Abstract
The intestine plays a pivotal role in cholesterol homeostasis by functioning as an absorptive and secretory organ in the reverse cholesterol transport pathway. Enterocytes control cholesterol absorption, apoAI synthesis, HDL biogenesis, and nonbiliary cholesterol fecal disposal. Thus, intestine-based therapeutic interventions may hold promise in the management of diseases driven by cholesterol overload. Lipid-sensing nuclear receptors (NRs) are highly expressed in the intestinal epithelium and regulate transcriptionally the handling of cholesterol by the enterocytes. Here, we discuss the NR regulation of cholesterol fluxes across the enterocytes with special emphasis on NR exploitation as a bona fide novel HDL-raising strategy.
Keywords: atherosclerosis, gene expression, lipoprotein, transport, transcription, high density lipoprotein
THE ENTEROCYTE, CHOLESTEROL HOMEOSTASIS, AND HDL
Cholesterol is essential in all mammalian cells as a structural component of cell membranes and as a precursor of a large variety of molecules critical in biological functions such as bile acids (BAs), steroid hormones, and vitamin D (1). Cellular cholesterol requirements are met through de novo synthesis; however, in the presence of plasma lipoproteins, hepatocytes and steroidogenic cells obtain cholesterol through internalization of exogenous cholesterol (2). At the cellular level, de novo cholesterol synthesis and uptake of lipoprotein cholesterol are modulated through a negative feedback loop responding to elevations in intracellular cholesterol and regulated by a family of membrane-bound transcription factors named sterol-regulatory element binding proteins (SREBPs) (3). Earlier studies by Dietschy, Spady, and colleagues (4–6) provided evidence that, although virtually every tissue can synthesize sterol from acetyl-CoA, cholesterol may also be absorbed into the body from dietary sources, thus supporting an intestinal route of sterol influx. The elucidation of the molecular mechanisms underlying cholesterol absorption and the identification of pharmacological compounds able to interfere with the absorptive process have greatly endorsed the intestinal apical and basolateral proteins as promising targets to modulate cholesterol metabolism (7, 8).
The handling of cholesterol by the enterocyte controls the metabolic fate of dietary and biliary cholesterol. In the lumen of the small intestine, free cholesterol (FC) from dietary intake and biliary secretion is solubilized in mixed micelles containing BAs and phospholipids. The apical protein Niemann-Pick C1-like 1 (NPC1L1) is both the crucial and major determinant of the amount of cholesterol absorbed by the enterocytes, as both NPC1L1 deficiency and treatment with the inhibitor ezetimibe result in a markedly reduced intestinal cholesterol absorption (9–13). Furthermore, BA presence in the intestinal lumen is an essential prerequisite for absorption to occur and, accordingly, cholesterol-7α-hydroxylase (CYP7A1)-deficient mice, which are unable to synthesize BAs in the liver, display virtually zero cholesterol absorption (14).
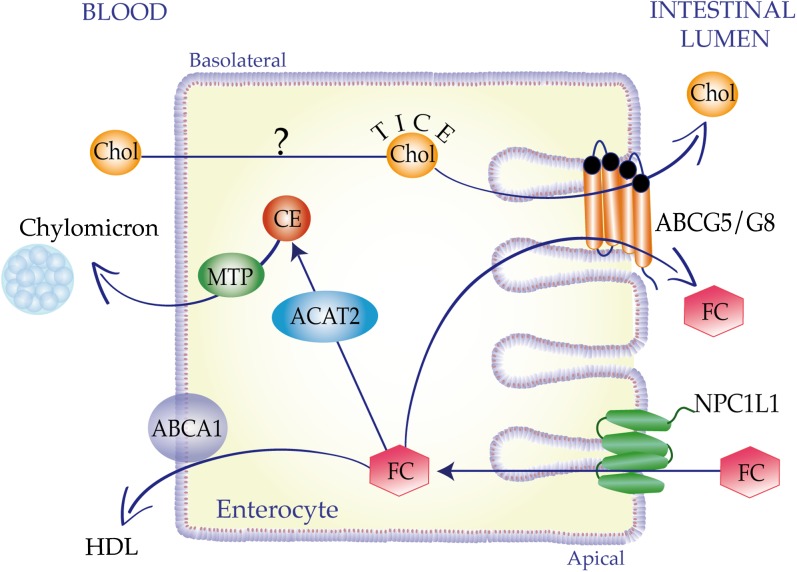
The metabolic fate of the absorbed cholesterol within the enterocyte involves an integrated network consisting of apical and basolateral uptake proteins, microsomal proteins, and transcriptional programs. Newly absorbed FC can be transported to the endoplasmic reticulum where it can be converted in cholesteryl ester (CE) by the microsomal enzyme ACAT2 (15–18) or be effluxed back into the intestinal lumen by the heterodimer ABCG5/G8 (19–23). The role of ACAT2 in intestinal sterol absorption is of special interest because the physicochemical state of the cholesterol molecule determines its fate in the body. FC is soluble in membrane phospholipids and represents an important structural component of cellular membranes; by contrast, CE is mostly insoluble in membranes and must be packaged into lipid droplets or incorporated into the core of lipoprotein particles for export out of the cell. Studies in ACAT2 knockout mice have reported that the lack of CE formation results in reduced cholesterol absorption (24, 25), and that CE formation by ACAT2 selectively utilizes chylomicron particles for quantitative transport of newly absorbed cholesterol out of the enterocyte into the lymphatic system, and subsequently into the body (26, 27). Along the basolateral membrane of the enterocyte, CE gets to be assembled into the core of chylomicron particles and be secreted into the lymph in a microsomal transfer protein (MTP)-dependent manner. The assembly of CE into chylomicron particles is critically dependent on MTP and apoB48; accordingly, absence of either of these proteins completely abolishes cholesterol absorption (28). Along the apical membrane of enterocytes, the heterodimer ABCG5/G8 counteracts the NPC1L1-mediated sterol uptake (29–32) by promoting FC efflux back into the intestinal lumen, with the net result of cholesterol absorption inhibition. Thoracic lymph duct cannulation studies have provided clear evidence that efficient cholesterol absorption requires both ACAT2 and ABCG5/G8 heterodimer, with the latter being responsible for the apical efflux of newly absorbed FC into the gut lumen, thus limiting the substrate availability for the esterification reaction (27).
