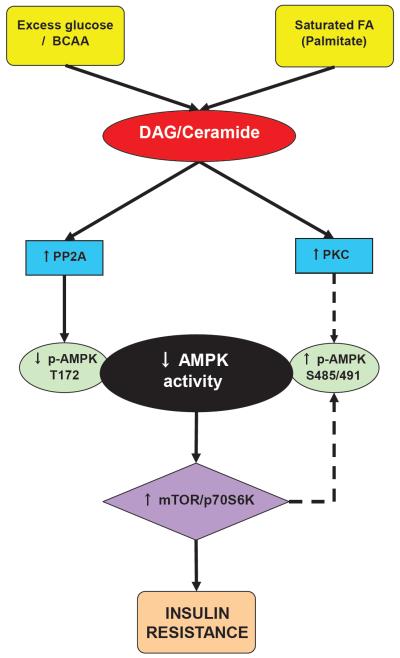
The precise mechanism for the suppressed activity of AMPK is unknown; however, prolonged exposure to excess nutrients (glucose, branched chain amino acids (BCAA), and fatty acids (FA)) has been shown to cause diminished AMPK activity [7] (Figure 1). Many factors may contribute to this decrease, such as reduced phosphorylation of αAMPK Thr172 (either by reduced activity of upstream kinases or increased phosphatase activity), an increase in phosphorylation of αAMPK Ser485/491, a site that is presumed to be inhibitory, changes in adenine nucleotide levels (decreased AMP/ATP ratio), a change in redox state, or other factors that may alter other aspects of this pathway [23]. This review will focus on the some of the mechanisms responsible for AMPK downregulation and the relative sequence and importance of these events.
Figure 1.
Hypothetical mechanisms by which nutrient excess downregulates AMPK and leads to insulin resistance (IR). High glucose and leucine increase DAG, whereas saturated FA elevates both DAG and ceramide, leading to increased PKC and phosphatase (PP2A) activity. PKC may phophorylate AMPK at Ser485/491, while PP2A dephosphorylates Thr172, inhibiting its activity. Reduced AMPK activity allows for activation of mTOR/p70S6K, which, in turn, contributes to IR. Other factors not shown here may also play a role in the downregulation of AMPK, including changes in cellular energy, redox state, or reductions in activity of upstream AMPK kinases. Dashed arrows represent unconfirmed pathways in insulin-sensitive tissues.
AMPK REGULATION
As previously mentioned, AMPK is a heterotrimeric protein consisting of a catalytic α subunit, and regulatory β and γ subunits [6,24]. There exist two isoforms of the α subunit, two β, and three γ isoforms. Some of the α/β/γ complexes are specific to certain tissues, and the different combinations of subunits may preferentially localize to certain subcellular compartments [25]. The γ subunit contains four CBS domains (each pair is referred to as a Bateman domain) to which adenine nucleotides bind [26]. Only three of the four CBS domains bind adenine nucleotides; AMP is always bound to one of the three binding sites, while the other two can bind to AMP, ADP, or ATP depending on their relative concentrations [26]. Under normal conditions, ATP is bound to these domains; however, when the AMP:ATP ratio is increased, AMP replaces ATP at the Bateman domains, causing an allosteric change that contributes to AMPK activation [26]. Recently, it has been proposed that ADP, as well as AMP, may be able to activate AMPK [27]. The allosteric change caused by binding of AMP (or ADP) makes AMPK a better substrate for its upstream kinases LKB1, CaMKKβ, and TAK1 to phosphorylate it at Thr172 of the α subunit [28-30]. The combination of allosteric activation and phosphorylation of this site leads to greater than a 1000-fold increase in kinase activity [31].
