Abstract
Angiopoietin-like protein 3 (ANGPTL3) is a circulating protein synthesized exclusively in the liver that inhibits LPL and endothelial lipase (EL), enzymes that hydrolyze TGs and phospholipids in plasma lipoproteins. Here we describe the development and testing of a fully human monoclonal antibody (REGN1500) that binds ANGPTL3 with high affinity. REGN1500 reversed ANGPTL3-induced inhibition of LPL activity in vitro. Intravenous administration of REGN1500 to normolipidemic C57Bl/6 mice increased LPL activity and decreased plasma TG levels by ≥50%. Chronic administration of REGN1500 to dyslipidemic C57Bl/6 mice for 8 weeks reduced circulating plasma levels of TG, LDL-cholesterol (LDL-C), and HDL-cholesterol (HDL-C) without any changes in liver, adipose, or heart TG contents. Studies in EL knockout mice revealed that REGN1500 reduced serum HDL-C through an EL-dependent mechanism. Finally, administration of a single dose of REGN1500 to dyslipidemic cynomolgus monkeys caused a rapid and pronounced decrease in plasma TG, nonHDL-C, and HDL-C. REGN1500 normalized plasma TG levels even in monkeys with a baseline plasma TG greater than 400 mg/dl. Collectively, these data demonstrate that neutralization of ANGPTL3 using REGN1500 reduces plasma lipids in dyslipidemic mice and monkeys, and thus provides a potential therapeutic agent for treatment of patients with hyperlipidemia.
Keywords: lipoprotein lipase, endothelial lipase, triglycerides, cholesterol, hyperlipidemia, dyslipidemia, angiopoietin-like protein 3
LPL plays a central role in the maintenance of normal lipid levels in the blood. LPL is located at the luminal surface of the capillary endothelium and is the key enzyme for hydrolysis of core TGs in plasma chylomicron and VLDL particles, and release of fatty acids to adjacent tissues for storage and energy production (1, 2). The activity of LPL is regulated at the transcriptional and posttranscriptional level in a tissue-specific manner (2). One of the posttranslational regulators of LPL activity is angiopoietin-like protein 3 (ANGPTL3), which belongs to a family of eight secreted proteins (3).
ANGPTL3 is secreted from the liver (4). Because the adult liver expresses little to no LPL, it is presumed that ANGPTL3 functions as a circulating inhibitor of LPL. ANGPTL3 inhibits LPL activity in vitro and in vivo, and mice deficient in Angptl3 have increased LPL activity and low plasma TG levels (5, 6). ANGPTL3 inhibits LPL activity by inducing a conformational change in LPL, resulting in increased susceptibility to cleavage by proprotein convertases, dissociation of LPL from the cell surface, and inhibition of its catalytic activity (7). In addition to inhibiting LPL, ANGPTL3 also inhibits the activity of endothelial lipase (EL), which hydrolyzes HDL phospholipids (8, 9).
Genetic studies have shown that humans with sequence variations in ANGPTL3 have reduced plasma lipid levels (10–15). In particular, individuals who have mutations in both ANGPTL3 alleles have pan-hypolipidemia with low plasma TG, LDL-cholesterol (LDL-C), and HDL-cholesterol (HDL-C) levels and increased plasma LPL activity (16). These findings confirm the importance of ANGPTL3 in human lipoprotein metabolism and make blocking ANGPTL3 with a monoclonal antibody a potential therapy to treat hyperlipidemia. In this study, we describe the fully human monoclonal antibody, REGN1500, that binds with high affinity to ANGPTL3 and effectively inhibits its activity in vivo, leading to robust lowering of plasma lipids in dyslipidemic mice and nonhuman primates.
MATERIALS AND METHODS
Antibodies and protein reagents
REGN1500 was derived using Regeneron’s Velocimmune® technology platform (17) and is a fully human monoclonal antibody with high affinity to ANGPTL3 from multiple species (mouse, rat, monkey, and human). REGN1500 has a human IgG4 constant region with a stabilizing mutation in the hinge region (serine to proline in position 108 in GenBank #P01864) to minimize half-antibody formation, which is known to occur for the natural IgG4 isotype (18). An isotype-matched antibody with irrelevant specificity was used as control.
The following proteins were obtained from R&D Systems, where “HisN” indicates a C-terminal oligohistidine tag (N is the number of His residues): hANGPTL3 (S17-E460)-His10 and mANGPTL3 (S17-T455)-His10. Additional recombinant epitope-tagged proteins were produced in Chinese hamster ovarian cells after stable transfection using vectors that substituted nonnative for endogenous signal peptides. Chinese hamster ovarian-expressed proteins were purified using immobilized metal affinity chromatography and dialyzed into Tris-buffered saline (pH 7.5) or PBS containing 5% glycerol (pH 7.4). These proteins included hANGPTL3 (S17-K170)-His6, MfANGPTL3 (S17-K170)-myc-myc-His6 (mmH) (at the C terminus), rANGPTL3 (S17-D240)-mmH, and mANGPTL3 (S17-T455)-His6.
Surface plasmon resonance-Biacore
Surface plasmon resonance experiments were performed on a Biacore T200 instrument using a dextran-coated (CM4) chip at 25°C. The running buffer was filtered HBS-T [10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, and 0.05% polysorbate 20 (pH 7.4)]. A capture sensor surface was prepared by covalently immobilizing α-histidine antibody (Qiagen) to the chip surface using (1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride)/N-hydroxysuccinimide (EDC/NHS) coupling chemistry. Following surface activation, α-histidine antibody in coupling buffer [0.1 M acetate buffer (pH 4.5)] was injected over the activated chip surface until a resonance unit (RU) signal of about 4,000 RU (α-penta histidine mouse monoclonal antibody; Qiagen) was reached. The activated coupled chip surfaces were then washed and treated with 10 mM glycine-HCl (pH 1.5) to remove uncoupled residual proteins. Recombinant proteins derived from human and nonhuman species ANGPTL3 were diluted into the running buffer and captured through the histidine tag on the coupled α-histidine antibody chip surface at low density (6–28 RU). One flow cell of the biosensor chip was left without captured protein to provide a reference surface for the kinetic experiment. The capture protocol was designed to yield capture levels of mAb that resulted in an Rmax no greater than 80 RU. Following the capture step, α-ANGPTL3 antibody was serially diluted 2-fold at concentration (50 nM to 0.39 nM) in the running buffer and injected in duplicate over various recombinant ANGPTL3 protein captured surfaces for 3 min at 50 μl/min followed by 5 min of dissociation. All capture surfaces were regenerated with one 15 s pulse of 10 mM glycine-HCl (pH 1.5). This format used a saturating concentration of REGN1500 for binding. Dissociation was monitored by a double referencing procedure (19) (Table 1). The data were processed and kinetic analyses performed using T200 Evaluation (version 1.0, Biacore). The equilibrium dissociation constant (KD) was calculated from the ratio of the dissociation rate constant divided by the association rate constant (KD = kd/ka).