The intestine is devoted to the tight control of whole-body cholesterol homeostasis, not only by functioning as absorptive organ but also by participating in the removal of excess cholesterol from the periphery via both the reverse cholesterol transport (RCT) pathway and, to a smaller extent, via trans-intestinal cholesterol excretion (TICE) (33). RCT has been originally described as a process by which extra-hepatic (peripheral) cholesterol is returned to the liver for biliary excretion and subsequent loss through the feces (34, 35); of note, the participation of the intestine in the regulation of RCT has been substantiated by the crucial role of this organ in the maintenance of plasma HDL (36–38) and cholesterol absorption (39). A critical checkpoint during RCT occurs in the hepatocytes, where cholesterol can be converted to BA or directly secreted via bile as FC into the intestine and ultimately to feces (40, 41). Thus, the classical RCT concept relies on two principles: a) HDL is the primary lipoprotein involved in RCT; and b) biliary secretion is the sole route for intestinal removal of plasma-derived cholesterol. The basolateral protein ABCA1 plays a cardinal role in RCT, as it mediates the cellular efflux of cholesterol and phospholipids to acceptor apoA1 (42–45), thus being responsible for nascent HDL particles (46, 47). According to the classical RCT concept, plasma HDL should be predictive of both biliary sterol secretion and fecal sterol loss. However, studies in mice lacking ABCA1 or apoAI indicated that, in the face of a near complete absence of HDL-cholesterol, biliary and fecal cholesterol levels were unchanged (48–50), thus suggesting that plasma HDL levels do not determine the amount of cholesterol ultimately excreted into the feces. Another caveat in the classical RCT framework is represented by the mounting evidence that biliary cholesterol does not predict its fecal disposal (33, 51–54). Thus, emerging evidence supports an unexpected role for the small intestine in actively excreting plasma-derived cholesterol in a process known as TICE (55–58). However, this pathway appears smaller compared with the classical RCT and does not require HDL (59).
The nonbiliary RCT pathway involves the targeting of plasma cholesterol to the proximal part of the small intestine and the subsequent cellular cholesterol secretion into the lumen of this organ. Intestinal perfusion studies indicated that plasma cholesterol can transverse the small intestine in a basolateral to apical direction thanks to the luminal presence of acceptors such as BAs and phospholipids (60). Although TICE accounts for a small amount of total fecal sterol loss (61), it is a highly dynamic pathway that can be efficiently upregulated under conditions of biliary insufficiency via activation of nuclear receptor (NR)-mediated transcriptional programs (52, 53, 62, 63). Indeed, there is an emerging interest in the molecular elucidation of the lipoproteins delivering cholesterol to the small intestine, the potential receptors involved in intestinal lipoprotein cholesterol uptake, factors controlling the trafficking of cholesterol across enterocyte and into the intestinal lumen, and luminal acceptor molecules promoting TICE. So far, the molecular mechanisms underlying TICE are not fully explored, although some progress has been made with regard to the role of donor particles delivering cholesterol for TICE, as well as the potential role of the heterodimer ABCG5/G8 as a TICE contributor (57, 58, 64–69) (Fig. 1).
Fig. 1.
Sterol fluxes across the enterocyte. An integrated network consisting of apical and basolateral proteins, microsomal enzymes, and transcriptional programs primes the metabolic fate of the cholesterol (Chol) fluxes within the enterocytes. At the apical membrane, FC is taken up by NPC1L1 protein and can be directed to ACAT2-mediated esterification and subsequent secretion in chylomicrons via MTP. FC can be secreted as a HDL component by the basolateral transporter ABCA1 or can be effluxed back into the intestinal lumen by the apical heterodimer ABCG5/G8. Enterocytes are also actively excreting plasma-derived cholesterol in a process named TICE. TICE underlying mechanisms are poorly explored with the only exception in the proposed contribution of the heterodimer ABCG5/G8.
A complex network consisting of cellular components (including sterol transporters and cholesterol-metabolizing enzymes) and transcriptional sensors balances intestinal cholesterol absorption and synthesis with biliary excretion and conversion to BAs. Mounting evidence supports a crucial role of intestinal NRs in cholesterol whole-body homeostasis, as they orchestrate the cholesterol fluxes across the enterocytes and out of the body by transcriptionally regulating cholesterol absorption, HDL biogenesis, RCT, and TICE (70–76). Thus, intestinal lipid-sensing NRs could be exploited as a therapeutic means in the management of chronic disorders where deregulated sterol metabolism drives the pathogenesis, including atherosclerosis, inflammation, and cancer.
THE NR SUPERFAMILY: FOCUS ON THE RETINOID X RECEPTOR HETERODIMERS
NRs are gaining increasing importance in the lexicon of functional biology because they represent a well-characterized, powerful, and fast-track bridge between pharmacology and physiology. NRs compose a large superfamily of evolutionarily related DNA transcription factors that transduce different metabolic signals into modulation of gene transcription. Thus, NRs control a broad range of biological processes and genetic programs, including development, cell growth and differentiation, metabolism, and immune response. An increasing understanding of the role of NRs in the physiology and pathophysiology of common and chronic diseases, including diabetes, obesity, and cancer, fostered the transition of NRs through the tipping point as therapeutic targets in the treatment of human diseases.
As elegantly discussed by Evans, Mangelsdorf, and colleagues, the discovery of the retinoid X receptor (RXR) and its endogenous ligand (9-cis-retinoic acid, a vitamin A metabolite) triggered a “Big Bang” of molecular endocrinology and led to the discovery of RXR heterodimerization with other NRs as a mechanism to control gene-specific transcription (77–82). On its own, RXR functions as a self-sufficient homodimer, binding to a direct repeat of half-sites separated by one nucleotide (DR1), and displays a ligand binding domain (LBD) that allows the adoption of multiple conformations and the dimerization with different NRs. Interestingly, the RXR heterodimer partners, with the exception of the NR-related 1 protein (NURR1), are all ligand dependent (77, 83–85).
As transcription factors, NRs rely on DNA sequence-specific binding to transactivate their target genes. NRs possess a DNA binding domain composed of two zinc finger motifs which bind a specific response element that can differ in terms of extension, duplication, and orientation of the repeat; accordingly response elements can be selective for a given NR or a class of receptors (86). Moreover NRs exhibit a LBD containing a ligand-dependent activation function-2 (AF-2) motif that mediates coactivator recruitment. In the absence of ligand, the LBD is bound to transcriptional corepressor complexes, while ligand binding to the NR triggers changes in the NR three-dimensional conformation which results in the dissociation of corepressor and in a subsequent recruitment of tissue-specific coregulators (87). Of note, the conserved modular structure of NRs is completed by an N-terminal ligand-independent activation function domain (AF-1) and a carbossi-terminal domain. Among the RXR heterodimers, lipid sensing NRs, including liver X receptor (LXR), farnesoid X receptor (FXR), and PPAR, are abundantly expressed along the gastrointestinal system (88), thus suggesting a crucial role of these transcription factors in the intestine with relevance to regulation of HDL-cholesterol metabolism, RCT, and TICE. Indeed, it is tempting to hypothesize that the intestinal epithelium may take advantage of these molecular sensors of nutrients and lipids to monitor cholesterol fluxes across enterocytes, as they sit at the crossroad of sterol disposal.
