Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that acts either as an intracellular messenger or as a ligand for its membrane receptors. S1P is a normal constituent of blood, where it is found both in plasma and blood cells. Compared with other cell types, sphingolipid metabolism in erythrocytes and platelets has unique features that allow the erythrocytes and platelets to accumulate S1P. In plasma, S1P is bound mainly to HDLs and albumin. Of note, metabolism and biological activity of S1P is to a large extent affected by the type of its carrier. Plasma S1P is characterized by a short half-life, indicating rapid clearance by degradative enzymes and the presence of high-capacity sources involved in maintaining its high concentration. These sources include blood cells, vascular endothelium, and hepatocytes. However, the extent to which each of these contributes to the plasma pool of S1P is a matter of debate. Circulating S1P plays a significant physiological role. It was found to be the key regulator of lymphocyte trafficking, endothelial barrier function, and vascular tone. The purpose of this review is to summarize the present state of knowledge on the metabolism, transport, and origin of plasma S1P, and to discuss the mechanisms regulating its homeostasis in blood.
Keywords: dihydrosphingosine-1-phosphate, endothelial cells, high density lipoprotein, red blood cells, thrombocytes
Sphingosine-1-phosphate (S1P) is a bioactive intermediate of sphingolipid metabolism consisting of two main components: a polar head group (phosphate) and a long-chain sphingoid base backbone (sphingosine) (1). S1P acts not only intracellularly as a second messenger, but also extracellularly as a ligand for its membrane receptors (S1PRs). Moreover, intracellularly generated S1P can be exported and act in paracrine or autocrine fashion (2). S1P induces a wide spectrum of cellular effects, including proliferation, differentiation, survival, and migration (3). It is also implicated in several diseases, including cancer, myocardial infarction, autoimmunity, osteoporosis, and atherosclerosis (4, 5).
Although S1P is constitutively present in all cells, its level in most tissues is low. This is in contrast to plasma where S1P is found in relatively high concentration (2). Circulating S1P serves an important physiological function and is a key regulator of lymphocyte trafficking, endothelial barrier function, and vascular tone (6–8). It also plays an important role in bone homeostasis (9). This review summarizes the present state of knowledge on the metabolism, transport, sources, and regulation of S1P in the plasma and blood cells.
OVERVIEW OF S1P METABOLISM
Sphingosine, the substrate for S1P synthesis, is produced by degradation of ceramide, not de novo biosynthesis. Subsequently sphingosine can be either re-acylated back to ceramide or phosphorylated by sphingosine kinase (SPHK) 1 and 2 to form S1P. Although both SPHK isoforms synthesize the same product, they display different catalytic properties, subcellular locations, tissue distribution, and possibly have unique and specific functions (2). SPHK1 is highly specific for sphingosine and dihydrosphingosine as substrates (10). It resides in the cytosol but translocates to the plasmalemma upon activation (11). A significant fraction of cellular SPHK1 (∼8%) is constitutively released to the extracellular space. The secreted enzyme is active, which enables local production of S1P in the vicinity of its cell surface receptors (2, 12). SPHK2 is mainly found in the nucleus, but it is also present in the cytosol, internal membranes, and plasmalemma (13). SPHK2 is not secreted and is able to phosphorylate a broader range of substrates, including sphingosine, dihydrosphingosine, phytosphingosine, and FTY720 (14, 15).
S1P is degraded by three types of enzymes: S1P phosphatase (SPP), S1P lyase (S1PL), and lipid phosphate phosphohydrolase (LPP) (16). In most cells, S1P is irreversibly degraded by S1PL to hexadecenal and ethanolamine-1-phosphate (17). S1P can also be dephosphorylated by two isoforms of SPP (SPP1 and SPP2), which are highly selective for sphingoid base-1-phosphates as substrates, to yield sphingosine (18).
SPPs and S1PL are localized in the endoplasmic reticulum, thus these enzymes cannot degrade S1P found in the extracellular space. The only enzymes that have the ability to degrade extracellular S1P belong to a family of three LPP isoforms. LPPs show broad substrate specificity and can degrade many phosphorylated lipids, including phosphatidic acid, lysophosphatidic acid, S1P, FTY720, and ceramide-1-phosphate. LPP2 resides intracellularly, whereas LPP1 and LPP3 are mainly localized to the plasma membrane and function as ecto-enzymes, degrading lipid phosphate substrates in the extracellular space (19–24).
S1P METABOLISM IN BLOOD CELLS
Platelets
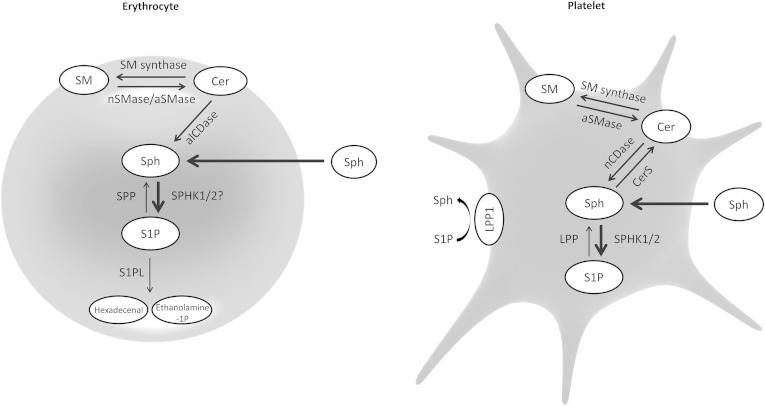
Platelets are characterized by high SPHK activity and lack of S1PL, which allows them to accumulate large amounts of S1P (Fig. 1) (25). Thrombocytes express both SPHK1 and SPHK2, however, the former isoform is responsible for 75% of total SPHK activity (26). Other, yet unidentified, enzyme possessing SPHK activity with high specificity toward phytosphingosine may also be present in platelets (27). Activity of serine palmitoyltransferase, the rate-limiting enzyme of the de novo sphingolipid biosynthesis pathway, in thrombocytes is very low. Therefore, in order to synthesize S1P, platelets have to incorporate sphingosine from the plasma. Alternatively, sphingosine can be generated on the outer leaflet of the plasmalemma (28). This process is initiated by degradation of the membrane sphingomyelin to ceramide (by acid sphingomyelinase released from activated platelets or by secretory sphingomyelinase present in the plasma), which is then deacylated to sphingosine by neutral ceramidase localized to the plasma membrane of thrombocytes (28–30).
Fig. 1.
Schematic representation of sphingolipid metabolism in erythrocytes and platelets. alCDase, alkaline ceramidase; aSMase, acid SMase; Cer, ceramide; CerS, ceramide synthase; nCDase, neutral ceramidase; nSMase, neutral SMase; Sph, sphingosine.
The concentration of S1P in human platelets decreases upon storage, which is associated with elevation in ceramide level (31). It was shown that thrombocytes are able to dephosphorylate S1P (most likely with the help of LPP) and incorporate released sphingosine into ceramide and then sphingomyelin via the action of ceramide synthase and sphingomyelin synthase (25, 28). Human platelets are likely also able to degrade extracellular S1P because they express LPP1 on the outer surface of their plasma membrane (32).
Erythrocytes
Compared with platelets, red blood cells (RBCs) are characterized by 25-fold lower SPHK activity per milligram of protein (3-fold lower per cell) (33). One study reported that erythrocytes express only SPHK1, whereas in another study, both SPHK isoforms were found to be present (34, 35). Similarly to thrombocytes, RBCs obtain sphingosine either by incorporation from the plasma or by generation on the outer leaflet of the cell membrane (Fig. 1). Human erythrocytes express alkaline ceramidase activity, and it was suggested that this enzyme plays an important role in generation of sphingosine used for S1P synthesis (36). Neutral and acid sphingomyelinase, as well as sphingomyelin synthase, were also found to be present in human erythrocytes (35, 37).