TABLE 1.
Summary of kinetic binding parameters for the interaction of REGN1500 with human, monkey, rat, and mouse ANGPTL3 proteins
| Protein | Kinetic Binding Parameters | |||
| ka (M−1s−1) | kd (s−1) | KD (M) | T1/2 (min) | |
| hANGPTL3 (S17-K170)-His6 | 1.09 × 106 | 1.39 × 10−3 | 1.28 × 10−9 | 8.3 |
| hANGPTL3 (S17-E460)-His10 | 1.01 × 106 | 9.22 × 10−4 | 9.15 × 10−10 | 12.5 |
| MfANGPTL3 (S17-K170)-mmH | 2.30 × 106 | 5.99 × 10−4 | 2.61 × 10−10 | 19.3 |
| rANGPTL3 (S17-D240)-mmH | 2.00 × 106 | 7.71 × 10−4 | 3.85 × 10−10 | 15.0 |
| mANGPTL3 (S17-T455)-His6 | 1.83 × 106 | 6.29 × 10−4 | 3.44 × 10−10 | 18.4 |
hANGPTL3 (S17-K170)-His6, N-terminal domain human ANGPTL3, amino acids 17-170, with a C-terminal hexahistidine tag; hANGPTL3 (S17-E460)-His10, full-length human ANGPTL3 with a C-terminal decahistidine tag; MfANGPTL3 (S17-K170)-mmH, N-terminal domain of monkey (Macaca fascicularis) ANGPTL3, amino acids 17-170, with a C-terminal myc-myc-hexahistidine tag; rANGPTL3 (S17-D240)-mmH, N-terminal domain human ANGPTL3, amino acids 17-240, with a C-terminal myc-myc-hexahistidine tag; mANGPTL3 (S17-T455)-His6, full-length mouse ANGPTL3 with a C-terminal hexahistidine tag; mANGPTL3 (S17-T455)-His6, full-length mouse ANGPTL3 with a C-terminal hexahistidine tag. ANGPTL3 proteins were immobilized onto the sensor surface by anti-penta-histidine capture. REGN1500 (12.5–0.39 nM) was injected across the captured protein chip surface and antibody-dependent changes in RUs were monitored.
Studies in mice
Male C57Bl/6 mice (8 weeks of age) were purchased from Jackson Laboratory or Taconic Farms. Lipg−/− mice were generated on 75% C578Bl/6Tac and 25% 129S6/SvEvTac background using VelociGene® technology (20). VelociGene® allele identification number is VG12135. All procedures were conducted in compliance with protocols approved by the Regeneron Pharmaceuticals Institutional Animal Care and Use Committee. REGN1500 and isotype control antibody were diluted with sterile PBS for sc injection into mice.
Single administration studies.
Mice were maintained on a regular chow diet (5001, LabDiet). To establish a baseline for serum chemistry parameters, serum samples were collected 7 days prior to antibody administration after a 4 h fast. On study day 0, mice were sorted into treatment groups based on their serum TG levels. Mice (n = 5–6 per group) were then administered a single sc injection of REGN1500 or isotype control antibody at the indicated doses. Subsequent serum samples were collected after a 4 h fast over the duration of the experiment and analyzed for serum chemistry parameters and human Fc levels. Postheparin plasma LPL activity was measured as described previously (21). A lipid tolerance test was performed 4 days after a single dose of REGN1500 (10 mg/kg). Mice were fasted for 2 h following intraperitoneal administration of 20% intralipid (Baxter Healthcare) at 10 μl/g body weight. TG level was evaluated in blood collected from the tail at subsequent time points.
Multiple administration studies.
Eight days prior to antibody administration (day −8), baseline serum chemistries were measured after a 4 h fast. On day −3, mice were placed on a high-fat high-cholesterol diet [21% fat, 0.21% cholesterol (wt/wt); catalog #12079B, Research Diets]. On study day 0, mice were sorted into treatment groups based on their serum TG levels (n = 10 per group). Mice were injected with REGN1500 or isotype control antibody once a week for 8 weeks. Body weights were measured every week. Mice were euthanized 1 week after the last injection and livers, epididymal white adipose tissue, and hearts were collected, weighed, and frozen for subsequent TG content measurements.
Serum chemistry analyses
Circulating TG, total cholesterol (TC), LDL-C, HDL-C, NEFA, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were determined in serum using an ADVIA® 1800 blood chemistry analyzer (Bayer, Leverkusen, Germany). NonHDL-C levels were calculated by subtracting HDL-C from TC values.
Lipoprotein separation using HPLC
Lipoprotein particles were separated from serum samples by dual detection HPLC system (Waters Corp.) using a tandem Superose 6 HR 10/300GL column (22). Cholesterol and TG lipoprotein concentrations were calculated as the area under the curve using VLDL, LDL, HDL (Biomedical Technologies), and TG (Teco Diagnostics) standards. The cholesterol or TG concentrations in the standards were determined using enzymatic methods (Teco Diagnostics).
TG tissue content
Lipids were extracted from tissues as described (23). Lipids were solubilized as outlined earlier (24). The levels of TG were measured using enzymatic assays (Infinity; Thermo Fisher Scientific) and normalized to wet tissue weight.