The protein phosphatases PP2A and PP2C have been shown to dephosphorylate AMPK at Thr172 [32]. The allosteric change caused by binding of AMP to the Bateman domains inhibits dephosphorylation of Thr172 by making it a poor substrate for the phosphatases [33]. Whether increased phosphatase activity contributes to AMPK downregulation due to nutrient excess remains to be determined. Although Thr172 is regarded as the main phosphorylation/activation site, there are several other phosphorylation sites with less defined functions on the α and β subunits. Phosphorylation of Ser485/491 on α1/α2 has been shown to be inhibitory in select tissues [34], such as heart [35], vascular smooth muscle cells (VSMC) [36,37] brown adipose tissue (BAT) [38], and hypothalamus [39], although its physiological importance in the insulin responsive tissues skeletal muscle and liver remains to be elucidated. It has been proposed that this phosphorylation impairs the physical association of LKB1 with AMPK, thus preventing phosphorylation of Thr172 and inhibiting activation [40]. AKT has been reported to phosphorylate Ser485/491 in heart [35], VSMCs [36,37], and BAT [38]; however, recently it was shown that p70S6K is responsible for directly phosphorylating α2 Ser491 in hypothalamus [39].
A physiological response to feeding is reduced AMPK activity and a corresponding transition from catabolic to anabolic processes. For example, in fasted rats with elevated AMPK, refeeding caused an acute reduction in AMPK activity and Thr172 phosphorylation in liver [41] and muscle [42], concomitant with an increase in plasma insulin levels [42]. In addition to the contributions of nutrients, postprandial insulin secretion may have relevance in vivo, as insulin has been shown to decrease AMPK activation in the heart [43,44] and hepatoma cells [45]. The proposed mechanism is that insulin-stimulated AKT downregulates AMPK [46,47], perhaps by directly phosphorylating it on Ser485/491 [35]. This physiological reduction in AMPK activity may have detrimental effects on insulin sensitivity in sustained hyperinsulinemia. The following sections will highlight the roles of specific nutrients on the regulation of AMPK.
AMPK inhibition by High Glucose
Exposure to excess glucose has been shown to decrease AMPK activity in several cell types and tissues, such as muscle [7,11,48], liver [11], HepG2 hepatocytes [49], kidney [50], pancreatic β cells [51], and human umbilical vein endothelial cells [52]. Our lab has shown that a one hour incubation of rat extensor digitorum longus (EDL) muscle with glucose dose dependently diminishes p-AMPK Thr172, α2 activity, and phosphorylation of its downstream substrate acetyl-coA carboxylase (ACC) [7]. Phosphorylation of ACC inhibits its ability to convert Acetyl CoA to malonyl CoA, allowing fatty acid oxidation to occur. Similarly, 5 hours of glucose infusion to maintain plasma glucose levels at 17-18mM reduced α2 AMPK activity in rat red gastrocnemius [11]. Importantly, the reductions in AMPK activity in response to high glucose were not accompanied by a change in energy state (AMP/ATP ratio); however, an increase in the lactate/pyruvate ratio, indicating a change in redox state (decreased NAD+/NADH ratio) was observed; however, this change was observed later (unpublished data). Recently, the redox sensitive protein SIRT1 was shown to play a role in modulating activity of LKB1, which in turn, regulates AMPK. [53,54]. Therefore, a decreased NAD+/NADH ratio could lead to diminished SIRT1 and LKB1 activity, which may partially explain the decrease in AMPK activity. Increases in DAG content and p-AMPK Ser485/491 were also observed in EDL incubated in high glucose; however, these also occurred later than the decrease in Thr172 phosphorylation (unpublished data). Although this suggests that DAG and Ser485/491 are not responsible for the initial drop in AMPK activity, they may contribute to the sustained reduction seen with pathological conditions.
Another change that was caused by incubation of rat EDL with excess glucose was an increase in mammalian Target of Rapamycin (mTOR) signaling and protein synthesis. mTOR is a protein kinase involved in many signaling pathways, including cell growth and protein synthesis. The mTOR Complex 1 (mTORC1) consists of the proteins mTOR, Raptor, MLST8, PRAS40, and DEPTOR [55,56]. mTORC1 is activated in response to normal insulin signaling and activates its downstream effectors p70S6K and 4E-BP1, which, among other things, stimulate protein synthesis. Overactivation of mTOR/p70S6K has been shown to cause insulin resistance [57-63]. Although activation of these proteins is a physiological response to insulin, prolonged activation impairs insulin signaling through a feedback loop in which S6K causes degradation of insulin-receptor substrates (IRS) and mTOR decreases glucose uptake [64,65]. Interestingly, the AMPK activators AICAR and α-lipoic acid prevented the increase in mTOR/p70S6K phosphorylation, protein synthesis, and the development of insulin resistance in EDL incubated with high glucose. Gleason et al. also saw an inverse correlation of decreased AMPK activation and increased mTOR signaling in response to high glucose in pancreatic β-cells [51], while rats fed a high fat diet (HFD) had decreased aortic AMPK activity, with a concurrent upregulation of mTOR [66]. Whether AMPK downregulation is causal to the increase in mTOR/p70S6K activation and protein synthesis requires further study.