Early studies reported that RBCs lack S1PL and SPP and are, therefore, unable to degrade S1P (33, 38). However, low activity of these enzymes was recently detected in erythrocyte membranes, which explains a marked decrease in S1P content in RBCs observed after prolonged storage. This phenomenon likely contributes to adverse clinical events, such as pulmonary edema and impaired immune cell function occurring after transfusion (39).
We recently provided the first evidence that S1P synthesis in erythrocytes may be regulated. We found that prolonged reduction in plasma S1P concentration in patients with myocardial infarction is associated with increased erythrocyte SPHK activity and enhanced SPHK1 protein expression (40). This observation suggests that RBCs may be able to increase the rate of S1P production in response to reduction in plasma S1P concentration. In addition, increased activity of acid and neutral sphingomyelinase and accumulation of sphingosine and S1P were reported for erythrocytes of patients with sickle cell disease (35). Degradation of membrane sphingolipids may represent another regulatory point of S1P metabolism in RBCs.
Leukocytes
White blood cells (WBCs) are also able to incorporate extracellular sphingosine and to convert it into S1P (41). As nuclear cells, leukocytes possess a full set of enzymes involved in sphingolipid metabolism. They are able to efficiently convert sphingosine into ceramide and then sphingomyelin. In addition, ceramidase and serine palmitoyltransferase were also found to be present in WBCs (41, 42). Both SPHK activity and S1P content in leukocytes are much lower than in platelets, and comparable to that of erythrocytes (33, 43, 44). In addition, considerable activity of S1P-degrading enzymes was found to be present in WBCs (38).
S1P CONCENTRATION AND DISTRIBUTION IN DIFFERENT BLOOD COMPARTMENTS
Plasma is characterized by a relatively high S1P level compared with solid tissues, and it was proposed that a significant S1P concentration gradient exists between plasma and interstitial fluid (45). In our studies, mean plasma S1P concentration in healthy subjects ranged from 100 ± 22 nM to 372 ± 142 nM depending on the population (40, 46–51). This is in line with the results of most other groups (44, 52–54). However, some authors reported much higher S1P concentrations of approximately 1 μM (55, 56). These differences are likely related to the protocol used to isolate plasma. In order to prevent S1P release from erythrocytes, blood should be put on ice immediately after sampling, and then centrifuged at 4°C (38). In addition, plasma separated by routine one-step centrifugation usually contains some platelets, which can lead to overestimation of S1P concentration as well (M. Baranowski, unpublished observation).
S1P concentration in murine plasma is higher compared with its concentration in human plasma; the reported values range from 471 ± 59 nM to 1.35 ± 0.19 μM (36, 53, 56–58). It should be noted that the serum S1P level is 2- to 3-fold higher than in plasma, which is a consequence of S1P release from platelets activated during blood clotting (59, 60). There is no difference in plasma S1P between fasting and nonfasting subjects (52). However, S1P concentration is increased after 12 h of fasting in mice (61).
One study showed that plasma S1P level is higher in males than in females (62), whereas another study found the opposite in both mice and humans (63). However, other authors did not observe any gender difference (52, 56). This discrepancy is likely related to differences in the age of populations studied, as plasma S1P concentration in women decreases markedly after menopause (63). It was also reported that the S1P level is elevated in obese humans and mice, and that it is negatively correlated with age (61, 63).
Ito et al. (33) found that one human platelet contains approximately 9-fold more S1P than one RBC. However, because erythrocytes constitute about 95% of total blood cell number, the overall contribution of RBCs to the S1P pool in whole blood is considerably higher than that of platelets. According to the results of the above study (33), 14% of the total blood S1P is found in plasma, 32% in platelets, and 54% in erythrocytes.
S1P CARRIERS IN THE PLASMA
Most of plasma S1P is transported bound to HDLs (50–60%). The remaining part binds to albumin (30–40%), LDLs (∼8%), and VLDLs (2–3%) (56, 64). However, in some subjects more than 60% of S1P is transported by albumin (55). Although HDL is the major S1P carrier, there is no straightforward relationship between plasma HDL and total S1P level. We observed comparable S1P concentration in healthy subjects and patients with ischemic heart disease despite a 2-fold difference in HDL-cholesterol level (49). Similarly, there is no difference in total serum S1P level between groups of subjects with high and low HDL-cholesterol concentration (65). However, HDL-bound S1P is strongly positively correlated with plasma HDL-cholesterol, apoA-I, and apoA-II content (66).
Interaction with plasma proteins seems to reduce the bioavailability of S1P. Exposition of S1PRs to the S1P concentration that is normally found in the plasma should result in their full activation because their Kd values are in the range of 2–30 nM (64). However, the estimated level of bioactive S1P in plasma is approximately 10 nM (67).
S1P is not evenly distributed between HDL subclasses. Small dense HDL3 particles carry ∼78% of the lipoprotein-associated S1P, whereas HDL2 binds only 16% (52). Christoffersen et al. (68) found that apoM is the carrier of S1P in HDL. They showed that the apoM-deficient fraction of human HDL contains no S1P, and that apoM−/− mice are characterized by a lack of HDL-bound S1P, whereas transgenic mice overexpressing human apoM show increased S1P content in HDL. However, according to a recent study, apoM may not be the only HDL protein able to bind S1P (69). It was estimated that, on average, only 1–10% of HDL particles in human plasma transport S1P, and in mice the molar ratio between HDL-bound S1P and plasma apoM is ∼1:3 (56, 68). The above data are in line with the fact that human apoM binds S1P with an IC50 of 0.9 μM (70), a concentration that is 2- to 3-fold higher compared with the plasma S1P level reported in most studies.
It is very likely that the biological activity and metabolism of plasma S1P are highly dependent on its carrier. The half-life of HDL-bound S1P, upon incubation with human umbilical vein endothelial cells (HUVECs), is 4-fold longer compared with albumin-bound S1P (71). This finding indicates that HDL prevents S1P degradation by ecto-phosphatase. In addition, protection against myocardial ischemia/reperfusion injury is induced by HDL- and albumin-bound S1P, whereas LDL-associated S1P is ineffective (72). Moreover, although both albumin- and HDL-bound S1P improve endothelial barrier function, the duration of the barrier promotion elicited by HDL-bound S1P is much longer due to specific effects on S1PR1 trafficking that prolong receptor signaling (73).
DEGRADATION OF PLASMA S1P
The half-life of albumin-bound C17-S1P (a 17-carbon analog of S1P), injected intravenously into mice, is ∼15 min (57). An even shorter half-life of ∼1 min was reported in another study (74). The above data indicate that circulating S1P is rapidly cleared by degradative enzymes and imply the presence of high-capacity sources of S1P involved in maintaining its high concentration in the plasma. Interestingly, S1P is stable in isolated plasma, but not in whole blood or in the presence of HUVECs (75). Zhao et al. (22) reported that HUVECs, as well as endothelial cells (ECs), from macro- and microvessels of human pulmonary circulation (but not epithelial cells, monocytes, or macrophages) rapidly degrade extracellular S1P via the action of LPP1. Released sphingosine is then taken up and used for synthesis of intracellular S1P by SPHK1 (but not SPHK2). Although extracellular dihydrosphingosine-1-phosphate (dhS1P) undergoes the same pathway, S1P is the preferred substrate in human pulmonary artery ECs (HPAECs) (22). As mentioned earlier, platelets express LPP1 (25, 32). However, plasma S1P concentration in NF-E2-deficient mice that lack thrombocytes is the same as in wild-type animals, suggesting that platelets do not play a significant role in degradation of S1P in vivo (76). Moreover, in mice, as much as 86% of intravenously administered C17-S1P accumulates in the liver, which strongly indicates the primary role of hepatic sinusoidal ECs in degradation of plasma S1P (74).
