Abstract
Among more than 30 members of the phospholipase A2 (PLA2) superfamily, secreted PLA2 (sPLA2) enzymes represent the largest family, being Ca2+-dependent low-molecular-weight enzymes with a His-Asp catalytic dyad. Individual sPLA2s exhibit unique tissue and cellular distributions and enzymatic properties, suggesting their distinct biological roles. Recent studies using transgenic and knockout mice for nearly a full set of sPLA2 subtypes, in combination with sophisticated lipidomics as well as biochemical and cell biological studies, have revealed distinct contributions of individual sPLA2s to various pathophysiological events, including production of pro- and anti-inflammatory lipid mediators, regulation of membrane remodeling, degradation of foreign phospholipids in microbes or food, or modification of extracellular noncellular lipid components. In this review, we highlight the current understanding of the in vivo functions of sPLA2s and the underlying lipid pathways as revealed by a series of studies over the last decade.
Keywords: arachidonic acid, eicosanoids, fatty acid, immunology, inflammation, lipidomics, lysophospholipid, membranes, obesity, phospholipids/metabolism
More than one third of the phospholipase A2 (PLA2) enzymes belong to the secreted PLA2 (sPLA2) family, which contains 10 catalytically active isoforms (IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XIIA) and one inactive isoform (XIIB) in mammals (1–4). Individual sPLA2s exhibit unique tissue and cellular distributions and substrate selectivity, suggesting their distinct biological roles. Because sPLA2s are secreted and require millimolar Ca2+ for their catalytic action, they principally target phospholipids in the extracellular space. Individual sPLA2s participate in diverse biological events through generation of a variety of lipid mediators, promotion of membrane remodeling, modification of extracellular noncellular lipid components such as surfactant, microparticles and lipoproteins, or degradation of foreign phospholipids in microbes and dietary components in response to given microenvironmental cues. The biological effects of sPLA2s may also be driven or counter-regulated by binding to soluble and membrane-bound M-type sPLA2 receptor (PLA2R1). Therefore, the phenotypes displayed in sPLA2 gene-manipulated mice may not rely merely on the changes in lipid mediator signaling (more particularly eicosanoid signaling), but may also involve one or a combination of the above possibilities. Here, we overview the latest knowledge regarding the pathophysiological roles of individual sPLA2s as revealed by studies using gene-manipulated mice over the past decade, focusing particularly on their target substrates and products in vivo. The classification and biochemical properties of sPLA2s have also been detailed in other elegant reviews (1–6).
GENERAL ASPECTS
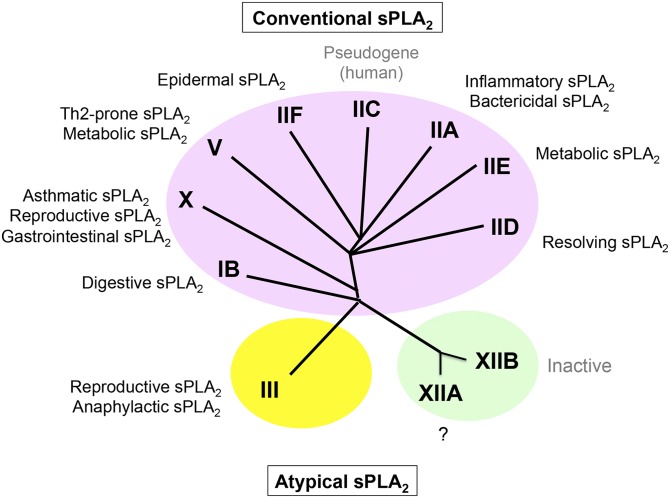
Conventional sPLA2s (group I/II/V/X) are closely related low-molecular-weight enzymes with a highly conserved Ca2+-binding loop and a His/Asp catalytic dyad as well as conserved disulfide bonds, while atypical sPLA2s (group III and XII) are each classified into distinct classes (Fig. 1). Of these, sPLA2-IB and -IIA are two prototypic sPLA2s that were originally identified by classical protein purification from pancreas and inflamed sites, respectively (7–10). Structurally, sPLA2-IB and -IIA are similar to snake venom group I and II sPLA2s, respectively. Among the conventional sPLA2s, sPLA2-IB is evolutionally the oldest sPLA2 isoform in the animal kingdom because three genes encoding IB-like sPLA2s are present in nematode (Caenorhabditis elegans), whereas group II, V, and X sPLA2s exist only in vertebrates (3). sPLA2-V does not possess the key features of group I and II sPLA2s, yet it is often classified into the group II subfamily of sPLA2s because its gene is mapped to the group II sPLA2 cluster locus (11, 12). sPLA2-X has both group I- and group II-like structural features, suggesting that it emerged during the diversification from group I to II sPLA2s (13). sPLA2-III is an atypical sPLA2 that is more similar to bee venom group III sPLA2 than to other mammalian sPLA2s (14). Another atypical sPLA2-XII subfamily, XIIA and XIIB, has very unique structural and functional features (15, 16), and the preservation of sPLA2-XII members from bacteria to human indicates that they emerged early in evolution prior to Eubacteria (17). Currently known sPLA2 inhibitors can inhibit conventional sPLA2s to various degrees, yet an agent that specifically inhibits sPLA2-III or -XIIA has not yet become available. Otoconin-90/95, which has two sPLA2-IB-like domains, can also be classified into the sPLA2 family, yet we do not describe it in detail here because it is a structural protein of the inner ear and is unrelated to phospholipid metabolism (18, 19).
Fig. 1.
A phylogenetic tree of the sPLA2 family and the functions of individual sPLA2s as revealed by studies using gene-manipulated mice. Several examples for the functions of individual sPLA2s, in which underlying lipid metabolisms have been clarified (see text), are illustrated on the phylogenetic tree. The overall functions of sPLA2s reported so far are summarized in Table 1. Although sPLA2-XIIB, which is catalytically inactive, has been implicated in steatohepatitis, the mechanistic action is unknown.
Biochemical analyses using pure sPLA2s have shown that individual sPLA2s have distinct substrate selectivity in terms of the polar head groups or sn-2 fatty acids of phospholipids. For instance, sPLA2-X is very active on phosphatidylcholine (PC), while sPLA2-IIA has much higher affinity for phosphatidylethanolamine (PE) or phosphatidylserine (PS) than for PC, and this substrate selectivity is partly attributable to their crystal structures (20, 21). With regard to sn-2 fatty acid specificity, sPLA2-IB, -IIA, and -III do not discriminate fatty acid species, sPLA2-V tends to prefer those with a lower degree of unsaturation such as oleic acid, and sPLA2-X tends to prefer PUFAs including arachidonic acid (AA) and DHA (22–26). It should be noted that the enzyme activity is influenced by the assay conditions employed, such as the composition of the substrate phospholipids (pure phospholipid vesicles or mixed micelles comprising multiple phospholipid species), the concentrations of the sPLA2s, presence of detergents, pH, and so on. Hence, the enzymatic properties of individual sPLA2s determined in different studies are not entirely identical. The use of excess super-physiological amounts of sPLA2 in in vitro experiments often masks the substrate selectivity. As membranes comprising a single phospholipid molecular species do not exist and detergent is absent under most physiological conditions, a result obtained using artificial phospholipid membranes may not reflect the true enzymatic properties of a given sPLA2. An exception is sPLA2-IB, for which a detergent (bile acid) is important for full enzymatic activity in the intestinal lumen (27). Ideally, the enzymatic activity of each sPLA2 isoform should be evaluated at a physiologically relevant enzyme concentration and with a physiologically relevant membrane on which the enzyme acts intrinsically. Nonetheless, the overall selectivity of sPLA2s for the various phospholipid head groups and for saturated versus unsaturated fatty acids has been well-depicted by several in vitro enzymatic studies, and the in vivo data using lipidomics have revealed an even more selective pattern of hydrolysis. In some aspects, the use of transgenic versus knockout mice is similar to the in vitro versus in vivo studies regarding the sPLA2 selectivity toward the full diversity of phospholipids with various head groups and sn-2 fatty acids.
Some of the biological actions of sPLA2s in vivo have been investigated using sPLA2-overexpressing transgenic mice (28–38). However, the results should be interpreted with caution, as a super-physiological level of sPLA2, even in tissues or cells where the enzyme is not intrinsically expressed, could result in an artificial phenotype. Nevertheless, studies using transgenic mice have yielded informative insights into some of the pathophysiological roles of sPLA2s. If mice transgenic for a certain sPLA2 display a particular phenotype opposite to that in knockout mice lacking the same sPLA2, it can be concluded that this phenotype represents the intrinsic function of this sPLA2 isoform. In cases such as this, transgenic mice are useful when searching for lipid-metabolic processes driven by a particular sPLA2 in vivo, because lipid mobilization in the transgenic mice is typically prominent and easy to chase using a lipidomics approach. Another noteworthy issue is that the overall phenotypes of mice transgenic for different sPLA2s are not entirely identical (28–38). If different sPLA2s have similar enzymatic properties, then the output phenotypes of mice transgenic for them should be similar. However, this is not actually the case. Why do mice transgenic for different sPLA2s display distinct phenotypes? The most likely explanation is that individual sPLA2s have distinct enzymatic properties, acting on different phospholipid substrates and mobilizing different lipid metabolites in vivo. Likewise, while it is undeniable that knockout mice have provided much insight into the pathophysiological role of sPLA2s, there is often the potential problem of compensatory mechanisms (i.e., that one enzyme compensates the absence of a related one by increasing its expression, activity, and/or function). However, accumulating evidence obtained from knockout mice for different sPLA2s suggests that it is also not the case in most situations, likely because each sPLA2 displays unique substrate selectivity and tissue distribution. This point implies that sPLA2s are not “functional” isozymes in vivo.
In order to comprehensively understand the specific biological roles of this enzyme family, it is important to consider as to when and where different sPLA2s are expressed, which isoforms are involved in specific types of pathophysiology, and how the sPLA2s exhibit their unique functions by driving specific types of lipid metabolism. In subsequent sections, we will describe the functions of individual sPLA2s as revealed by studies using knockout and/or transgenic mice along with lipidomics approaches to clarify their in vivo substrates and metabolites. The roles of individual sPLA2s, and the underlying lipid-metabolic pathways in which they are involved, are summarized in Table 1, and several examples are illustrated in Fig. 1 and Fig. 2.