Study in cynomolgus monkeys
The study was performed by Crown Bioscience (Taicang, Jiangsu, China). Seventeen spontaneous hypertriglyceridemic monkeys were selected for the study based on their overnight fasted serum TG, nonHDL-C, and HDL-C profile (supplementary Table 1). The monkeys were individually housed, had free access to water and were fed twice daily with a complete nutritionally balanced diet (Shanghai Shilin Biotechnology Inc., Shanghai, China), which was enriched with seasonal fruits and vegetables. All animal procedures were approved by the Crown Bioscience Institutional Animal Care and Use Committee and performed according to guidelines approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
The seventeen animals were divided into three groups. On day 0, the monkeys were given either vehicle or REGN1500 (3 mg/kg or 10 mg/kg). Blood (4 ml) was collected following a 16 h fast with 1 to 5 day intervals up to day 55 via venipuncture into BD sterile venous blood collection tubes for serum preparation and blood glucose measurements (ACCU-CHEK Active, Roche). Serum TG, TC, LDL-C, HDL-C, NEFA, ALT, AST, and insulin were assayed in a Beckman Coulter UniCelDxC 800 Synchron clinical system (Beckman Coulter Inc.). The serum chemistry methods were validated (25).
Data analyses
All data are mean ± SEM. Statistical analyses were performed utilizing GraphPad software Prism 6.0. LPL and hepatic lipase (HL) activities in REGN1500- and control antibody-treated mice were compared by Student’s t-test. All other parameters were analyzed by two-way ANOVA; a threshold of P < 0.05 was considered statistically significant. If a significant F ratio was obtained with two-way ANOVA, post hoc analysis was conducted between groups with Bonferroni posttests. In the monkey study, the average of each parameter on days −15, −7, −2, and 0 was used as the baseline value. Mean values and standard errors of each parameter in each group at a specific date were calculated. In addition, the percentage change of the mean value of each parameter in each experimental group compared with the baseline value was calculated. Student’s t-tests (two tails, paired) were performed in each group to compare the differences of the value at a specific date and the baseline value.
RESULTS
In vitro characterization of anti-ANGPTL3 antibody REGN1500
The relative affinity of REGN1500 to human, mouse, rat, and monkey ANGPTL3 was compared using surface plasmon resonance. REGN1500 bound ANGPTL3 from the four species with comparable affinities (KD = 0.26–1.28 nM) (Table 1). No binding was detected between REGN1500 and human ANGPTL4, ANGPTL5, or ANGPTL8 (data not shown).
We used an in vitro assay to evaluate the effect of REGN1500 on the inhibition of LPL by ANGPTL3. REGN1500 effectively blocked the inhibition of LPL by ANGPTL3 at a concentration that was within 2.5-fold of the EC50 value for each species with IC50 values from 1.0 to 13.6 nM (Table 2, supplementary Fig. 1). Because REGN1500 bound human and mouse ANGPTL3 with comparable affinities and effectively blocked the inhibitory action of mouse ANGPTL3, we selected mice as the first in vivo model to test the pharmacological efficacy of the antibody.
TABLE 2.
Summary of IC50 values for REGN1500 blockade of ANGPTL3-mediated LPL inhibition
| Parameter | Protein | ||||
| hANGPTL3 (S17-E460)-His10 | hANGPTL3 (S17-K170)-His6 | MfANGPTL3 (S17-K170)-mmH | rANGPTL3 (S17-D240)-mmH | mANGPTL3 (S17-T455)-His10 | |
| REGN1500, IC50 (nM)a | 9.6 | 2.9 | 10.4 | 1.0 | 13.6 |
| Control Ab, IC50 (nM)b | NB | NB | NB | NB | NB |
hANGPTL3, human ANGPTL3; MfANGPTL3, M. fascicularis ANGPTL3; rANGPTL3, rat ANGPTL3; mANGPTL3, mouse ANGPTL3; His10, decahistidine tag; His6, hexahistidine tag; mmH, myc-myc-hexahistidine tag; NB, no detectable blocking under the assay conditions used.
IC50 values were determined using constant concentrations of ANGPTL proteins that were within 2.5-fold of the measured EC50 values for the ANGPTL protein inhibiting LPL lipase activity.
Control Ab, hIgG4 isotype control antibody.
REGN1500 lowers serum TGs in normolipidemic C57Bl/6 mice
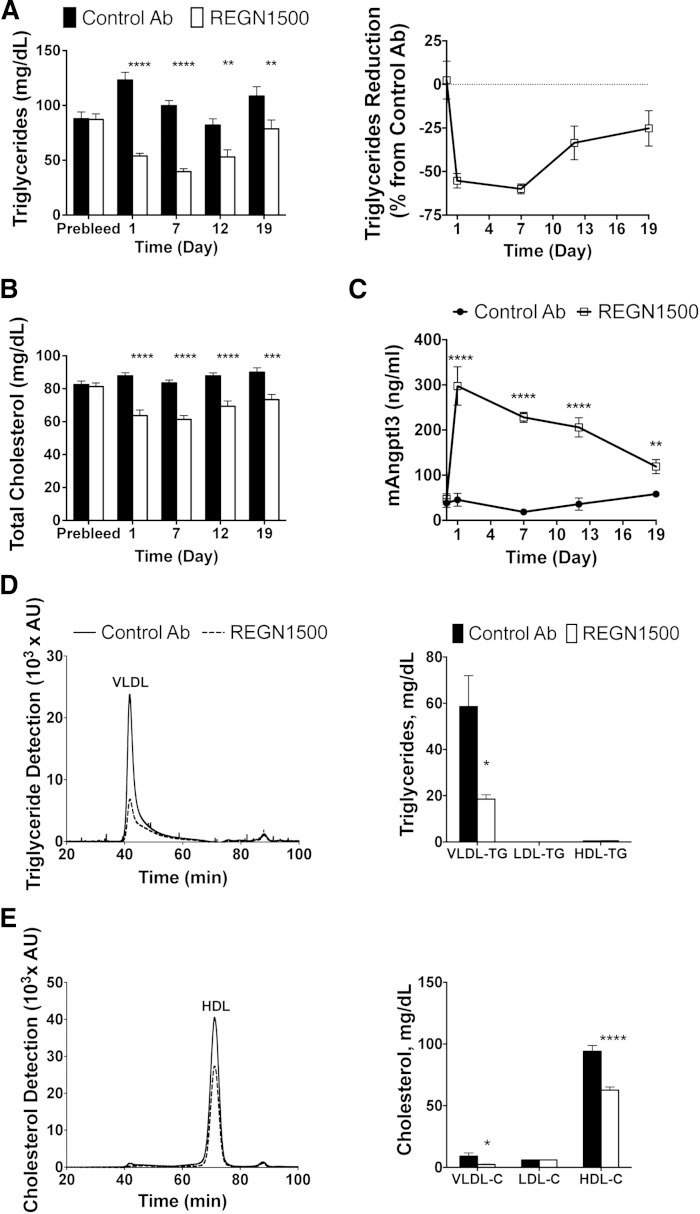
To assess the effect of REGN1500 on serum lipid levels, normolipidemic chow-fed C57Bl/6 mice were treated with a single sc injection of REGN1500 or isotype control antibody (10 mg/kg). Blood samples were collected after a 4 h fast at the indicated times. REGN1500 caused a rapid reduction in TG levels, with the maximal mean level of serum TG 55% lower in the antibody-treated group than in the mice given the control antibody. At the end of the experiment (day 19), the mice receiving REGN1500 still had a significantly lower mean TG level (Fig. 1A). TC levels were also reduced up to 26% in REGN1500-treated mice (Fig. 1B). Plasma levels of total ANGPTL3 (free and bound) increased within 1 day of treatment with REGN1500 and then progressively fell over the course of the experiment (Fig. 1C). Angptl3 liver expression did not change in mice administered REGN1500 (supplementary Fig. 2). Serum was separated on a Superose 6 column using HPLC to determine TG (Fig. 1D) and cholesterol (Fig. 1E) distribution among lipoproteins. Quantification of the lipoprotein distributions revealed that REGN1500 significantly reduced VLDL-TG, VLDL-cholesterol, and HDL-C levels in normolipidemic C57Bl/6 mice (Fig. 1D, E).