Pharmacological inhibition or knockout of SPHK1 decreases the S1P level in whole blood and plasma by approximately 50%, suggesting that both SPHK isoforms are equally important in generation of the blood pool of this sphingolipid (77, 78). Unexpectedly, genetic or pharmacological inhibition of SPHK2 results in a striking increase in plasma and blood cell S1P concentration (77, 79). This observation indicates that regulation of plasma S1P level is more complex than initially presumed. Sensken et al. (79) proposed that the accumulation of S1P in blood observed in mice lacking SPHK2 is a consequence of impaired intracellular rephosphorylation of sphingosine released from blood-borne S1P degraded by LPP1. Their results strongly suggest that SPHK2 plays a key role in regulation of S1P redistribution from erythrocytes into ECs.
S1P RELEASE FROM BLOOD CELLS
Erythrocytes
S1P release seems to be cell specific, and the amount of exported S1P is much higher for blood cells than for other cell types (80). Although both platelets and erythrocytes store large amounts of sphingoid base-1-phosphates, only RBCs are considered to be an important source of plasma S1P because they release S1P spontaneously without any stimulation (38). Transfusion of wild-type erythrocytes (but not leukocytes or platelets) to SPHK1/2-deficient mice is sufficient to restore normal plasma S1P level, which proves the key role of RBCs in blood S1P homeostasis (76). Consistently, there is a strong positive correlation between RBC-related parameters and S1P level in human plasma, and anemic patients show lower S1P concentration in plasma (but not in blood cells) than healthy subjects (39, 62, 81). Interestingly, anemia selectively depletes the HDL-bound pool of S1P (62). However, in mice, neither severe hemolytic anemia nor blood loss affects plasma S1P level (82). These findings suggest that, compared with humans, mice possess more robust mechanisms to replenish circulating S1P when erythrocyte number declines.
When radiolabeled sphingosine was incubated with erythrocytes, it was efficiently taken up and converted to S1P, which was then released without the rate of export being affected by Ca2+, protein kinase C (PKC) activator, or thrombin (83). S1P release is proportional to RBC number and is temperature dependent (it does not efficiently occur at 4°C). It also requires the presence of S1P acceptors. Erythrocytes do not release S1P in plasma/serum-free medium, and the rate of export decreases upon dilution of plasma (38). HDL and albumin are major endogenous triggers of S1P release from erythrocytes. However, HDL is much more effective in this regard compared with albumin, which extracts only two S1P molecules per thousand molecules of albumin. VLDL and LDL are very weak inducers of S1P release (81). apoM plays a significant role in HDL-induced S1P efflux from erythrocytes. Compared with normal lipoproteins, HDLs isolated from mice overexpressing apoM induce markedly higher (and nonsaturable) S1P release from RBCs. Surprisingly, HDLs isolated from apoM-deficient mice do not exhibit impaired effectiveness in this regard. This finding strongly indicates the presence of an additional apoM-independent mechanism (most likely involving apoA-I) responsible for HDL-induced release of S1P from erythrocytes (69). It was also demonstrated that S1P transfer between RBCs and HDL is facilitated by phospholipid transfer protein, and phospholipid transfer protein-deficient mice show a 60% decrease in plasma S1P concentration (84).
Binding capacity of an acceptor determines the amount of S1P released from RBCs. A highly specific anti-S1P antibody extracts S1P at a molar ratio of 1:1 (81). Interestingly, when erythrocytes are incubated in plasma/serum-free medium containing S1P, large amounts of the compound are taken up by the RBCs (but not the WBCs or platelets) (38). Moreover, addition of C17-S1P to erythrocytes incubated in the presence of albumin results in partial uptake of the compound (81). These findings suggest that there is a balance between the amount of S1P found in RBCs and bound to acceptors in the medium, and that incorporation or release of S1P occurs when this balance is disturbed (81). This hypothesis is supported by the data obtained from mice transfused with erythrocytes with either high or low S1P content. After 24 h, the amount of S1P in these cells equalized with that of endogenous RBCs (38).
Another mechanism of S1P export is its transcellular transportation from erythrocytes to ECs that is not mediated by plasma proteins. Both fluorescent analog of S1P and C17-S1P are readily exchanged between RBCs, as well as between erythrocytes and HUVECs, when they are co-incubated in plasma/serum-free medium (81). This transcellular transportation is, however, prevented by the presence of albumin or HDL. The blocking effect of albumin can be circumvented by tight contact between erythrocytes and HUVECs. It is, therefore, very likely that RBC-associated S1P is transferred to vascular ECs during erythrocyte passage through the capillaries. This exchange is facilitated by the fact that RBCs store S1P predominantly in their plasma membrane (38).
Recent studies identified several members of the ABC transporter family and spinster homolog 2 (SPNS2) as putative S1P transporters in various cell types (85). SPNS2 is not expressed in murine erythrocytes, and the rate of S1P release from RBCs is not affected by SPNS2 deficiency (58, 86, 87). The transporter involved in S1P export from rat erythrocytes is ATP dependent; hydrolysis of ATP is, however, not required for its activity. It is also sensitive to glyburide (nonspecific ABC transporter inhibitor) and to vanadate (a phosphate analog, inhibitor of ATPases), but not to the ABCB1 inhibitor cyclosporin or the ABCC1/ABCC4 inhibitor MK571. Nevertheless, ABCC1, which is expressed in RBCs, was suggested as a candidate for erythrocyte S1P transporter (83). However, plasma S1P level is not affected by deficiency of ABCC1 (88). Interestingly, transport of S1P in erythrocyte inside-out membrane vesicles is not inhibited by the presence of closely related compounds such as dhS1P and ceramide-1-phosphate. This finding indicates that the transporter involved in S1P export from RBCs is highly specific to this sphingoid base-1-phosphate (83). Identification of the precise nature of the erythrocyte S1P transporter requires further investigation. Analysis of the rate of S1P release from RBCs isolated from mice deficient in different types of ABC transporters could shed some light on this matter.
Platelets
In contrast to erythrocytes, platelets require activation to release significant amounts of S1P, and the rate of release depends on the agonist potency. Strong platelet agonists, like thrombin, collagen, or convulxin, induce greater release compared with weak agonists such as ADP, which is barely effective in this regard (89, 90). S1P export from stimulated platelets is enhanced by increasing the concentration of albumin in the medium (89). Interestingly, HDL extracts S1P from activated human thrombocytes less efficiently than albumin (75), suggesting that platelets and erythrocytes differ in their preferences for extracellular S1P acceptors.
There is some controversy as to whether thrombocytes show constitutive release of S1P. Two studies found no evidence for spontaneous S1P release (38, 91), whereas in other reports some S1P was released constitutively (90, 92, 93). However, Jonnalagadda et al. (89) found that considerable amounts of S1P are released without stimulation from human thrombocytes, especially at higher albumin concentration in the medium. They also showed that increasing albumin concentration results in elevation of S1P stores in human and murine platelets. The authors postulated that albumin, by extracting S1P from resting thrombocytes, stimulates a homeostatic mechanism that maintains platelet S1P levels. A similar mechanism is likely activated upon thrombocyte stimulation, as evidenced by accumulation of sphingosine and depletion of ceramide in murine platelets under these conditions (94). Jonnalagadda et al. (89) concluded that thrombocytes contain two pools of S1P, a passively extractable one, similar to the pool of S1P in erythrocytes, and a pool that is acutely mobilized by platelet activation. It was also reported that S1P concentration in plasma from platelet concentrates increases markedly upon storage, and that the incidence of transfusion reactions is associated with a higher S1P level in the concentrate (95).
It is generally thought that thrombocytes do not represent an important source of plasma S1P under normal conditions. This notion is supported by the fact that the concentration of S1P in human plasma is not correlated with platelet count (62). This parameter is, however, the major determinant of S1P level in serum, which is reasonable considering that platelets are fully activated during blood clotting (96). Moreover, thrombocytopenic, as well as platelet-deficient, mice are characterized by normal plasma S1P concentration (57, 76). It should be noted, however, that murine thrombocytes, unlike those of humans, store mostly dhS1P and contain only a small (if any) amount of S1P (89, 94). Interestingly, the proportion between these two sphingoid base-1-phosphates in rat platelets resembles that of human thrombocytes (M. Baranowski, unpublished observation).