AMPK inhibition by Amino Acids
It has been established that branched chain amino acids (BCAA), particularly leucine, increase mTOR signaling, protein synthesis, and insulin resistance. More recently, it has been shown that BCAAs also decrease AMPK activity at the same time as increasing mTOR/p70S6K signaling and protein synthesis. For example, incubation of rat EDL with 100 or 200μM leucine for one hour significantly increased phosphorylation of mTOR at Ser2448, p70S6K at Thr389, and protein synthesis [7]. At the same time, AMPK phosphorylation at Thr172 and AMPK α2 activity were significantly reduced. These effects are specific to BCAAs, as isoleucine, another BCAA, had similar but lesser effects, while glutamine had no effect. In addition to increasing mTOR/p70S6K signaling and protein synthesis, leucine incubation also caused insulin resistance in rat EDL, as evaluated by a decrease in insulin-stimulated phosphorylation of AKT at Ser473 [7]. Just as with high glucose, we observed an increase in AMPK Ser485/491 phosphorylation, but this change was subsequent to reduced AMPK Thr172 phosphorylation (unpublished data). Similar studies using the C2C12 mouse muscle cell line showed that incubation with 2mM leucine decreased AMPK Thr172 phosphorylation and increased mTOR phosphorylation and protein synthesis as early as 30 minutes [55], whereas treatment with an amino acid mixture had the same effects in pancreatic-cells [51]. In humans, infusion of amino acids activated mTOR/p70S6K and caused IR [67,68], while rapamycin treatment prevented both the activation of mTOR and the IR in healthy men [69].
As with high glucose, treatment with the AMPK activators AICAR and α-lipoic acid prevented the increase in mTOR/p70S6K phosphorylation, protein synthesis, and the development of IR in EDL incubated with physiological concentrations of leucine [7]. Similarly, AICAR treatment prevented the increase in mTOR phosphorylation and protein synthesis in C2C12 cells, and AICAR or phenformin treatment prevented phosphorylation of p70S6K in β-cells [51]. In contrast to these data, in EDL, we did not see a decrease in Raptor on Ser792 or TSC2 on Ser1387, two substrates of AMPK that lead to inhibition of mTORC1 [7]. However, since basal levels of phosphorylation are low to begin with, a further decrease may have been too small to detect [7]. It is interesting to note that in the C2C12 cells, a decrease in the AMP/ATP ratio was detected with leucine exposure, while no difference was detected in the EDL incubations. Differences in dosing and/or incubation time may explain this discrepancy; however, further studies are needed to determine whether a change in adenine nucleotides or another factor is responsible for the initial downregulation of AMPK activity.
AMPK inhibition by Fatty Acids
It is known that excess dietary fat leads to an accumulation of DAG and ceramides, both of which are toxic lipid derivatives, in muscle [1,5]. Palmitate, the most abundant dietary saturated fat [5], leads to de novo ceramide synthesis. It has been proposed that lipotoxicity resulting from DAG and ceramide accumulation is a primary cause of IR. Prolonged exposure to palmitate decreases AMPK activity in a variety of tissues and cells. For example, exposure of cultured bovine aortic endothelial cells (BAECs) to palmitate (0.4 mM) but not to palmitoleic or oleic acid (0.4 mM) for 40 h significantly reduced Thr172 phosphorylation of AMPK without altering the phosphorylation (activation) of its upstream kinase LKB1 on Ser428 [70]. Additionally, palmitate significantly increased the activity of PP2A, while inhibition of PP2A with okadaic acid or PP2A siRNA prevented the decrease in AMPK Thr172 phosphorylation. Moreover, aortas from mice fed a HFD rich in palmitate had decreased AMPK Thr172 phosphorylation and increased PP2A activity, whereas those fed a HFD rich in oleate did not [70]. These data suggest that saturated, but not unsaturated FA decrease AMPK activity. Interestingly, prevention of de novo ceramide synthesis with the inhibitor fumonisin B1 prevented the effects of palmitate on AMPK and PP2A, suggesting that ceramides, at least in part, contribute to downregulation of AMPK [70]. In rodents, HFD has been shown to decrease phosphorylation of AMPK Thr172 in both liver and muscle [71,72]. Interestingly, acute exposure to palmitate has been shown to increase both AMPK Thr172 and ACC phosphorylation in L6 muscle cells [73,74]. We have also observed this acute increase in AMPK activation in C2C12 myotubes (up to 6h), whereas prolonged exposure (24h) to palmitate decreases AMPK Thr 172 phosphorylation, concomitant with reduced insulin sensitivity (unpublished data).
