Abstract
Humans and mice lacking angiopoietin-like protein 3 (ANGPTL3) have pan-hypolipidemia. ANGPTL3 inhibits two intravascular lipases, LPL and endothelial lipase, and the low plasma TG and HDL-cholesterol levels in ANGPTL3 deficiency reflect increased activity of these enzymes. The mechanism responsible for the low LDL-cholesterol levels associated with ANGPTL3 deficiency is not known. Here we used an anti-ANGPTL3 monoclonal antibody (REGN1500) to inactivate ANGPTL3 in mice with genetic deficiencies in key proteins involved in clearance of ApoB-containing lipoproteins. REGN1500 treatment consistently reduced plasma cholesterol levels in mice in which Apoe, Ldlr, Lrp1, and Sdc1 were inactivated singly or in combination, but did not alter clearance of rabbit 125I-βVLDL or mouse 125I-LDL. Despite a 61% reduction in VLDL-TG production, VLDL-ApoB-100 production was unchanged in REGN1500-treated animals. Hepatic TG content, fatty acid synthesis, and fatty acid oxidation were similar in REGN1500 and control antibody-treated animals. Taken together, our findings indicate that inactivation of ANGPTL3 does not affect the number of ApoB-containing lipoproteins secreted by the liver but alters the particles that are made such that they are cleared more rapidly from the circulation via a noncanonical pathway(s). The increased clearance of lipolytic remnants results in decreased production of LDL in ANGPTL3-deficient animals.
Keywords: cholesterol, dyslipidemias, lipase/endothelial, lipase/lipoprotein, angiopoietin-like protein 3, very low density lipoprotein
An elevated plasma level of LDL-cholesterol (LDL-C) is the cardinal risk factor for coronary heart disease (CHD), and reducing plasma LDL-C levels is the cornerstone for CHD prevention (1). Inhibitors of HMG-CoA reductase, an enzyme that catalyzes an early step in cholesterol biosynthesis, effectively lower plasma LDL-C levels and prevent CHD (2). Human genetic studies have provided several new targets for LDL-C lowering. Individuals who lack ApoB (3), or microsomal transfer protein (MTP), the enzyme that transfers TG to TG-rich lipoproteins in the liver and intestine (4), have very low LDL levels. Agents that suppress ApoB expression (5) or inhibit MTP activity (6) are now available for treatment of severe hypercholesterolemia in individuals with homozygous familial hypercholesterolemia. Loss-of-function mutations in PCSK9, a secreted proprotein convertase that promotes degradation of the LDL receptor (LDLR), cause a 30% reduction in LDL-C and substantial protection from CHD (7). Anti-PCSK9 antibodies lower circulating LDL-C levels (8) and clinical trials are now underway to determine whether there is an associated reduction in CHD (9).
More recently, several families with inactivating mutations in angiopoietin-like protein 3 (ANGPTL3) were identified (10–12). Family members with two loss-of-function mutations in ANGPTL3 have striking pan-hypolipidemia; plasma levels of TGs, NEFAs, VLDL-cholesterol (VLDL-C), LDL-C, and HDL-cholesterol (HDL-C) are all markedly reduced. The mechanisms by which ANGPTL3 modulates TG metabolism have been extensively investigated (13). ANGPTL3 inhibits the activity of two intravascular lipases: LPL, which catalyzes hydrolysis of TGs in TG-rich lipoproteins, and endothelial lipase (EL), which hydrolyzes lipoprotein phospholipids (14–16). Thus, increased activity of LPL and EL may account for the low plasma levels of TG and HDL-C associated with ANGPTL3 deficiency. The finding that LPL and EL activities are increased in Angptl3−/− mice (14, 15) is consistent with this hypothesis. Corresponding data from humans is limited to a single study of four individuals who were homozygous for a nonsense mutation (S17X) in ANGPTL3 (17). Postheparin plasma LPL activity was higher in the individuals harboring the nonsense mutation than in family members who did not carry mutations in ANGPTL3, but no significant differences in EL activity were observed.
In contrast, the mechanism responsible for the lower plasma levels of LDL-C associated with ANGPTL3 deficiency remains enigmatic. Plasma LDL-C levels are determined by the relative rates of production and clearance of LDL (18). LDL is produced in the circulation from VLDL, a TG-rich lipoprotein assembled in the liver. The surface of VLDL is decorated with multiple proteins, including several copies of ApoE as well as a single copy of ApoB. Following lipolysis by LPL, VLDL and its metabolic intermediate, IDL, have two possible fates: clearance by the liver or further metabolism to LDL. Hepatic uptake of VLDL remnants (and intestine-derived chylomicron remnants) is mediated by ApoE, which is bound by LDLRs (19) and by LDLR related protein 1 (LRP1) (20) or by proteoglycans (syndecan 1) (21). The remaining VLDL remnant particles mature into LDL, which is removed from the blood primarily by LDLR-mediated endocytosis in the liver. Inactivation of ANGPTL3 in mice by gene targeting or by anti-ANGPTL3 antibodies reduces plasma cholesterol levels in mice lacking functional ApoE (22) or LDLR (23). These data indicate that ANGPTL3 lowers LDL by a mechanism that is independent of both a major ligand for receptor-mediated clearance of lipoproteins and the major receptor that mediates clearance of circulating LDL.
To investigate the mechanism by which ANGPTL3 deficiency lowers plasma LDL-C levels, we used REGN1500 (24), a fully human monoclonal antibody that binds with high affinity to both human and mouse ANGPTL3, to inactivate ANGPTL3 in mice with genetically defined defects in the major pathways through which circulating lipoproteins are cleared. Here we report the effect of ANGPTL3 inactivation on the synthesis and clearance of LDL in mice.
MATERIALS AND METHODS
Materials
REGN1500, a fully human monoclonal antibody, was derived using Regeneron’s Velocimmune® technology platform (24). An isotype-matched antibody with irrelevant specificity was used as control. All other chemicals were purchased from Sigma unless otherwise indicated.
Studies in mice
WT, Apoe−/−, and Ldlr−/− C57BL/6J mice were purchased from Jackson Laboratory. Mice deficient in syndecan 1 (Sdc1−/−) were provided by P. Park (Harvard University, Cambridge, MA) (25). Liver-specific Lrp1 KO mice were from Joachim Herz (University of Texas Southwestern, Dallas, TX) (26). Ldlr−/−;Lrp1−/− mice were generated by crossing liver-specific Lrp1−/− mice with Ldlr−/− animals. Angptl3−/− mice were developed by homologous recombination using Regeneron’s Velocigene® technology (27). All Angptl3−/− mice were on a mixed background (25% of 129/Sv and 75% of C57BL/6). F1 heterozygous mice were bred to generate WT and KO mice, which were used for all experiments. All animal procedures were conducted in compliance with protocols approved by the University of Texas Southwestern Medical Center Animal Care and Use Committee and in accordance with federal animal welfare policies and regulations. Mice were fed a chow diet (4% fat and 0.04% cholesterol) (Teklad 7001) and maintained on a 12 h light (7:00 AM to 7:00 PM)/12 h dark (7:00 PM to 7:00 AM) cycle unless otherwise indicated. Fat-free diet was obtained from MP Biomedicals, LLC (901683). REGN1500 and isotype control antibodies were diluted in sterile saline to a concentration of 1 mg/ml. Mice were injected with 10 mg/kg body weight of either the control antibody or REGN1500 via the tail vein.
Blood chemistries
Circulating TG, total cholesterol, alanine aminotransferase, and aspartate aminotransferase levels were determined using an enzymatic method (Infinity; Thermo Scientific). NEFAs were measured by use of an HR Series NEFA-HR kit (Wako Diagnostics).
Lipoprotein profiling
Plasma lipoprotein particles were size fractionated by fast performance LC (FPLC) using a Superose 6 column (GE Healthcare) and the cholesterol and TG content of each fraction was measured enzymatically (Infinity; Thermo Scientific) as described (28).