TABLE 1.
sPLA2-driven lipid-metabolic pathways in homeostasis and diseases
| sPLA2s | Distributions | Target Membranes | Products | Mechanistic Insights | Homeostasis and Diseases | References |
| IB | Pancreatic acinar cells | Dietary and biliary PC in the gastrointestinal tract | LPC | Phospholipid digestion in the gastrointestinal lumen | Metabolic disorders, atherosclerosis | 39–43 |
| IIA | Platelets, leukocytes, Paneth cells | Bacterial membranes | Lipid mediator-independent | Killing of Gram-positive bacteria | Host defense | 31, 32, 53–55 |
| Extracellular mitochondria | Eicosanoids | Amplification of inflammation | Arthritis | 63, 64 | ||
| Unknown | Unknown | Regulation of intestinal microflora? | Anti-colon cancer | 57 | ||
| IID | Lymph tissue DCs | DHA-containing PE | DHA and resolvin D1 | Resolution of Th1 immunity | Anti-contact dermatitis | 70 |
| IIE | Hypertrophic adipocytes | PE and PS in lipoproteins | Lipid mediator-independent | Fat deposition in adipose tissue and liver | Obesity | 74 |
| IIF | Keratinocytes | Unknown | Unknown | Unknown | Epidermal barrier | 79 |
| V | Bronchial epithelial cells | Surfactant dipalmitoyl-PC | Lipid mediator-independent | Surfactant degradation | Airway injury | 34 |
| Macrophages, DCs | Unknown | Unknown | M2 macrophage polarization and Th2 immunity | Asthma | 84–86, 92–95 | |
| LPE | Phagocytosis of microorganisms | Host defense | 96, 97 | |||
| LPE? | Phagocytosis of immune complexes | Anti-arthritis | 63 | |||
| Hematopoietic cells | Unknown | Unknown | Atherosclerotic plaque formation | Atherosclerosis | 99 | |
| Cardiomyocytes | Unknown | Eicosanoids (indirect?) | Apoptosis of injured myocardial cells | Myocardial infarction | 101 | |
| Aorta | Unknown | Unknown | Aortic inflammation | Aneurysm | 102 | |
| Hypertrophic adipocytes | PC in LDL | Oleic and linoleic acids | Reduced adipose tissue inflammation by unsaturated fatty acids | Anti-obesity | 74 | |
| X | Airway epithelium | Infiltrating eosinophils, airway epithelial cells | Eicosanoids (indirect?) | Airway inflammation | Asthma, influenza infection | 94, 112, 113, 116 |
| Macrophages? | Unknown | PUFAs? | Enhanced lipid accumulation and TLR4 signaling in macrophages | Macrophage function | 127 | |
| Adrenal glands | Unknown | PUFAs? | Reduced corticosteroid synthesis by downregulating adrenal steroidogenic acute regulatory protein | Hypercorticosteronemia | 122 | |
| Dorsal root ganglia | Unknown | LPC? | Neuritogenesis and pain transmission | Pain | 121 | |
| Hematopoietic cells | Unknown | Unknown | Reduced Th1 immunity and atherosclerotic plaque formation | Anti-atherosclerosis | 124 | |
| Neutrophils | Unknown | Eicosanoids (indirect?) | Tissue damage by neutrophils | Aneurysm, myocardial infarction | 118–120 | |
| Pancreatic β cells | Unknown | PGE2 | Suppression of insulin secretion | Diabetes | 123 | |
| Gut epithelium | Dietary and biliary PC in the gastrointestinal tract | LPC | Phospholipid digestion in the gastrointestinal lumen | Adiposity | 121 | |
| Adipocytes? | Unknown | PUFAs? | Repression of adipogenesis by inhibiting liver X receptor activation | Anti-obesity | 128 | |
| Sperm acrosome | PC in sperm membrane | LPC | Boosting acrosome reaction | Male fertility | 132 | |
| Hair follicles | Unknown | Unknown | Hair homeostasis | Alopecia | 36 | |
| III | Mast cells | Adjacent fibroblasts | PGD2 | Promotion of mast cell maturation | Anaphylaxis | 134 |
| Epididymal epithelium | PC in sperm membrane | Lipid mediator-independent | Sperm maturation by membrane phospholipid remodeling | Male fertility | 135 | |
| XIIB | Hepatocytes | Unknown | Unknown | Hepatic secretion of VLDL | Steatohepatitis | 145 |
Distributions, target membranes and products, underlying mechanisms, and related diseases for sPLA2s are summarized. Their key substrates and products have been identified in several, but not in many other, cases.
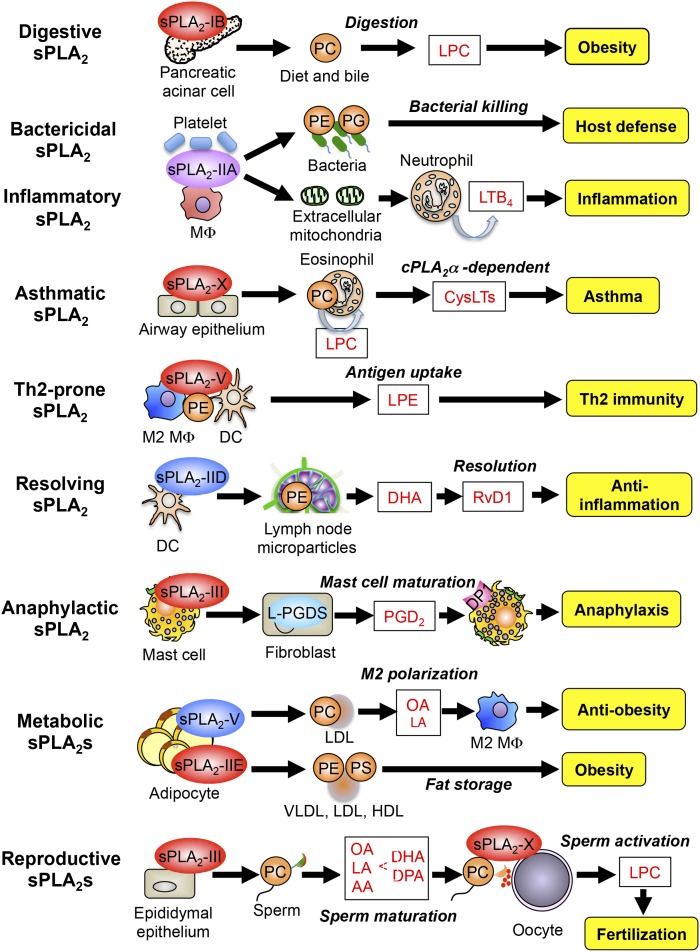
Fig. 2.
Examples of the sPLA2-driven lipid pathways. Individual sPLA2s are involved in distinct biological processes or diseases through driving unique lipid pathways that involve or do not depend on lipid mediators. In all cases, sPLA2s act on extracellular phospholipids (e.g., adjacent cells, lipoproteins, microparticles, diet, and bacteria membranes) after secretion. For details, please see the text. RvD1, resolvin D1; OA, oleic acid; LA, linoleic acid.
CONVENTIONAL sPLA2s
PLA2G1B/sPLA2-IB
sPLA2-IB is abundantly expressed in the pancreas, and to a much lesser extent in the lung and kidney. After secretion from pancreatic acinar cells into the duodenal lumen, an N-terminal heptapeptide of the inactive zymogen is cleaved by trypsin to yield an active enzyme (7, 8). Gene disruption of sPLA2-IB (Pla2g1b−/−) results in decreased digestion of dietary and biliary phospholipids in the gastrointestinal tract (39). Accordingly, the reduced gastrointestinal production and absorption of lysophosphatidylcholine (LPC), a causal factor for insulin resistance, confers protection against diet-induced obesity, glucose intolerance, hyperlipidemia, and atherosclerosis in Pla2g1b−/− mice (40–43). On the other hand, pancreatic acinar cell-specific Pla2g1b-transgenic mice develop more severe obesity and insulin resistance (28). Oral supplementation with methyl-indoxam, a pan-sPLA2 inhibitor, prevents diet-induced obesity and diabetes in mice, most probably through inhibition of sPLA2-IB (44). Moreover, the PLA2G1B gene maps to a locus for obesity susceptibility in humans (45). Thus, pharmacological inhibition of sPLA2-IB, a “digestive sPLA2,” could be an effective oral therapeutic option for treatment of metabolic diseases.
PLA2G2A/sPLA2-IIA
sPLA2-IIA is the only isoform detectable in the circulation, particularly under pathological conditions. Because sPLA2-IIA expression is induced by pro-inflammatory stimuli in various cells and because its levels in sera or inflammatory exudates are correlated with the severity of inflammation (9, 10, 46), it is often referred to as an “inflammatory sPLA2.” However, the precise role of sPLA2-IIA in inflammation has remained unknown until recently, because a frame-shift mutation in the Pla2g2a gene in C57BL/6 and 129Sv mice has prevented adequate evaluation of its functions by gene targeting (47, 48). Up to now, therefore, the in vivo functions of sPLA2-IIA have been addressed mainly using transgenic mice.
The most probable physiological role of sPLA2-IIA is degradation of bacterial membranes, thereby providing a first line of antimicrobial defense (49). Indeed, sPLA2-IIA kills bacteria (Gram-positive in particular) at physiological concentrations (50). Bacterial membranes are rich in PE and phosphatidylglycerol (PG), whereas PC is a major phospholipid in the outer leaflet of the plasma membrane of mammalian cells. sPLA2-IIA has a much higher affinity for PE and PG than PC, thus accounting for the preferential action of this enzyme on bacterial cells rather than on mammalian cells. In addition to this substrate specificity, the highly cationic nature of sPLA2-IIA, which is not shared with other sPLA2s, is essential for bacterial membrane hydrolysis by this enzyme (51, 52). As such, PLA2G2A-transgenic mice, or wild-type mice treated with recombinant sPLA2-IIA, are resistant to pneumonia and sepsis following bacterial infection (31, 32, 53–55). For this reason, sPLA2-IIA can be regarded as a “bactericidal sPLA2.” Some bacteria such as Pseudomonas aeruginosa and Bacillus anthracis have developed a resistance mechanism against sPLA2-IIA by inhibiting its induction in macrophages (55, 56).
Mouse strains with natural disruption of the Pla2g2a gene (see above) are more sensitive to intestinal tumorigenesis (48). Transgenic transfer of the Pla2g2a gene into these mice reduces the incidence of intestinal polyposis (57), indicating that sPLA2-IIA acts as a tumor suppressor in the gastrointestinal tract. Consistently, there is an inverse relationship between PLA2G2A expression and gastric cancer in humans (58), and polymorphisms in the PLA2G2A gene are associated with fundic gland polyposis in patients with familial adenomatous polyposis (59). Given its function as a “bactericidal sPLA2,” sPLA2-IIA secreted from intestinal Paneth cells might control the gastrointestinal microflora, thereby preventing tumor development. In contrast, sPLA2-IIA expression shows a positive correlation with several types of cancer, including prostate cancer (60–62), suggesting distinct impacts of sPLA2-IIA on different types of cancer.
In a recent study, the mutated Pla2g2a allele in the C57BL/6 strain was backcrossed onto the BALB/c strain to produce Pla2g2a−/− BALB/c mice. These Pla2g2a−/− mice are protected from autoantibody-induced arthritis, while PLA2G2A-transgenic mice display more severe symptoms in the same model (63), thus providing compelling evidence for the bona fide pro-inflammatory role of sPLA2-IIA. Mechanistically, sPLA2-IIA targets phospholipids in microparticles, particularly in extracellular mitochondria, thereby amplifying inflammation (64). Mitochondria, which were originated from bacteria during evolution, are released from activated platelets or leukocytes to accumulate at inflamed sites (65). Hydrolysis of the mitochondrial membrane by sPLA2-IIA yields inflammatory mediators including eicosanoids and lysophospholipids as well as mitochondrial DNA as a danger-associated molecular pattern, which promotes leukocyte activation. Moreover, sPLA2-IIA-targeted extracellular mitochondria interact with neutrophils, triggering adhesion of these cells to the vascular wall. This breakthrough finding explains a long-sought mechanism for the function of sPLA2-IIA as an “inflammatory sPLA2.” Thus, sPLA2-IIA is primarily involved in host defense by both killing bacteria and alarming the innate immunity response, and over-amplification of the response can lead to excessive inflammation. In the latter case, sPLA2-IIA can be viewed as a “double-edged sword.”
Transgenic overexpression of sPLA2-IIA results in skin abnormalities manifested by hair loss and epidermal hyperplasia (29), and by increased carcinogen-induced skin cancer (33). Importantly, sPLA2-IIA has long been implicated in atherosclerosis as a potential causal factor or as a biomarker in many studies, which are summarized in previous reviews (1–6). For instance, in line with clinical evidence that PLA2G2A gene polymorphisms are associated with atherosclerosis (66) and that serum sPLA2-IIA levels show a positive correlation with cardiovascular diseases (67), PLA2G2A-transgenic mice develop more advanced atherosclerotic lesions (30, 68). However, conclusive evidence for the offensive roles of sPLA2-IIA in skin and atherosclerosis will await future studies using Pla2g2a−/− mice on the proper genetic background.