INTESTINAL MUCOSA EXPRESSION PATTERN OF NRs
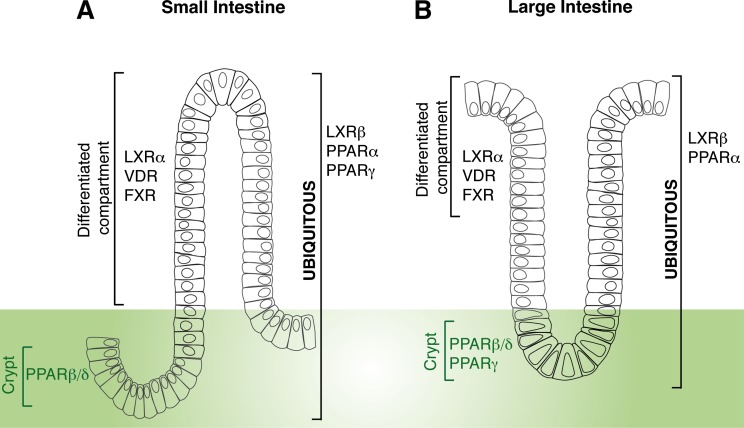
The relationship between NR expression, function, and physiology has been explored by clustering NR tissue expression distribution profiles and has revealed the existence of a hierarchical network tying NR function to development, basal metabolic functions, dietary lipid metabolism, and energy homeostasis. The NR expression pattern was first investigated in normal mouse samples, and has mined initial information about the regulatory elements required to govern a specific network of receptors sharing common expression profiles (88). Lipid-sensing NRs, including LXRs, FXRs, PPARs, and vitamin D receptors (VDRs), appeared expressed at moderate to high levels along the murine gastro-enteric system, thus supporting the concept of the intestine as gatekeeper of lipid homeostasis being the intestinal mucosa responsible for sensing luminal contents. Thus, an “enteric NR team” composed of LXRs, FXRs, PPARs, and other NRs sits at the crossroad of nutritional and hormonal signals modulating the intertwined interactions between dietary lipid fluxes across the enterocyte and the intestinal epithelium homeostasis. The intestine is the most rapidly self-renewing tissue, and the homeostasis of its epithelium relies on the integrated control of proliferation, differentiation, and apoptosis, as well as on the functional architecture of the enterocytes. The permanent renewal takes place in the crypt-to-villus axis and is accomplished by the stem cells located at the bottom of the crypts that generate four differentiated cell types: Paneth cells, goblet cells, enteroendocrine cells, and the absorptive enterocytes. The last three cell types differentiate during their migration upward from crypts toward the tip of the villi, where they die by apoptosis and are shed into the lumen (89–92). The mechanisms controlling cell transition from crypt-to-villus tip involve transcription factors that switch on and off compartment- and cell-specific genes (93). By using in situ hybridization and systematic real-time quantitative polymerase chain reaction, the enteric NR family has been clustered into different quantitative expression and qualitative cell-type-specific networks (93). The fatty acid sensor, PPARβ/δ, is mostly localized between the proliferative progenitor cells and the lower third of the crypt, while a group of NRs including LXRβ, PPARα, (and PPARγ only in the small intestine) is more ubiquitously expressed in the intestinal mucosal epithelium. Finally, the BA and vitamin D sensors, namely FXR and VDR, as well as the oxysterol sensor, LXRα, are mostly expressed in the fully differentiated cells lining the intestinal epithelium (Fig. 2). Importantly, the NR expression pattern was found to predict the modulation of its expression in tumors (93). The degree of NR enteric expression is suggestive of their great potential as targets with regard to the transcriptional modulation of the multifaceted functions of the small intestine as both a cholesterol-absorbing and an excretory organ.
Fig. 2.
Overview of intestinal expression pattern of NRs relevant to cholesterol trafficking across enterocytes. In the differentiated cells lining the epithelium of both small (A) and large (B) intestine oxysterol-, BA-, and vitamin D-sensing receptors (LXRα, FXR, and VDR) are mostly expressed, while a fatty acid-sensing receptor, such as PPARβδ, is mostly localized to the proliferative progenitor cells in the crypt. A more ubiquitous expression pattern has been found for LXRβ and PPARα. The fatty acid sensor PPARγ displays a more ubiquitous expression pattern in the small intestine, while being mostly confined to the crypt in the large intestine (93).
NRs IN THE ENTEROCYTE
LXR
The NRs LXRα and LXRβ are oxysterol-activated transcription factors and function as chief regulators of cholesterol homeostasis by controlling cholesterol influx, transport, and efflux (94, 95). Studies in LXR-deficient mice (96, 97) and use of LXR agonists have provided compelling evidence that LXRs are critical for cholesterol homeostasis and that they exert atheroprotective effects including promotion of RCT, elevation of HDL-cholesterol levels, inhibition of cholesterol absorption, and stimulation of TICE (8, 31, 61, 98, 99). However, the use of LXR agonists in therapy has been hampered by the observation that LXR-driven atheroprotection is accompanied by hypertriglyceridemia, which has been attributed to the LXRα isoform, mostly expressed in the liver (100). As LXRs are expressed in multiple tissues involved in RCT, including macrophages, liver, and intestine, there has been an increasing interest in the development of tissue-selective LXR modulators. An earlier study in LDL receptor-deficient mice showed the ability of a LXR agonist, named ATI-829, to selectively activate LXR target gene expression in mouse intestine and macrophages, but not in the liver, thus providing initial evidence of the feasibility of a tissue-specific LXR activation (100). A few years later, Dan Rader’s group reported the identification of an intestine-specific LXR agonist, named GW6340, which was able to promote macrophage RCT in vivo, thus suggesting that macrophage LXR itself plays a nondominant role in promoting RCT in response to a LXR agonist (101). Finally, constitutive intestinal LXRα activation has been found to reduce cholesterol absorption, increase preβ HDL particles, and protect from diet-induced atherosclerosis without any side effects such as liver steatosis and increased fatty acid synthesis (71).
In the intestine, LXRs control both cholesterol excretion and luminal reabsorption by transcriptionally regulating critical uptake and delivery proteins involved in RCT and TICE (33, 38, 61, 103). Studies with LXR agonists clarified that regulation of sterol loss by LXR depends on the function of the ABCG5/G8 heterodimer (31) while being independent from ABCA1 (102). In contrast, studies in intestine-specific ABCA1-deficient mice implicated ABCA1 in the LXR-dependent increase in plasma HDL (99). These studies highlighted the importance of intestine-specific cholesterol absorption and efflux pathways in regulating HDL biogenesis and cholesterol homeostasis. Whether cholesterol absorption per se is required for the HDL-raising properties of the LXR agonist has been studied in NPC1L1-deficient mice (103) and was recently evaluated in intestine-specific MTP-deficient mice (73). Xie et al. (73) demonstrated that LXR agonist administration led to an increase in HDL biogenesis in the absence of chylomicron secretion, thus illustrating the complex and interrelated pathways that modulate cholesterol efflux pathways in vivo. Because two isoforms exist, the significance of LXRα and LXRβ on cholesterol absorption was investigated (72). Hu et al. (72) reported that selective LXRβ activation increased cholesterol absorption and apoB-containing lipoprotein secretion, which seem to be counteracted by LXRα isoform; thus, these data underscored the relevance of an isoform-specific LXR modulation.
Collectively, strategies targeting LXR in an isoform-specific or tissue-selective manner may hold promise in our understanding of how we can exploit LXR activation as a powerful tool in the management of cholesterol overload-induced chronic disorders.