VLDL production
Mice were injected via the tail vein with Triton WR1339 (500 mg/kg) (Tyloxapol, Sigma-Aldrich) in 200 μl of sterile saline to inhibit the hydrolysis and clearance of plasma VLDL (29). At the indicated time points, blood samples were collected in EDTA-coated tubes from the tail vein and the TG content was measured enzymatically. In a parallel set of antibody-treated mice, plasma samples collected at 90 min were pooled and subjected to FPLC fractionation. A total of 30 μl of each fraction (total volume of 300 μl) was subjected to immunoblotting using a rabbit anti-mouse ApoB polyclonal antibody that was developed in our laboratory.
ApoB secretion
ApoB secretion rates were measured as described previously (30). Briefly, each mouse was injected with 200 μCi of [35S]methionine (1,175 Ci/mmol) (PerkinElmer Life Sciences) and Triton WR1339 (500 mg/kg). Blood was collected from the tail vein before and 90 min after the injection into EDTA-coated tubes that contained aprotinin. Plasma was isolated immediately, delipidated with 2 vol of butanol:diisopropyl-ether (4:6) at room temperature for 20 min and on ice for another 20 min. Then plasma was centrifuged for 5 min at 4,000 rpm and the lower aqueous phase was carefully removed and diluted 10-fold with saline. The sample was mixed with 6× SDS sample buffer, heated for 5 min at 95°C, and size fractionated by 5% SDS-PAGE. The gels were dried and exposed to X-ray film (BIOMAX XAR; Kodak, catalog number 1651579) for 4 days at −80°C to visualize the ApoB-100 and ApoB-48. The films were scanned using a HP Scanjet 5590 and quantified using ImageJ. The intensity of each band was corrected for background using a blank from the same film. In a separate gel, 10 μl of each diluted sample was subjected to immunoblot analysis with an anti-ApoB polyclonal antibody. The immunoblots were scanned with an Odyssey fluorescent imaging system (LI-COR, Inc.) to quantify the intensity of the ApoB bands.
LDL and β-VLDL clearance
LDL was isolated by sequential ultracentrifugation (31) from Ldlr−/− mice fed a high-cholesterol (1%) diet for 2 weeks and labeled with 125I using the iodine monochloride method. A total of 15 μg of 125I-LDL (specific activity: 170 cpm/ng) in 10 mM TrisCl (pH 7.4), 150 mM NaCl, and 0.2% BSA was injected into the tail veins of mice treated with REGN1500 or control antibody. Blood samples were collected from the tail vein at 2 min, 10 min, 30 min, 1 h, 2 h, and 4 h after the injection. Plasma was isolated from each sample and radioactivity was measured by γ-counting (10 μl aliquot of plasma). A second 10 μl aliquot was precipitated with isopropanol and the supernatant was counted. ApoB labeling was calculated as the difference between total counts and the supernatant counts. The amount of 125I-LDL remaining in the plasma was expressed as a percentage of the initial concentration, measured as the amount of 125I-radioactivity in the plasma 2 min after injection. The half-life of LDL was calculated by fitting an exponential to the decay curve of 125I-ApoB plotted against time.
β-VLDL was isolated by ultracentrifugation from rabbits fed a chow diet containing 2% cholesterol for 10 days. The particles were radiolabeled with 125I using the iodine monochloride method (32). A total of 15 μg of 125I-β-VLDL (specific activity: 567 cpm/ng) in 10 mM TrisCl (pH 7.4), 150 mM NaCl, and 0.2% BSA was injected into tail veins of mice treated with REGN1500 or with control antibody. Blood samples were collected from the tail vein at 2 min, 10 min, 30 min, 1 h, and 2 h after the injection. The mice were then euthanized and perfused with 10 ml of cold saline through the left ventricle before the tissues were collected. The radioactivity of each tissue was quantified in a γ-counter (total uptake). A sample was also subjected to lysis using RIPA buffer [50 mM TrisCl (pH 7.4), 0.1% SDS, 1% Trition-100, 0.1% sodium deoxycholate, 250 mM NaCl, 20 mM NaF] plus protease inhibitor cocktail (Roche) and the proteins were precipitated with acetone. The supernatant was subjected to γ-counting. Tissue 125I-ApoB uptake was calculated by subtracting the counts in the supernatant from the total counts. Data was expressed as percentage uptake of total injected dose per 100 mg of tissue. Plasma samples were processed as described for the LDL clearance experiment.
Fatty acid uptake
Tritiated bromopalmitate and 14C-palmitate were complexed with fatty acid-free BSA (Sigma) as described (33). Briefly, 10 μCi of 3H-bromopalmitate and 10 μCi of 14C-palmitate were dried under N2 and then resuspended in 150 μl of ethanol. The solution was added drop-wise to 0.6 ml of 4% fatty acid-free BSA and stirred continuously. Normal saline was added up to a final volume of 2 ml. A total of 200 μl was injected into each mouse via the tail vein; and 5 min later, the mice were perfused with 10 ml of cold saline and euthanized. Tissues were collected, lysed, and the radioactivity quantitated as described (27).
Fatty acid infusion
On day 1, a cannula was placed in the jugular vein. From day 2 to day 5, the mice were fasted from 5:00 PM to 7:00 AM and refed from 7:00 AM to 5:00 PM. On day 5, mice were refed at 7:00 AM and at 9:00 AM were injected with control antibody or REGN1500. Two hours after antibody treatment, 3H-palmitate was infused into the mice as described (33). Briefly, 50 μCi of 3H-palmitate and 305 nmol palmitic acid were dried under N2 and resuspended in 2 ml of fatty acid-free BSA (1%). The mixture was infused at a rate of 100 μl/min/30 g body weight over 30 s and then for 4.5 min at a rate of 20 μl/min/30 g body weight. Blood was sampled every minute from the tail vein during the infusion period and twice after infusion. Plasma was isolated and subjected to scintillation counting.
Transcriptome sequencing
Male WT and Angptl3−/− mice (age 12 weeks, n = 4–6 per group) were entrained to a synchronized feeding schedule (12 h fasting and 12 h feeding) for 3 days and euthanized at the end of the dark cycle of the third day. Livers were harvested and total RNA was extracted using a Qiagen RNeasy Midi kit. RNA samples were pooled and subjected to whole transcriptome shotgun sequencing as described (27).
Fatty acid synthesis
Feeding was synchronized for 5 days prior to measurement of fatty acid synthesis in the liver and kidney (fasting, light cycle: 9:00 AM to 9:00 PM and refeeding, dark cycle: 9:00 PM to 9:00 AM). Fatty acid synthesis was measured at the end of the refeeding cycle using 3H2O incorporation (34). Briefly, 3H-labeled water (50 mCi in 0.25 ml of isotonic saline) was injected intraperitoneally. After 30 min, the liver and kidneys were collected and the incorporation of 3H into fatty acids was quantitated as previously described (34).
Fatty acid oxidation
WT mice were treated with either REGN1500 or control antibody (10 mg/kg). Four days after treatment, primary hepatocytes were isolated from the mice at 9:00 AM. XF-24 cell culture plates (Seahorse Bioscience) were coated with rat tail collagen (Sigma) and primary hepatocytes were seeded at a density of 2 × 104 cells per well in five replicates for each mouse. Cells were allowed to adhere for 5 h and then oxygen consumption was measured as described previously (35). Basal oxygen consumption was recorded for 20 min before and after addition of 200 μm palmitate-BSA and then etomoxir (500 μm) for 40 min. The rate of oxygen consumption was normalized for the protein content of the cells.
Statistics
Data are expressed as mean ± standard error. Mean values were compared using unpaired t-tests as implemented in GraphPad Prism (GraphPad Software, Inc.).
RESULTS
As a first step toward investigating the mechanism by which inactivation of ANGPTL3 lowers plasma LDL-C levels, we used REGN1500 to inactivate circulating ANGPTL3 in mice with defects in the major clearance pathways for LDL and its biosynthetic precursors: LDLR, the predominant vehicle for the clearance of LDL (19, 36); LRP1, a receptor for ApoE-containing lipoproteins (37); and syndecan 1, a heparan sulfate proteoglycan on the surfaces of hepatocytes that facilitates clearance of some ApoE-containing lipoproteins, such as chylomicron remnants (21).