PLA2G2D/sPLA2-IID
sPLA2-IID shows the closest structural relationship to sPLA2-IIA (69). This isoform is expressed preferentially in dendritic cells (DCs) in secondary lymphoid organs such as the spleen and lymph nodes of mice and humans (70), suggesting its regulatory role in adaptive immunity. In a model of Th1-dependent contact hypersensitivity, resolution of inflammation is compromised in the skin and lymph nodes of Pla2g2d−/− mice (70). sPLA2-IID in regional lymph nodes mobilizes a pool of ω3 PUFAs that are metabolized to pro-resolving lipid mediators such as DHA-derived resolvin D1, which suppresses Th1 cytokine production and DC activation. sPLA2-IID preferentially hydrolyzes DHA-containing PE in lymph node membranes (possibly in microparticles). Consistent with its anti-inflammatory role, sPLA2-IID expression in DCs is downregulated after cell activation. Furthermore, administration of sPLA2-IID-Fc protein attenuates autoimmune diseases in mice (71). Thus, sPLA2-IID is a “resolving sPLA2” that ameliorates inflammation by mobilizing DHA-derived pro-resolving lipid mediators. In humans, a PLA2G2D polymorphism is associated with body weight loss in chronic obstructive pulmonary disease (72).
PLA2G2E/sPLA2-IIE
Like sPLA2-IID, sPLA2-IIE is structurally most homologous to sPLA2-IIA (73). Expression of sPLA2-IIE is markedly induced in adipocytes after high-fat feeding in vivo and during adipogenesis in vitro. Pla2g2e−/− mice are modestly protected from diet-induced obesity, fatty liver, and hyperlipidemia (74). Mechanistically, sPLA2-IIE hydrolyzes minor lipoprotein phospholipids, PE, and PS, with no apparent fatty acid selectivity. As such, sPLA2-IIE alters lipid composition in lipoproteins, thereby affecting fat accumulation in adipose tissue and liver. Thus, sPLA2-IIE is a “metabolic sPLA2” that regulates systemic metabolic states by modifying lipoprotein phospholipids. However, expression of sPLA2-IIE in human adipose tissue is very low, revealing a species difference. In humans, a polymorphism in the PLA2G2E gene is associated with ulcerative colitis (75).
PLA2G2C/sPLA2-IIC and PLA2G2F/sPLA2-IIF
sPLA2-IIC and -IIF have structural characteristics of group II sPLA2s, but possess an extra sequence in the middle and C-terminal regions, respectively (73, 76). A cell biological study using Pla2g2c knockdown has shown that sPLA2-IIC is upregulated in hepatitis B-infected mouse hepatocytes to produce lysophosphatidylethanolamine (LPE), which is then presented to CD1d on natural killer T cells, leading to propagation of an anti-virus immune response (77). sPLA2-IIC is also expressed in meiotic cells in rodent testis (78). However, as sPLA2-IIC is a pseudogene in humans (12), analysis of Pla2g2c−/− mice has not been performed.
sPLA2-IIF is abundantly expressed in the suprabasal epidermis (79, 80). Gene disruption of sPLA2-IIF (Pla2g2f−/−) has been reported to impair the acidification of the stratum corneum and delay recovery of the skin barrier after tape-stripping (79), although a mechanistic insight is currently obscure and it should be confirmed or expanded in other ongoing studies.
PLA2G5/sPLA2-V
Because sPLA2-V is able to hydrolyze PC more efficiently than is sPLA2-IIA (81), most investigators in this research field have focused on the potential role of this enzyme in inflammation in the context of AA metabolism. It should be noted, however, that sPLA2-V releases fatty acids with a low degree of unsaturation, such as palmitic, oleic, and linoleic acids, in preference to AA from cellular membranes, lipoproteins, and even pure phospholipid vesicles (22, 23, 25, 26). Therefore, the possibility that sPLA2-V mobilizes lipid metabolites other than AA-derived eicosanoids should be taken into consideration to explain the biological actions of this enzyme.
Zymosan-induced peritonitis or lipopolysaccharide (LPS)-induced air pouch inflammation is partially ameliorated in mice lacking sPLA2-V (Pla2g5−/−) (82, 83). sPLA2-V is expressed in bronchial epithelial cells and alveolar macrophages, and Pla2g5−/− mice are protected from airway disorders such as antigen-induced asthma and LPS- or ventilator-induced alveolar injury (84–86). These studies lend support to the offensive roles of sPLA2-V, yet the underlying mechanisms remain uncertain. Although these phenotypes in Pla2g5−/− mice are often accompanied by reduced levels of eicosanoids, it is unclear whether sPLA2-V indeed drives AA metabolism by itself in vivo because of its fatty acid selectivity as noted above. Considering that the inflammatory responses are often accompanied by activation of cytosolic PLA2α (cPLA2α), a major AA-releasing PLA2 (87), the observed alterations in eicosanoid levels in Pla2g5−/− mice might merely reflect the disease-associated changes in cPLA2α activation, rather than hydrolytic liberation of AA by sPLA2-V. In relation to this, there is evidence suggesting that sPLA2-V regulates cPLA2α phosphorylation (88, 89). Moreover, transgenic overexpression of sPLA2-V leads to respiratory distress and neonatal death with no or only a modest increase in pulmonary eicosanoid levels (34). This transgenic phenotype is attributable to aberrant hydrolysis of surfactant phospholipids (dipalmitoyl-PC) and is apparently eicosanoid-independent.
Although sPLA2-V was previously thought to be upregulated by pro-inflammatory stimuli (as in the case of sPLA2-IIA) (90, 91), it has recently become obvious that its expression is induced by the Th2 cytokines, IL-4 and IL-13, much more potently than by pro-inflammatory stimuli including LPS, zymosan, and Th1 cytokines (74, 92, 93). Indeed, sPLA2-V is expressed in IL-4-driven M2 macrophages and Th2 cells, which facilitate Th2-type immunity while attenuating Th1- or Th17-type immunity. Notably, Th2 responses such as IL-4 expression and IgE production are reduced in Pla2g5−/− mice (74, 92, 94), which accounts for the reduced allergic response in the absence of sPLA2-V (84, 94, 95). In this regard, sPLA2-V can be referred to as a “Th2-prone sPLA2.”
Thus, researchers should consider a bi-faceted action for sPLA2-V, which could play both pro- and anti-inflammatory (“Th2-prone”) roles depending on conditions, cell types, and species. In the process of Th2-dependent asthma, sPLA2-V appears to function in antigen-presenting cells to regulate antigen processing and thereby the Th2 response, as well as in airway epithelial cells to promote airway injury that may involve surfactant degradation (34, 92, 94, 95). In contrast, Pla2g5−/− mice are more susceptible to Candida albicans or Escherichia coli infection (Th1 immunity) and arthritis (Th17 immunity) accompanied by reduced clearance of harmful materials (microorganisms and immune complex, respectively) by macrophages (63, 96, 97). As M2 macrophages have greater phagocytic activity, the reduced phagocytosis in Pla2g5−/− macrophages could also be partly explained by the ability of sPLA2-V to promote M2 macrophage polarization in Th2 immunity and therefore to counteract Th1/Th17 immunity. Alternatively, sPLA2-V may produce a certain lipid metabolite that directly regulates macrophage phagocytosis. In fact, it has recently been reported that IL-4-induced sPLA2-V promotes phagocytosis in human macrophages through production of LPE, which fully restores defective phagocytosis of zymosan and bacteria in sPLA2-V-knockdown cells (93).
Because hydrolysis of phospholipids in LDL by sPLA2-V is capable of promoting foam cell formation by macrophages in vitro (98), sPLA2-V (and several other sPLA2s) has currently been implicated in the development of atherosclerosis and related cardiovascular disorders. However, the roles of sPLA2-V in cardiovascular diseases, particularly in the context of lipoprotein metabolism, are controversial. Ldlr−/− mice given transplants of Pla2g5−/− bone marrow cells are mildly protected from atherosclerosis (99); yet neither the plaque formation nor plasma LDL levels are affected by global Pla2g5 deficiency on the Apoe−/− background (100). Pla2g5 ablation attenuates myocardial infarction (101), while it worsens angiotensin II-induced cardiac fibrosis (102). Moreover, it has been reported that varespladib, a sPLA2 inhibitor that broadly inhibits conventional sPLA2s, failed to show efficacy in a phase III clinical trial for cardiovascular diseases (103). Thus, it appears that sPLA2-V is not a major contributor to atherosclerosis and associated diseases, even though it may promote these diseases in certain situations. Rather, it has recently been clarified that LDL phospholipid hydrolysis by sPLA2-V is associated with obesity-related metabolic syndrome.
In obesity, sPLA2-V is induced in hypertrophic adipocytes (74). When fed a high-fat diet, Pla2g5−/− mice display hyperlipidemia with higher plasma levels of lipid-rich LDL and increased obesity, fatty liver, and insulin resistance. sPLA2-V plays a protective role in metabolic disorders by hydrolyzing and thereby normalizing PC in LDL and by tipping the immune balance toward an Th2/M2 state that counteracts adipose tissue inflammation. Mechanistically, sPLA2-V-driven oleic and linoleic acids from PC in LDL dampen M1 macrophage polarization by saturated fatty acids (e.g., palmitic acid), probably through attenuation of endoplasmic reticulum stress. Together, these studies have underscored the physiological relevance of lipoprotein hydrolysis by sPLA2s, highlighted two adipocyte-driven “metabolic sPLA2s” (sPLA2-IIE and -V) as integrated regulators of immune and metabolic responses, and brought about a paradigm shift toward a better understanding of the roles of the sPLA2 family as metabolic coordinators (74).
In humans, PLA2G5 gene polymorphisms are correlated with LDL levels in subjects with type 2 diabetes (104). In vitro sPLA2-V susceptibility of LDL from patients with type 2 diabetes is greater than that of LDL from healthy controls (105). Moreover, PLA2G5 expression in human visceral adipose tissue inversely correlates with plasma LDL levels (74). These results imply a human relevance for the metabolic role of sPLA2-V. Additionally, biallelic mutations in the PLA2G5 gene cause benign fleck retina (106). Loss of LPC acyltransferase 1 (LPCAT1) also causes retinal degeneration (107), suggesting a potential link between sPLA2-V and LPCAT1 in PC metabolism for retina homeostasis.
PLA2G10/sPLA2-X
As in the case of sPLA2-IB, sPLA2-X is synthesized as a zymogen, and removal of an N-terminal propeptide produces an active mature enzyme (13). This processing occurs either before secretion intracellularly by furin-like convertase or after secretion extracellularly (108, 109). Among the sPLA2s, sPLA2-X has the highest affinity for PC and thus exhibits the most potent ability to hydrolyze plasma membrane phospholipids in intact cells (110, 111). Because of this property, many investigators have speculated that sPLA2-X plays a pro-inflammatory role, although conflicting evidence also exists (see below).