Fig. 1.
REGN1500 reduces serum lipid levels in chow-fed C57Bl/6 mice. Serum samples were collected after a 4 h fast from male C57Bl/6 mice (n = 6 per group) 7 days before (Prebleed) and at the indicated days following a single subcutaneous injection of REGN1500 or isotype control antibody (Control Ab) (10 mg/kg). Serum TG (A) and cholesterol (B) were measured enzymatically. C: Plasma levels of mouse ANGPTL3 were measured by ELISA. Serum (20 μl) from each mouse collected 7 days after treatment with REGN1500 or Control Ab (10 mg/kg) were size-fractionated by HPLC. TG (D) and cholesterol (E) were measured in each fraction. Values shown are mean ± SEM, except for the chromatograms where only mean values are shown. Statistical analysis was conducted by two-way ANOVA with Bonferroni correction posttest. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
A dose-response study was performed in chow-fed C57Bl/6 mice. Administration of a single dose of REGN1500 at 1 mg/kg was not associated with significant changes in plasma TG levels. However, doses of REGN1500 at 5, 10, and 25 mg/kg elicited a progressive dose-dependent reduction in circulating TG levels with maximal effects observed at day 7 (supplementary Fig. 3A). The greatest reduction of TG (−66%) occurred with the highest antibody dose (25 mg/kg) (supplementary Fig. 3B). No change in LDL-C was observed at any dose (data not shown). The corresponding human Fc levels in serum are shown in supplementary Fig. 3C, indicating that a serum REGN1500 concentration of between 5 and 15 μg/ml is required to induce significant pharmacological effects on circulating TG levels in normolipidemic C57Bl/6 mice.
REGN1500 increases postheparin plasma LPL activity
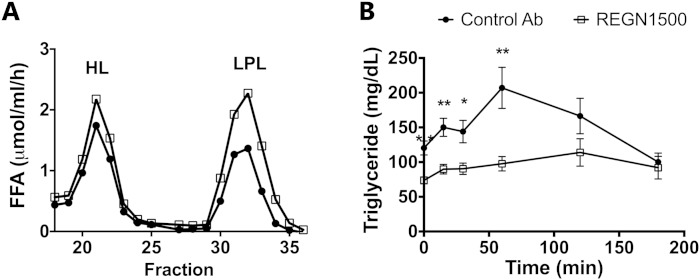
First, we confirmed that inhibition of ANGPTL3 by treatment with REGN1500 was associated with an increase in postheparin plasma LPL activity in both C57Bl/6 mice (Fig. 2A) and in dyslipidemic db/db mice (supplementary Fig. 4A). The postheparin LPL activity was increased by 1.8-fold in C57Bl/6 mice and 2.5-fold in the db/db mice with REGN1500 treatment, whereas HL activity remained unchanged (Fig. 2A, supplementary Fig. 4B). In the db/db mice, REGN1500 significantly reduced serum TG levels (−48%; P < 0.0001), TC levels (−37%; P < 0.01), and NEFAs (−35%; P < 0.01) (supplementary Fig. 4C–E). REGN1500 treatment did not change body weight, glucose, or insulin levels in the db/db mice (supplementary Fig. 4F–H). Thus, REGN1500 reduces circulating TG by upregulating LPL activity in vivo through blocking of ANGPTL3.
Fig. 2.
REGN1500 increases postheparin plasma LPL and improves lipid tolerance. A: Postheparin plasma LPL and HL activity of chow-fed C57Bl/6 mice treated with REGN1500 or control antibody (Control Ab) (10 mg/kg, n = 5 per group). Postheparin plasma was pooled and fractionated on a heparin column to separate HL and LPL, and TG hydrolase activity was measured as described in the Materials and Methods. B: Effect of REGN1500 treatment on plasma TG levels following a lipid tolerance test. Male C57Bl/6 mice were treated with a single dose of REGN1500 or control antibody (10 mg/kg, n = 5 per group) 4 days prior to the intraperitoneal administration of Intralipid (10 μl/g of 20% Intralipid). Values are mean ± SEM. Statistical analysis was conducted by Student’s t-test comparing the groups at each time point. *P < 0.05; **P < 0.01.
The effect of ANGPTL3 inhibition with REGN1500 on TG clearance was evaluated by acute fat loading. C57Bl/6 mice were treated with either REGN1500 or control antibody (10 mg/kg). Four days later, they were injected intraperitoneally with an intralipid emulsion (20%) after a 2 h fast. Plasma TG levels were significantly lower at baseline and increased only minimally in response to fat challenge in mice treated with REGN1500 (Fig. 2B).