REGN1500 is a monoclonal antibody developed against human ANGPTL3 that binds mouse, rat, and monkey ANGPTL3 with a similar affinity. A full description of REGN1500 is provided in the companion article (24).
Immunological inactivation of ANGPTL3 lowers plasma cholesterol levels in the absence of LDLR or hepatic LRP1
Previously, Lee et al. (23) showed that antibody-mediated inactivation of ANGPTL3 decreases plasma cholesterol levels in Ldlr−/− mice, thus indicating that ANGPTL3 inhibition lowers LDL-C levels independently of the LDLR. Consistent with these earlier findings, REGN1500 lowered cholesterol and TG by 33 and 40%, respectively, in Ldlr−/− and WT animals (supplementary Fig. 1A). Treatment with REGN1500 lowered TG and cholesterol in both the VLDL and LDL fractions of the Ldlr−/− mice (supplementary Fig. 1B). Quantitative immunoblot analysis was performed to determine the amount of ApoB in each fraction. ApoB-48 levels were reduced in all fractions from the REGN1500-treated animals. ApoB-100 levels were similar in the VLDL size range but were reduced in the LDL fractions. Thus, inactivation of ANGPTL3 was associated with changes in both the lipid and protein content of the ApoB-containing lipoproteins.
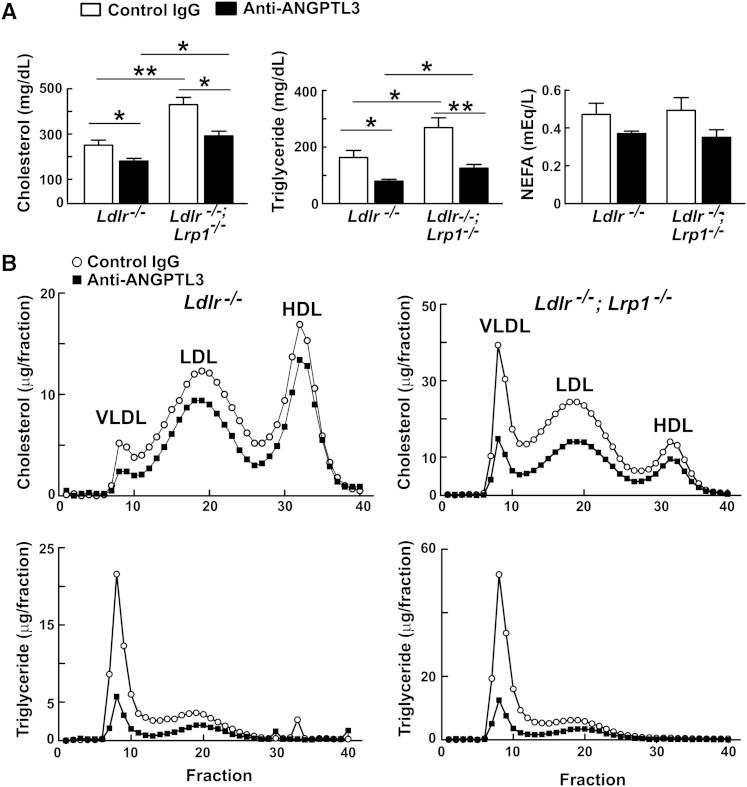
It remains possible that in the absence of ANGPTL3, LDL precursor particles are cleared more rapidly by LRP1. To determine whether LRP1 contributes to the lower lipid levels in ANGPTL3-deficient animals, we compared the lipid-lowering effects of REGN1500 in Ldlr−/−, and Ldlr−/−;Lrp1−/− mice (Fig. 1A). Plasma levels of cholesterol and TG were significantly higher in Ldlr−/−;Lrp1−/− animals than in Ldlr−/− mice, but antibody treatment lowered both lipids to a comparable extent (cholesterol by 23 and 30%, and TG by 46 and 51%, respectively) in the two lines of mice (Fig. 1A). REGN1500 also reduced plasma levels of NEFAs, although the reduction was not significant in this experiment. To determine the effect of inactivating ANGPTL3 on lipoprotein levels in these animals, plasma from each group of mice was fractionated by FPLC (Fig. 1B). The cholesterol was reduced in all the ApoB-containing lipoprotein fractions, as well as in the HDL fractions, in both the single and double KO animals (Fig. 1B).
Fig. 1.
Effects of anti-ANGPTL3 antibody (REGN1500) on levels and distribution of plasma cholesterol and TG in Ldlr−/− and Ldlr−/−;Lrp1−/− (liver-specific Lrp1 KO) mice. A: Ldlr−/− and Ldlr−/−;Lrp1−/− mice (n = 4–6 males per group, age 10–15 weeks) were injected with control IgG or REGN1500 (10 mg/kg) via the tail vein. Four days later, at the end of the dark cycle, the mice were fasted for 2 h prior to blood sampling. Plasma lipid levels were measured as described in the Materials and Methods. B: Distribution of plasma lipids. Pooled plasma from each group of mice was fractionated by FPLC. Cholesterol and TG levels were measured enzymatically in each fraction. The experiment was repeated and the results were similar. *P < 0.05, **P < 0.01.
Immunological inactivation of ANGPTL3 lowers plasma cholesterol levels in the absence of both ApoE and LDLR
To determine whether inactivation of ANGPTL3 promotes ApoE-mediated clearance of lipoproteins independently of LDLR or LRP1, we treated Apoe−/−;Ldlr−/− mice with REGN1500. Inactivation of ANGPTL3 lowered plasma cholesterol levels in Apoe−/− mice (Fig. 2A), as was reported previously (22, 23). Similar reductions were observed in Apoe−/−;Ldlr−/− mice. The levels of cholesterol were reduced in the VLDL and LDL fractions, but not in the HDL fractions (where they were already very low) on FPLC analysis (Fig. 2B).
Fig. 2.
REGN1500 reduces plasma levels of cholesterol and TG in Apoe−/− and Apoe−/−;Ldlr−/− mice. A: Apoe−/− and Apoe−/−;Ldlr−/− mice were injected with control IgG or REGN1500 (10 mg/kg) by tail vein (n = 4–5 males per group, 11 weeks old). Four days after the injection, mice were fasted for 2 h at the end of the dark cycle, and blood was collected. Plasma lipid levels were measured enzymatically as described in the Materials and Methods. B: Pooled plasma from each group of mice was fractionated by FPLC and both cholesterol and TG levels were measured in each fraction. *P < 0.05, **P < 0.01.
Immunological inactivation of ANGPTL3 lowers plasma cholesterol levels in the absence of syndecan 1
An alternate pathway for hepatic clearance of LDL precursor particles is by heparan sulfate glycoproteins. These surface glycoproteins, particularly syndecan 1, contribute to clearance of remnant lipoproteins arising from LPL-mediated lipolysis (21, 38). To determine whether ANGPTL3 inactivation accelerates syndecan 1-mediated lipoprotein clearance, we examined the effect of REGN1500 treatment on plasma lipid levels in Sdc1−/− mice at the end of the feeding cycle. Plasma levels of both cholesterol and TG were increased in the Sdc1−/− mice, which is consistent with what has been reported previously (21). Administration of REGN1500 to Sdc1−/− mice led to significant decreases in plasma cholesterol and TG levels that were similar to those observed in WT animals (Fig. 3A). The cholesterol levels were reduced in the VLDL and HDL fractions, which are the major cholesterol-carrying lipoproteins in these mice (Fig. 3B). The TG content of the VLDL fraction was dramatically reduced by antibody treatment in Sdc1−/− and WT mice (85 and 65%, respectively).
Fig. 3.
REGN1500 reduces circulating levels of cholesterol and TG in Sdc1−/− mice. A: Sdc1−/− and WT C57BL/6J mice were injected with control IgG or REGN1500 (10 mg/kg) by tail vein (n = 6 males per group, 10–12 weeks old). Four days later mice were fasted for 2 h at the end of the dark cycle and blood was collected and plasma lipid levels were measured. B: Pooled plasma from each group of mice was fractionated by FPLC and the cholesterol and TG levels were measured enzymatically in each fraction. The experiment was repeated and the results were similar. *P < 0.05, **P < 0.01.