PPARs
PPARs are ligand-activated transcription factors that act as fatty acid sensors to control metabolic programs and to regulate energy homeostasis (104). PPAR ligands are represented by native and modified (oxidized and nitrated) fatty acids, eicosanoids, derivatives of polyunsaturated fatty acids, fibrates, and thiazolidinedione. Three isoforms exist in mammals (α, β/δ, and γ), characterized by a very specific tissue distribution and physiological functions with the PPARα isoform mediating fibrate action on HDL-cholesterol levels via transcriptional induction of synthesis of the major HDL apolipoproteins, apoAI and apoAII (105–110). With regard to intestinal expression, PPARα and PPARβ/δ are localized to the small intestine while PPARγ is mostly confined in the large intestine (colon) (86). Thus, PPARα and PPARδ activation has been found to stimulate both RCT and TICE (62, 70, 74, 75). PPARα’s ability to increase HDL cholesterol levels has been typically attributed to activation of PPARα in the liver; however, an earlier study by Colin et al. (74) highlighted the role of PPARα in the intestine in increasing HDL and showed that upon PPARα activation, apoAI and ABCA1 mRNA were increased, thus leading to augmented HDL lipidation and number of HDL particles secreted by the enterocytes. More recently, studies in mice receiving PPARα/δ modulator (named GFT505) suggested that intestinal PPARα upregulation regulates HDL production by inducing ABCA1 expression and apoAI secretion, and reducing cholesterol esterification (74).
Of the several PPAR isoforms, PPARβ/δ is the least understood, but the recent discovery of PPARδ agonists and their ability to increase HDL cholesterol levels and RCT (111, 112) shed light on the therapeutic potential of PPARδ modulators in cholesterol overload-induced clinical conditions. As PPARδ agonists increase HDL-cholesterol concentrations, they therefore have the potential to stimulate macrophage-to-feces RCT. In vivo studies have shown that PPARδ agonists promote RCT by reducing NPC1L1 expression rather than by stimulating macrophage cholesterol efflux, and increase HDL cholesterol without any ABCA1 upregulation either in the liver or in the small intestine (70). Similarly, van der Veen et al. (69) reported that PPARδ activation is associated with elevated plasma HDL and reduced cholesterol absorption efficiency that may be related to downregulation of NPC1L1 expression.
The role of PPARγ in the intestine with regard to cholesterol homeostasis has been poorly investigated. Recent studies indicate close intriguing links between microbial communities and regulation of intestinal PPARγ expression by colonocytes, and suggests that lipopolysaccharide from Gram-negative bacteria is critical in colonic steady state PPARγ expression through Toll-like receptor 4 (112). Studies in vitro have shown that PPARγ agonist (troglitazone) treatment was able to lower cholesterol synthesis via reduced concentration of nuclear SREBP2 (113), while studies in vivo reported that combined deficiency of MTP and ABCA1 was associated with increased PPARγ expression and accompanied with a significant reduction of plasma cholesterol levels (114). Further studies are ongoing to assess commensal bacteria ability to induce PPARγ expression and activation and to evaluate whether this may have relevance with regard to cholesterol homeostasis. In mammals, it has long been known that luminal cholesterol of both exogenous and endogenous origin is metabolized by the intestinal microbial community and the end-product is mostly the poorly absorbable coprostanol, whose formation facilitates cholesterol removal from the body. Whether PPARγ may participate in cholesterol homeostasis via involvement in bacterial conversion to coprostanol may be the focus of future studies. Moreover, the ability of the PPARγ agonist, pioglitazone, to inhibit cholesterol absorption in rats (115) and to increase HDL-cholesterol levels in diabetic patients (116, 117) provides the impetus to further investigate the hypocholesterolemic potential of PPARγ activation.
FXR
FXR is the master regulator of BA homeostasis by controlling BA synthesis, efflux, influx, and detoxification in the gut/liver axis (118–121). In the liver, FXR stimulates BA and phospholipid secretion into bile and BA conjugation and detoxification, and represses the BA-synthesizing enzyme CYP7A1 (122–124). In the intestine FXR regulates fibroblast growth factor 15 (FGF15) expression (124) as well as genes involved in apical BA uptake [apical sodium BA cotransporter (ASBT)] (125), intra-enterocyte BA transport [ileal BA binding protein (IBABP)] (126–129), and basolateral BA secretion [organic solute transporter α and β (OSTαβ)] (129). Although FXR activation has been reported to decrease serum cholesterol and apoAI concentrations while increasing phospholipid transfer protein (PLTP)-mediated HDL remodeling (130, 131), the role of intestinal-specific FXR activation has not yet been studied with regard to changes in cholesterol metabolism. Indeed, constitutive intestinal FXR activation has been reported to protect mice from cholestasis and to lower both biliary BA and cholesterol (132). These data seem to implicate a link between upregulation of intestinal FXR transcriptome and modulation of cell and tissue sterol content. In line with this, a recent study highlighted a relationship among cholesterol levels, SREBP2, and intestinal FXR transcriptional machinery, thus suggesting that SREBP2 negatively regulates FXR-mediated induction of the FGF19 gene in human intestinal cells (133). Future studies aiming at investigating how intestinal FXR-mediated CYP7A1 repression along with modulation of luminal BA content may impact cholesterol homeostasis are urgently awaited.
PERSPECTIVES FROM PHYSIOLOGY TO PHARMACOLOGY
The role of the intestine in the regulation of whole-body cholesterol homeostasis has been underestimated for a long time, but during the last 10 years a compelling body of evidence has greatly contributed to the appreciation of the gatekeeper role of the intestine in cholesterol homeostasis maintenance. The intestine has thus emerged as a dynamic organ with tremendous therapeutic potential, as it houses a large variety of proteins that sense (NRs), modify (microsomal enzymes), and transport (apical and basolateral transporters) cholesterol across the intestinal epithelium, thus priming its metabolic fate. The intestine functions as an absorptive and excretory organ regulating cholesterol fluxes across the enterocytes and out of the body. While the intestinal cholesterol absorption mechanisms have been largely explored, thus providing the knowledge necessary for the development of pharmacological tools able to interfere with the numerous cellular factors involved, only recently, the intestinal role as a cholesterol excretory organ in RCT and partly via TICE has received attention. The process of RCT involves principally HDL-mediated delivery of peripheral cholesterol to the liver for biliary secretion. However, the observation that a small percentage of RCT persists in genetic or surgical models of biliary insufficiency (33, 56, 134) supports the existence of a nonbiliary fecal cholesterol disposal pathway (60). However, HDL lipoproteins do not appear to be involved in this nonbiliary RCT (58). Nevertheless, the biliary and nonbiliary RCT pathway appeared to be highly dynamic and amenable to NR-based pharmacologic interventions (52, 53, 56, 62, 63). Pharmacological activation of both LXR and PPARδ has been reported to independently stimulate each of these intestinal pathways for cholesterol removal and promote the identification of the lipoproteins delivering cholesterol to the small intestine, the potential receptors involved in intestinal lipoprotein cholesterol uptake, and the factors controlling the trafficking of cholesterol across enterocyte and into the intestinal lumen. Unfortunately, most of these vexing questions are still unanswered and recent studies linking microbiota, RCT, and atherosclerosis (135) have opened new possibilities of targeting the intestine as a source of molecules displaying a pro/anti-atherogenic potential. To this end, a deeper knowledge of PPARγ function in the colon and its potential involvement in the regulation of the bacterial communities, particularly those able to convert cholesterol to coprostanol, may hold promise and deserve further investigation. Moreover, the intestinal NR network includes members whose functions in the intestine with regard to RCT have never been investigated, including FXR and VDR. Finally, targeting the multifaceted functions of the intestine as a solid gatekeeper of plasma cholesterol homeostasis may be a burgeoning area for pharmaceutical efforts with the goal of managing the clinical conditions arising from or subsequent to deregulated sterol metabolism.