Although platelets do not seem to determine plasma sphingoid base-1-phosphate concentration in healthy subjects, it is possible that under pathological conditions, in which thrombocytes are activated, they play some role in this regard. For instance, in vivo platelet activation by anti-CD41 antibody resulted in elevation of plasma dhS1P in mice. The S1P level remained stable, which is not surprising considering the fact that murine platelets lack this compound (94). In addition, rabbits with hypercholesterolemic atherosclerosis, a condition associated with platelet activation, are characterized by a markedly increased S1P level in plasma and thrombocytes, as well as by enhanced S1P release from activated platelets (97). In humans, plasma S1P is correlated (albeit weakly) with concentrations of serotonin and β-thromboglobulin, which are well-known in vivo platelet activation markers (62).
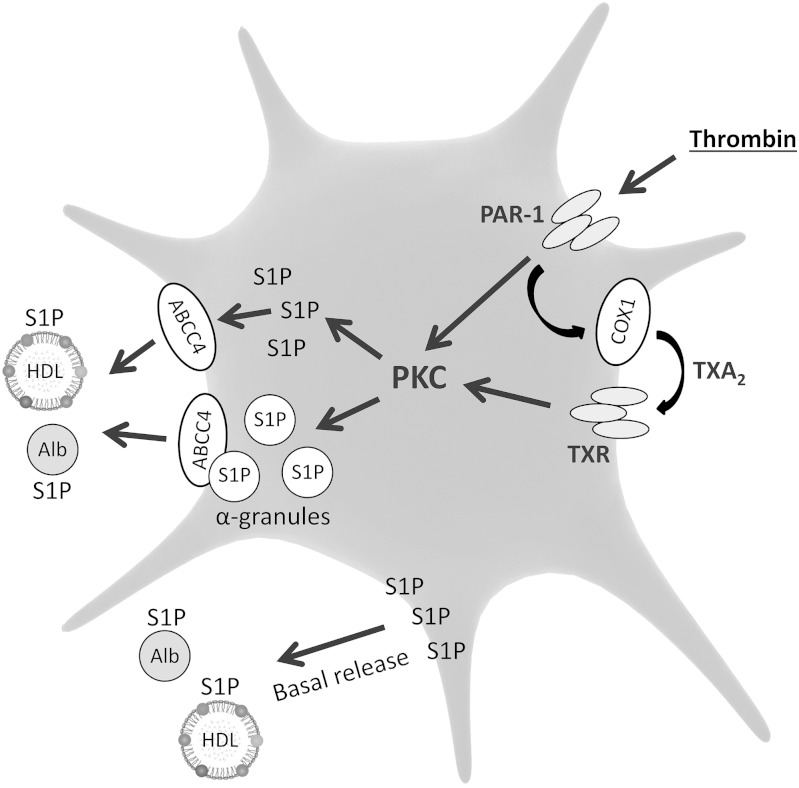
There is only limited information on the signaling pathways mediating S1P release from platelets upon stimulation (Fig. 2). Thrombin-induced S1P release requires PKC activation, as evidenced by the fact that it is mimicked by 12-O-tetradecanoylphorbol-13-acetate (PKC stimulator) and attenuated by staurosporine (PKC inhibitor) (93). There is conflicting evidence as to whether extracellular calcium affects thrombin-induced S1P release from platelets (89, 92).
Fig. 2.
Mechanism of S1P release from platelets. Alb, albumin; ABC, ABC transporter; COX, cyclooxygenase; PAR, protease-activated receptor; TXA2, thromboxane A2; TXR, thromboxane receptor.
Ulrych et al. (90) reported that S1P release from human platelets is mediated by thromboxane formation and subsequent activation of its receptor. They found that thrombin- and collagen-induced S1P release is suppressed by acetylsalicylic acid and thromboxane receptor antagonist, whereas thromboxane receptor activator stimulates S1P release from platelets in an acetylsalicylic acid-independent manner. Moreover, ADP, that caused only a minor increase in thromboxane formation, did not induce S1P release. They also found evidence for the presence of an additional thromboxane-independent mechanism of thrombin-induced S1P release. Constitutive S1P release was not affected by inhibition of thromboxane synthesis. In addition, thromboxane receptor-deficient mice showed normal plasma S1P concentration (90). The above data indicate that thromboxane mediates S1P release only under conditions of platelet activation. However, we (50) and others (98) found that aspirin and celecoxib reduce the plasma S1P level in healthy subjects, suggesting a role for cyclooxygenase in S1P homeostasis in blood.
Two studies concluded that S1P export from platelets is not mediated by exocytosis, and that S1P is stored in the plasma membrane and cytoplasm rather than in secretory granules (90, 93). However, recently Jonnalagadda et al. (89) provided strong evidence for the involvement of exocytosis in the release of S1P from stimulated thrombocytes. They found that the kinetics of S1P release closely mirrored those of the α-granule marker, platelet factor 4, and subcellular fractionation confirmed the presence of S1P in α-granules, but not in dense granules of human thrombocytes. Moreover, Unc13dJinx mice, characterized by defective exocytosis, showed attenuated thrombin-induced S1P release from platelets. In the above study (89), newly synthesized S1P required more than an hour to enter the stimulation-dependent releasable pool. Of note, in the previous reports, where release of [3H]S1P, instead of the natural compound, was measured, platelets were incubated with [3H]sphingosine for not longer than ten minutes before stimulation (90, 93), which may explain why these authors did not find any evidence for granule-mediated S1P release.
Similarly to erythrocytes, the precise nature of the platelet S1P transporter remains unclear. S1P release from stimulated rat thrombocytes is inhibited by glyburide, suggesting involvement of an ABC transporter; however, cyclosporin or MK571 shows no effect (93). On the other hand, S1P release from stimulated human platelets is suppressed by MK571. Indomethacin and dipyridamole, which also inhibit ABCC4, show a similar effect. It should be noted that basal S1P release is not affected by these compounds (90). ABCC4 is expressed in human thrombocytes at a high level, which further indicates that this isoform may mediate agonist-induced S1P release (99). It was also reported that SPNS2 transporter is not involved in S1P release from stimulated murine platelets (58).
Leukocytes
In human leukocytes, S1P is the major sphingoid base-1-phosphate, whereas in murine WBCs, dhS1P predominates. However, unlike platelets, murine leukocytes also contain significant amounts of S1P (94). Hänel, Andréani, and Gräler (38) reported that human WBCs effectively degrade extracellular S1P but are not able to release it, whereas Yang et al. (41) found that human neutrophils and mononuclear cells show significant basal S1P release. This discrepancy likely results from the fact that Yang et al. (41) determined the release of [3H]S1P, whereas Hänel, Andréani, and Gräler (38) measured changes in S1P concentration in the incubation medium. The results of the above studies suggest that the rate of degradation of extracellular S1P by leukocytes exceeds the rate of its release by these cells. Plasma S1P concentration is not correlated with WBC count in healthy subjects (62), and is not affected by deficiency of both T and B cells in mice (76). These findings suggest that leukocytes do not play a significant role in S1P homeostasis in blood.
Stimulation of mast cells with IgE induces rapid activation of SPHKs, accumulation of intracellular S1P, and its release into the extracellular space (100). In murine and human mast cells, the export of S1P is markedly reduced by MK571 and by silencing of ABCC1 expression, suggesting an important role for this transporter in S1P release (101). However, it remains obscure whether similar mechanisms operate in circulating WBCs upon their activation. Murine lymphocytes lack SPNS2, suggesting that this transporter is not involved in S1P export from leukocytes (86).