Immunologic inactivation of ANGPTL3 does not alter the clearance of exogenous LDL or β-VLDL
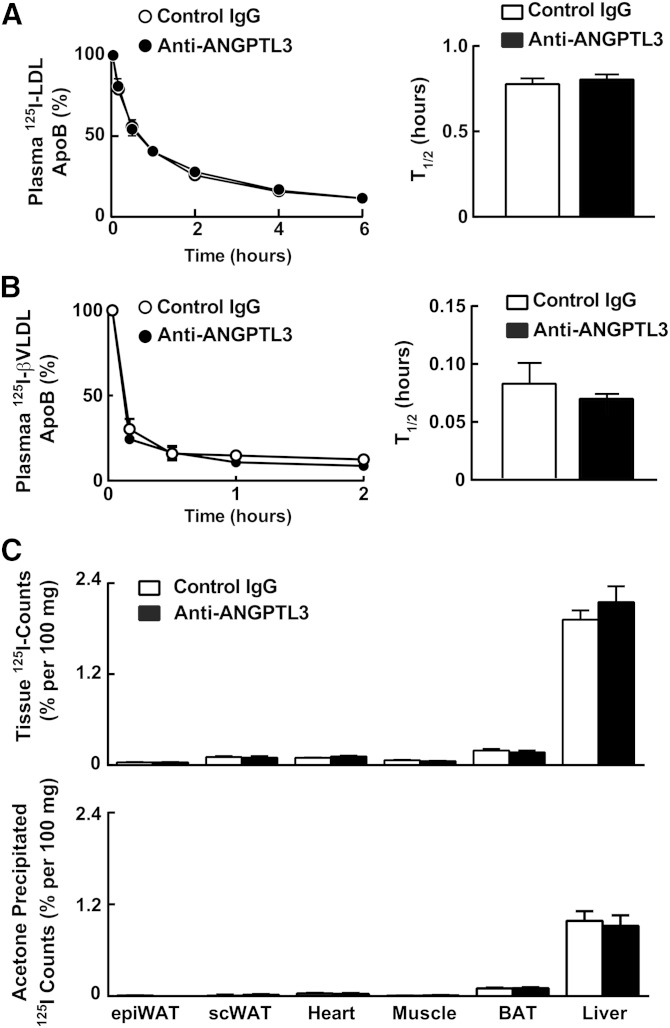
These data suggest that inactivation of ANGPTL3 does not promote clearance of LDL or its biosynthetic precursors via known lipoprotein receptors or ligands. To directly assess the effect of ANGPTL3 inactivation on LDL clearance, we injected radiolabeled mouse LDL into WT mice treated with either REGN1500 or with control antibody. The decay curves of radiolabeled LDL were superimposable (Fig. 4A) and the half-lives virtually identical in the mice treated with control antibody and REGN1500 (0.78 ± 0.03 h vs. 0.80 ± 0.03 h, P = 0.56).
Fig. 4.
REGN1500 does not alter LDL and β-VLDL turnover in WT mice. A: LDL was isolated from Ldlr−/− mice and radiolabeled with iodine as described in the Materials and Methods. The labeled LDL (15 μg, specific activity: 170 cpm/ng) was injected into WT mice 4 days after injection of REGN1500, as described in the legend to Fig. 1 (n = 6 males per group, 10 weeks old). Blood was obtained from the tail vein at the indicated times after LDL injection. ApoB was precipitated from plasma using isopropanol and the radioactivity was quantitated in a scintillation counter. The percentage of injected label remaining at each time point was determined using the value obtained at 2 min as the starting value. The half-life was calculated from the decay curve of ApoB activity plotted against time. B: Rabbit β-VLDL was isolated and labeled with iodine as described in the Materials and Methods. The labeled β-VLDL (15 μg, specific activity: 567 cpm/ng) was injected into WT mice 4 days after treatment with REGN1500, as described in the legend to Fig. 1 (n = 5 males per group, 10 weeks old). Blood was collected and processed as described in (A). C: Two hours after the β-VLDL was injected into the mice, the mice were euthanized and tissues were collected. Tissue total uptake and acetone precipitated counts were measured as described in the Materials and Methods. The experiments were repeated once with similar results. BAT, brown adipose tissue; epiWAT, epidydimal white adipose tissue (WAT); scWAT, subcutaneous WAT.
We then performed a similar experiment using radiolabeled rabbit β-VLDL as a model ApoE-rich lipoprotein in WT mice. The results were similar to those obtained with LDL. No differences were found in the rates of particle clearance between the two groups of mice (Fig. 4B). To determine whether remnants were being cleared by tissues other than the liver, as suggested by Bartelt et al. (39), we measured tissue uptake of radiolabeled β-VLDL by quantifying the amount of total and protein-associated (acetone precipitated) radioactivity in tissues (Fig. 4C, top and bottom, respectively). As expected, the tissue with the greatest uptake was the liver. No differences were seen between the two groups of mice in the amount of total protein-associated radioactive counts in liver, heart, muscle, epididymal fat, subcutaneous fat, or brown adipose tissue.
These findings indicate that inactivation of ANGPTL3 does not increase the functional activity of pathways that clear LDL or ApoE-containing lipoproteins either in the liver or in peripheral tissues.
Both immunological and genetic inactivation of ANGPTL3 reduces secretion of VLDL-TG but not VLDL-ApoB-100
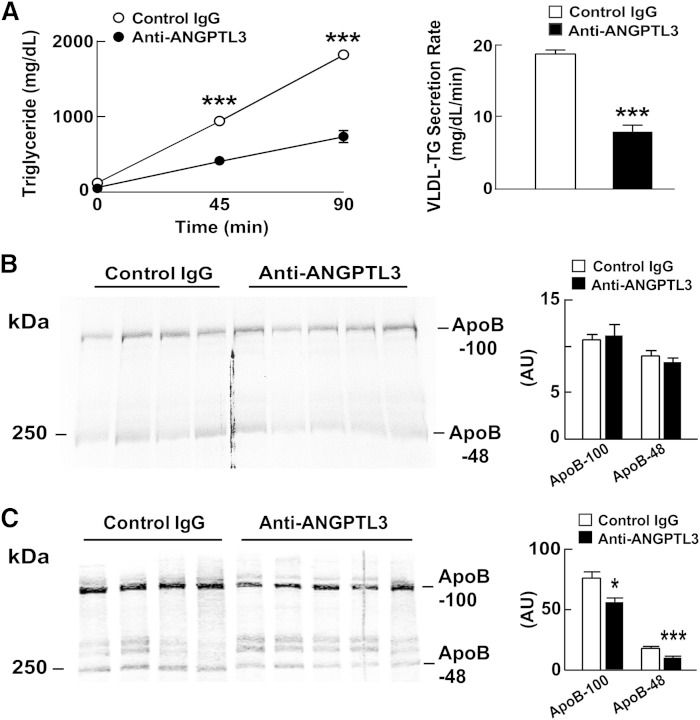
One mechanism by which ANGPTL3 inactivation might lower LDL is by decreasing the secretion of VLDL particles by the liver, which would result in fewer VLDL remnants being processed to LDL. To determine whether inactivating ANGPTL3 reduces VLDL-TG secretion, we used Triton WR1339 to inhibit LPL in WT and Ldlr−/− mice treated with REGN1500 or with control antibody (29). The experiment was performed first in WT mice 4 h after refeeding, because ANGPTL3 appears to be most active in the fed state (40). The rate of accumulation of plasma TG following Triton injection was reduced by 61% (from 12.5 to 4.9 mg TG/dl plasma/min) in REGN1500-treated mice (Fig. 5A) relative to animals treated with the control antibody. Blood was collected at the end of the experiment and subjected to FPLC analysis. The cholesterol and TG content of the VLDL fractions were both lower in the mice treated with REGN1500. Moreover, the level of ApoB-100 and ApoB-48 in the VLDL fractions from FPLC-fractionated plasma was reduced by a similar proportion in the REGN1500-treated animals (Fig. 5B).