Acknowledgments
The authors apologize to their distinguished colleagues whose work has not been cited owing to space limitations. The authors are indebted to Dr. Roberta Le Donne for the art work.
Footnotes
Abbreviations:
- BA
- bile acid
- CE
- cholesteryl ester
- CYP7A1
- cholesterol-7α-hydroxylase
- FC
- free cholesterol
- FXR
- farnesoid X receptor
- LBD
- ligand binding domain
- LXR
- liver X receptor
- MTP
- microsomal transfer protein
- NPC1L1
- Niemann-Pick C1-like 1
- NR
- nuclear receptor
- RCT
- reverse cholesterol transport
- RXR
- retinoid X receptor
- SREBP
- sterol-regulatory element binding protein
- TICE
- trans-intestinal cholesterol efflux
- VDR
- vitamin D receptor
This work was funded by NR-NET FP7 Marie Curie ITN, Italian Association for Cancer Research (AIRC, IG 14732), Italian Ministry of University and Education (Finanziamenti per la Ricerca di Base IDEAS RBID08C9N7; PRIN 2010FHH32M-002), Italian Ministry of Health (Young Researchers Grant GR-2008-1143546; GR-2010-2314703), and University of Bari (IDEA GRBA0802SJ-2008).
REFERENCES
- 1.Ikonen E. 2008. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9: 125–138. [DOI] [PubMed] [Google Scholar]
- 2.Liscum L., Dahl N. K. 1992. Intracellular cholesterol transport. J. Lipid Res. 33: 1239–1254. [PubMed] [Google Scholar]
- 3.Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietschy J. M., Siperstein M. D. 1967. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J. Lipid Res. 8: 97–104. [PubMed] [Google Scholar]
- 5.Spady D. K., Dietschy J. M. 1983. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J. Lipid Res. 24: 303–315. [PubMed] [Google Scholar]
- 6.Spady D. K., Woollett L. A., Dietschy J. M. 1993. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu. Rev. Nutr. 13: 355–381. [DOI] [PubMed] [Google Scholar]
- 7.Kruit J. K., Groen A. K., van Berkel T. J., Kuipers F. 2006. Emerging roles of the intestine in control of cholesterol metabolism. World J. Gastroenterol. 12: 6429–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonamassa B., Moschetta A. 2013. Atherosclerosis: lessons from LXR and the intestine. Trends Endocrinol. Metab. 24: 120–128. [DOI] [PubMed] [Google Scholar]
- 9.Altmann S. W., Davis H. R., Jr, Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maquire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 10.Davies J. P., Scott C., Oishi K., Liapis A., Ioannou Y. A. 2005. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 280: 12710–12720. [DOI] [PubMed] [Google Scholar]
- 11.Davis H. R., Jr, Zhu L. J., Hoos L. M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S. P., Lam M. H., Lund E. G., et al. 2004. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279: 33586–33592. [DOI] [PubMed] [Google Scholar]
- 12.Clader J. W. 2004. The discovery of ezetimibe: a view from outside the receptor. J. Med. Chem. 47: 1–9. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Calvo M., Lisnock J., Bull H. G., Hawes B. E., Burnett D. A., Braun M. P., Crona J. H., Davis H. R., Jr, Dean D. C., Detmers P. A., et al. 2005. The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA. 102: 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz M., Russell D. W., Dietschy J. M., Turley S. D. 1998. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39: 1833–1843. [PubMed] [Google Scholar]
- 15.Anderson R. A., Joyce C., Davis M., Reagan J. W., Clark M., Shelness G. S., Rudel L. L. 1998. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 273: 26747–26754. [DOI] [PubMed] [Google Scholar]
- 16.Rudel L. L., Lee R. G., Cockman T. L. 2001. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr. Opin. Lipidol. 12: 121–127. [DOI] [PubMed] [Google Scholar]
- 17.Willner E. L., Tow B., Buhman K. K., Wilson M., Sanan D. A., Rudel L. L., Farese R. V., Jr 2003. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA. 100: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee R. G., Shah R., Sawyer J. K., Hamilton R. L., Parks J. S., Rudel L. L. 2005. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl ester to LDL in mice. J. Lipid Res. 46: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 19.Lee M. H., Lu K., Hazard S., Yu H., Shulenin S., Hidaka H., Kojima H., Allikmets R., Sakuma N., Pegoraro R., et al. 2001. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 27: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775. [DOI] [PubMed] [Google Scholar]
- 21.Repa J. J., Berge K. E., Pomajzl C., Richardson J. A., Hobbs H., Mangelsdorf D. J. 2002. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 277: 18793–18800. [DOI] [PubMed] [Google Scholar]
- 22.Graf G. A., Li W. P., Gerard R. D., Gelissen I., White A., Cohen J. C., Hobbs H. H. 2002. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Invest. 110: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graf G. A., Yu L., Li W. P., Gerard R., Tuma P. L., Cohen J. C., Hobbs H. H. 2003. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278: 48275–48282. [DOI] [PubMed] [Google Scholar]
- 24.Buhman K. K., Accad M., Novak S., Choi R. S., Wong J. S., Hamilton R. L., Turley S., Farese R. V., Jr 2000. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 6: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 25.Repa J. J., Buhman K. K., Farese R. V., Jr, Dietschy J. M., Turley S. D. 2004. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 40: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen T. M., Sawyer J. K., Kelley K. L., Davis M. A., Rudel L. L. 2012. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen T. M., Sawyer J. K., Kelley K. L., Davis M. A., Kent C. R., Rudel L. L. 2012. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53: 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain M. M., Leung T. M., Zhou L., Abu-Merhi S. 2013. Regulating intestinal function to reduce atherogenic lipoproteins. Clin. Lipidol. 8: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L., York J., von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2003. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J. Biol. Chem. 278: 15565–15570. [DOI] [PubMed] [Google Scholar]
- 31.Yu L., Gupta S., Xu F., Liverman A. D., Moschetta A., Mangelsdorf D. J., Repa J. J., Hobbs H. H., Cohen J. C. 2005. Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J. Biol. Chem. 280: 8742–8747. [DOI] [PubMed] [Google Scholar]
- 32.Temel R. E., Sawyer J. K., Yu L., Lord C., Degirolamo C., McDaniel A., Marshall S. M., Wang N., Shah R., Rudel L. L., et al. 2010. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 12: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glomset J. A. 1968. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 9: 155–167. [PubMed] [Google Scholar]
- 34.Glomset J. A., Norum K. R. 1973. The metabolic role of lecithin:cholesterol acyltransferase: perspectives form pathology. Adv. Lipid Res. 11: 1–65. [PubMed] [Google Scholar]