REGN1500 lowers serum TG and cholesterol levels in dyslipidemic C57Bl/6 mice
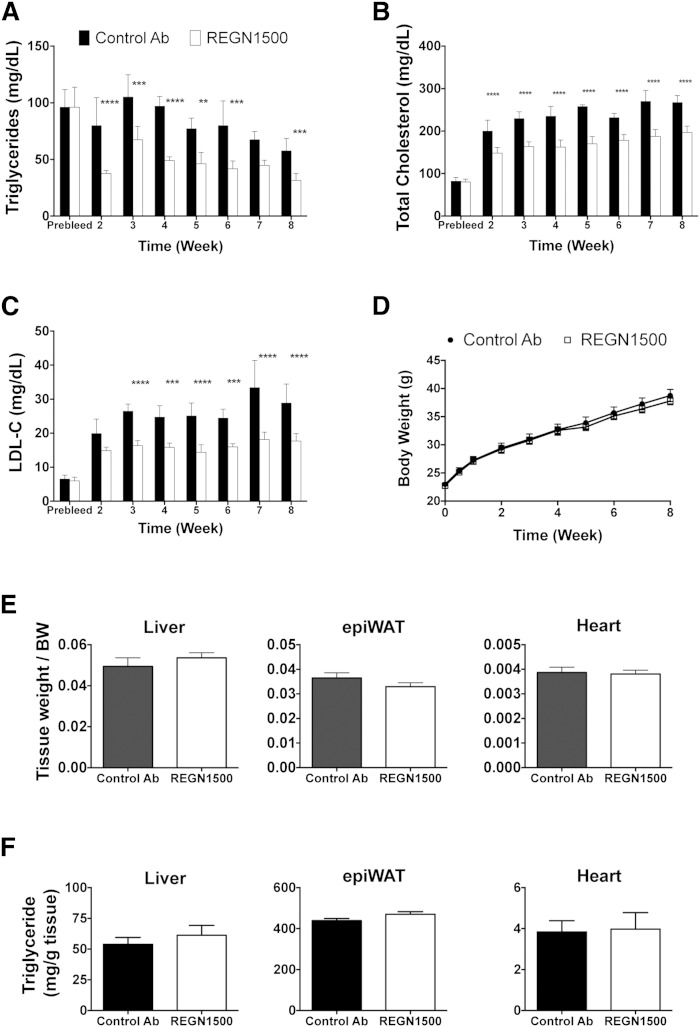
C57Bl/6 mice were placed on a high-fat high-cholesterol diet 3 days prior to initiating treatment with REGN1500. REGN1500 or the control antibody were administered once weekly (25 mg/kg) for 8 weeks. Serum samples were collected after a 4 h fast, 6 days after each injection. A marked and sustained reduction in circulating TG levels (up to −53%) was seen in the mice given REGN1500 compared with the control antibody (Fig. 3A). Hepatic TG secretion rate, assessed following administration of Triton WR1339, was reduced by 58% in mice receiving REGN1500 compared with control antibody (supplementary Fig. 5). The reduction in hepatic TG secretion rate was not produced by changes in de novo lipogenesis or fatty acid oxidation, as no differences were found in the gene expression of the major enzymes of these pathways in livers from mice administrated REGN1500 or control antibody (supplementary Fig. 6). Significant reductions in TC (up to −35%; Fig. 3B) and LDL-C (up to −45%; Fig. 3C) were also observed, showing that REGN1500 can reduce cholesterol levels originally elevated by a high-fat high-cholesterol diet. The reductions in circulating lipids were sustained throughout the 8 weeks of the study.
Fig. 3.
Weekly administration of REGN1500 reduces serum lipid levels in mice maintained on a high-fat high-cholesterol diet for 8 weeks. Serum samples (Prebleed) were collected from male C57Bl/6 mice (n = 10 per group, 8 weeks old) after a 4 h fast. After 5 days, the mice were placed on a high-fat high-cholesterol diet. Three days later, the mice started receiving a weekly injection of with REGN1500 or isotype control antibody (Control Ab) (25 mg/kg). Serum samples were collected 6 days after each injection following a 4 h fast. Changes in TG (A), TC (B), and LDL-C (C) serum levels are shown. D: Body weights were monitored weekly. E: Liver, epididymal white adipose tissue (epiWAT), and hearts were collected at the end of the study and weighed. F: TG content of liver, epiWAT, and hearts. All values are mean ± SEM. Statistical analysis was conducted by two-way ANOVA with Bonferroni correction posttest. **P < 0.01; ***P < 0.001; ****P < 0.0001
No effects of weekly REGN1500 administration were seen on body weights (Fig. 3D) or on liver, epididymal fat, or heart weights (Fig. 3E) or TG contents (Fig. 3F). Weekly administration of REGN1500 had no effect on liver transaminases (supplementary Fig. 7A–C). No changes were observed in Oil Red O staining of livers from control antibody- or REGN1500-treated mice on the high-fat high-cholesterol diet despite the marked increase in lipids staining in both groups of mice when compared with livers from mice on a chow diet (supplementary Fig. 7D). In summary, these data show that weekly administration of REGN1500 effectively lowers serum TG and LDL-C levels without affecting body weight or TG content of the major metabolic tissues in mice fed a high-fat high-cholesterol diet.