Fig. 5.
Decreased VLDL-TG production in REGN1500-treated WT mice. A: WT mice were synchronized for 3 days (fasting: 5:00 PM to 7:00 AM and feeding: 7:00 AM to 5:00 PM). On day 4, mice were refed at 7:00 AM and injected at 9:00 AM with either a control antibody or REGN1500 (10 mg/kg) (n = 5 male mice per group, 10 weeks old). Two hours after the injection, Triton WR1339 (500 mg/kg) was injected into the tail vein. Blood samples were drawn from the tail veins at the indicated time points and then the mice were euthanized and blood was collected. Plasma TG levels were measured enzymatically. Plasma collected at the 90 min time point was pooled and fractionated by FPLC as described in the Materials and Methods, and TG and cholesterol were measured enzymatically in each fraction. The total TG and cholesterol content of the pooled VLDL fractions (7, 8, and 9) is shown below. B: ApoB-100 and ApoB-48 protein levels in the pooled VLDL fractions (7, 8, and 9) were assayed by immunoblotting (top) and quantified using ImageJ. ***, P < 0.001.
We repeated the experiment in Angptl3−/− mice and the results were remarkably similar (supplementary Fig. 2A, left panel). Thus, the reduction in VLDL-TG secretion is not a transient consequence of acute inactivation of ANGPTL3. Angptl3−/− mice did not have increased hepatic TG levels (supplementary Fig. 2B), as would be expected if ANGPTL3 was required for the efficient secretion of VLDL.
REGN1500 also lowered VLDL-TG secretion in fed Ldlr−/− mice (supplementary Fig. 2C).
The consistent reduction in VLDL-TG secretion associated with inactivation of ANGPTL3 in this study contrasts with a previous report (14) in which similar rates of VLDL secretion were observed in KK-San mice, which lack ANGPTL3, and KK mice, which are WT for ANGPTL3. Both studies used the Triton WR1339 method to assess VLDL production, but Shimizugawa et al. (14) fasted the mice before Triton injection. To determine whether differences in nutritional status could account for the differences in the two studies, we repeated the experiment in fasting mice. The effects of REGN1500 on TG accumulation were markedly attenuated when Triton WR1339 was administered to fasting WT animals (supplementary Fig. 2D). Whereas, VLDL-TG secretion was reduced 61% in REGN1500-treated fed mice (Fig. 5A), it was only reduced 15% in similarly treated fasted animals (supplementary Fig. 2D).
In mice fed regular chow (4% fat), circulating TG-rich lipoproteins are derived from both the intestine and the liver. To obviate confounding by intestine-derived lipoproteins, we repeated the Triton WR1339 experiment in WT mice refed with fat-free chow (supplementary Fig. 2E). In these animals the effect of ANGPTL3 inactivation on accumulation of circulating TG was comparable to that observed in animals refed chow.
The reduction in VLDL-TG secretion together with the decrease in plasma levels of VLDL-ApoB and VLDL-C is consistent with a model in which inactivation of ANGPTL3 decreases the secretion of VLDL particles. To directly test this hypothesis, we administered [35S]methionine by intravenous injection to WT mice pretreated with either control IgG or REGN1500 and then measured the appearance of radiolabeled ApoB-100 in the circulation 90 min after Triton WR1339. VLDL-TG secretion was decreased by REGN1500 treatment (Fig. 6A), but no differences were seen in the rates of appearance of labeled ApoB-100 or ApoB-48 (Fig. 6B). Immunoblot analysis of plasma revealed lower levels of both ApoB proteins in the REGN1500-treated animals (Fig. 6C).
Fig. 6.
ApoB secretion in REGN1500-treated WT mice. A: WT mice were synchronized for 3 days (fasting: 5:00 PM to 7:00 AM and feeding: 7:00 AM to 5:00 PM). On day 4, mice were refed at 7:00 AM and injected at 9:00 AM with either a control antibody or REGN1500 (10 mg/kg) (n = 6 male mice per group, 10 weeks old). Two hours after the injection, Triton WR1339 (500 mg/kg) and 200 μCi of [35S]methionine were injected into the tail vein. Blood samples were drawn from the tail veins before and 45 min after the injection. The mice were euthanized at 90 min and blood was collected. Plasma TG levels were measured at the indicated time after Triton WR1339 injection. B: Plasma collected at the 90 min time point was delipidated and size fractionated by SDS-PAGE (5%). Gels were dried and exposed to X-ray film (BIOMAX XAR; Kodak, catalog number 1651579) for 4 days at −80°C. The films were scanned using a HP Scanjet 5590 and quantified using ImageJ. The intensity of each band was corrected for background using a blank from the same film. C: The same plasma samples were subjected to immunoblot analysis with an anti-ApoB antibody and the bands were quantified using an Odyssey Image Analyzer (Li-Cor). *P < 0.05, ***P < 0.001.
Thus, inactivation of ANGPTL3 reduced the secretion of TG, but not of ApoB-100 or ApoB-48.
Inactivation of ANGPTL3 does not alter the rate of fatty acid synthesis or fatty acid oxidation in mice
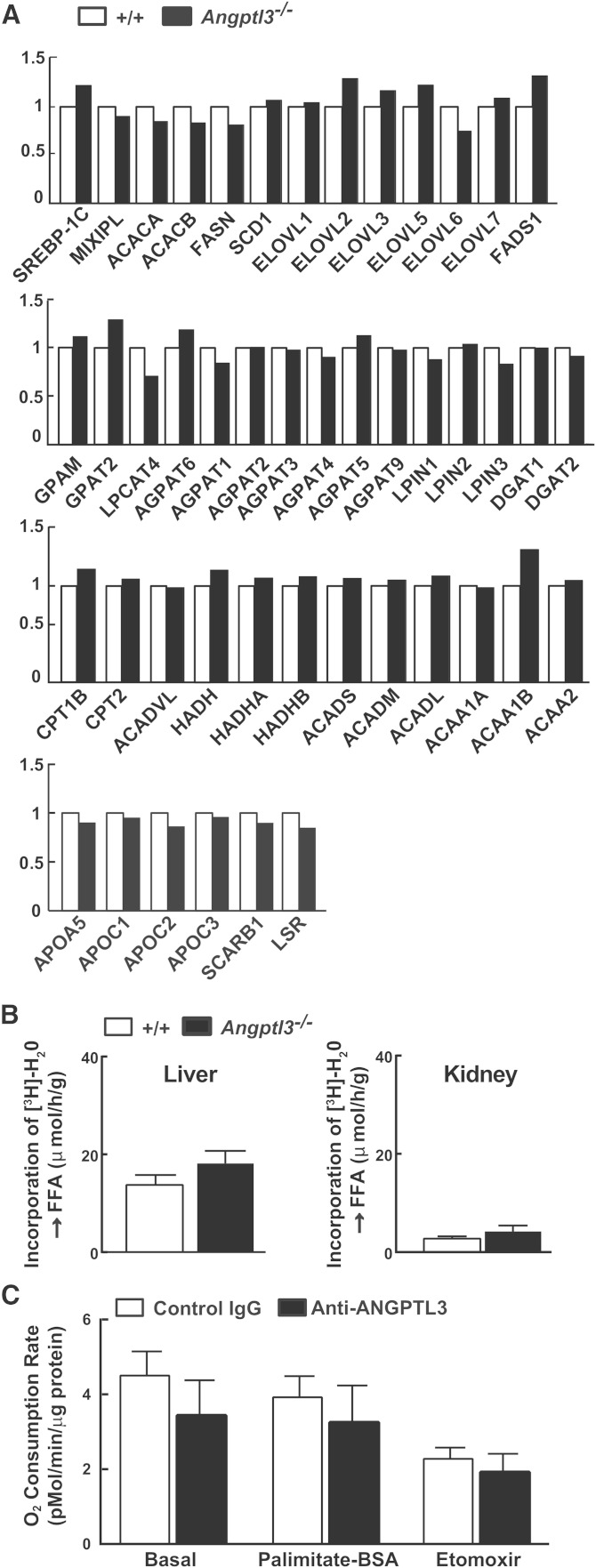
As a first step toward determining whether either reduced TG synthesis or increased fatty acid oxidation contributed to the reduction in VLDL-TG secretion in the Angptl3−/− mice, we compared the hepatic RNA expression profiles of Angptl3−/− and WT mouse liver using whole transcriptome sequencing (RNA-Seq). No major differences were seen in the levels of several mRNAs that encode proteins with key roles in hepatic fatty acid synthesis (ACC, FAS, SCD1), TG lipolysis (ATGL, CGI-58), fatty acid oxidation (CPT-1α, CPT-2, ACO), or VLDL catabolism (APOA5, APOC1, APOC2, APOC3, SCARB1, LSR) (Fig. 7A).