OTHER SOURCES OF CIRCULATING S1P
Vascular endothelium
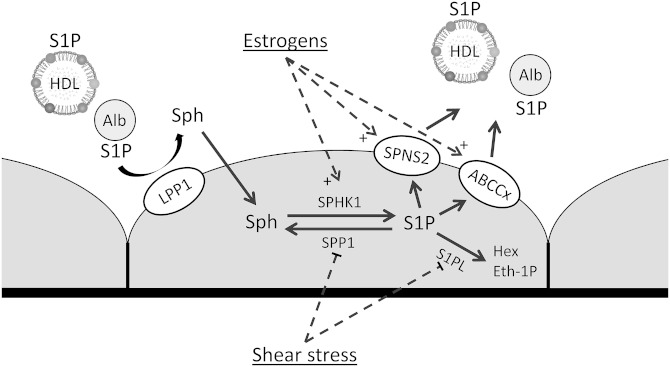
Similarly to erythrocytes, vascular ECs also spontaneously release S1P (Fig. 3). This was confirmed for HUVECs, HPAECs, ECs from murine aorta, and mouse embryonic ECs (57, 58, 63, 87, 88). However, in one study S1P export from HPAECs was not observed (22).
Fig. 3.
Mechanism and regulation of S1P export from human vascular ECs. Alb, albumin; ABC, ABC transporter, Eth-1P, ethanolamine-1-phosphate; Hex, hexadecenal; Sph, sphingosine.
There is an increasing body of evidence that S1P release from ECs is mediated by SPNS2. ECs isolated from the aorta of SPNS2-deficient mice are unable to release S1P, and silencing of SPNS2 expression markedly attenuates S1P export from HUVECs and HPAECs (58, 87). Consistently, whole-body or endothelium-specific SPNS2 deficiency reduces plasma S1P concentration in mice by approximately 40% (58, 86, 87). However, one study found no difference in plasma S1P level between SPNS2-null mice and wild-type animals (102).
Hisano et al. (58) reported that murine peripheral blood vessels express SPNS2 exclusively in the endothelium. In addition, they concluded that SPNS2 expression in the venous ECs is higher than in the arterial ECs. In mice, the presence of this transporter in vascular ECs was confirmed for the thymus, heart, lungs, and hypothalamus, but not for the kidneys. SPNS2 is also expressed in human venous, aortic, and microvascular ECs (87).
S1P release from HUVECs is attenuated by glyburide and MK571, which indicates involvement of an ABC transporter in addition to SPNS2. However, deficiency of ABCA1, ABCA7, or ABCC1 does not affect plasma S1P concentration in mice (88). This observation, together with the finding that murine ECs are not able to export S1P in the absence of SPNS2, indicates that ABC transporters are not involved in S1P release by the vascular endothelium in mice.
There is only limited information on the mechanisms regulating S1P export from ECs. Estradiol increases intracellular content and release of S1P from HUVECs, which explains lower plasma S1P concentration in postmenopausal women. The effect of estradiol is related to enhanced expression of SPHK1, SPNS2, ABCC1, and ABCG2 (63). In addition, shear stress acutely increases S1P release from murine ECs, which is associated with downregulated expression of S1PL and SPP1 (57). Export of S1P from ECs is also enhanced by silencing of S1PL expression (57). Interestingly, overexpression of SPHK1 increases release of dhS1P but not S1P (88).
The extent to which vascular endothelium contributes to the plasma pool of S1P is a matter of debate. S1P concentration in lethally irradiated wild-type mice transplanted with SPHK1/2-deficient bone marrow is reduced by 90%, and transplantation of SPHK1/2-deficient mice, that lack S1P in plasma, with wild-type bone marrow restores its concentration back to normal (76). These findings would indicate that the endothelium is only a minor contributor to the plasma S1P pool in mice. However, it was also shown that adenoviral-mediated transduction of SPHK1 to SPHK1-null mice, characterized by 40% lower plasma S1P level, restores its normal concentration (57). The fact that recombinant SPHK1 is expressed in ECs (mainly in the liver), and not in blood cells, suggests an important role for the vascular endothelium in S1P homeostasis. This notion is also supported by the previously mentioned studies showing significant reduction in plasma S1P concentration in SPNS2-deficient mice. Recently, Xiong et al. (103) provided strong evidence that the vascular endothelium and RBCs are equally important sources of plasma S1P in mice. In their study, erythrocyte-specific loss of three alleles of SPHK (SPHK1fl/fl SPHK2+/−) reduced the S1P content in RBCs by 98%, whereas its plasma concentration decreased by only ∼50%. Furthermore, endothelial-specific SPHK1/2 deletion caused a 30% reduction in plasma S1P.
We recently provided some in vivo evidence that S1P may be released by the vascular endothelium in humans (104). We found that plasma is enriched in S1P (but not in dhS1P) upon its passage through the vasculature of the leg. Interestingly, this effect was no longer present after 30 min of exercise, suggesting acute changes in the rate of S1P release and/or degradation by the endothelium.
Liver
Murine primary hepatocytes and HepG2 cells are able to export S1P, although at a lower rate than ECs (57, 105, 106). Moreover, plasma S1P concentration is reduced after partial hepatectomy in mice, upon the development of carbon tetrachloride-induced liver fibrosis in rats, and in chronic hepatitis C patients (105, 107). These findings suggest that the liver is important for the maintenance of a normal plasma S1P level. In addition to mere S1P export, the role of this organ in S1P homeostasis may also be related to the fact that the liver is the source of plasma apoM, the major carrier of circulating S1P (108). Indeed, hepatic overexpression of apoM induces a striking increase in plasma S1P concentration (105). This increase may result from several effects. First of all, isolated murine hepatocytes and HepG2 cells overexpressing apoM are characterized by enhanced export of S1P (105, 106). However, apoM overexpression not only stimulates S1P release from hepatocytes, but also protects it from degradation by ecto-phosphatase (105). In addition, increased plasma apoM concentration may stimulate S1P export from extrahepatic sources.
It was recently found that insulin affects the plasma S1P level via its action on hepatic apoM production. S1P and apoM concentration is elevated in mice with streptozotocin-induced diabetes, and administration of insulin decreases their levels in both healthy and diabetic mice. In addition, incubation of HepG2 cells with insulin reduces apoM content in the medium and cells (109).
Secretory SPHK
Apart from being released to the circulation by various cell types, S1P can also be produced directly in the plasma by secreted SPHK. Enzymatically active SPHK1 is secreted by HUVECs, and SPHK activity was detected in murine and human plasma (14). In mice, contribution of plasma to total SPHK activity in blood is comparable to that of platelets. However, human plasma is characterized by 20-fold lower SPHK activity, which argues against a significant role of secreted SPHK1 in the generation of S1P. This notion is supported by the fact that phosphorylation of sphingosine was not detected in isolated human plasma (75).
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Studies conducted over the last decade substantially expanded our knowledge on the origin, transport, and metabolism of S1P in blood. Several new sources of circulating S1P were identified, and it is now evident that platelets do not play a major role in this regard. However, the relative contribution of erythrocytes, vascular endothelium, and hepatocytes to the plasma S1P pool remains unclear. Moreover, many fundamental questions about the mechanisms maintaining S1P homeostasis remain to be resolved. Which transporter is involved in S1P release from erythrocytes, and is it regulated? Are other sources able to “sense” abnormal S1P concentration and adjust the rate of its export? What exactly is regulated, total plasma S1P concentration or the level of its specific pool (e.g., HDL-bound)? Answering these questions is essential for development of pharmacological tools to manipulate plasma S1P concentration for therapeutic purposes. Another important area for future research is better characterization of the biological activity of different pools of plasma S1P and factors that affect the relative distribution of S1P between these pools.
It is, however, important to note that the results of studies in mice cannot be directly translated to humans due to significant differences in metabolism, and possibly also the origin of circulating S1P. First of all, in murine blood cells, in contrast to the human blood cells, dhS1P is the dominant sphingoid base-1-phosphate. Second, plasma S1P concentration is correlated with RBC-related parameters only in humans. In addition, S1P release from human ECs and activated platelets seems to be mediated by distinct types of transporters than those in mice. Yet another difference is high activity of secretory SPHK in the plasma of mice. Therefore, future studies in this area should be conducted on humans whenever possible.