- 35.Glickman R. M., Green P. H. 1977. The intestine as a source of apolipoprotein A1. Proc. Natl. Acad. Sci. USA. 74: 2569–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunham L. R., Kruit J. K., Pape T. D., Parks J. S., Kuipers F., Hayden M. R. 2006. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ. Res. 99: 672–674. [DOI] [PubMed] [Google Scholar]
- 38.Sehayek E., Hazen S. L. 2008. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler. Thromb. Vasc. Biol. 28: 1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietschy J. M., Turley S. D. 2002. Control of cholesterol turnover in the mouse. J. Biol. Chem. 277: 3801–3804. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Rader D. J. 2007. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 22: 368–372. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz G., Kaminski W. E., Porsch-Ozcurumez M., Klucken J., Orso E., Bodzioch M., Buchler C., Drobnik W. 1999. ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism? Pathobiology. 67: 236–240. [DOI] [PubMed] [Google Scholar]
- 42.Oram J. F., Vaughan A. M. 2000. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr. Opin. Lipidol. 11: 253–260. [DOI] [PubMed] [Google Scholar]
- 43.Wade D. P., Owen J. S. 2001. Regulation of the cholesterol efflux gene, ABCA1. Lancet. 357: 161–163. [DOI] [PubMed] [Google Scholar]
- 44.Wang N., Tall A. R. 2003. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26: 20–27. [DOI] [PubMed] [Google Scholar]
- 46.Murthy S., Born E., Mathur S. N., Field F. J. 2002. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J. Lipid Res. 43: 1054–1064. [DOI] [PubMed] [Google Scholar]
- 47.Groen A. K., Bloks V. W., Bandsma R. H., Ottenhoff R., Chimini G., Kuipers F. 2001. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J. Clin. Invest. 108: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jolley C. D., Woollett L. A., Turley S. D., Dietschy J. M. 1998. Centripetal cholesterol flux to the liver is dictated by events in the peripheral organs and not by the plasma high density lipoprotein or apolipoprotein A-I concentration. J. Lipid Res. 39: 2143–2149. [PubMed] [Google Scholar]
- 49.Xie C., Turley S. D., Dietschy J. M. 2009. ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J. Lipid Res. 50: 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voshol P. J., Havinga R., Wolters H., Ottenhoff R., Princen H. M., Oude Elferink R. P., Groen A. K., Kuipers F. 1998. Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P-glycoprotein-deficient mice. Gastroenterology. 114: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 51.Kruit J. K., Plosch T., Havinga R., Boverhof R., Groot P. H., Groen A. K., Kuipers F. 2005. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology. 128: 147–156. [DOI] [PubMed] [Google Scholar]
- 52.Temel R. E., Tang W., Ma Y., Rudel L. L., Willingham M. C., Ioannou Y. A., Davies J. P., Nilsson L. M., Yu L. 2007. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 117: 1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown J. M., Bell T. A., III, Alger H. M., Sawyer J. K., Smith T. L., Kelley K., Shah R., Wilson M. D., Davis M. A., Lee R. G., et al. 2008. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J. Biol. Chem. 283: 10522–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Velde A. E., Vrins C. L., van den Oever K., Kunne C., Oude Elferink R. P., Kuipers F., Groen A. K. 2007. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 133: 967–975. [DOI] [PubMed] [Google Scholar]
- 55.van der Velde A. E., Brufau G., Groen A. K. 2010. Transintestinal cholesterol efflux. Curr. Opin. Lipidol. 21: 167–171. [DOI] [PubMed] [Google Scholar]
- 56.Brufau G., Kuipers F., Lin Y., Trautwein E. A., Groen A. K. 2011. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS ONE. 6: e21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vrins C. L. 2010. From blood to gut: direct secretion of cholesterol via transintestinal cholesterol efflux. World J. Gastroenterol. 16: 5953–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrins C. L., Ottenhoff R., van den Oever K., de Waart D. R., Kruyt J. K., Zhao Y., van Berkel T. J., Havekes L. M., Aerts J. M., van Eck M., et al. 2012. Trans-intestinal cholesterol efflux is not mediated through high density lipoprotein. J. Lipid Res. 53: 2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groen A. K., Bloks V. W., Verkade H., Kuipers F. 2014. Cross-talk between liver and intestine in control of cholesterol and energy homeostasis. Mol. Aspects Med. 37: 77–88. [DOI] [PubMed] [Google Scholar]
- 60.van der Veen J. N., van Dijk T. H., Vrins C. L., van Meer H., Havinga R., Bijsterveld K., Tietge U. J., Groen A. K., Kuipers F. 2009. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284: 19211–19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vrins C. L., van der Velde A. E., van den Oever K., Levels J. H., Huet S., Oude Elferink R. P., Kuipers F., Groen A. K. 2009. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J. Lipid Res. 50: 2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Velde A. E., Vrins C. L., van den Oever K., Seemann I., Oude Elferink R. P., van Eck M., Kuipers F., Groen A. K. 2008. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G203–G208. [DOI] [PubMed] [Google Scholar]
- 63.Le May C., Berger J. M., Lespine A., Pillot B., Prieur X., Letessier E., Hussain M. M., Collet X., Cariou B., Costet P. 2013. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 33: 1484–1493. [DOI] [PubMed] [Google Scholar]
- 64.Jakulj L., Vissers M. N., van Roomen C. P., van der Veen J. N., Vrins C. L., Kunne C., Stellaard F., Kastelein J. J., Groen A. K. 2010. Ezetimibe stimulates faecal neutral sterol excretion depending on abcg8 function in mice. FEBS Lett. 584: 3625–3628. [DOI] [PubMed] [Google Scholar]
- 65.Yamanashi Y., Takada T., Yoshikado T., Shoda J., Suzuki H. 2011. NPC2 regulates biliary cholesterol secretion via stimulation of ABCG5/G8-mediated cholesterol transport. Gastroenterology. 140: 1664–1674. [DOI] [PubMed] [Google Scholar]
- 66.Bura K. S., Lord C., Marshall S., McDaniel A., Thomas G., Warrier M., Zhang J., Davis M. A., Sawyer J. K., Shah R., et al. 2013. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J. Lipid Res. 54: 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall S. M., Kelley K. L., Davis M. A., Wilson M. D., McDaniel A. L., Lee R. G., Crooke R. M., Graham M. J., Rudel L. L., Brown J. M., et al. 2014. Reduction of VLDL secretion decreases cholesterol excretion in niemann-pick C1-like 1 hepatic transgenic mice. PLoS ONE. 9: e84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall S. M., Gromovsky A. D., Kelley K. L., Davis M. A., Wilson M. D., Lee R. G., Crooke R. M., Graham M. J., Rudel L. L., Brown J. M., et al. 2014. Acute sterol O-acyltransferase 2 (SOAT2) knockdown rapidly mobilizes hepatic cholesterol for fecal excretion. PLoS ONE. 9: e98953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Veen J. N., Kruit J. K., Havinga R., Baller J. F., Chimini G., Lestavel S., Staels B., Groot P. H., Groen A. K., Kuipers F. 2005. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J. Lipid Res. 46: 526–534. [DOI] [PubMed] [Google Scholar]