Mice lacking sPLA2-X (Pla2g10−/−) are refractory to antigen-induced asthma, with marked reductions in infiltration of eosinophils, hyperplasia of goblet cells, thickening of the smooth muscle layer, and levels of Th2 cytokines and eicosanoids (112). The attenuated asthmatic responses in Pla2g10−/− mice are restored by knock-in of human sPLA2-X, and treatment of the knock-in mice with an inhibitor specific for human sPLA2-X suppresses airway inflammation (113). Mechanistically, sPLA2-X secreted from the airway epithelium may act on infiltrating eosinophils to augment leukotriene production in a process involving LPC-dependent activation of cPLA2α (114). In addition, sPLA2-X expression is increased during in vitro epithelial differentiation and directly participates in AA release by epithelial cells (115). Pla2g10−/− mice are also partially protected from the early phase of lung inflammation in a model of pandemic influenza infection (116), further underlining the pro-inflammatory role of this enzyme in the airway. Moreover, sPLA2-X is one of the major sPLA2 isoforms detected in the airway of patients with asthma (117), thus directing attention to sPLA2-X, an “asthmatic sPLA2,” as a novel therapeutic target for asthma. Unlike sPLA2-V, however, sPLA2-X does not influence the Th2 response itself, because antigen-sensitized Pla2g10−/− and wild-type mice have similar IgE and IL-4 levels (94).
Pla2g10−/− mice are also protected from myocardial infarction or aneurysm (118–120), show a reduced inflammatory pain (121), have an increased adrenal steroidogenesis (122), and exhibit alteration in insulin secretion by pancreatic β cells, perhaps as a result of reduced prostaglandin E2 (PGE2) synthesis (123). However, several of the phenotypes reported for Pla2g10−/− mice are controversial. Although sPLA2-X (like sPLA2-V) has been implicated in atherosclerosis, different groups have reported opposite (exacerbated or attenuated) atherosclerotic phenotypes in Pla2g10−/− mice (119, 124). In humans, polymorphisms in the PLA2G10 gene are linked to a decreased risk of recurrent cardiovascular events (125), or not associated with plasma sPLA2 activity or with coronary heart disease risk (126). Additionally, in different studies, Pla2g10−/− mice display altered or unaltered macrophage functions (127) or increased or decreased adiposity (121, 128). Although some of these studies were performed under the assumption that sPLA2-X is expressed in macrophages or adipocytes, our own investigations have shown that its expression in these cells is low or almost undetectable. Rather, sPLA2-X might be expressed in a limited subset of these cells or supplied from proximal or even distal cells in a paracrine manner. As sPLA2-X is abundantly expressed in the gut epithelium (a “gastrointestinal sPLA2”), it is likely that the decreased digestion and absorption of dietary and biliary phospholipids are eventually linked to the reduced adiposity in Pla2g10−/− mice (121), a situation similar to that in Pla2g1b−/− mice (see above). Alternatively, the intestinal expression of sPLA2-X might alter the microbiota, which could secondarily influence both immune and metabolic balances (129–131). This might account for some of the discrepancies observed in Pla2g10−/− mice maintained in different facilities. Another feature of note is that sPLA2-X is able to release ω3 PUFAs, such as DHA, in addition to ω6 AA (26, 37). Hence, when assessing the biological roles of sPLA2-X, researchers should consider the balance between ω6 and ω3 PUFA metabolism, rather than focusing only on AA metabolism.
In addition to the gastrointestinal tract, sPLA2-X is abundantly expressed in the testis, where it is stored in acrosomes in the head of sperm cells (132). sPLA2-X is released from activated sperm cells during the acrosome reaction. Pla2g10−/− spermatozoa display a poorer acrosome reaction and lower fertility, despite showing normal maturation and motility (121, 132). Thus, sPLA2-X, a “reproductive sPLA2,” plays a specific role in sperm activation, boosting the acrosome reaction probably through production of some lipid products from sperm membranes in a paracrine or autocrine manner. LPC is a candidate product responsible for the action of sPLA2-X, because it can partially restore the defective fertilization of wild-type sperm treated with anti-sPLA2-X antibody (132).
Lastly, a striking skin phenotype characterized by alopecia in Pla2g10-transgenic mice points to a unique role of sPLA2-X in hair homeostasis (36). Although the coat hairs of Pla2g10−/− mice appear grossly normal, they have ultrastructural abnormalities including a hypoplasic outer root sheath and reduced melanin granules in their hair follicles. However, considering that the expression of endogenous sPLA2-X in mouse skin is very low, it is possible that the transgenic overexpression of sPLA2-X might have mimicked the intrinsic action of a specific skin-resident sPLA2 (e.g., sPLA2-IIF).
ATYPICAL sPLA2s
PLA2G3/sPLA2-III
sPLA2-III, an atypical sPLA2, has a central sPLA2 domain with a typical group III feature that is flanked by unique N- and C-terminal domains (14). The N- and C-terminal domains are removed to give rise to a mature sPLA2 domain-only form (133). Transgenic overexpression of sPLA2-III in Apoe−/− mice results in increased atherosclerosis due to accelerated LDL hydrolysis and increased thromboxane A2 synthesis (37). These mice also develop systemic inflammation as they age due to increased eicosanoid formation (38). Thus, beyond the overexpression strategy, sPLA2-III has pro-inflammatory potential.
Microenvironmental alterations in mast cell phenotypes affect susceptibility to allergy, yet the mechanisms underlying the proper maturation of mast cells toward an allergy-sensitive phenotype have been poorly understood. sPLA2-III is released from mast cell granules, and mast cell-associated anaphylactic responses are markedly attenuated in Pla2g3−/− mice and conversely augmented in Pla2g3-transgenic mice (134). Tissue mast cells in Pla2g3−/− mice are immature, and therefore resistant to IgE-dependent and even IgE-independent activation. Similar mast cell abnormalities are also seen in mice lacking lipocalin-type prostaglandin D2 (PGD2) synthase (L-PGDS) or those lacking the PGD2 receptor DP1, suggesting their functional relationship. Indeed, genetic or pharmacological inhibition of DP1 in mast cells or L-PGDS in fibroblasts phenocopies that of sPLA2-III in mast cells in terms of defective mast cell maturation and anaphylaxis. Mechanistically, sPLA2-III secreted from immature mast cells is coupled with fibroblastic L-PGDS to provide PGD2, which in turn promotes mast cell maturation via DP1. It has long been believed that mast cell maturation requires some unknown factor(s) derived from microenvironmental fibroblasts. The PGD2 driven by the sPLA2-III/L-PGDS/DP1 loop provides a missing microenvironmental cue that underlies the proper maturation of mast cells (134). This paracrine loop also appears to be operative for maturation of human mast cells.
sPLA2-III is highly expressed in the epididymal epithelium, where it acts on immature sperm cells passing through the duct in a paracrine manner to regulate phospholipid remodeling (135). During epididymal transit of spermatozoa, PC in the sperm membrane undergoes a dramatic shift in its acyl groups from oleic acid and AA to docosapentaenoic acid (DPA) and DHA, and the increased proportion of DPA/DHA consequently contributes to increased sperm membrane fluidity and thereby flagellar motility. This sperm membrane remodeling is severely compromised in Pla2g3−/− mice, whose spermatozoa, with a low proportion of DPA/DHA, have aberrant acrosomes and flagella with an abnormal axoneme configuration and display reduced motility and fertility (135). Thus, the two “reproductive sPLA2s” (sPLA2-III and -X), which are expressed in different locations within male genital organs, exert nonredundant but interrelated functions in two major steps of male fertility; the former during sperm maturation in the epididymis and the latter during capacitation and acrosome reaction, likely after ejaculation in the female genital duct.
In humans, sPLA2-III is a candidate biomarker for colon cancer (136), and a PLA2G3 haplotype is correlated with a higher risk of colon cancer (137). PLA2G3 polymorphisms are associated with acquired immune deficiency syndrome (138). sPLA2-III is induced in a human neuronal model of oxidative stress, and PLA2G3 polymorphisms are associated with Alzheimer’s disease (139). Lastly, a functional genomic screen for modulators of ciliogenesis has identified sPLA2-III as a negative ciliogenesis regulator probably through regulation of the endocytic recycling pathway (140).
PLA2G12/sPLA2-XII subfamily
The atypical group XII subfamily contains two isoforms, sPLA2-XIIA and -XIIB. Although sPLA2-XIIA is highly expressed in various tissues, its physiological functions are largely obscure because studies using Pla2g12a−/− mice have not yet been conducted. Reportedly, sPLA2-XIIA kills Gram-negative bacteria such as Helicobacter pylori even more efficiently than sPLA2-IIA in vitro (50, 141). Ectopic overexpression of sPLA2-XIIA in Xenopus laevis embryos leads to neurogenesis toward olfactory sensory structures (142). sPLA2-XIIA is present in axon terminals and dendrites in rat brain, and injection of its antisense oligonucleotide into the prefrontal cortex results in deficits of working memory and attention (143). In humans, there is a suggestive association between a PLA2G12A polymorphism and response to anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration (144).
sPLA2-XIIB, preferentially expressed in the liver, is catalytically inactive due to the replacement of the catalytic histidine by a leucine residue (16). Hepatic expression of sPLA2-XIIB is induced by the transcription factor HNF-4α and its coactivator PGC-1α, and Pla2g12b−/− mice display steatohepatitis due to impaired hepatic secretion of VLDL (145). However, the molecular mechanism underlying the action of this catalytically inactive sPLA2 remains fully unknown.
PLA2R1/sPLA2 RECEPTOR
PLA2R1, also known as Clec13c belonging to the C-type lectin family, binds to several conventional sPLA2s with distinct affinities (146). PLA2R1 exists as an integral membrane protein with a very large extracellular region comprising 10 distinct domains and only a short cytoplasmic domain, or as a soluble protein produced by alternative splicing or shedding from the membrane-bound receptor (147–149). PLA2R1 may act in three modes: i) as a clearance receptor that inactivates sPLA2s; ii) as a signaling receptor that transduces sPLA2-dependent signals in a catalytic activity-independent fashion; or iii) as a pleiotropic receptor that binds to nonsPLA2 ligands.
Pla2r1−/− mice show lower inflammation after LPS challenge through some unknown mechanism (150). In a model of allergen-induced asthma, the lungs of Pla2r1−/− mice show greater infiltration of immune cells and higher levels of eicosanoids and Th2 cytokines, accompanied by greater levels of sPLA2-IB and -X proteins, than those of wild-type mice (151), providing the first in vivo evidence that PLA2R1 serves as a clearance receptor for these sPLA2s. In a model of myocardial infarction, Pla2r1−/− mice exhibit higher rates of cardiac rupture, with impaired collagen-dependent migration, growth, and activation of myofibroblasts (152). Mechanistically, binding of sPLA2-IB to PLA2R1 augments the migration and growth of myofibroblasts, and thereby wound healing, through functional interaction with integrin, supporting the signaling role of PLA2R1. However, as the cardiac expression of sPLA2-IB is very low, other sPLA2(s) or unknown component(s) might act as a PLA2R1 ligand in this situation. Alternatively, considering that ablation of sPLA2-V or -X ameliorates myocardial infarction (101, 118), the lower clearance of these sPLA2s might explain the observed phenotypes in Pla2r1−/− mice. PLA2R1 may also function as a tumor suppressor by inducing cellular senescence (153–155). In line with this, Pla2r1−/− mice have increased susceptibility to skin tumorienesis due to escape from senescence (155). Although the anti-tumor function of PLA2R1 may be sPLA2-independent, it is also possible that the protective effect of PLA2R1 against skin cancer is due to the clearance of a skin-resident sPLA2.
Recently, PLA2R1 has been identified as a major autoantigen in membranous nephropathy, a severe autoimmune disease leading to podocyte injury and high levels of proteinuria (156, 157), suggesting that PLA2R1 is a key protein expressed in human renal podocytes. However, it is not clear whether the role of PLA2R1 in podocytes is sPLA2-dependent or -independent, or whether sPLA2s may play some roles in the microenvironment of the glomerulus by being supplied from the circulation or from neighboring cells such as mesangial cells, which are known to secrete sPLA2-IIA under inflammatory conditions (158).