Footnotes
Abbreviations:
- dhS1P
- dihydrosphingosine-1-phosphate
- EC
- endothelial cell
- HPAEC
- human pulmonary artery endothelial cell
- HUVEC
- human umbilical vein endothelial cell
- LPP
- lipid phosphate phosphohydrolase
- PKC
- protein kinase C
- RBC
- red blood cell
- S1P
- sphingosine-1-phosphate
- SPHK
- sphingosine kinase
- S1PL
- sphingosine-1-phosphate lyase
- SPNS2
- spinster homolog 2
- SPP
- sphingosine-1-phosphate phosphatase
- S1PR
- sphingosine-1-phosphate receptor
- WBC
- white blood cell
This study was supported by the Polish Ministry of Science and Higher Education (grant number IP2012 011472).
REFERENCES
- 1.Saba J. D., Hla T. 2004. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 94: 724–734. [DOI] [PubMed] [Google Scholar]
- 2.Takabe K., Paugh S. W., Milstien S., Spiegel S. 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabasinezhad M., Samadi N., Ghanbari P., Mohseni M., Saei A. A., Sharifi S., Saeedi N., Pourhassan A. 2013. Sphingosine 1-phosphate contributes in tumor progression. J. Cancer Res. Ther. 9: 556–563. [DOI] [PubMed] [Google Scholar]
- 4.Maceyka M., Harikumar K. B., Milstien S., Spiegel S. 2012. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knapp M. 2011. Cardioprotective role of sphingosine-1-phosphate. J. Physiol. Pharmacol. 62: 601–607. [PubMed] [Google Scholar]
- 6.Simmons S., Ishii M. 2014. Sphingosine-1-phosphate: a master regulator of lymphocyte egress and immunity. Arch. Immunol. Ther. Exp. (Warsz.). 62: 103–115. [DOI] [PubMed] [Google Scholar]
- 7.Wilkerson B. A., Argraves K. M. 2014. The role of sphingosine-1-phosphate in endothelial barrier function. Biochim. Biophys. Acta. 1841: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerage D., Brindley D. N., Hemmings D. G. 2014. Review: novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta. 35(Suppl): S86–S92. [DOI] [PubMed] [Google Scholar]
- 9.Kikuta J., Kawamura S., Okiji F., Shirazaki M., Sakai S., Saito H., Ishii M. 2013. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc. Natl. Acad. Sci. USA. 110: 7009–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. 1998. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273: 23722–23728. [DOI] [PubMed] [Google Scholar]
- 11.Chan H., Pitson S. M. 2013. Post-translational regulation of sphingosine kinases. Biochim. Biophys. Acta. 1831: 147–156. [DOI] [PubMed] [Google Scholar]
- 12.Ancellin N., Colmont C., Su J., Li Q., Mittereder N., Chae S. S., Stefansson S., Liau G., Hla T. 2002. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 277: 6667–6675. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi N., Okada T., Hayashi S., Fujita T., Jahangeer S., Nakamura S. 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278: 46832–46839. [DOI] [PubMed] [Google Scholar]
- 14.Venkataraman K., Thangada S., Michaud J., Oo M. L., Ai Y., Lee Y. M., Wu M., Parikh N. S., Khan F., Proia R. L., et al. 2006. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 397: 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275: 19513–19520. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Zhang Q. H., Yi G. H. 2012. Regulation of metabolism and transport of sphingosine-1-phosphate in mammalian cells. Mol. Cell. Biochem. 363: 21–33. [DOI] [PubMed] [Google Scholar]
- 17.Bandhuvula P., Saba J. D. 2007. Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends Mol. Med. 13: 210–217. [DOI] [PubMed] [Google Scholar]
- 18.Mandala S. M. 2001. Sphingosine-1-phosphate phosphatases. Prostaglandins Other Lipid Mediat. 64: 143–156. [DOI] [PubMed] [Google Scholar]
- 19.Nanjundan M., Possmayer F. 2001. Pulmonary lipid phosphate phosphohydrolase in plasma membrane signalling platforms. Biochem. J. 358: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai M., Sakane F., Jia Y. J., Imai S., Yasuda S., Kanoh H. 2006. Lipid phosphate phosphatases 1 and 3 are localized in distinct lipid rafts. J. Biochem. 140: 677–686. [DOI] [PubMed] [Google Scholar]
- 21.Alderton F., Darroch P., Sambi B., McKie A., Ahmed I. S., Pyne N., Pyne S. 2001. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 276: 13452–13460. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y., Kalari S. K., Usatyuk P. V., Gorshkova I., He D., Watkins T., Brindley D. N., Sun C., Bittman R., Garcia J. G., et al. 2007. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 282: 14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jasinska R., Zhang Q. X., Pilquil C., Singh I., Xu J., Dewald J., Dillon D. A., Berthiaume L. G., Carman G. M., Waggoner D. W., et al. 1999. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 340: 677–686. [PMC free article] [PubMed] [Google Scholar]
- 24.Mechtcheriakova D., Wlachos A., Sobanov J., Bornancin F., Zlabinger G., Baumruker T., Billich A. 2007. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 581: 3063–3068. [DOI] [PubMed] [Google Scholar]
- 25.Yatomi Y., Yamamura S., Hisano N., Nakahara K., Igarashi Y., Ozaki Y. 2004. Sphingosine 1-phosphate breakdown in platelets. J. Biochem. 136: 495–502. [DOI] [PubMed] [Google Scholar]
- 26.Anada Y., Igarashi Y., Kihara A. 2007. The immunomodulator FTY720 is phosphorylated and released from platelets. Eur. J. Pharmacol. 568: 106–111. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda Y., Kihara A., Igarashi Y. 2003. Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem. Biophys. Res. Commun. 309: 155–160. [DOI] [PubMed] [Google Scholar]
- 28.Tani M., Sano T., Ito M., Igarashi Y. 2005. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J. Lipid Res. 46: 2458–2467. [DOI] [PubMed] [Google Scholar]
- 29.Simon C. G., Chatterjee S., Gear A. R. 1998. Sphingomyelinase activity in human platelets. Thromb. Res. 90: 155–161. [DOI] [PubMed] [Google Scholar]
- 30.Marathe S., Schissel S. L., Yellin M. J., Beatini N., Mintzer R., Williams K. J., Tabas I. 1998. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J. Biol. Chem. 273: 4081–4088. [DOI] [PubMed] [Google Scholar]
- 31.Pienimaeki-Roemer A., Ruebsaamen K., Boettcher A., Orsó E., Scherer M., Liebisch G., Kilalic D., Ahrens N., Schmitz G. 2013. Stored platelets alter glycerophospholipid and sphingolipid species, which are differentially transferred to newly released extracellular vesicles. Transfusion. 53: 612–626. [DOI] [PubMed] [Google Scholar]
- 32.Smyth S. S., Sciorra V. A., Sigal Y. J., Pamuklar Z., Wang Z., Xu Y., Prestwich G. D., Morris A. J. 2003. Lipid phosphate phosphatases regulate lysophosphatidic acid production and signaling in platelets: studies using chemical inhibitors of lipid phosphate phosphatase activity. J. Biol. Chem. 278: 43214–43223. [DOI] [PubMed] [Google Scholar]
- 33.Ito K., Anada Y., Tani M., Ikeda M., Sano T., Kihara A., Igarashi Y. 2007. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 357: 212–217. [DOI] [PubMed] [Google Scholar]
- 34.Kihara A., Igarashi Y. 2008. Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim. Biophys. Acta. 1781: 496–502. [DOI] [PubMed] [Google Scholar]
- 35.Awojoodu A. O., Keegan P. M., Lane A. R., Zhang Y., Lynch K. R., Platt M. O., Botchwey E. A. 2014. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood. 124: 1941–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu R., Sun W., Jin J., Obeid L. M., Mao C. 2010. Role of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes. FASEB J. 24: 2507–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López D. J., Egido-Gabas M., López-Montero I., Busto J. V., Casas J., Garnier M., Monroy F., Larijani B., Goñi F. M., Alonso A. 2012. Accumulated bending energy elicits neutral sphingomyelinase activity in human red blood cells. Biophys. J. 102: 2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hänel P., Andréani P., Gräler M. H. 2007. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 21: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 39.Selim S., Sunkara M., Salous A. K., Leung S. W., Berdyshev E. V., Bailey A., Campbell C. L., Charnigo R., Morris A. J., Smyth S. S. 2011. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin. Sci. (Lond.). 121: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knapp M., Lisowska A., Zabielski P., Musiał W., Baranowski M. 2013. Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 106: 53–61. [DOI] [PubMed] [Google Scholar]