- 70.Briand F., Naik S. U., Fuki I., Millar J. S., Macphee C., Walker M., Billheimer J., Rothblat G., Rader D. J. 2009. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin. Transl. Sci. 2: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lo Sasso G., Murzilli S., Salvatore L., D’Errico I., Petruzzelli M., Conca P., Jiang Z. Y., Calabresi L., Parini P., Moschetta A. 2010. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 12: 187–193. [DOI] [PubMed] [Google Scholar]
- 72.Hu X., Steffensen K. R., Jiang Z. Y., Parini P., Gustafsson J. A., Gafvels M., Eggertsen G. 2012. LXRbeta activation increases intestinal cholesterol absorption, leading to an atherogenic lipoprotein profile. J. Intern. Med. 272: 452–464. [DOI] [PubMed] [Google Scholar]
- 73.Xie Y., Kennedy S., Sidhu R., Luo J., Ory D. S., Davidson N. O. 2012. Liver X receptor agonist modulation of cholesterol efflux in mice with intestine-specific deletion of microsomal triglyceride transfer protein. Arterioscler. Thromb. Vasc. Biol. 32: 1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colin S., Briand O., Touche V., Wouters K., Baron M., Pattou F., Hanf R., Tailleux A., Chinetti G., Staels B., et al. 2013. Activation of intestinal peroxisome proliferator-activated receptor-alpha increases high-density lipoprotein production. Eur. Heart J. 34: 2566–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Out C., Dikkers A., Laskewitz A., Boverhof R., van der Ley C., Kema I. P., Wolters H., Havinga R., Verkade H. J., Kuipers F., et al. 2014. Prednisolone increases enterohepatic cycling of bile acids by induction of Asbt and promotes reverse cholesterol transport. J. Hepatol. 61: 351–357. [DOI] [PubMed] [Google Scholar]
- 76.Evans R. M., Mangelsdorf D. J. 2014. Nuclear receptors, RXR, and the Big Bang. Cell. 157: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. 1990. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 345: 224–229. [DOI] [PubMed] [Google Scholar]
- 78.Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., et al. 1995. The nuclear receptor superfamily: the second decade. Cell. 83: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 1992. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 68: 397–406. [DOI] [PubMed] [Google Scholar]
- 80.Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. 1992. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 358: 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A., et al. 1992. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 355: 359–361. [DOI] [PubMed] [Google Scholar]
- 82.Aarnisalo P., Kim C. H., Lee J. W., Perlmann T. 2002. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J. Biol. Chem. 277: 35118–35123. [DOI] [PubMed] [Google Scholar]
- 83.Lou X., Toresson G., Benod C., Suh J. H., Philips K. J., Webb P., Gustafsson J. A. 2014. Structure of the retinoid X receptor α-liver X receptor β (RXRα-LXRβ) heterodimer on DNA. Nat. Struct. Mol. Biol. 21: 277–281. [DOI] [PubMed] [Google Scholar]
- 84.Sacchetti P., Dwornik H., Formstecher P., Rachez C., Lefebvre P. 2002. Requirements for heterodimerization between the orphan nuclear receptor Nurr1 and retinoid X receptors. J. Biol. Chem. 277: 35088–35096. [DOI] [PubMed] [Google Scholar]
- 85.Vacca M., Degirolamo C., Mariani-Costantini R., Palasciano G., Moschetta A. 2011. Lipid-sensing nuclear receptors in the pathophysiology and treatment of the metabolic syndrome. Wiley Interdiscip. Rev. Syst. Biol. Med. 3: 562–587. [DOI] [PubMed] [Google Scholar]
- 86.McKenna N. J., O’Malley B. W. 2001. Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann. N. Y. Acad. Sci. 949: 3–5. [DOI] [PubMed] [Google Scholar]
- 87.Bookout A. L., Jeong Y., Downes M., Yu R. T., Evans R. M., Mangelsdorf D. J. 2006. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 126: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Flier L. G., Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71: 241–260. [DOI] [PubMed] [Google Scholar]
- 89.Barker N. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15: 19–33. [DOI] [PubMed] [Google Scholar]
- 90.Simons B. D., Clevers H. 2011. Stem cell self-renewal in intestinal crypt. Exp. Cell Res. 317: 2719–2724. [DOI] [PubMed] [Google Scholar]
- 91.Crosnier C., Stamataki D., Lewis J. 2006. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7: 349–359. [DOI] [PubMed] [Google Scholar]
- 92.D’Errico I., Moschetta A. 2008. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell. Mol. Life Sci. 65: 1523–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Modica S., Gofflot F., Murzilli S., D’Orazio A., Salvatore L., Pellegrini F., Nicolucci A., Tognoni G., Copetti M., Valanzano R., et al. 2010. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 138:636–48, 648. [DOI] [PubMed] [Google Scholar]
- 94.Peet D. J., Janowski B. A., Mangelsdorf D. J. 1998. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8: 571–575. [DOI] [PubMed] [Google Scholar]
- 95.Peet D. J., Turley S. D., Ma W., Janowski B. A., Lobaccaro J. M., Hammer R. E., Mangelsdorf D. J. 1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 93: 693–704. [DOI] [PubMed] [Google Scholar]
- 96.Alberti S., Schuster G., Parini P., Feltkamp D., Diczfalusy U., Rudling M., Angelin B., Bjorkhem I., Pettersson S., Gustafsson J. A. 2001. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J. Clin. Invest. 107: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tontonoz P., Mangelsdorf D. J. 2003. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17: 985–993. [DOI] [PubMed] [Google Scholar]
- 98.Hong C., Tontonoz P. 2014. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13: 433–444. [DOI] [PubMed] [Google Scholar]
- 99.Schultz J. R., Tu H., Luk A., Repa J. J., Medina J. C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D. J., et al. 2000. Role of LXRs in control of lipogenesis. Genes Dev. 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng D., Hiipakka R. A., Dai Q., Guo J., Reardon C. A., Getz G. S., Liao S. 2008. Antiatherosclerotic effects of a novel synthetic tissue-selective steroidal liver X receptor agonist in low-density lipoprotein receptor-deficient mice. J. Pharmacol. Exp. Ther. 327: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yasuda T., Grillot D., Billheimer J. T., Briand F., Delerive P., Huet S., Rader D. J. 2010. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 30: 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Plösch T., Kok T., Bloks V. W., Smit M. J., Havinga R., Chimini G., Groen A. K., Kuipers F. 2002. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 277: 33870–33877. [DOI] [PubMed] [Google Scholar]