Several features of PLA2R1 pose questions regarding the signaling role of this protein. Although various sPLA2s bind to mouse PLA2R1 with high to moderate affinity, this ligand specificity is not conserved in other species, including humans (146). Furthermore, unlike most signaling receptors that have a long cytoplasmic region with one or more signaling motifs, PLA2R1 possesses only a short stretch in the cytoplasmic tail without any known signaling module except for an endocytosis motif (159). With this structural property, it is difficult to envisage that PLA2R1 itself would act as a signaling receptor. Hence, the presence of a second, as yet unknown, signaling subunit that could form a functional complex with PLA2R1 should be taken into consideration. It is interesting to note that several C-type lectins can act cooperatively with other signaling receptors (160, 161). For instance, mannose-binding lectin enhances TLR2/TLR6 signaling (162), dectin-1, which recognizes a fungal component, can collaborate with TLR2 (163), and dectin-2, which does not possess an intracellular signaling motif, can transmit signals by interacting with ITAM motif-bearing receptors such as FcRγ and DAP12 (164). By analogy, PLA2R1, as a member of the C-type lectins, might be functionally coupled with other signaling receptors leading to cellular responses.
CONCLUDING REMARKS
Studies during the last decade have revealed the pathophysiological functions of various sPLA2s, as exemplified by sPLA2-IB, IIA, IID, IIE, V, X, and III acting as “digestive,” “inflammatory or bactericidal,” “resolving,” “metabolic,” “reproductive or anaphylactic,” “Th2-prone or metabolic,” and “asthmatic, reproductive, or gastrointestinal” sPLA2s, respectively (Figs. 1 and 2) (64, 70, 74, 112, 121, 132, 134, 135). It is now obvious that individual sPLA2s play unique and tissue-specific roles by acting on extracellular phospholipids, which include adjacent cell membranes, noncellular lipid components, and foreign phospholipids, such as those in microbes and food. The diversity of target phospholipids and products may explain why the sPLA2 family contains multiple isoforms. However, as most of our knowledge on sPLA2 functions has been obtained from mouse studies, it is important to translate these studies to humans with caution. Indeed, not all of these studies might be translated into humans (as exemplified by sPLA2-IIA in humans versus sPLA2-IIE in mice (74)), and evidence also exists that knockout mice for the same enzyme on different backgrounds behave differently (165). Nonetheless, several functions of sPLA2s in mice, as depicted in Fig. 2, appear to be conserved in humans (55, 70, 74, 93, 117, 134). Further advances in this research field and their integration for therapeutic applications are expected to benefit from improved lipidomics that will allow monitoring of individual sPLA2s and associated lipid metabolisms within specific tissue niches. Hopefully, the next decade will yield a comprehensive map of the sPLA2-driven lipid networks, thus allowing the therapeutic application of inhibitors for some sPLA2s central to human diseases.
Acknowledgments
In the interest of brevity, the authors have referenced other reviews whenever possible and apologize to the authors of the numerous original papers that were not explicitly cited.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- DC
- dendritic cell
- DPA
- docosapentaenoic acid
- LPC
- lysophosphatidylcholine
- LPCAT1
- lysophosphatidylcholine acyltransferase 1
- LPE
- lysophosphatidylethanolamine
- L-PGDS
- lipocalin-type prostaglandin D2 synthase
- LPS
- lipopolysaccharide
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PGD2
- prostaglandin D2
- PGE2
- prostaglandin E2
- PS
- phosphatidylserine
- PLA2
- phospholipase A2
- cPLA2α
- cytosolic phospholipase A2α
- sPLA2
- secreted phospholipase A2
- PLA2R1
- M-type secreted phospholipase A2 receptor
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and CREST from the Japan Science and Technology Agency. The authors have no conflicting financial interests.
REFERENCES
- 1.Lambeau G., Gelb M. H. 2008. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 77: 495–520. [DOI] [PubMed] [Google Scholar]
- 2.Dennis E. A., Cao J., Hsu Y. H., Magrioti V., Kokotos G. 2011. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111: 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. 2011. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 50: 152–192. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M., Taketomi Y., Girard C., Yamamoto K., Lambeau G. 2010. Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie. 92: 561–582. [DOI] [PubMed] [Google Scholar]
- 5.Murakami M., Taketomi Y., Sato H., Yamamoto K. 2011. Secreted phospholipase A2 revisited. J. Biochem. 150: 233–255. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M., Taketomi Y., Miki Y., Sato H., Yamamoto K., Lambeau G. 2014. Emerging roles of secreted phospholipase A2 enzymes: the 3rd edition. Biochimie. 107: 105–113. [DOI] [PubMed] [Google Scholar]
- 7.Verheij H. M., Westerman J., Sternby B., De Haas G. H. 1983. The complete primary structure of phospholipase A2 from human pancreas. Biochim. Biophys. Acta. 747: 93–99. [DOI] [PubMed] [Google Scholar]
- 8.Seilhamer J. J., Randall T. L., Yamanaka M., Johnson L. K. 1986. Pancreatic phospholipase A2: isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 5: 519–527. [DOI] [PubMed] [Google Scholar]
- 9.Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. 1989. Structure and properties of a human non-pancreatic phospholipase A2. J. Biol. Chem. 264: 5768–5775. [PubMed] [Google Scholar]
- 10.Seilhamer J. J., Pruzanski W., Vadas P., Plant S., Miller J. A., Kloss J., Johnson L. K. 1989. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J. Biol. Chem. 264: 5335–5338. [PubMed] [Google Scholar]
- 11.Chen J., Engle S. J., Seilhamer J. J., Tischfield J. A. 1994. Cloning and recombinant expression of a novel human low molecular weight Ca2+-dependent phospholipase A2. J. Biol. Chem. 269: 2365–2368. [PubMed] [Google Scholar]
- 12.Tischfield J. A., Xia Y. R., Shih D. M., Klisak I., Chen J., Engle S. J., Siakotos A. N., Winstead M. V., Seilhamer J. J., Allamand V., et al. 1996. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 32: 328–333. [DOI] [PubMed] [Google Scholar]
- 13.Cupillard L., Koumanov K., Mattei M. G., Lazdunski M., Lambeau G. 1997. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J. Biol. Chem. 272: 15745–15752. [DOI] [PubMed] [Google Scholar]
- 14.Valentin E., Ghomashchi F., Gelb M. H., Lazdunski M., Lambeau G. 2000. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 275: 7492–7496. [DOI] [PubMed] [Google Scholar]
- 15.Gelb M. H., Valentin E., Ghomashchi F., Lazdunski M., Lambeau G. 2000. Cloning and recombinant expression of a structurally novel human secreted phospholipase A2. J. Biol. Chem. 275: 39823–39826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouault M., Bollinger J. G., Lazdunski M., Gelb M. H., Lambeau G. 2003. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry. 42: 11494–11503. [DOI] [PubMed] [Google Scholar]
- 17.Nevalainen T. J., Cardoso J. C. 2012. Conservation of group XII phospholipase A2 from bacteria to human. Comp. Biochem. Physiol. Part D Genomics Proteomics. 7: 340–350. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Kowalski P. E., Thalmann I., Ornitz D. M., Mager D. L., Thalmann R. 1998. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2. Proc. Natl. Acad. Sci. USA. 95: 15345–15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X., Yang H., Yamoah E. N., Lundberg Y. W. 2007. Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix. Dev. Biol. 304: 508–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott D. L., White S. P., Browning J. L., Rosa J. J., Gelb M. H., Sigler P. B. 1991. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science. 254: 1007–1010. [DOI] [PubMed] [Google Scholar]
- 21.Pan Y. H., Yu B. Z., Singer A. G., Ghomashchi F., Lambeau G., Gelb M. H., Jain M. K., Bahnson B. J. 2002. Crystal structure of human group X secreted phospholipase A2. Electrostatically neutral interfacial surface targets zwitterionic membranes. J. Biol. Chem. 277: 29086–29093. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Dennis E. A. 1998. Expression and characterization of human group V phospholipase A2. Biochim. Biophys. Acta. 1394: 57–64. [DOI] [PubMed] [Google Scholar]
- 23.Murakami M., Shimbara S., Kambe T., Kuwata H., Winstead M. V., Tischfield J. A., Kudo I. 1998. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 273: 14411–14423. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M., Masuda S., Shimbara S., Bezzine S., Lazdunski M., Lambeau G., Gelb M. H., Matsukura S., Kokubu F., Adachi M., et al. 2003. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (groups III and XII). J. Biol. Chem. 278: 10657–10667. [DOI] [PubMed] [Google Scholar]
- 25.Pruzanski W., Lambeau L., Lazdunsky M., Cho W., Kopilov J., Kuksis A. 2005. Differential hydrolysis of molecular species of lipoprotein phosphatidylcholine by groups IIA, V and X secretory phospholipases A2. Biochim. Biophys. Acta. 1736: 38–50. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuishi M., Masuda S., Kudo I., Murakami M. 2007. Human group III phospholipase A2 suppresses adenovirus infection into host cells. Evidence that group III, V and X phospholipase A2s act on distinct cellular phospholipid molecular species. Biochim. Biophys. Acta. 1771: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 27.Hille J. D., Egmond M. R., Dijkman R., van Oort M. G., Jirgensons B., de Haas G. H. 1983. Aggregation of porcine pancreatic phospholipase A2 and its zymogen induced by submicellar concentrations of negatively charged detergents. Biochemistry. 22: 5347–5353. [DOI] [PubMed] [Google Scholar]
- 28.Cash J. G., Kuhel D. G., Goodin C., Hui D. Y. 2011. Pancreatic acinar cell-specific overexpression of group 1B phospholipase A2 exacerbates diet-induced obesity and insulin resistance in mice. Int. J. Obes. (Lond). 35: 877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grass D. S., Felkner R. H., Chiang M. Y., Wallace R. E., Nevalainen T. J., Bennett C. F., Swanson M. E. 1996. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J. Clin. Invest. 97: 2233–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivandic B., Castellani L. W., Wang X. P., Qiao J. H., Mehrabian M., Navab M., Fogelman A. M., Grass D. S., Swanson M. E., de Beer M. C., et al. 1999. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler. Thromb. Vasc. Biol. 19: 1284–1290. [DOI] [PubMed] [Google Scholar]
- 31.Laine V. J., Grass D. S., Nevalainen T. J. 1999. Protection by group II phospholipase A2 against Staphylococcus aureus. J. Immunol. 162: 7402–7408. [PubMed] [Google Scholar]
- 32.Laine V. J., Grass D. S., Nevalainen T. J. 2000. Resistance of transgenic mice expressing human group II phospholipase A2 to Escherichia coli infection. Infect. Immun. 68: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulherkar R., Kirtane B. M., Ramchandani A., Mansukhani N. P., Kannan S., Naresh K. N. 2003. Expression of enhancing factor/phospholipase A2 in skin results in abnormal epidermis and increased sensitivity to chemical carcinogenesis. Oncogene. 22: 1936–1944. [DOI] [PubMed] [Google Scholar]