Fig. 7.
Hepatic de novo lipogenesis and fatty acid oxidation did not change after ANGPTL3 depletion. A: mRNA levels of genes involved in fatty acid synthesis or oxidation. Livers were collected from refed mice used in the experiment shown in supplementary Fig. 2A. Total RNA was extracted and subjected to whole transcriptome shotgun sequencing using an Illumina 2500 Hi-Seq as described previously (27). B: Rates of de novo lipogenesis in livers and kidneys of Angptl3−/− mice and littermate controls (n = 8 males per group, 12–16 weeks old). Mice were synchronized for 5 days. De novo lipogenesis was measured at the end of the refeeding cycle as described in the Materials and Methods. C: Fatty acid oxidation in primary hepatocytes isolated from REGN1500- or control antibody-treated WT mice (n = 4 males per group, 12 weeks old). Mice were treated with REGN1500 or control antibody (10 mg/kg) for 4 days. Primary hepatocytes were isolated and oxygen consumption rates were measured using a Seahorse XF analyzer as described in the Materials and Methods.
We also measured and compared the rate of synthesis of fatty acids from tritiated water in the livers of Angptl3−/− mice and littermate controls and found no differences between the two groups (Fig. 7B).
Finally, we measured fatty acid oxidation in primary hepatocytes isolated from the REGN1500- and control antibody-treated animals using a Seahorse oxygen analyzer. No systematic differences were found in O2 consumption after treatment with the antibody (Fig. 7C). As a positive control for the experiment, we added etomoxir, a fatty acid oxidation inhibitor, which reduced O2 consumption in hepatocytes from both groups of mice.
Inactivation of ANGPTL3 does not affect fatty acid uptake by the liver or other tissues
To determine whether the reduction in TG secretion is caused by reduced delivery of NEFAs to the liver, we measured the uptake of 14C-palmitate and its nonmetabolizable analog, 3H-bromopalmitate, into tissues of animals treated with REGN1500 or control antibody. Inactivation of ANGPTL3 significantly reduced circulating TG and NEFA levels (supplementary Fig. 3A), but did not affect the tissue distributions of palmitate and bromopalmitate (supplementary Fig. 3B). To determine whether the reduction in NEFA levels reflects increased clearance of NEFAs, we infused a priming dose followed by a constant infusion of labeled palmitate to achieve a stable level of labeled NEFAs in the plasma (supplementary Fig. 3C). No differences were seen in the levels of 14C counts over the time course of the experiment. Thus, the reduction in circulating NEFA levels after inactivation of ANGPTL3 cannot be explained by increased clearance, and presumably reflects reduced influx of fatty acids into the circulation.
DISCUSSION
In this study we examined the rates of production and clearance of ApoB-containing lipoproteins in mice with antibody-mediated inactivation of ANGPTL3. Our data show that inactivation of ANGPTL3 reduces hepatic secretion of TG in fed mice, which contributes to the low plasma TG levels in these animals. Inactivation of ANGPTL3 also reduced plasma levels of LDL-C and ApoB, despite not reducing hepatic ApoB secretion. These findings imply increased clearance of ApoB-containing lipoproteins in ANGPTL3-deficient animals. However, we found no increase in the clearance of exogenous LDL or β-VLDL in the REGN1500-treated mice. Moreover, genetic disruption of the known clearance pathways for ApoB-containing lipoproteins failed to block the reductions in plasma lipids associated with ANGPTL3 inactivation. Thus, inactivation of ANGPTL3 does not upregulate the pathways that normally remove these lipoproteins. The reduction in VLDL-C in REGN1500-treated Apoe−/− KO mice (Fig. 2B) indicates that the cholesterol-lowering effects of ANGPTL3 inactivation are mediated (or least initiated) prior to the formation of LDL. Taken together, our data are most consistent with a model in which inactivation of ANGPTL3 increases the clearance of ApoB-containing lipoproteins as they progress through the lipolytic cascade, thereby decreasing the fraction of VLDL that is converted to LDL and reducing LDL production.
The nature of the pathway by which ANGPTL3 inactivation promotes the clearance of ApoB is not known. ANGPTL3 deficiency is associated with lower cholesterol levels in mice lacking either ApoE or LDLR (22, 23), and the present study extends these findings to LRP1 and syndecan 1. Thus, the increased clearance of ApoB-containing lipoproteins presumably involves a noncanonical pathway that does not require ApoE, the LDLR, LRP1, or syndecan 1. Reductions in plasma cholesterol have also been observed in other mouse models in which LPL expression is increased either directly (41–43) or indirectly (44). The cholesterol-lowering effects were observed with increases in LPL that are comparable (∼2- to 3-fold) to those associated with inactivation of ANGPTL3 in the present study (43). Therefore, it is possible that an increase in LPL promotes the clearance of ApoB-containing lipoproteins either by catalyzing changes in lipid content that increase binding of the lipoprotein particles to nonspecific binding sites on the cell surface, such as proteoglycans (45), or by acting as a molecular bridge between lipoproteins and cell surface receptors or proteoglycans (46), as originally proposed by Felts, Itakura, and Crane (47). The finding that inactivation of ANGPTL3 did not increase the clearance of exogenous LDL or β-VLDL suggests that LPL must engage lipoproteins early in the catalytic cascade in order to mediate their clearance.
If the cholesterol- and ApoB-lowering effects of ANGPTL3 inactivation are mediated by LPL, then REGN1500 should be ineffective in LPL-deficient animals. Direct evaluation of this hypothesis is complicated because Lpl−/− mice die shortly after birth. Sonnenburg et al. (48) found that genetic ablation or antibody-mediated inactivation of ANGPTL3 decreased plasma TG levels by 26% in fed mice lacking GPIHBP1, a protein required for the transport of LPL to the capillary endothelial surface (49). Unfortunately, those authors did not report plasma levels of cholesterol for this experiment. Furthermore, careful time-course studies indicate that LPL activity may be as much as 25% of normal in these animals (50). Thus, it is possible that the reduction in TG levels in these mice is due to residual LPL activity. Further studies will therefore be required to determine the role of LPL in the reductions in plasma cholesterol levels that result from inactivation of ANGPTL3.
ANGPTL3 also inhibits EL (15, 16), so inactivation of ANGPTL3 is associated with increased EL activity and reduced plasma HDL-C levels. Inactivation of ANGPTL3 was not associated with reduced cholesterol levels in the EL KO mice (24). Broedl et al. (51) reported that overexpression of EL markedly reduces circulating levels of VLDL, LDL, and ApoB in Ldlr−/−, Apoe−/−, and APOBTg mice. In contrast to LPL, overexpression of a catalytically inactive EL did not lower plasma lipid levels. These findings indicate that EL promotes the clearance of ApoB-containing lipoproteins by enzymatic modification rather than by bridging, but they also imply a noncanonical pathway for the clearance of the resulting lipoproteins. Therefore, increased expression of EL may also contribute to the reduction in the cholesterol content of ApoB-containing lipoproteins in ANGPTL3-deficient animals.