The mechanism by which FA causes IR is not fully understood. The protein kinase C (PKC) family of serine/threonine kinases can be activated by both DAG and ceramides, and various PKC isoforms have been implicated to play a role in the development of FA induced IR [1,75]. Several PKC isoforms are activated in the muscle of various species in response to FA metabolites; for example, PKCε, θ, and δ in rats [76-78], PKCθ, δ, and βII in humans [76-78], and PKCγ and ζ in C2C12 myotubes [79]. One proposed mechanism by which PKC activation leads to IR is by phosphorylating insulin receptor and insulin receptor substrate 1 (IRS-1) on serine/threonine residues, thus preventing its normal tyrosine phosphorylation and downstream signaling [5,78,80-85]. It has also been reported that novel PKC isoforms can activate mTOR by phosphorylation [56], which could potentially contribute to IR as it does in response to excess glucose or BCAA. Additionally, Cazzolli et al. showed that ceramides can activate PP2A and increase PKCζ in C2C12 cells treated with palmitate [79]. The upregulation of PP2A was proposed to hinder insulin signaling by dephosphorylating AKT [79], however, it could potentially also contribute to dephosphorylation of AMPK. PKC activation may also lead to IR and reduced AMPK activity through phosphorylation of AMPK Ser485/491. Kodiha and Stochaj proposed that PKC and AKT phosphorylate Ser485/491 which diminishes AMPK activity [25]. Further studies are needed to determine whether the upregulation of PP2A or various PKC isoforms plays a role in AMPK downregulation in lipid induced IR.
CONCLUSION
Decreased AMPK activity and AMPK Thr172 phosphorylation seem to be early and seminal events in the path to IR due to nutrient excess. Glucose and leucine may diminish AMPK activity through a similar but unidentified pathway, whereas FA may do so through a different mechanism involving DAG, ceramide, and PKCs. In addition to nutrients, AMPK activity can also be inhibited by numerous hormones and inflammatory cytokines, as reviewed by Viollet et al. [86]. As AMPK downregulation is a common event resulting from all of three of these nutrient stimuli, its activation is naturally a target for therapeutic intervention. Indeed, current therapies for treating T2D and the metabolic syndrome include diet, exercise, and insulin sensitizing pharmacological agents such as metformin, all of which activate AMPK [6]. While these treatments are helpful, they are often not sufficient by themselves to manage patients’ blood glucose levels, leaving room for the development of more effective medications. As none of the currently available therapies are direct AMPK activators, pharmaceutical agents that act directly on this enzyme may be a promising addition to the currently available medications. Combination therapy may prove to be more beneficial, as it may augment AMPK activity to a greater extent and could potentially prevent the reduction in AMPK activity due to overnutrition in obese humans.
As previously mentioned, acute IR may be a beneficial adaptation to a glucose, amino acid, or lipid load, but chronic IR leads to detrimental changes in the tissue. Identification of events marking a transition from physiological (easily reversible) to pathological (less reversible) IR, which is accompanied by further damaging processes such as inflammation and oxidative stress, would help determine sites for intervention. The initiating event responsible for decreasing AMPK activity and AMPK Thr172 phosphorylation remains to be elucidated. Some have reported that it is due to a decrease in AMP/ATP ratio, while others attributed it to an increase in PP2A activity. However, we have seen decreased AMPK activation prior to changes in either of these measures. Additionally, the significance of changes in AMPK Ser485/491 phosphorylation as well as redox state and SIRT1 activity require further study. These events help to maintain the inhibition of AMPK or they may signal to other molecules to affect AMPK or other aspects of insulin signaling. A better understanding of AMPK regulation and the factors contributing to its down regulation could help identify novel ways to design chemical AMPK activators, as well as other molecules in the pathway that could be targeted to act in concert with AMPK activators to enhance amelioration of IR.