- 34.Ohtsuki M., Taketomi Y., Arata S., Masuda S., Ishikawa Y., Ishii T., Takanezawa Y., Aoki J., Arai H., Yamamoto K., et al. 2006. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J. Biol. Chem. 281: 36420–36433. [DOI] [PubMed] [Google Scholar]
- 35.Curfs D. M., Ghesquiere S. A., Vergouwe M. N., van der Made I., Gijbels M. J., Greaves D. R., Verbeek J. S., Hofker M. H., de Winther M. P. 2008. Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J. Biol. Chem. 283: 21640–21648. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto K., Taketomi Y., Isogai Y., Miki Y., Sato H., Masuda S., Nishito Y., Morioka K., Ishimoto Y., Suzuki N., et al. 2011. Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin. J. Biol. Chem. 286: 11616–11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato H., Kato R., Isogai Y., Saka G., Ohtsuki M., Taketomi Y., Yamamoto K., Tsutsumi K., Yamada J., Masuda S., et al. 2008. Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J. Biol. Chem. 283: 33483–33497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato H., Taketomi Y., Isogai Y., Masuda S., Kobayashi T., Yamamoto K., Murakami M. 2009. Group III secreted phospholipase A2 transgenic mice spontaneously develop inflammation. Biochem. J. 421: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huggins K. W., Boileau A. C., Hui D. Y. 2002. Protection against diet-induced obesity and obesity- related insulin resistance in group 1B PLA2-deficient mice. Am. J. Physiol. Endocrinol. Metab. 283: E994–E1001. [DOI] [PubMed] [Google Scholar]
- 40.Labonté E. D., Kirby R. J., Schildmeyer N. M., Cannon A. M., Huggins K. W., Hui D. Y. 2006. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 55: 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labonté E. D., Pfluger P. T., Cash J. G., Kuhel D. G., Roja J. C., Magness D. P., Jandacek R. J., Tschöp M. H., Hui D. Y. 2010. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 24: 2516–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollie N. I., Hui D. Y. 2011. Group 1B phospholipase A2 deficiency protects against diet-induced hyperlipidemia in mice. J. Lipid Res. 52: 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollie N. I., Konaniah E. S., Goodin C., Hui D. Y. 2014. Group 1B phospholipase A2 inactivation suppresses atherosclerosis and metabolic diseases in LDL receptor-deficient mice. Atherosclerosis. 234: 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui D. Y., Cope M. J., Labonte E. D., Chang H. T., Shao J., Goka E., Abousalham A., Charmot D., Buysse J. 2009. The phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br. J. Pharmacol. 157: 1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson S. G., Adam G., Langdown M., Reneland R., Braun A., Andrew T., Surdulescu G. L., Norberg M., Dudbridge F., Reed P. W., et al. 2006. Linkage and potential association of obesity-related phenotypes with two genes on chromosome 12q24 in a female dizygous twin cohort. Eur. J. Hum. Genet. 14: 340–348. [DOI] [PubMed] [Google Scholar]
- 46.Pruzanski W., Vadas P. 1991. Phospholipase A2–a mediator between proximal and distal effectors of inflammation. Immunol. Today. 12: 143–146. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy B. P., Payette P., Mudgett J., Vadas P., Pruzanski W., Kwan M., Tang C., Rancourt D. E., Cromlish W. A. 1995. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 270: 22378–22385. [DOI] [PubMed] [Google Scholar]
- 48.MacPhee M., Chepenik K. P., Liddell R. A., Nelson K. K., Siracusa L. D., Buchberg A. M. 1995. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 81: 957–966. [DOI] [PubMed] [Google Scholar]
- 49.Weinrauch Y., Abad C., Liang N. S., Lowry S. F., Weiss J. 1998. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J. Clin. Invest. 102: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koduri R. S., Gronroos J. O., Laine V. J., Le Calvez C., Lambeau G., Nevalainen T. J., Gelb M. H. 2002. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277: 5849–5857. [DOI] [PubMed] [Google Scholar]
- 51.Weiss J., Inada M., Elsbach P., Crowl R. M. 1994. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J. Biol. Chem. 269: 26331–26337. [PubMed] [Google Scholar]
- 52.Koprivnjak T., Peschel A., Gelb M. H., Liang N. S., Weiss J. P. 2002. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277: 47636–47644. [DOI] [PubMed] [Google Scholar]
- 53.Piris-Gimenez A., Paya M., Lambeau G., Chignard M., Mock M., Touqui L., Goossens P. L. 2005. In vivo protective role of human group IIa phospholipase A2 against experimental anthrax. J. Immunol. 175: 6786–6791. [DOI] [PubMed] [Google Scholar]
- 54.Movert E., Wu Y., Lambeau G., Touqui L., Areschoug T. 2011. A novel bacterial resistance mechanism against human group IIA-secreted phospholipase A2: role of Streptococcus pyogenes sortase A. J. Immunol. 187: 6437–6446. [DOI] [PubMed] [Google Scholar]
- 55.Pernet E., Guillemot L., Burgel P. R., Martin C., Lambeau G., Sermet-Gaudelus I., Sands D., Leduc D., Morand P. C., Jeammet L., et al. 2014. Pseudomonas aeruginosa eradicates Staphylococcus aureus by manipulating the host immunity. Nat. Commun. 5: 5105. [DOI] [PubMed] [Google Scholar]
- 56.Raymond B., Leduc D., Ravaux L., Le Goffic R., Candela T., Raymondjean M., Goossens P. L., Touqui L. 2007. Edema toxin impairs anthracidal phospholipase A2 expression by alveolar macrophages. PLoS Pathog. 3: e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cormier R. T., Hong K. H., Halberg R. B., Hawkins T. L., Richardson P., Mulherkar R., Dove W. F., Lander E. S. 1997. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat. Genet. 17: 88–91. [DOI] [PubMed] [Google Scholar]
- 58.Leung S. Y., Chen X., Chu K. M., Yuen S. T., Mathy J., Ji J., Chan A. S., Li R., Law S., Troyanskaya O. G., et al. 2002. Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proc. Natl. Acad. Sci. USA. 99: 16203–16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomlinson I. P., Beck N. E., Neale K., Bodmer W. F. 1996. Variants at the secretory phospholipase A2 (PLA2G2A) locus: analysis of associations with familial adenomatous polyposis and sporadic colorectal tumours. Ann. Hum. Genet. 60: 369–376. [DOI] [PubMed] [Google Scholar]
- 60.Graff J. R., Konicek B. W., Deddens J. A., Chedid M., Hurst B. M., Colligan B., Neubauer B. L., Carter H. W., Carter J. H. 2001. Expression of group IIa secretory phospholipase A2 increases with prostate tumor grade. Clin. Cancer Res. 7: 3857–3861. [PubMed] [Google Scholar]
- 61.Scott K. F., Sajinovic M., Hein J., Nixdorf S., Galettis P., Liauw W., de Souza P., Dong Q., Graham G. G., Russell P. J. 2010. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie. 92: 601–610. [DOI] [PubMed] [Google Scholar]
- 62.Brglez V., Lambeau G., Petan T. 2014. Secreted phospholipases A2 in cancer: diverse mechanisms of action. Biochimie. 107: 114–123. [DOI] [PubMed] [Google Scholar]
- 63.Boilard E., Lai Y., Larabee K., Balestrieri B., Ghomashchi F., Fujioka D., Gobezie R., Coblyn J. S., Weinblatt M. E., Massarotti E. M., et al. 2010. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol. Med. 2: 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boudreau L. H., Duchez A. C., Cloutier N., Soulet D., Martin N., Bollinger J., Pare A., Rousseau M., Naika G. S., Levesque T., et al. 2014. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 124: 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boilard E., Nigrovic P. A., Larabee K., Watts G. F., Coblyn J. S., Weinblatt M. E., Massarotti E. M., Remold-O’Donnell E., Farndale R. W., Ware J., et al. 2010. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 327: 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wootton P. T., Drenos F., Cooper J. A., Thompson S. R., Stephens J. W., Hurt-Camejo E., Wiklund O., Humphries S. E., Talmud P. J. 2006. Tagging-SNP haplotype analysis of the secretory PLA2IIa gene PLA2G2A shows strong association with serum levels of sPLA2IIa: results from the UDACS study. Hum. Mol. Genet. 15: 355–361. [DOI] [PubMed] [Google Scholar]
- 67.Kugiyama K., Ota Y., Takazoe K., Moriyama Y., Kawano H., Miyao Y., Sakamoto T., Soejima H., Ogawa H., Doi H., et al. 1999. Circulating levels of secretory type II phospholipase A2 predict coronary events in patients with coronary artery disease. Circulation. 100: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 68.Webb N. R., Bostrom M. A., Szilvassy S. J., van der Westhuyzen D. R., Daugherty A., de Beer F. C. 2003. Macrophage-expressed group IIA secretory phospholipase A2 increases atherosclerotic lesion formation in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 23: 263–268. [DOI] [PubMed] [Google Scholar]
- 69.Valentin E., Koduri R. S., Scimeca J. C., Carle G., Gelb M. H., Lazdunski M., Lambeau G. 1999. Cloning and recombinant expression of a novel mouse-secreted phospholipase A2. J. Biol. Chem. 274: 19152–19160. [DOI] [PubMed] [Google Scholar]
- 70.Miki Y., Yamamoto K., Taketomi Y., Sato H., Shimo K., Kobayashi T., Ishikawa Y., Ishii T., Nakanishi H., Ikeda K., et al. 2013. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 210: 1217–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Allmen C. E., Schmitz N., Bauer M., Hinton H. J., Kurrer M. O., Buser R. B., Gwerder M., Muntwiler S., Sparwasser T., Beerli R. R., et al. 2009. Secretory phospholipase A2-IID is an effector molecule of CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 106: 11673–11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takabatake N., Sata M., Inoue S., Shibata Y., Abe S., Wada T., Machiya J., Ji G., Matsuura T., Takeishi Y., et al. 2005. A novel polymorphism in secretory phospholipase A2-IID is associated with body weight loss in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 172: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 73.Valentin E., Ghomashchi F., Gelb M. H., Lazdunski M., Lambeau G. 1999. On the diversity of secreted phospholipases A2. Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 274: 31195–31202. [DOI] [PubMed] [Google Scholar]
- 74.Sato H., Taketomi Y., Ushida A., Isogai Y., Kojima T., Hirabayashi T., Miki Y., Yamamoto K., Nishito Y., Kobayashi T., et al. 2014. The adipocyte-inducible secreted phospholipases PLA2G5 and PLA2G2E play distinct roles in obesity. Cell Metab. 20: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang S. K., Hong M., Zhao W., Jung Y., Tayebi N., Ye B. D., Kim K. J., Park S. H., Lee I., Shin H. D., et al. 2013. Genome-wide association study of ulcerative colitis in Koreans suggests extensive overlapping of genetic susceptibility with Caucasians. Inflamm. Bowel Dis. 19: 954–966. [DOI] [PubMed] [Google Scholar]
- 76.Chen J., Engle S. J., Seilhamer J. J., Tischfield J. A. 1994. Cloning and characterization of novel rat and mouse low molecular weight Ca2+-dependent phospholipase A2s containing 16 cysteines. J. Biol. Chem. 269: 23018–23024. [PubMed] [Google Scholar]
- 77.Zeissig S., Murata K., Sweet L., Publicover J., Hu Z., Kaser A., Bosse E., Iqbal J., Hussain M. M., Balschun K., et al. 2012. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat. Med. 18: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J., Shao C., Lazar V., Srivastava C. H., Lee W. H., Tischfield J. A. 1997. Localization of group IIc low molecular weight phospholipase A2 mRNA to meiotic cells in the mouse. J. Cell. Biochem. 64: 369–375. [DOI] [PubMed] [Google Scholar]