An alternative hypothesis is that inactivation of ANGPTL3 reduces plasma cholesterol and ApoB levels by decreasing VLDL secretion. This hypothesis provides a single simple explanation for the robust lipid-lowering effects of ANGPTL3 inactivation in diverse murine models of hyperlipidemia. Our present results suggest that the effects of ANGPTL3 inactivation on VLDL secretion are more complex; secretion of TG is decreased but secretion of ApoB is not. The mechanism underlying the selective decrease in secretion of TG is not known. Inactivation of proteins required for VLDL assembly, such as ApoB and MTP, decreases VLDL secretion (52, 53) but is invariably associated with hepatic steatosis, which is not observed in Angptl3−/− mice (54) or in humans with ANGPTL3 deficiency (55). Therefore, it is unlikely that inactivation of ANGPTL3 disrupts VLDL assembly. An alternative possibility is that increased activity of EL (or another as yet unidentified extracellular lipase) in ANGPTL3-deficient mice may result in hydrolysis of TG from VLDL before the VLDL can acquire enough Triton WR1339 to inhibit lipolysis. Whereas it seems unlikely that the large differences in VLDL-TG secretion observed in this study can be explained by increased intra-hepatic TG hydrolysis in the Angptl3−/− mice, we cannot formally exclude this possibility.
Whereas inactivation of ANGPTL3 did not decrease ApoB secretion, as determined by incorporation of 35S-methionine, plasma levels of ApoB were lower in the REGN1500-treated mice. We hypothesize that this difference arises because the 35S-methionine measures only the newly secreted ApoB, whereas the antibody measures all of the ApoB in plasma, which includes the ApoB present prior to Triton WR1339 administration plus the newly secreted ApoB. The plasma levels of ApoB are already appreciably lower in the REGN1500-treated mice by the time the Triton WR1339 is injected. Therefore, after the influx of equal amounts of ApoB into the plasma compartment into the two lines, the levels of ApoB will still be lower in the KO animals.
The effect of inactivating ANGPTL3 on VLDL secretion was most apparent in fed animals (supplementary Fig. 2), as was observed previously for LPL activity (40). The coupling of ANGPTL3 function to nutritional state is surprising because expression of the protein is only modestly affected by fasting and refeeding. We have proposed that ANGPTL3 acts together with ANGPTL8 to inhibit LPL (27), and that the activity of the complex is limited by expression of ANGPTL8, which is very low in fasting animals and is strongly upregulated by feeding. VLDL-TG secretion is also markedly reduced in fed Angptl8−/− mice.
The liver derives fatty acids for VLDL-TG synthesis from intracellular lipid droplets, de novo lipogenesis, lipoprotein remnants, and circulating free fatty acids. In the present study, inactivation of ANGPTL3 did not affect hepatic lipid content or de novo lipogenesis, but circulating free fatty acids, the major substrate for hepatic VLDL-TG, were consistently lower in mice treated with REGN1500 (although the decrease did not reach statistical significance in some experiments), as reported previously in mice lacking ANGPTL3 (17, 56). We found no change in the fractional uptake of exogenously administered palmitate or bromopalmitate into the livers of the antibody-treated mice. This finding, together with the decrease in circulating fatty acid concentrations, suggests that the total uptake of fatty acids by the liver is decreased by ANGPTL3 inactivation. The decrease in hepatic TG secretion in animals lacking ANGPTL3 may therefore be due to a decrease in the supply of free fatty acids to the liver. This hypothesis is not consistent with data from Zhang et al. (57), who showed that a 6 h infusion of 6 mM oleate into mice altered secretion of ApoB, but not TG.
The effects on plasma lipids of inactivating ANGPTL3 resemble those observed in mice lacking ApoC3 (58). Like ANGPTL3, ApoC3 has been studied primarily as a circulating inhibitor of LPL activity. However, a recent report that inactivation of ApoC3 using an antisense inhibitor markedly reduced plasma levels of TG in patients with LPL deficiency (59) confirmed a broader role for ApoC3 in human lipoprotein metabolism. The antibody described in this report will allow similar studies to be undertaken for ANGPTL3.
Supplementary Material
Acknowledgments
The authors wish to thank Christina Zhao for excellent technical assistance.
Footnotes
Abbreviations:
- ANGPTL3
- angiopoietin-like protein 3
- CHD
- coronary heart disease
- EL
- endothelial lipase
- FPLC
- fast performance LC
- HDL-C
- HDL-cholesterol
- LDL-C
- LDL-cholesterol
- LDLR
- LDL receptor
- LRP1
- LDL receptor related protein 1
- MTP
- microsomal transfer protein
- VLDL-C
- VLDL-cholesterol
This work was supported by the following National Institutes of Health Grants: PO1 HL20948, RO1 DK090066, and UL1TR001105.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel. 2014. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia–full report. J. Clin. Lipidol. 8: 29–60. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 3.Linton M. F., Farese R. V., Jr, Young S. G. 1993. Familial hypobetalipoproteinemia. J. Lipid Res. 34: 521–541. [PubMed] [Google Scholar]
- 4.Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. [DOI] [PubMed] [Google Scholar]
- 5.Stein E. A., Dufour R., Gagne C., Gaudet D., East C., Donovan J. M., Chin W., Tribble D. L., McGowan M. 2012. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 126: 2283–2292. [DOI] [PubMed] [Google Scholar]
- 6.Cuchel M., Rader D. J. 2013. Microsomal transfer protein inhibition in humans. Curr. Opin. Lipidol. 24: 246–250. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. C., Boerwinkle E., Mosley T. H., Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 8.Stein E. A., Mellis S., Yancopoulos G. D., Stahl N., Logan D., Smith W. B., Lisbon E., Gutierrez M., Webb C., Wu R., et al. 2012. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 366: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 9.Weinreich M., Frishman W. H. 2014. Antihyperlipidemic therapies targeting PCSK9. Cardiol. Rev. 22: 140–146. [DOI] [PubMed] [Google Scholar]
- 10.Musunuru K., Pirruccello J. P., Do R., Peloso G. M., Guiducci C., Sougnez C., Garimella K. V., Fisher S., Abreu J., Barry A. J., et al. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 363: 2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisciotta L., Favari E., Magnolo L., Simonelli S., Adorni M. P., Sallo R., Fancello T., Zavaroni I., Ardigo D., Bernini F., et al. 2012. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ Cardiovasc Genet. 5: 42–50. [DOI] [PubMed] [Google Scholar]
- 12.Minicocci I., Montali A., Robciuc M. R., Quagliarini F., Censi V., Labbadia G., Gabiati C., Pigna G., Sepe M. L., Pannozzo F., et al. 2012. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 97: E1266–E1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arca M., Minicocci I., Maranghi M. 2013. The angiopoietin-like protein 3: a hepatokine with expanding role in metabolism. Curr. Opin. Lipidol. 24: 313–320. [DOI] [PubMed] [Google Scholar]
- 14.Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R., Ueda K., Inaba T., Minekura H., Kohama T., et al. 2002. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 277: 33742–33748. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 27: 366–372. [DOI] [PubMed] [Google Scholar]
- 16.Jin W., Wang X., Millar J. S., Quertermous T., Rothblat G. H., Glick J. M., Rader D. J. 2007. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 6: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robciuc M. R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Oorni K., Metso J., Minicocci I., Ciociola E., Ceci F., et al. 2013. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 33: 1706–1713. [Erratum. 2013. Arterioscler. Thromb. Vasc. Biol. 33: e124.] [DOI] [PubMed] [Google Scholar]
- 18.Kane J. P., Havel R. J. 2001. Structure and metabolism of plasma lipoproteins. In Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, D. Valle, et al., editors. McGraw Hill, New York. 2717–2752. [Google Scholar]
- 19.Brown M. S., Goldstein J. L. 1976. Receptor-mediated control of cholesterol metabolism. Science. 191: 150–154. [DOI] [PubMed] [Google Scholar]