ACKNOWLEDGEMENTS
The work in the author’s laboratory was supported by US PHS grants RO1DK19514 and RO1DK67509 and HL-007224 (RV).
REFERENCES
- 1.Amati F. Revisiting the diacylglycerol-induced insulin resistance hypothesis. Obes Rev. 2012;13(Suppl 2):40–50. doi: 10.1111/j.1467-789X.2012.01036.x. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee-Young RS, Bonner JS, Mayes WH, Iwueke I, Barrick BA, Hasenour CM, et al. AMP-activated protein kinase (AMPK)α2 plays a role in determining the cellular fate of glucose in insulin-resistant mouse skeletal muscle. Diabetologia. 2013;56:608–617. doi: 10.1007/s00125-012-2787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, et al. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 5.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 7.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW. Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. Am J Physiol. 1999;277:E1070–1076. doi: 10.1152/ajpendo.1999.277.6.E1070. [DOI] [PubMed] [Google Scholar]
- 9.Liang B, Wang S, Wang Q, Zhang W, Viollet B, Zhu Y, et al. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-alpha2 in vivo. Arterioscler Thromb Vasc Biol. 2013;33:595–604. doi: 10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol. 1999;276:E1–1E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 11.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, et al. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290:E471–479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 12.Beall C, Piipari K, Al-Qassab H, Smith MA, Parker N, Carling D, et al. Loss of AMP-activated protein kinase alpha2 subunit in mouse beta-cells impairs glucose-stimulated insulin secretion and inhibits their sensitivity to hypoglycaemia. Biochem J. 2010;429:323–333. doi: 10.1042/BJ20100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foretz M, Guihard S, Leclerc J, Fauveau V, Couty JP, Andris F, et al. Maintenance of red blood cell integrity by AMP-activated protein kinase alpha1 catalytic subunit. FEBS Lett. 2010;584:3667–3671. doi: 10.1016/j.febslet.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Liang B, Viollet B, Zou MH. Inhibition of the AMP-activated protein kinase-α2 accentuates agonist-induced vascular smooth muscle contraction and high blood pressure in mice. Hypertension. 2011;57:1010–1017. doi: 10.1161/HYPERTENSIONAHA.110.168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH, A, et al. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol. 2013;182:1021–1030. doi: 10.1016/j.ajpath.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, et al. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase α subunits. Arterioscler Thromb Vasc Biol. 2011;31:1757–1765. doi: 10.1161/ATVBAHA.111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Zhang X, Wang H, Guo X, Li H, Wang Y, et al. AMP-activated protein kinase α1 protects against diet-induced insulin resistance and obesity. Diabetes. 2012;61:3114–3125. doi: 10.2337/db13-rt03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K, Kobayashi S, Xu X, Viollet B, Liang Q. AMP activated protein kinase is indispensable for myocardial adaptation to caloric restriction in mice. PLoS One. 2013;8:e59682. doi: 10.1371/journal.pone.0059682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kröller-Schön S, Jansen T, Hauptmann F, Schüler A, Heeren T, Hausding M, et al. α1AMP-activated protein kinase mediates vascular protective effects of exercise. Arterioscler Thromb Vasc Biol. 2012;32:1632–1641. doi: 10.1161/ATVBAHA.111.243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier MS, O’Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, et al. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell Cycle. 2011;10:3447–3451. doi: 10.4161/cc.10.20.17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Kodiha M, Stochaj U. In: Targeting AMPK for Therapeutic Intervention in Type 2 Diabetes, in Medical Complications of Type 2 Diabetes. Croniger C, editor. 2011. [Google Scholar]
- 26.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 27.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 32.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 33.Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- 34.Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, et al. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278:28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 35.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 36.Stone JD, Narine A, Tulis DA. Inhibition of vascular smooth muscle growth via signaling crosstalk between AMP-activated protein kinase and cAMP-dependent protein kinase. Front Physiol. 2012;3:409. doi: 10.3389/fphys.2012.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ning J, Xi G, Clemmons DR. Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology. 2011;152:3143–3154. doi: 10.1210/en.2011-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulinilkunnil T, He H, Kong D, Asakura K, Peroni OD, Lee A, et al. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286:8798–8809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab. 2012;16:104–112. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, 4th, Schlattner U, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 41.Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- 42.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, et al. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301:E1236–1242. doi: 10.1152/ajpendo.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamble J, Lopaschuk GD. Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism. 1997;46:1270–1274. doi: 10.1016/s0026-0495(97)90229-8. [DOI] [PubMed] [Google Scholar]