- 41.Yang L., Yatomi Y., Miura Y., Satoh K., Ozaki Y. 1999. Metabolism and functional effects of sphingolipids in blood cells. Br. J. Haematol. 107: 282–293. [DOI] [PubMed] [Google Scholar]
- 42.Dedov V. N., Dedova I. V., Nicholson G. A. 2004. Hypoxia causes aggregation of serine palmitoyltransferase followed by non-apoptotic death of human lymphocytes. Cell Cycle. 3: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim F. B., Pang S. J., Melendez A. J. 2004. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J. Biol. Chem. 279: 44802–44811. [DOI] [PubMed] [Google Scholar]
- 44.Yatomi Y., Igarashi Y., Yang L., Hisano N., Qi R., Asazuma N., Satoh K., Ozaki Y., Kume S. 1997. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 121: 969–973. [DOI] [PubMed] [Google Scholar]
- 45.Schwab S. R., Cyster J. G. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 46.Knapp P., Baranowski M., Knapp M., Zabielski P., Błachnio-Zabielska A. U., Górski J. 2010. Altered sphingolipid metabolism in human endometrial cancer. Prostaglandins Other Lipid Mediat. 92: 62–66. [DOI] [PubMed] [Google Scholar]
- 47.Kułakowska A., Zendzian-Piotrowska M., Baranowski M., Konończuk T., Drozdowski W., Górski J., Bucki R. 2010. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci. Lett. 477: 149–152. [DOI] [PubMed] [Google Scholar]
- 48.Baranowski M., Charmas M., Długołęcka B., Górski J. 2011. Exercise increases plasma levels of sphingoid base-1 phosphates in humans. Acta Physiol. (Oxf.). 203: 373–380. [DOI] [PubMed] [Google Scholar]
- 49.Knapp M., Baranowski M., Lisowska A., Musiał W. 2012. Decreased free sphingoid base concentration in the plasma of patients with chronic systolic heart failure. Adv. Med. Sci. 57: 100–105. [DOI] [PubMed] [Google Scholar]
- 50.Knapp M., Lisowska A., Knapp P., Baranowski M. 2013. Dose-dependent effect of aspirin on the level of sphingolipids in human blood. Adv. Med. Sci. 58: 274–281. [DOI] [PubMed] [Google Scholar]
- 51.Baranowski M., Górski J., Klapcinska B., Waskiewicz Z., Sadowska-Krepa E. 2014. Ultramarathon run markedly reduces plasma sphingosine-1-phosphate concentration. Int. J. Sport Nutr. Exerc. Metab. 24: 148–156. [DOI] [PubMed] [Google Scholar]
- 52.Hammad S. M., Pierce J. S., Soodavar F., Smith K. J., Al Gadban M. M., Rembiesa B., Klein R. L., Hannun Y. A., Bielawski J., Bielawska A. 2010. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J. Lipid Res. 51: 3074–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andréani P., Gräler M. H. 2006. Comparative quantification of sphingolipids and analogs in biological samples by high-performance liquid chromatography after chloroform extraction. Anal. Biochem. 358: 239–246. [DOI] [PubMed] [Google Scholar]
- 54.Sattler K. J., Elbasan S., Keul P., Elter-Schulz M., Bode C., Gräler M. H., Bröcker-Preuss M., Budde T., Erbel R., Heusch G., et al. 2010. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res. Cardiol. 105: 821–832. [DOI] [PubMed] [Google Scholar]
- 55.Hammad S. M., Al Gadban M. M., Semler A. J., Klein R. L. 2012. Sphingosine 1-phosphate distribution in human plasma: associations with lipid profiles. J. Lipids. 2012: 180705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karuna R., Park R., Othman A., Holleboom A. G., Motazacker M. M., Sutter I., Kuivenhoven J. A., Rohrer L., Matile H., Hornemann T., et al. 2011. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 219: 855–863. [DOI] [PubMed] [Google Scholar]
- 57.Venkataraman K., Lee Y. M., Michaud J., Thangada S., Ai Y., Bonkovsky H. L., Parikh N. S., Habrukowich C., Hla T. 2008. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hisano Y., Kobayashi N., Yamaguchi A., Nishi T. 2012. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 7: e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. 2000. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352: 809–815. [PMC free article] [PubMed] [Google Scholar]
- 60.Caligan T. B., Peters K., Ou J., Wang E., Saba J., Merrill A. H. 2000. A high-performance liquid chromatographic method to measure sphingosine 1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal. Biochem. 281: 36–44. [DOI] [PubMed] [Google Scholar]
- 61.Kowalski G. M., Carey A. L., Selathurai A., Kingwell B. A., Bruce C. R. 2013. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS ONE. 8: e72449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohkawa R., Nakamura K., Okubo S., Hosogaya S., Ozaki Y., Tozuka M., Osima N., Yokota H., Ikeda H., Yatomi Y. 2008. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann. Clin. Biochem. 45: 356–363. [DOI] [PubMed] [Google Scholar]
- 63.Guo S., Yu Y., Zhang N., Cui Y., Zhai L., Li H., Zhang Y., Li F., Kan Y., Qin S. 2014. Higher level of plasma bioactive molecule sphingosine 1-phosphate in women is associated with estrogen. Biochim. Biophys. Acta. 1841: 836–846. [DOI] [PubMed] [Google Scholar]
- 64.Okajima F. 2002. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim. Biophys. Acta. 1582: 132–137. [DOI] [PubMed] [Google Scholar]
- 65.Argraves K. M., Sethi A. A., Gazzolo P. J., Wilkerson B. A., Remaley A. T., Tybjaerg-Hansen A., Nordestgaard B. G., Yeatts S. D., Nicholas K. S., Barth J. L., et al. 2011. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B., Tomura H., Kuwabara A., Kimura T., Miura S., Noda K., Okajima F., Saku K. 2005. Correlation of high density lipoprotein (HDL)-associated sphingosine 1-phosphate with serum levels of HDL-cholesterol and apolipoproteins. Atherosclerosis. 178: 199–205. [DOI] [PubMed] [Google Scholar]
- 67.Hla T., Venkataraman K., Michaud J. 2008. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta. 1781: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnström J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 108: 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutter I., Park R., Othman A., Rohrer L., Hornemann T., Stoffel M., Devuyst O., von Eckardstein A. 2014. Apolipoprotein M modulates erythrocyte efflux and tubular reabsorption of sphingosine-1-phosphate. J. Lipid Res. 55: 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sevvana M., Ahnström J., Egerer-Sieber C., Lange H. A., Dahlbäck B., Muller Y. A. 2009. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J. Mol. Biol. 393: 920–936. [DOI] [PubMed] [Google Scholar]
- 71.Kimura T., Sato K., Kuwabara A., Tomura H., Ishiwara M., Kobayashi I., Ui M., Okajima F. 2001. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 276: 31780–31785. [DOI] [PubMed] [Google Scholar]
- 72.Theilmeier G., Schmidt C., Herrmann J., Keul P., Schäfers M., Herrgott I., Mersmann J., Larmann J., Hermann S., Stypmann J., et al. 2006. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 114: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 73.Wilkerson B. A., Grass G. D., Wing S. B., Argraves W. S., Argraves K. M. 2012. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem. 287: 44645–44653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salous A. K., Panchatcharam M., Sunkara M., Mueller P., Dong A., Wang Y., Graf G. A., Smyth S. S., Morris A. J. 2013. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J. Lipid Res. 54: 2775–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aoki S., Yatomi Y., Ohta M., Osada M., Kazama F., Satoh K., Nakahara K., Ozaki Y. 2005. Sphingosine 1-phosphate-related metabolism in the blood vessel. J. Biochem. 138: 47–55. [DOI] [PubMed] [Google Scholar]