- 20.Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. 1989. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. USA. 86: 5810–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanford K. I., Bishop J. R., Foley E. M., Gonzales J. C., Niesman I. R., Witztum J. L., Esko J. D. 2009. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 119: 3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando Y., Shimizugawa T., Takeshita S., Ono M., Shimamura M., Koishi R., Furukawa H. 2003. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipid Res. 44: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 23.Lee E. C., Desai U., Gololobov G., Hong S., Feng X., Yu X. C., Gay J., Wilganowski N., Gao C., Du L. L., et al. 2009. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J. Biol. Chem. 284: 13735–13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gusarova V., Alexa C. A., Wang Y., Rafique A., Kim J. H., Buckler D., Mintah I. J., Shihanian L. M., Cohen J. C., Hobbs H. H., et al. 2015. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 56: 1308 – 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park P. W., Pier G. B., Hinkes M. T., Bernfield M. 2001. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 411: 98–102. [DOI] [PubMed] [Google Scholar]
- 26.Rohlmann A., Gotthardt M., Hammer R. E., Herz J. 1998. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest. 101: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D. M., Cohen J. C., Hobbs H. H. 2013. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 110: 16109–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quagliarini F., Wang Y., Kozlitina J., Grishin N. V., Hyde R., Boerwinkle E., Valenzuela D. M., Murphy A. J., Cohen J. C., Hobbs H. H. 2012. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 109: 19751–19756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millar J. S., Cromley D. A., McCoy M. G., Rader D. J., Billheimer J. T. 2005. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 30.Ota T., Gayet C., Ginsberg H. N. 2008. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest. 118: 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein J. L., Basu S. K., Brown M. S. 1983. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 98: 241–260. [DOI] [PubMed] [Google Scholar]
- 33.Oakes N. D., Kjellstedt A., Forsberg G. B., Clementz T., Camejo G., Furler S. M., Kraegen E. W., Olwegard-Halvarsson M., Jenkins A. B., Ljung B. 1999. Development and initial evaluation of a novel method for assessing tissue-specific plasma free fatty acid utilization in vivo using (R)-2-bromopalmitate tracer. J. Lipid Res. 40: 1155–1169. [PubMed] [Google Scholar]
- 34.Shimano H., Horton J. D., Hammer R. E., Shimomura I., Brown M. S., Goldstein J. L. 1996. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Invest. 98: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pike L. S., Smift A. L., Croteau N. J., Ferrick D. A., Wu M. 2011. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta. 1807: 726–734. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein J. L., Brown M. S. 2009. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herz J., Strickland D. K. 2001. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foley E. M., Esko J. D. 2010. Hepatic heparan sulfate proteoglycans and endocytic clearance of triglyceride-rich lipoproteins. Prog. Mol. Biol. Transl. Sci. 93: 213–233. [DOI] [PubMed] [Google Scholar]
- 39.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., et al. 2011. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17: 200–205. [DOI] [PubMed] [Google Scholar]
- 40.Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., et al. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 146: 4943–4950. [DOI] [PubMed] [Google Scholar]
- 41.Shimada M., Ishibashi S., Inaba T., Yagyu H., Harada K., Osuga J. I., Ohashi K., Yazaki Y., Yamada N. 1996. Suppression of diet-induced atherosclerosis in low density lipoprotein receptor knockout mice overexpressing lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 93: 7242–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zsigmond E., Kobayashi K., Tzung K. W., Li L., Fuke Y., Chan L. 1997. Adenovirus-mediated gene transfer of human lipoprotein lipase ameliorates the hyperlipidemias associated with apolipoprotein E and LDL receptor deficiencies in mice. Hum. Gene Ther. 8: 1921–1933. [DOI] [PubMed] [Google Scholar]
- 43.Hu L., van der Hoogt C. C., Espirito Santo S. M., Out R., Kypreos K. E., van Vlijmen B. J., Van Berkel T. J., Romijn J. A., Havekes L. M., van Dijk K. W., et al. 2008. The hepatic uptake of VLDL in lrp-ldlr-/-vldlr-/- mice is regulated by LPL activity and involves proteoglycans and SR-BI. J. Lipid Res. 49: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 44.Adachi H., Kondo T., Koh G. Y., Nagy A., Oike Y., Araki E. 2011. Angptl4 deficiency decreases serum triglyceride levels in low-density lipoprotein receptor knockout mice and streptozotocin-induced diabetic mice. Biochem. Biophys. Res. Commun. 409: 177–180. [DOI] [PubMed] [Google Scholar]
- 45.Olin K. L., Potter-Perigo S., Barrett P. H., Wight T. N., Chait A. 1999. Lipoprotein lipase enhances the binding of native and oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. J. Biol. Chem. 274: 34629–34636. [DOI] [PubMed] [Google Scholar]
- 46.Merkel M., Kako Y., Radner H., Cho I. S., Ramasamy R., Brunzell J. D., Goldberg I. J., Breslow J. L. 1998. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proc. Natl. Acad. Sci. USA. 95: 13841–13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felts J. M., Itakura H., Crane R. T. 1975. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants - a new theory. Biochem. Biophys. Res. Commun. 66: 1467–1475. [DOI] [PubMed] [Google Scholar]
- 48.Sonnenburg W. K., Yu D., Lee E. C., Xiong W., Gololobov G., Key B., Gay J., Wilganowski N., Hu Y., Zhao S., et al. 2009. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J. Lipid Res. 50: 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beigneux A. P., Davies B. S., Bensadoun A., Fong L. G., Young S. G. 2009. GPIHBP1, a GPI-anchored protein required for the lipolytic processing of triglyceride-rich lipoproteins. J. Lipid Res. 50(Suppl): S57–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinstein M. M., Yin L., Beigneux A. P., Davies B. S., Gin P., Estrada K., Melford K., Bishop J. R., Esko J. D., Dallinga-Thie G. M., et al. 2008. Abnormal patterns of lipoprotein lipase release into the plasma in GPIHBP1-deficient mice. J. Biol. Chem. 283: 34511–34518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broedl U. C., Maugeais C., Millar J. S., Jin W., Moore R. E., Fuki I. V., Marchadier D., Glick J. M., Rader D. J. 2004. Endothelial lipase promotes the catabolism of ApoB-containing lipoproteins. Circ. Res. 94: 1554–1561. [DOI] [PubMed] [Google Scholar]
- 52.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanoli T., Yue P., Yablonskiy D., Schonfeld G. 2004. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J. Lipid Res. 45: 941–947. [DOI] [PubMed] [Google Scholar]
- 54.Inaba T., Matsuda M., Shimamura M., Takei N., Terasaka N., Ando Y., Yasumo H., Koishi R., Makishima M., Shimomura I. 2003. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J. Biol. Chem. 278: 21344–21351. [DOI] [PubMed] [Google Scholar]
- 55.Yue P., Tanoli T., Wilhelm O., Patterson B., Yablonskiy D., Schonfeld G. 2005. Absence of fatty liver in familial hypobetalipoproteinemia linked to chromosome 3p21. Metabolism. 54: 682–688. [DOI] [PubMed] [Google Scholar]
- 56.Koishi R., Ando Y., Ono M., Shimamura M., Yasumo H., Fujiwara T., Horikoshi H., Furukawa H. 2002. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 30: 151–157. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y. L., Hernandez-Ono A., Ko C., Yasunaga K., Huang L. S., Ginsberg H. N. 2004. Regulation of hepatic apolipoprotein B-lipoprotein assembly and secretion by the availability of fatty acids. I. Differential response to the delivery of fatty acids via albumin or remnant-like emulsion particles. J. Biol. Chem. 279: 19362–19374. [DOI] [PubMed] [Google Scholar]
- 58.Jong M. C., Rensen P. C., Dahlmans V. E., van der Boom H., van Berkel T. J., Havekes L. M. 2001. Apolipoprotein C–III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J. Lipid Res. 42: 1578–1585. [PubMed] [Google Scholar]
- 59.Gaudet D., Brisson D., Tremblay K., Alexander V. J., Singleton W., Hughes S. G., Geary R. S., Baker B. F., Graham M. J., Crooke R. M., et al. 2014. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 371: 2200–2206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.