- 44.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 45.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–2867. [PubMed] [Google Scholar]
- 46.Clark H, Carling D, Saggerson D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur J Biochem. 2004;271:2215–2224. doi: 10.1111/j.1432-1033.2004.04151.x. [DOI] [PubMed] [Google Scholar]
- 47.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 48.Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- 49.Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 50.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 51.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 52.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 53.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 54.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85:919–927. doi: 10.2527/jas.2006-342. [DOI] [PubMed] [Google Scholar]
- 56.Moschella PC, Rao VU, McDermott PJ, Kuppuswamy D. Regulation of mTOR and S6K1 activation by the nPKC isoforms, PKCepsilon and PKCdelta, in adult cardiac muscle cells. J Mol Cell Cardiol. 2007;43:754–766. doi: 10.1016/j.yjmcc.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012;4:350–358. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 59.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 60.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, et al. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 62.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 63.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 64.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villén J, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma L, Ma S, He H, Yang D, Chen X, Luo Z, et al. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res. 2010;33:446–453. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- 67.Tremblay F, Brûlé S, Hee Um S, Li Y, Masuda K, Roden M, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 69.Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 71.Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, et al. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkes JJ, Nguyen MT, Bandyopadhyay GK, Nelson E, Olefsky JM. Topiramate treatment causes skeletal muscle insulin sensitization and increased Acrp30 secretion in high-fat-fed male Wistar rats. Am J Physiol Endocrinol Metab. 2005;289:E1015–1022. doi: 10.1152/ajpendo.00169.2005. [DOI] [PubMed] [Google Scholar]
- 73.Fediuc S, Gaidhu MP, Ceddia RB. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J Lipid Res. 2006;47:412–420. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Pimenta AS, Gaidhu MP, Habib S, So M, Fediuc S, Mirpourian M, et al. Prolonged exposure to palmitate impairs fatty acid oxidation despite activation of AMP-activated protein kinase in skeletal muscle cells. J Cell Physiol. 2008;217:478–485. doi: 10.1002/jcp.21520. [DOI] [PubMed] [Google Scholar]
- 75.Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL. Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1692–1699. doi: 10.1152/ajpregu.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, et al. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 77.Dey D, Mukherjee M, Basu D, Datta M, Roy SS, Bandyopadhyay A, et al. Inhibition of insulin receptor gene expression and insulin signaling by fatty acid: interplay of PKC isoforms therein. Cell Physiol Biochem. 2005;16:217–228. doi: 10.1159/000089847. [DOI] [PubMed] [Google Scholar]
- 78.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 79.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50:2210–2218. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 80.Bollag GE, Roth RA, Beaudoin J, Mochly-Rosen D, Koshland DE., Jr Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986;83:5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chin JE, Dickens M, Tavare JM, Roth RA. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993;268:6338–6347. [PubMed] [Google Scholar]
- 82.De Fea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997;36:12939–12947. doi: 10.1021/bi971157f. [DOI] [PubMed] [Google Scholar]
- 83.Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes. 2000;49:1353–1358. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- 84.Ravichandran LV, Esposito DL, Chen J, Quon MJ. Protein kinase C-zeta phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J Biol Chem. 2001;276:3543–3549. doi: 10.1074/jbc.M007231200. [DOI] [PubMed] [Google Scholar]
- 85.Takayama S, White MF, Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988;263:3440–3447. [PubMed] [Google Scholar]
- 86.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]