- 76.Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., et al. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 316: 295–298. [DOI] [PubMed] [Google Scholar]
- 77.Kharel Y., Raje M., Gao M., Gellett A. M., Tomsig J. L., Lynch K. R., Santos W. L. 2012. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem. J. 447: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kharel Y., Mathews T. P., Gellett A. M., Tomsig J. L., Kennedy P. C., Moyer M. L., Macdonald T. L., Lynch K. R. 2011. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem. J. 440: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sensken S. C., Bode C., Nagarajan M., Peest U., Pabst O., Gräler M. H. 2010. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J. Immunol. 184: 4133–4142. [DOI] [PubMed] [Google Scholar]
- 80.Tani M., Ito M., Igarashi Y. 2007. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 19: 229–237. [DOI] [PubMed] [Google Scholar]
- 81.Bode C., Sensken S. C., Peest U., Beutel G., Thol F., Levkau B., Li Z., Bittman R., Huang T., Tölle M., et al. 2010. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell. Biochem. 109: 1232–1243. [DOI] [PubMed] [Google Scholar]
- 82.Dong A., Sunkara M., Panchatcharam M., Salous A., Selim S., Morris A. J., Smyth S. S. 2012. Synergistic effect of anemia and red blood cells transfusion on inflammation and lung injury. Adv. Hematol. 2012: 924042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kobayashi N., Yamaguchi A., Nishi T. 2009. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J. Biol. Chem. 284: 21192–21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Y., Guo S., Feng Y., Feng L., Cui Y., Song G., Luo T., Zhang K., Wang Y., Jiang X. C., et al. 2014. Phospholipid transfer protein deficiency decreases the content of S1P in HDL via the loss of its transfer capability. Lipids. 49: 183–190. [DOI] [PubMed] [Google Scholar]
- 85.Nagahashi M., Takabe K., Terracina K. P., Soma D., Hirose Y., Kobayashi T., Matsuda Y., Wakai T. 2014. Sphingosine-1-phosphate transporters as targets for cancer therapy. Biomed. Res. Int. 2014: 651727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendoza A., Bréart B., Ramos-Perez W. D., Pitt L. A., Gobert M., Sunkara M., Lafaille J. J., Morris A. J., Schwab S. R. 2012. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Reports. 2: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J., et al. 2012. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee Y. M., Venkataraman K., Hwang S. I., Han D. K., Hla T. 2007. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC). Prostaglandins Other Lipid Mediat. 84: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jonnalagadda D., Sunkara M., Morris A. J., Whiteheart S. W. 2014. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim. Biophys. Acta. 1841: 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ulrych T., Böhm A., Polzin A., Daum G., Nüsing R. M., Geisslinger G., Hohlfeld T., Schrör K., Rauch B. H. 2011. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J. Thromb. Haemost. 9: 790–798. [DOI] [PubMed] [Google Scholar]
- 91.Yatomi Y., Ohmori T., Rile G., Kazama F., Okamoto H., Sano T., Satoh K., Kume S., Tigyi G., Igarashi Y., et al. 2000. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 96: 3431–3438. [PubMed] [Google Scholar]
- 92.Sano T., Baker D., Virag T., Wada A., Yatomi Y., Kobayashi T., Igarashi Y., Tigyi G. 2002. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 277: 21197–21206. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi N., Nishi T., Hirata T., Kihara A., Sano T., Igarashi Y., Yamaguchi A. 2006. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J. Lipid Res. 47: 614–621. [DOI] [PubMed] [Google Scholar]
- 94.Dahm F., Nocito A., Bielawska A., Lang K. S., Georgiev P., Asmis L. M., Bielawski J., Madon J., Hannun Y. A., Clavien P. A. 2006. Distribution and dynamic changes of sphingolipids in blood in response to platelet activation. J. Thromb. Haemost. 4: 2704–2709. [DOI] [PubMed] [Google Scholar]
- 95.Igarashi Y., Yatomi Y. 1998. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim. Pol. 45: 299–309. [PubMed] [Google Scholar]
- 96.Ono Y., Kurano M., Ohkawa R., Yokota H., Igarashi K., Aoki J., Tozuka M., Yatomi Y. 2013. Sphingosine 1-phosphate release from platelets during clot formation: close correlation between platelet count and serum sphingosine 1-phosphate concentration. Lipids Health Dis. 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Son D. J., Lee H. W., Shin H. W., Lee J. J., Yoo H. S., Kim T. J., Yun Y. P., Hong J. T. 2008. Enhanced release of sphingosine-1-phosphate from hypercholesterolemic platelets: role in development of hypercholesterolemic atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. 78: 383–390. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt H., Schmidt R., Geisslinger G. 2006. LC-MS/MS-analysis of sphingosine-1-phosphate and related compounds in plasma samples. Prostaglandins Other Lipid Mediat. 81: 162–170. [DOI] [PubMed] [Google Scholar]
- 99.Jedlitschky G., Tirschmann K., Lubenow L. E., Nieuwenhuis H. K., Akkerman J. W., Greinacher A., Kroemer H. K. 2004. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 104: 3603–3610. [DOI] [PubMed] [Google Scholar]
- 100.Olivera B. M. 2006. Conus peptides: biodiversity-based discovery and exogenomics. J. Biol. Chem. 281: 31173–31177. [DOI] [PubMed] [Google Scholar]
- 101.Mitra P., Oskeritzian C. A., Payne S. G., Beaven M. A., Milstien S., Spiegel S. 2006. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA. 103: 16394–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nijnik A., Clare S., Hale C., Chen J., Raisen C., Mottram L., Lucas M., Estabel J., Ryder E., Adissu H., et al. 2012. The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J. Immunol. 189: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiong Y., Yang P., Proia R. L., Hla T. 2014. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Invest. 124: 4823–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baranowski M., Błachnio-Zabielska A. U., Charmas M., Helge J. W., Dela F., Książek M., Długołęcka B., Klusiewicz A., Chabowski A., Górski J. 2015. Exercise increases sphingoid base-1-phosphate levels in human blood and skeletal muscle in a time- and intensity-dependent manner. Eur. J. Appl. Physiol 115: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kurano M., Tsukamoto K., Ohkawa R., Hara M., Iino J., Kageyama Y., Ikeda H., Yatomi Y. 2013. Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis. 229: 102–109. [DOI] [PubMed] [Google Scholar]
- 106.Sterner E., Masuko S., Li G., Li L., Green D. E., Otto N. J., Xu Y., DeAngelis P. L., Liu J., Dordick J. S., et al. 2014. Fibroblast growth factor-based signaling through synthetic heparan sulfate blocks copolymers studied using high cell density three-dimensional cell printing. J. Biol. Chem. 289: 9754–9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ikeda H., Ohkawa R., Watanabe N., Nakamura K., Kume Y., Nakagawa H., Yoshida H., Okubo S., Yokota H., Tomiya T., et al. 2010. Plasma concentration of bioactive lipid mediator sphingosine 1-phosphate is reduced in patients with chronic hepatitis C. Clin. Chim. Acta. 411: 765–770. [DOI] [PubMed] [Google Scholar]
- 108.Xu N., Dahlbäck B. 1999. A novel human apolipoprotein (apoM). J. Biol. Chem. 274: 31286–31290. [DOI] [PubMed] [Google Scholar]
- 109.Nojiri T., Kurano M., Tokuhara Y., Ohkubo S., Hara M., Ikeda H., Tsukamoto K., Yatomi Y. 2014. Modulation of sphingosine-1-phosphate and apolipoprotein M levels in the plasma, liver and kidneys in streptozotocin-induced diabetic mice. J. Diabetes Investig. 5: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]