REGN1500 lowers serum HDL-C by an EL-dependent mechanism
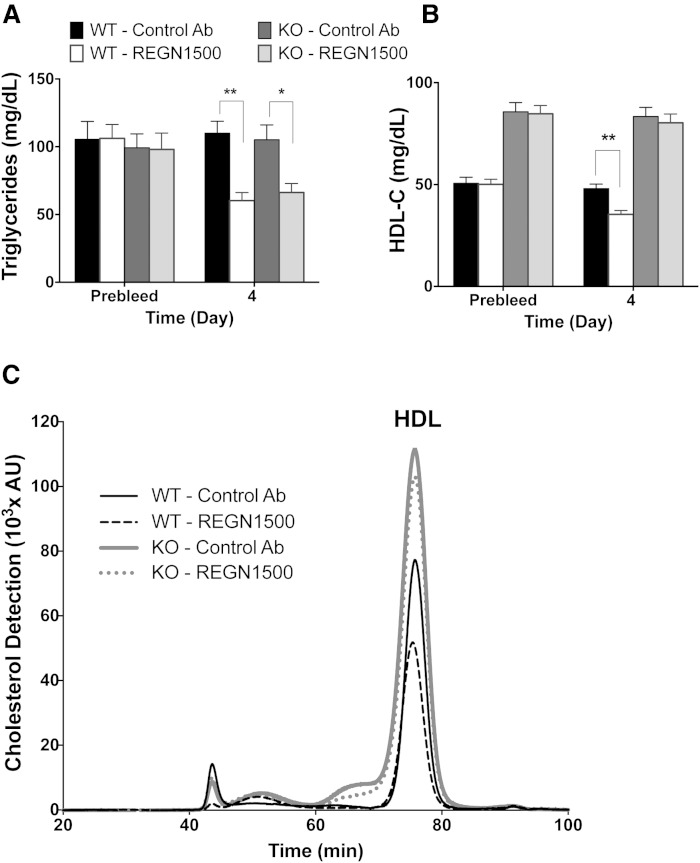
Besides LPL, ANGPTL3 also inhibits the activity of EL (8). Thus, REGN1500 would be expected to decrease HDL-C levels in wild-type mice, but not in mice that do not express EL (Lipg−/−). To test this hypothesis, we administered REGN1500 to Lipg−/− mice and their wild-type littermates. As expected, REGN1500 (10 mg/kg) led to significant reductions in plasma TG in both Lipg−/− and control mice (Fig. 4A). REGN1500 also markedly reduced HDL-C in wild-type mice, but did not affect HDL-C levels in Lipg−/− mice (Fig. 4B, C). These findings are consistent with in vitro data showing that REGN1500 blocks the inhibitory action of ANGPTL3 on EL with IC50 = 96 nM (supplementary Fig. 8) Thus, EL mediates the HDL-C lowering effects of REGN1500.
Fig. 4.
REGN1500 reduces HDL-C in WT but not in EL-deficient (Lipg−/−) mice. Serum samples were collected after a 4 h fast from male chow-fed Lipg−/− mice and their WT littermates (n = 7 per group) before (Prebleed; 7 days) and 4 days after a single administration of REGN1500 or isotype control antibody (Control Ab) at 10 mg/kg. Serum was analyzed for TG (A) and HDL-C (B). C: Chromatogram showing cholesterol distribution among lipoprotein subclasses after REGN1500 or control antibody administration in WT and Lipg−/− mice. All values are mean ± SEM, except chromatograms where only mean values are shown. Statistical analysis was by two-way ANOVA with Bonferroni correction posttest. *P < 0.05; **P < 0.01.
REGN1500 lowers serum lipids in dyslipidemic cynomolgus monkeys
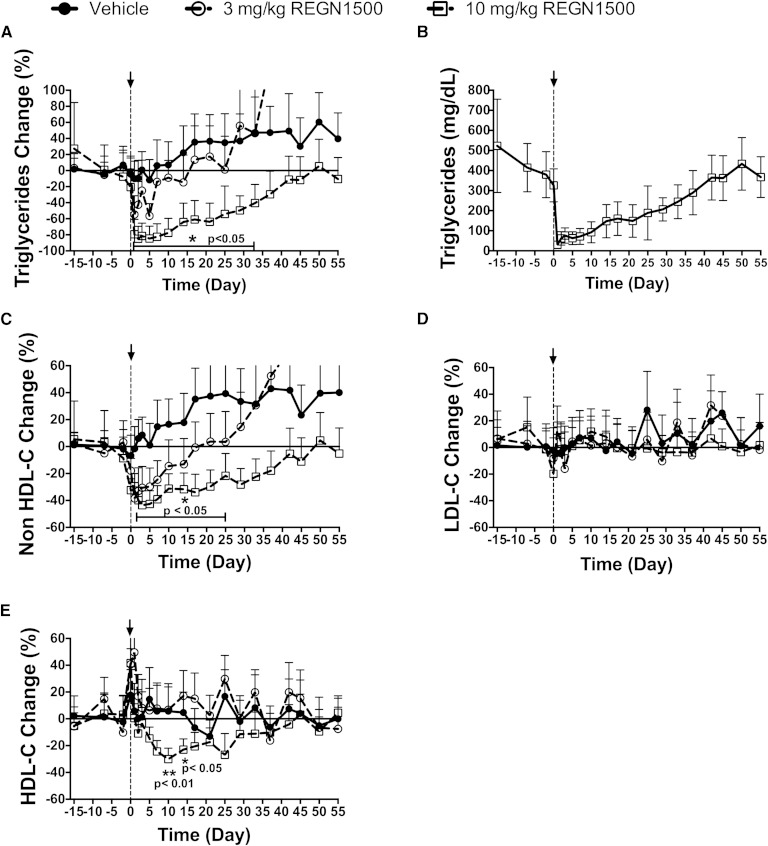
To extend our observations to nonhuman primates, we assessed the effect of REGN1500 on circulating lipid levels in cynomolgus monkeys. Seventeen dyslipidemic monkeys were selected for this study (supplementary Table 1). Three groups of five to six monkeys each were given a single dose of REGN1500 (3 mg/kg or 10 mg/kg) or vehicle. REGN1500 produced a robust and sustained dose-dependent reduction in circulating TG that reached a maximum within 1 to 2 days following the treatment (Fig. 5A). The 3 mg/kg dose of REGN1500 treatment resulted in a 48% reduction in mean plasma TG levels (from 320 ± 7 mg/dl to 165 ± 19 mg/dl for days 1 and 2; P < 0.05; n = 6), whereas the 10 mg/kg dose lowered plasma TG by 89% (from 411 ± 42 mg/dl to 46 ± 16 mg/dl for days 1 and 2; P < 0.05; n = 5). The higher dose of REGN1500 lowered serum TG to levels that were similar to the normolipidemic cynomolgus monkeys (58 ± 3 mg/dl; n = 60, unpublished observations). The reductions in plasma TG were maintained for 33 days before returning to baseline levels (Fig. 5A). The absolute reduction in plasma TG levels is provided for the higher dose treatment group (Fig. 5B). NonHDL-C was reduced by 44% and reached a nadir at day 3 (Fig. 5C). REGN1500 did not alter the plasma levels of LDL-C (Fig. 5D), perhaps because plasma LDL-C levels were low at baseline (58 ± 7 mg/dl; n = 17) and similar to those of normolipidemic monkeys (54 ± 4 mg/dl; n = 60). At the higher doses of REGN1500, the plasma HDL-C levels fell by ∼30% at days 10 and 14 (Fig. 5E). No changes were observed in liver enzyme levels (ALT and AST, supplementary Fig. 9) or fasting glucose or insulin (data not shown).