- 79.Ilic D., Bollinger J. M., Gelb M., Mauro T. M. 2014. sPLA2 and the epidermal barrier. Biochim. Biophys. Acta. 1841: 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Man M. Q., Lin T. K., Santiago J. L., Celli A., Zhong L., Huang Z. M., Roelandt T., Hupe M., Sundberg J. P., Silva K. A., et al. 2014. Basis for enhanced barrier function of pigmented skin. J. Invest. Dermatol. 134: 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han S. K., Kim K. P., Koduri R., Bittova L., Munoz N. M., Leff A. R., Wilton D. C., Gelb M. H., Cho W. 1999. Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2. J. Biol. Chem. 274: 11881–11888. [DOI] [PubMed] [Google Scholar]
- 82.Satake Y., Diaz B. L., Balestrieri B., Lam B. K., Kanaoka Y., Grusby M. J., Arm J. P. 2004. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J. Biol. Chem. 279: 16488–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lapointe S., Brkovic A., Cloutier I., Tanguay J. F., Arm J. P., Sirois M. G. 2010. Group V secreted phospholipase A2 contributes to LPS-induced leukocyte recruitment. J. Cell. Physiol. 224: 127–134. [DOI] [PubMed] [Google Scholar]
- 84.Muñoz N. M., Meliton A. Y., Arm J. P., Bonventre J. V., Cho W., Leff A. R. 2007. Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J. Immunol. 179: 4800–4807. [DOI] [PubMed] [Google Scholar]
- 85.Muñoz N. M., Meliton A. Y., Meliton L. N., Dudek S. M., Leff A. R. 2009. Secretory group V phospholipase A2 regulates acute lung injury and neutrophilic inflammation caused by LPS in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 296: L879–L887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meliton A. Y., Munoz N. M., Meliton L. N., Birukova A. A., Leff A. R., Birukov K. G. 2013. Mechanical induction of group V phospholipase A2 causes lung inflammation and acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 304: L689–L700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uozumi N., Kume K., Nagase T., Nakatani N., Ishii S., Tashiro F., Komagata Y., Maki K., Ikuta K., Ouchi Y., et al. 1997. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 390: 618–622. [DOI] [PubMed] [Google Scholar]
- 88.Kikawada E., Bonventre J. V., Arm J. P. 2007. Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse mast cells through amplification of ERK and cPLA2α activation. Blood. 110: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruipérez V., Astudillo A. M., Balboa M. A., Balsinde J. 2009. Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s. J. Immunol. 182: 3877–3883. [DOI] [PubMed] [Google Scholar]
- 90.Balsinde J., Dennis E. A. 1996. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 271: 6758–6765. [DOI] [PubMed] [Google Scholar]
- 91.Sawada H., Murakami M., Enomoto A., Shimbara S., Kudo I. 1999. Regulation of type V phospholipase A2 expression and function by proinflammatory stimuli. Eur. J. Biochem. 263: 826–835. [DOI] [PubMed] [Google Scholar]
- 92.Ohta S., Imamura M., Xing W., Boyce J. A., Balestrieri B. 2013. Group V secretory phospholipase A2 is involved in macrophage activation and is sufficient for macrophage effector functions in allergic pulmonary inflammation. J. Immunol. 190: 5927–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rubio J. M., Rodriguez J. P., Gil-de-Gomez L., Guijas C., Balboa M. A., Balsinde J. 2015. Group V secreted phospholipase A2 is upregulated by IL-4 in human macrophages and mediates phagocytosis via hydrolysis of ethanolamine phospholipids. J. Immunol. 194: 3327–3339. [DOI] [PubMed] [Google Scholar]
- 94.Henderson W. R., Jr, Ye X., Lai Y., Ni Z., Bollinger J. G., Tien Y. T., Chi E. Y., Gelb M. H. 2013. Key role of group v secreted phospholipase A2 in Th2 cytokine and dendritic cell-driven airway hyperresponsiveness and remodeling. PLoS ONE. 8: e56172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giannattasio G., Fujioka D., Xing W., Katz H. R., Boyce J. A., Balestrieri B. 2010. Group V secretory phospholipase A2 reveals its role in house dust mite-induced allergic pulmonary inflammation by regulation of dendritic cell function. J. Immunol. 185: 4430–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balestrieri B., Maekawa A., Xing W., Gelb M. H., Katz H. R., Arm J. P. 2009. Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J. Immunol. 182: 4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Degousee N., Kelvin D. J., Geisslinger G., Hwang D. M., Stefanski E., Wang X. H., Danesh A., Angioni C., Schmidt H., Lindsay T. F., et al. 2011. Group V phospholipase A2 in bone marrow-derived myeloid cells and bronchial epithelial cells promotes bacterial clearance after Escherichia coli pneumonia. J. Biol. Chem. 286: 35650–35662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wooton-Kee C. R., Boyanovsky B. B., Nasser M. S., de Villiers W. J., Webb N. R. 2004. Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler. Thromb. Vasc. Biol. 24: 762–767. [DOI] [PubMed] [Google Scholar]
- 99.Bostrom M. A., Boyanovsky B. B., Jordan C. T., Wadsworth M. P., Taatjes D. J., de Beer F. C., Webb N. R. 2007. Group V secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler. Thromb. Vasc. Biol. 27: 600–606. [DOI] [PubMed] [Google Scholar]
- 100.Boyanovsky B., Zack M., Forrest K., Webb N. R. 2009. The capacity of group V sPLA2 to increase atherogenicity of ApoE−/− and LDLR−/− mouse LDL in vitro predicts its atherogenic role in vivo. Arterioscler. Thromb. Vasc. Biol. 29: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yano T., Fujioka D., Saito Y., Kobayashi T., Nakamura T., Obata J. E., Kawabata K., Watanabe K., Watanabe Y., Mishina H., et al. 2011. Group V secretory phospholipase A2 plays a pathogenic role in myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 90: 335–343. [DOI] [PubMed] [Google Scholar]
- 102.Boyanovsky B. B., Bailey W., Dixon L., Shridas P., Webb N. R. 2012. Group V secretory phospholipase A2 enhances the progression of angiotensin II-induced abdominal aortic aneurysms but confers protection against angiotensin II-induced cardiac fibrosis in apoE-deficient mice. Am. J. Pathol. 181: 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nicholls S. J., Kastelein J. J., Schwartz G. G., Bash D., Rosenson R. S., Cavender M. A., Brennan D. M., Koenig W., Jukema J. W., Nambi V., et al. ; VISTA-16 Investigators. 2014. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 311: 252–262. [DOI] [PubMed] [Google Scholar]
- 104.Wootton P. T., Arora N. L., Drenos F., Thompson S. R., Cooper J. A., Stephens J. W., Hurel S. J., Hurt-Camejo E., Wiklund O., Humphries S. E., et al. 2007. Tagging SNP haplotype analysis of the secretory PLA2-V gene, PLA2G5, shows strong association with LDL and oxLDL levels, suggesting functional distinction from sPLA2-IIA: results from the UDACS study. Hum. Mol. Genet. 16: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 105.Pettersson C., Fogelstrand L., Rosengren B., Stahlman S., Hurt-Camejo E., Fagerberg B., Wiklund O. 2008. Increased lipolysis by secretory phospholipase A2 group V of lipoproteins in diabetic dyslipidaemia. J. Intern. Med. 264: 155–165. [DOI] [PubMed] [Google Scholar]
- 106.Sergouniotis P. I., Davidson A. E., Mackay D. S., Lenassi E., Li Z., Robson A. G., Yang X., Kam J. H., Isaacs T. W., Holder G. E., et al. 2011. Biallelic mutations in PLA2G5, encoding group V phospholipase A2, cause benign fleck retina. Am. J. Hum. Genet. 89: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedman J. S., Chang B., Krauth D. S., Lopez I., Waseem N. H., Hurd R. E., Feathers K. L., Branham K. E., Shaw M., Thomas G. E., et al. 2010. Loss of lysophosphatidylcholine acyltransferase 1 leads to photoreceptor degeneration in rd11 mice. Proc. Natl. Acad. Sci. USA. 107: 15523–15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masuda S., Murakami M., Takanezawa Y., Aoki J., Arai H., Ishikawa Y., Ishii T., Arioka M., Kudo I. 2005. Neuronal expression and neuritogenic action of group X secreted phospholipase A2. J. Biol. Chem. 280: 23203–23214. [DOI] [PubMed] [Google Scholar]
- 109.Jemel I., Ii H., Oslund R. C., Payre C., Dabert-Gay A. S., Douguet D., Chargui K., Scarzello S., Gelb M. H., Lambeau G. 2011. Group X secreted phospholipase A2 proenzyme is matured by a furin-like proprotein convertase and releases arachidonic acid inside of human HEK293 cells. J. Biol. Chem. 286: 36509–36521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bezzine S., Koduri R. S., Valentin E., Murakami M., Kudo I., Ghomashchi F., Sadilek M., Lambeau G., Gelb M. H. 2000. Exogenously added human group X secreted phospholipase A2 but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J. Biol. Chem. 275: 3179–3191. [DOI] [PubMed] [Google Scholar]
- 111.Murakami M., Koduri R. S., Enomoto A., Shimbara S., Seki M., Yoshihara K., Singer A., Valentin E., Ghomashchi F., Lambeau G., et al. 2001. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 276: 10083–10096. [DOI] [PubMed] [Google Scholar]
- 112.Henderson W. R., Jr, Chi E. Y., Bollinger J. G., Tien Y. T., Ye X., Castelli L., Rubtsov Y. P., Singer A. G., Chiang G. K., Nevalainen T., et al. 2007. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204: 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Henderson W. R., Jr, Oslund R. C., Bollinger J. G., Ye X., Tien Y. T., Xue J., Gelb M. H. 2011. Blockade of human group X secreted phospholipase A2 (GX-sPLA2)-induced airway inflammation and hyperresponsiveness in a mouse asthma model by a selective GX-sPLA2 inhibitor. J. Biol. Chem. 286: 28049–28055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai Y., Oslund R. C., Bollinger J. G., Henderson W. R., Jr, Santana L. F., Altemeier W. A., Gelb M. H., Hallstrand T. S. 2010. Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group X. J. Biol. Chem. 285: 41491–41500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hallstrand T. S., Lai Y., Altemeier W. A., Appel C. L., Johnson B., Frevert C. W., Hudkins K. L., Bollinger J. G., Woodruff P. G., Hyde D. M., et al. 2013. Regulation and function of epithelial secreted phospholipase A2 group X in asthma. Am. J. Respir. Crit. Care Med. 188: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kelvin A. A., Degousee N., Banner D., Stefanski E., Leomicronn A. J., Angoulvant D., Paquette S. G., Huang S. S., Danesh A., Robbins C. S., et al. 2014. Lack of group X secreted phospholipase A2 increases survival following pandemic H1N1 influenza infection. Virology. 454–455: 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hallstrand T. S., Lai Y., Ni Z., Oslund R. C., Henderson W. R., Jr, Gelb M. H., Wenzel S. E. 2011. Relationship between levels of secreted phospholipase A2 groups IIA and X in the airways and asthma severity. Clin. Exp. Allergy. 41: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fujioka D., Saito Y., Kobayashi T., Yano T., Tezuka H., Ishimoto Y., Suzuki N., Yokota Y., Nakamura T., Obata J. E., et al. 2008. Reduction in myocardial ischemia/reperfusion injury in group X secretory phospholipase A2-deficient mice. Circulation. 117: 2977–2985. [DOI] [PubMed] [Google Scholar]