- 103.Tang W., Ma Y., Jia L., Ioannou Y. A., Davies J. P., Yu L. 2008. Niemann-Pick C1-like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler. Thromb. Vasc. Biol. 28: 448–454. [DOI] [PubMed] [Google Scholar]
- 104.Tontonoz P., Spiegelman B. M. 2008. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77: 289–312. [DOI] [PubMed] [Google Scholar]
- 105.Vu-Dac N., Schoonjans K., Laine B., Fruchart J. C., Auwerx J., Staels B. 1994. Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J. Biol. Chem. 269: 31012–31018. [PubMed] [Google Scholar]
- 106.Vu-Dac N., Schoonjans K., Kosykh V., Dallongeville J., Fruchart J. C., Staels B., Auwerx J. 1995. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J. Clin. Invest. 96: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peters J. M., Hennuyer N., Staels B., Fruchart J. C., Fievet C., Gonzalez F. J., Auwerx J. 1997. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J. Biol. Chem. 272: 27307–27312. [DOI] [PubMed] [Google Scholar]
- 108.Jin F. Y., Kamanna V. S., Chuang M. Y., Morgan K., Kashyap M. L. 1996. Gemfibrozil stimulates apolipoprotein A-I synthesis and secretion by stabilization of mRNA transcripts in human hepatoblastoma cell line (Hep G2). Arterioscler. Thromb. Vasc. Biol. 16: 1052–1062. [DOI] [PubMed] [Google Scholar]
- 109.Saku K., Gartside P. S., Hynd B. A., Kashyap M. L. 1985. Mechanism of action of gemfibrozil on lipoprotein metabolism. J. Clin. Invest. 75: 1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oliver W. R., Jr, Shenk J. L., Snaith M. R., Russell C. S., Plunket K. D., Bodkin N. L., Lewis M. C., Winegar D. A., Sznaidman M. L., Lambert M. H., et al. 2001. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA. 98: 5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sprecher D. L., Massien C., Pearce G., Billin A. N., Perlstein I., Willson T. M., Hassall D. G., Ancellin N., Patterson S. D., Lobe D. C., et al. 2007. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler. Thromb. Vasc. Biol. 27: 359–365. [DOI] [PubMed] [Google Scholar]
- 112.Peyrin-Biroulet L., Beisner J., Wang G., Nuding S., Oommen S. T., Kelly D., Parmentier-Decrucq E., Dessein R., Merour E., Chavatte P., et al. 2010. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc. Natl. Acad. Sci. USA. 107: 8772–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klopotek A., Hirche F., Eder K. 2006. PPAR gamma ligand troglitazone lowers cholesterol synthesis in HepG2 and Caco-2 cells via a reduced concentration of nuclear SREBP-2. Exp. Biol. Med. (Maywood). 231: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 114.Iqbal J., Parks J. S., Hussain M. M. 2013. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J. Biol. Chem. 288: 30432–30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colca J. R., Dailey C. F., Palazuk B. J., Hillman R. M., Dinh D. M., Melchior G. W., Spilman C. H. 1991. Pioglitazone hydrochloride inhibits cholesterol absorption and lowers plasma cholesterol concentrations in cholesterol-fed rats. Diabetes. 40: 1669–1674. [DOI] [PubMed] [Google Scholar]
- 116.Al Majali K., Cooper M. B., Staels B., Luc G., Taskinen M. R., Betteridge D. J. 2006. The effect of sensitisation to insulin with pioglitazone on fasting and postprandial lipid metabolism, lipoprotein modification by lipases, and lipid transfer activities in type 2 diabetic patients. Diabetologia. 49: 527–537. [DOI] [PubMed] [Google Scholar]
- 117.Betteridge D. J. 2007. Effects of pioglitazone on lipid and lipoprotein metabolism. Diabetes Obes. Metab. 9: 640–647. [DOI] [PubMed] [Google Scholar]
- 118.Russell D. W. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 119.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Wilson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 120.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102: 731–744. [DOI] [PubMed] [Google Scholar]
- 121.Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., et al. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 6: 517–526. [DOI] [PubMed] [Google Scholar]
- 122.Lee Y. K., Moore D. D. 2008. Liver receptor homolog-1, an emerging metabolic modulator. Front. Biosci. 13: 5950–5958. [DOI] [PubMed] [Google Scholar]
- 123.Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., Mangelsdorf D. J. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 6: 507–515. [DOI] [PubMed] [Google Scholar]
- 124.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., McDonald J. G., Luo G., Jones S. A., Goodwin B., Richardson J. A., et al. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2: 217–225. [DOI] [PubMed] [Google Scholar]
- 125.Lazaridis K. N., Pham L., Tietz P., Marinelli R. A., deGroen P. C., Levine S., Dawson P. A., LaRusso N. F. 1997. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J. Clin. Invest. 100: 2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gong Y. Z., Everett E. T., Schwartz D. A., Norris J. S., Wilson F. A. 1994. Molecular cloning, tissue distribution, and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc. Natl. Acad. Sci. USA. 91: 4741–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tochtrop G. P., DeKoster G. T., Covey D. F., Cistola D. P. 2004. A single hydroxyl group governs ligand site selectivity in human ileal bile acid binding protein. J. Am. Chem. Soc. 126: 11024–11029. [DOI] [PubMed] [Google Scholar]
- 128.Neimark E., Chen F., Li X., Shneider B. L. 2004. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 40: 149–156. [DOI] [PubMed] [Google Scholar]
- 129.Dawson P. A., Hubbert M., Haywood J., Craddock A. L., Zerangue N., Christian W. V., Ballatori N. 2005. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 280: 6960–6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Claudel T., Sturm E., Duez H., Torra I. P., Sirvent A., Kosykh V., Fruchart J. C., Dallongeville J., Hum D. W., Kuipers F., et al. 2002. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 109: 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lambert G., Amar M. J., Guo G., Brewer H. B., Jr, Gonzalez F. J., Sinal C. J. 2003. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 278: 2563–2570. [DOI] [PubMed] [Google Scholar]
- 132.Modica S., Petruzzelli M., Bellafante E., Murzilli S., Salvatore L., Celli N., Di Tullio G., Palasciano G., Moustafa T., Halibasic E., et al. 2012. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 142: 355–365. [DOI] [PubMed] [Google Scholar]
- 133.Miyata M., Hata T., Yamazoe Y., Yoshinari K. 2014. SREBP-2 negatively regulates FXR-dependent transcription of FGF19 in human intestinal cells. Biochem. Biophys. Res. Commun. 443: 477–482. [DOI] [PubMed] [Google Scholar]
- 134.Brufau G., Groen A. K., Kuipers F. 2011. Reverse cholesterol transport revisited: contribution of biliary versus intestinal cholesterol excretion. Arterioscler. Thromb. Vasc. Biol. 31: 1726–1733. [DOI] [PubMed] [Google Scholar]
- 135.Wang D., Xia M., Yan X., Li D., Wang L., Xu Y., Jin T., Ling W. 2012. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 111: 967–981. [DOI] [PubMed] [Google Scholar]