Fig. 5.
REGN1500 reduces circulating TG, nonHDL-C, and HDL-C levels in dyslipidemic cynomolgus monkeys. Baseline serum samples were collected at days −15, −7, −2, and 0 following a 16 h fast. Seventeen monkeys were divided into three groups and were administered vehicle or REGN1500 (3 or 10 mg/kg) on day 0 of the study. Serum samples were collected on multiple days and analyzed for TG (A), nonHDL-C (C), LDL-C (D), and HDL-C (E), and are represented as a percent change from baseline. All values are mean ± SEM. Panel (B) shows average changes in absolute TG values before and following treatment with REGN1500 (10 mg/kg). For each parameter, Student’s t-test (two tails, paired) was performed in each group comparing the mean value at each time point to the baseline value. *P < 0.05; **P < 0.01.
DISCUSSION
In this paper we show that administration of a high affinity antibody to ANGPTL3 rapidly and effectively reduces serum TG and cholesterol levels in normolipidemic mice, as well as in dyslipidemic mice and monkeys. The phenotype of the treated animals resembles that seen in humans with genetic deficiency of ANGPTL3 (16, 26–31) and suggests that inhibition of ANGPTL3 may be beneficial for treatment of patients with mixed dyslipidemia.
A mouse antibody to ANGPTL3 was shown previously to lower circulating levels of plasma TG and TC in normolipidemic and hyperlipidemic mice (32, 33). Here we describe the development of a new fully human monoclonal antibody that binds human, monkey, mouse, and rat ANGPTL3 with high affinity. REGN1500 treatment protected LPL activity from ANGPTL3 inhibition in vitro and in vivo after the antibody was injected into the circulation of mice. The increase in LPL activity in mice treated with REGN1500 resembles that seen in Angptl3 knockout mice (5, 6) and could account for the rapid clearance of plasma TG following a lipid challenge. A single dose of REGN1500 (5 mg/kg or greater) effectively reduced circulating TG levels in both normolipidemic and hyperlipidemic mice, and in dyslipidemic cynomolgus monkeys. These findings indicate that REGN1500 reduces circulating TG levels by upregulating LPL activity through inhibition of ANGPTL3.
We also evaluated the effect of chronic treatment with REGN1500. Weekly administration of REGN1500 in C57Bl/6 mice fed a high-fat high-cholesterol diet for 8 weeks caused a sustained reduction in circulating TG, TC, and LDL-C levels without affecting body weight, liver enzymes, or hepatic, adipose, or heart TG content. The reduction in serum TG levels can in part be attributed to reduced hepatic TG secretion rate. We show that the ability of REGN1500 to lower serum lipid levels is independent of changes in genes involved in de novo lipogenesis or in fatty acid oxidation in the liver.
ANGPTL3 inactivation with REGN1500 reduced LDL-C levels only in animals fed a high-fat high-cholesterol diet, but not in normolipidemic mice or cynomolgus monkeys that have normally low baseline LDL-C levels. The mechanism responsible for the LDL-lowering effect of ANGPTL3 is currently not clear, but appears not to involve the major canonical pathways of clearance of ApoB-containing lipoproteins (34).
The administration of REGN1500 to normolipidemic and hyperlipidemic mice also caused a reduction in plasma levels of HDL-C. Humans who are homozygous for inactivating mutations in ANGPTL3 have lower plasma HDL-C levels (26–30). It has been proposed that the reduction in plasma HDL-C levels in ANGPTL3-deficient individuals is due to the inhibitory effects of ANGPTL3 on the phospholipase activity of EL (8). Plasma phospholipase activity is higher in Angptl3−/− mice than in wild-type mice and the EL activity can be inhibited by recombinant human ANGPTL3 in vitro (8). In this study, administration of REGN1500 to mice lacking EL resulted in a reduction in circulating TG levels, but not in plasma HDL-C levels, thus confirming that HDL-C reduction results from the relief of EL inhibition by ANGPTL3.
HDL-C plays a key role in the process of reverse cholesterol transport by promoting the efflux of excess cholesterol from peripheral tissues to the liver for biliary excretion (35). The possible therapeutic benefit of ANGPTL3 blockade using REGN1500 to lower both TG and LDL-C is possibly tempered by concern about its ability to decrease HDL-C. However, recent genetic and pharmacological findings have questioned the importance of HDL-C as a marker for cardiovascular risk prediction (36–38), particularly when reflecting changes due to EL action. Consistent with this scenario, it was recently shown that humans with a common inactivating mutation in LIPG and higher plasma levels of HDL-C are not protected from coronary heart disease (36). Measurement of reverse cholesterol transport and assessment of HDL functionality may help to better understand the potential consequences of reduced plasma HDL-C in humans.
Although very limited analysis is available on the prevalence of coronary heart disease in carriers of homozygous ANGPTL3 mutations, Pisciotta et al. (28) reported no clinical evidence of atherosclerosis in their patients, attributing these findings to markedly reduced levels of pro-atherogenic apoB-containing lipoproteins (VLDL, VLDL remnants, and LDL) in the mutation carriers.
In summary, biochemical characterization and in vivo studies in normolipidemic and dyslipidemic mice and monkeys demonstrate that inhibition of ANGPTL3 by REGN1500 lowers the circulating levels of both cholesterol and TG significantly. These data suggest that REGN1500 may provide a new therapeutic approach for lipid-lowering, especially in subjects with combined hyperlipidemia.