- 119.Zack M., Boyanovsky B. B., Shridas P., Bailey W., Forrest K., Howatt D. A., Gelb M. H., de Beer F. C., Daugherty A., Webb N. R. 2011. Group X secretory phospholipase A2 augments angiotensin II-induced inflammatory responses and abdominal aortic aneurysm formation in apoE-deficient mice. Atherosclerosis. 214: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Watanabe K., Fujioka D., Saito Y., Nakamura T., Obata J. E., Kawabata K., Watanabe Y., Mishina H., Tamaru S., Hanasaki K., et al. 2012. Group X secretory PLA2 in neutrophils plays a pathogenic role in abdominal aortic aneurysms in mice. Am. J. Physiol. Heart Circ. Physiol. 302: H95–H104. [DOI] [PubMed] [Google Scholar]
- 121.Sato H., Isogai Y., Masuda S., Taketomi Y., Miki Y., Kamei D., Hara S., Kobayashi T., Ishikawa Y., Ishii T., et al. 2011. Physiological roles of group X-secreted phospholipase A2 in reproduction, gastrointestinal phospholipid digestion, and neuronal function. J. Biol. Chem. 286: 11632–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shridas P., Bailey W. M., Boyanovsky B. B., Oslund R. C., Gelb M. H., Webb N. R. 2010. Group X secretory phospholipase A2 regulates the expression of steroidogenic acute regulatory protein (StAR) in mouse adrenal glands. J. Biol. Chem. 285: 20031–20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shridas P., Zahoor L., Forrest K. J., Layne J. D., Webb N. R. 2014. Group X secretory phospholipase A2 regulates insulin secretion through a cyclooxygenase-2-dependent mechanism. J. Biol. Chem. 289: 27410–27417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ait-Oufella H., Herbin O., Lahoute C., Coatrieux C., Loyer X., Joffre J., Laurans L., Ramkhelawon B., Blanc-Brude O., Karabina S., et al. 2013. Group X secreted phospholipase A2 limits the development of atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 33: 466–473. [DOI] [PubMed] [Google Scholar]
- 125.Gora S., Perret C., Jemel I., Nicaud V., Lambeau G., Cambien F., Ninio E., Blankenberg S., Tiret L., Karabina S. A. 2009. Molecular and functional characterization of polymorphisms in the secreted phospholipase A2 group X gene: relevance to coronary artery disease. J. Mol. Med. (Berl). 87: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guardiola M., Exeter H. J., Perret C., Folkersen L., Van’t Hooft F., Eriksson P., Franco-Cereceda A., Paulsson-Berne G., Palmen J., Li K. W., et al. PLA2G10 gene variants, sPLA2 activity and coronary heart disease risk. Circ. Cardiovasc. Genet. Epub ahead of print. January 12, 2015; 10.1161/CIRCGENETICS.114.000633. [DOI] [PubMed] [Google Scholar]
- 127.Shridas P., Bailey W. M., Talbott K. R., Oslund R. C., Gelb M. H., Webb N. R. 2011. Group X secretory phospholipase A2 enhances TLR4 signaling in macrophages. J. Immunol. 187: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li X., Shridas P., Forrest K., Bailey W., Webb N. R. 2010. Group X secretory phospholipase A2 negatively regulates adipogenesis in murine models. FASEB J. 24: 4313–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mazmanian S. K., Liu C. H., Tzianabos A. O., Kasper D. L. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 122: 107–118. [DOI] [PubMed] [Google Scholar]
- 130.Ley R. E., Backhed F., Turnbaugh P., Lozupone C. A., Knight R. D., Gordon J. I. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tremaroli V., Backhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature. 489: 242–249. [DOI] [PubMed] [Google Scholar]
- 132.Escoffier J., Jemel I., Tanemoto A., Taketomi Y., Payre C., Coatrieux C., Sato H., Yamamoto K., Masuda S., Pernet-Gallay K., et al. 2010. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 120: 1415–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murakami M., Masuda S., Shimbara S., Ishikawa Y., Ishii T., Kudo I. 2005. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A2. J. Biol. Chem. 280: 24987–24998. [DOI] [PubMed] [Google Scholar]
- 134.Taketomi Y., Ueno N., Kojima T., Sato H., Murase R., Yamamoto K., Tanaka S., Sakanaka M., Nakamura M., Nishito Y., et al. 2013. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 14: 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sato H., Taketomi Y., Isogai Y., Miki Y., Yamamoto K., Masuda S., Hosono T., Arata S., Ishikawa Y., Ishii T., et al. 2010. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 120: 1400–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mounier C. M., Wendum D., Greenspan E., Flejou J. F., Rosenberg D. W., Lambeau G. 2008. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br. J. Cancer. 98: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoeft B., Linseisen J., Beckmann L., Muller-Decker K., Canzian F., Husing A., Kaaks R., Vogel U., Jakobsen M. U., Overvad K., et al. 2010. Polymorphisms in fatty-acid-metabolism-related genes are associated with colorectal cancer risk. Carcinogenesis. 31: 466–472. [DOI] [PubMed] [Google Scholar]
- 138.Limou S., Coulonges C., Foglio M., Heath S., Diop G., Leclerc S., Hirtzig T., Spadoni J. L., Therwath A., Lambeau G., et al. 2008. Exploration of associations between phospholipase A2 gene family polymorphisms and AIDS progression using the SNPlex method. Biomed. Pharmacother. 62: 31–40. [DOI] [PubMed] [Google Scholar]
- 139.Martínez-García A., Sastre I., Recuero M., Aldudo J., Vilella E., Mateo I., Sanchez-Juan P., Vargas T., Carro E., Bermejo-Pareja F., et al. 2010. PLA2G3, a gene involved in oxidative stress induced death, is associated with Alzheimer’s disease. J. Alzheimers Dis. 22: 1181–1187. [DOI] [PubMed] [Google Scholar]
- 140.Kim J., Lee J. E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., Gleeson J. G. 2010. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 464: 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huhtinen H. T., Gronroos J. O., Gronroos J. M., Uksila J., Gelb M. H., Nevalainen T. J., Laine V. J. 2006. Antibacterial effects of human group IIA and group XIIA phospholipase A2 against Helicobacter pylori in vitro. APMIS. 114: 127–130. [DOI] [PubMed] [Google Scholar]
- 142.Muñoz-Sanjuán I., Brivanlou A. H. 2005. Induction of ectopic olfactory structures and bone morphogenetic protein inhibition by Rossy, a group XII secreted phospholipase A2. Mol. Cell. Biol. 25: 3608–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ee S. M., Lo Y. L., Shui G., Wenk M. R., Shin E. J., Kim H. C., Ong W. Y. 2014. Distribution of secretory phospholipase A2 XIIA in the brain and its role in lipid metabolism and cognition. Mol. Neurobiol. 50: 60–75. [DOI] [PubMed] [Google Scholar]
- 144.Wang V. M., Rosen R. B., Meyerle C. B., Kurup S. K., Ardeljan D., Agron E., Tai K., Pomykala M., Chew E. Y., Chan C. C., et al. 2012. Suggestive association between PLA2G12A single nucleotide polymorphism rs2285714 and response to anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration. Mol. Vis. 18: 2578–2585. [PMC free article] [PubMed] [Google Scholar]
- 145.Guan M., Qu L., Tan W., Chen L., Wong C. W. 2011. Hepatocyte nuclear factor-4 α regulates liver triglyceride metabolism in part through secreted phospholipase A2 GXIIB. Hepatology. 53: 458–466. [DOI] [PubMed] [Google Scholar]
- 146.Rouault M., Le Calvez C., Boilard E., Surrel F., Singer A., Ghomashchi F., Bezzine S., Scarzello S., Bollinger J., Gelb M. H., et al. 2007. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 46: 1647–1662. [DOI] [PubMed] [Google Scholar]
- 147.Ishizaki J., Hanasaki K., Higashino K., Kishino J., Kikuchi N., Ohara O., Arita H. 1994. Molecular cloning of pancreatic group I phospholipase A2 receptor. J. Biol. Chem. 269: 5897–5904. [PubMed] [Google Scholar]
- 148.Lambeau G., Ancian P., Barhanin J., Lazdunski M. 1994. Cloning and expression of a membrane receptor for secretory phospholipases A2. J. Biol. Chem. 269: 1575–1578. [PubMed] [Google Scholar]
- 149.Ancian P., Lambeau G., Mattei M. G., Lazdunski M. 1995. The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J. Biol. Chem. 270: 8963–8970. [DOI] [PubMed] [Google Scholar]
- 150.Hanasaki K., Yokota Y., Ishizaki J., Itoh T., Arita H. 1997. Resistance to endotoxic shock in phospholipase A2 receptor-deficient mice. J. Biol. Chem. 272: 32792–32797. [DOI] [PubMed] [Google Scholar]
- 151.Tamaru S., Mishina H., Watanabe Y., Watanabe K., Fujioka D., Takahashi S., Suzuki K., Nakamura T., Obata J. E., Kawabata K., et al. 2013. Deficiency of phospholipase A2 receptor exacerbates ovalbumin-induced lung inflammation. J. Immunol. 191: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 152.Mishina H., Watanabe K., Tamaru S., Watanabe Y., Fujioka D., Takahashi S., Suzuki K., Nakamura T., Obata J. E., Kawabata K., et al. 2014. Lack of phospholipase A2 receptor increases susceptibility to cardiac rupture after myocardial infarction. Circ. Res. 114: 493–504. [DOI] [PubMed] [Google Scholar]
- 153.Augert A., Payre C., de Launoit Y., Gil J., Lambeau G., Bernard D. 2009. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 10: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Augert A., Vindrieux D., Girard C. A., Le Calve B., Gras B., Ferrand M., Bouchet B. P., Puisieux A., de Launoit Y., Simonnet H., et al. 2013. PLA2R1 kills cancer cells by inducing mitochondrial stress. Free Radic. Biol. Med. 65: 969–977. [DOI] [PubMed] [Google Scholar]
- 155.Vindrieux D., Augert A., Girard C. A., Gitenay D., Lallet-Daher H., Wiel C., Le Calve B., Gras B., Ferrand M., Verbeke S., et al. 2013. PLA2R1 mediates tumor suppression by activating JAK2. Cancer Res. 73: 6334–6345. [DOI] [PubMed] [Google Scholar]
- 156.Beck L. H., Jr, Bonegio R. G., Lambeau G., Beck D. M., Powell D. W., Cummins T. D., Klein J. B., Salant D. J. 2009. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stanescu H. C., Arcos-Burgos M., Medlar A., Bockenhauer D., Kottgen A., Dragomirescu L., Voinescu C., Patel N., Pearce K., Hubank M., et al. 2011. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364: 616–626. [DOI] [PubMed] [Google Scholar]
- 158.Beck S., Lambeau G., Scholz-Pedretti K., Gelb M. H., Janssen M. J., Edwards S. H., Wilton D. C., Pfeilschifter J., Kaszkin M. 2003. Potentiation of tumor necrosis factor α-induced secreted phospholipase A2 (sPLA2)-IIA expression in mesangial cells by an autocrine loop involving sPLA2 and peroxisome proliferator-activated receptor α activation. J. Biol. Chem. 278: 29799–29812. [DOI] [PubMed] [Google Scholar]
- 159.Zvaritch E., Lambeau G., Lazdunski M. 1996. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J. Biol. Chem. 271: 250–257. [DOI] [PubMed] [Google Scholar]
- 160.Geijtenbeek T. B., Gringhuis S. I. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takeuchi O., Akira S. 2010. Pattern recognition receptors and inflammation. Cell. 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 162.Ip W. K., Takahashi K., Moore K. J., Stuart L. M., Ezekowitz R. A. 2008. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J. Exp. Med. 205: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sato K., Yang X. L., Yudate T., Chung J. S., Wu J., Luby-Phelps K., Kimberly R. P., Underhill D., Cruz P. D., Jr, Ariizumi K. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 281: 38854–38866. [DOI] [PubMed] [Google Scholar]
- 165.Diaz B. L., Satake Y., Kikawada E., Balestrieri B., Arm J. P. 2006. Group V secretory phospholipase A2 amplifies the induction of cyclooxygenase 2 and delayed prostaglandin D2 generation in mouse bone marrow culture-derived mast cells in a strain-dependent manner. Biochim. Biophys. Acta. 1761: 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]