Supplementary Material
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- ANGPTL3
- angiopoietin-like protein 3
- AST
- aspartate aminotransferase
- EL
- endothelial lipase
- HDL-C
- HDL-cholesterol
- HL
- hepatic lipase
- LDL-C
- LDL-cholesterol
- mmH
- myc-myc-His6
- RU
- resonance unit
- TC
- total cholesterol
This work was supported by National Institutes of Health Grant PO1 HL20948.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of nine figures, one table, and text.
REFERENCES
- 1.Merkel M., Eckel R. H., Goldberg I. J. 2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43: 1997–2006. [DOI] [PubMed] [Google Scholar]
- 2.Wang H., Eckel R. H. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297: E271–E288. [DOI] [PubMed] [Google Scholar]
- 3.Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R., Ueda K., Inaba T., Minekura H., Kohama T., et al. 2002. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 277: 33742–33748. [DOI] [PubMed] [Google Scholar]
- 4.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y., Koishi R., Furukawa H. 2003. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 278: 41804–41809. [DOI] [PubMed] [Google Scholar]
- 5.Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., et al. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 146: 4943–4950. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto K., Koishi R., Shimizugawa T., Ando Y. 2006. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp. Anim. 55: 27–34. [DOI] [PubMed] [Google Scholar]
- 7.Liu J., Afroza H., Rader D. J., Jin W. 2010. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J. Biol. Chem. 285: 27561–27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 27: 366–372. [DOI] [PubMed] [Google Scholar]
- 9.Jin W., Wang X., Millar J. S., Quertermous T., Rothblat G. H., Glick J. M., Rader D. J. 2007. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 6: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kathiresan S., Willer C. J., Peloso G. M., Demissie S., Musunuru K., Schadt E. E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. 2009. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegele R. A., Ban M. R., Hsueh N., Kennedy B. A., Cao H., Zou G. Y., Anand S., Yusuf S., Huff M. W., Wang J. 2009. A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia. Hum. Mol. Genet. 18: 4189–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romeo S., Yin W., Kozlitina J., Pennacchio L. A., Boerwinkle E., Hobbs H. H., Cohen J. C. 2009. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robciuc M. R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Oorni K., Metso J., Minicocci I., Ciociola E., Ceci F., et al. 2013. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 33: 1706–1713. [Erratum. 2013. Arterioscler. Thromb. Vasc. Biol. 33: e124.] [DOI] [PubMed] [Google Scholar]
- 17.Murphy A. J., Macdonald L. E., Stevens S., Karow M., Dore A. T., Pobursky K., Huang T. T., Poueymirou W. T., Esau L., Meola M., et al. 2014. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc. Natl. Acad. Sci. USA. 111: 5153–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrijn A. F., Buijsse A. O., van den Bremer E. T., Verwilligen A. Y., Bleeker W. K., Thorpe S. J., Killestein J., Polman C. H., Aalberse R. C., Schuurman J., et al. 2009. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat. Biotechnol. 27: 767–771. [DOI] [PubMed] [Google Scholar]
- 19.Myszka D. G. 1999. Improving biosensor analysis. J. Mol. Recognit. 12: 279–284. [DOI] [PubMed] [Google Scholar]
- 20.Valenzuela D. M., Murphy A. J., Frendewey D., Gale N. W., Economides A. N., Auerbach W., Poueymirou W. T., Adams N. C., Rojas J., Yasenchak J., et al. 2003. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat. Biotechnol. 21: 652–659. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D. M., Cohen J. C., Hobbs H. H. 2013. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 110: 16109–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usui S., Hara Y., Hosaki S., Okazaki M. 2002. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 43: 805–814. [PubMed] [Google Scholar]
- 23.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 24.Carr T. P., Andresen C. J., Rudel L. L. 1993. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 26: 39–42. [DOI] [PubMed] [Google Scholar]
- 25.Han S., Flattery A. M., McLaren D., Raubertas R., Lee S. H., Mendoza V., Rosa R., Geoghagen N., Castro-Perez J. M., Roddy T. P., et al. 2012. Comparison of lipoprotein separation and lipid analysis methodologies for human and cynomolgus monkey plasma samples. J. Cardiovasc. Transl. Res. 5: 75–83. [DOI] [PubMed] [Google Scholar]
- 26.Musunuru K., Pirruccello J. P., Do R., Peloso G. M., Guiducci C., Sougnez C., Garimella K. V., Fisher S., Abreu J., Barry A. J., et al. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 363: 2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Campos J. M., Roig R., Mayoral C., Martinez S., Marti G., Arroyo J. A., Julve J., Blanco-Vaca F. 2012. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin. Chim. Acta. 413: 552–555. [DOI] [PubMed] [Google Scholar]
- 28.Pisciotta L., Favari E., Magnolo L., Simonelli S., Adorni M. P., Sallo R., Fancello T., Zavaroni I., Ardigo D., Bernini F., et al. 2012. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ Cardiovasc Genet. 5: 42–50. [DOI] [PubMed] [Google Scholar]
- 29.Noto D., Cefalu A. B., Valenti V., Fayer F., Pinotti E., Ditta M., Spina R., Vigna G., Yue P., Kathiresan S., et al. 2012. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler. Thromb. Vasc. Biol. 32: 805–809. [DOI] [PubMed] [Google Scholar]
- 30.Minicocci I., Montali A., Robciuc M. R., Quagliarini F., Censi V., Labbadia G., Gabiati C., Pigna G., Sepe M. L., Pannozzo F., et al. 2012. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 97: E1266–E1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minicocci I., Santini S., Cantisani V., Stitziel N., Kathiresan S., Arroyo J. A., Marti G., Pisciotta L., Noto D., Cefalu A. B., et al. 2013. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J. Lipid Res. 54: 3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee E. C., Desai U., Gololobov G., Hong S., Feng X., Yu X. C., Gay J., Wilganowski N., Gao C., Du L. L., et al. 2009. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J. Biol. Chem. 284: 13735–13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonnenburg W. K., Yu D., Lee E. C., Xiong W., Gololobov G., Key B., Gay J., Wilganowski N., Hu Y., Zhao S., et al. 2009. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J. Lipid Res. 50: 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Gusarova V., Banfi S., Gromada J., Cohen J. C., Hobbs H. H. 2015. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J. Lipid Res. 56: 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voight B. F., Peloso G. M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M. K., Hindy G., Holm H., Ding E. L., Johnson T., et al. 2012. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 380: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 38.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.