Abstract
The surfactant proteins (SPs), SP-B and SP-C, are important components of pulmonary surfactant involved in the reduction of alveolar surface tension. Quantification of SP-B and SP-C in surfactant drugs is informative for their quality control and the evaluation of their biological activity. Western blot analysis enabled the quantification of SP-B, but not SP-C, in surfactant drugs. Here, we report a new procedure involving chemical treatments and LC-MS to analyze SP-C peptides. The procedure enabled qualitative analysis of SP-C from different species with discrimination of the palmitoylation status and the artificial modifications that occur during handling and/or storage. In addition, the method can be used to estimate the total amount of SP-C in pulmonary surfactant drugs. The strategy described here might serve as a prototype to establish analytical methods for peptides that are extremely hydrophobic and behave like lipids. The new method provides an easy measurement of SP-C from various biological samples, which will help the characterization of various experimental animal models and the quality control of surfactant drugs, as well as diagnostics of human samples.
Keywords: pulmonary surfactant, mass spectrometry, palmitoylation, proteomics, lung, drug therapy, surfactant drug, liquid chromatography
Pulmonary surfactant is a surface-active material secreted in the alveolar space. It reduces alveolar surface tension upon compression and prevents alveolar collapse at end-expiration. Neonatal insufficiency of pulmonary surfactant leads to respiratory distress syndrome. Pulmonary surfactant is a mixture of ∼90% lipids and ∼10% proteins, of which the major lipids are the phospholipids, phosphatidylcholine and phosphatidylglycerol (1). The most studied proteins of pulmonary surfactant are the surfactant proteins (SPs), SP-A, SP-B, SP-C, and SP-D (2, 3). Among them, SP-B and SP-C are hydrophobic peptides that act in concert with the major pulmonary surfactant phospholipid, dipalmitoylphosphatidylcholine (DPPC), and contribute to the surface tension-lowering property. Mature SP-C consists of 34 or 35 amino acid residues, and contains two palmitoylated cysteine residues at the N-terminal portion (4).
Multiple surfactant drugs are used for replacement therapy to prevent respiratory distress syndrome in preterm newborns (5, 6). Most of them are extracts from animals such as cows and pigs. It should be useful to quantify SP-B and SP-C for the quality control of surfactant drugs, and also for the understanding of their biological activity. It has been reported that SP-B and SP-C can be analyzed by Western blotting (7, 8). However, because the molecular mass of SP-C is extremely small (∼4 kDa), Western blot should be performed with precautions such as the usage of a Tris-Tricine system, and might be problematic. Another method that can be used for the quantification of SP-C is amino acid compositional analysis (9). However, this method requires the preparation of a relatively pure SP-C from samples. LC-MS is a useful method to quantify peptides, as well as metabolites such as lipids. LC-MS has been used to characterize SP-C from biological samples, but as far as we know, quantitative analysis has not been done (10, 11). Due to the extreme hydrophobic nature of SP-C, this peptide might behave like a lipid, and it is not known whether its quantitative analysis by LC-MS can be done using conventional settings that have been used for peptide analysis. In this study, we present a LC-MS-based method that enables qualitative analysis and relative quantification of SP-C, and show that this method can be applied to biological samples and quality assessment of surfactant drugs.
MATERIALS AND METHODS
Analysis of SP-B and SP-C by SDS-PAGE
Human SP-B and SP-C were obtained from bronchoalveolar lavage fluid of de-identified patients under a protocol approved by the institutional review board of the Cincinnati Children’s Hospital Medical Center (7, 12), purified by the method of Suzuki, Fujita, and Kogishi (13), and quantified by bicinchoninic acid protein assay (Pierce Chemicals) or amino acid compositional analysis as previously described (9). Surfacten (Mitsubishi Tanabe Pharma Corp.) or Infasurf (ONY Inc.) was rehydrated in saline, as is done clinically (4 ml for Surfacten to obtain a 30 mg/ml dispersion, and 3 ml for Infasurf to obtain a 35 mg phospholipid/ml dispersion), diluted as indicated in the figures, and separated by SDS-PAGE using precast 18% gels (Invitrogen). Gels were silver stained (Pierce Chemicals) or transferred to nitrocellulose membranes for Western blotting. SP-B and SP-C were detected using polyclonal rabbit antibodies as previously described (12), bands were quantitated by phosphorimage analysis (Storm, GE), and concentrations were calculated from standard curves of known amounts of human SP-B or SP-C.
Preparation of SP-C samples for LC-MS
Peptides corresponding to mature SP-C from mice or cows were obtained by custom synthesis (Sigma). SP-C was dissolved in trifluoroacetic acid (TFA) (in most cases at 1 mg/ml) and then diluted 1:10 in methanol. For comparison between different preparations, SP-C solutions in TFA were aliquoted and dried under a nitrogen stream until processing, as described above. All chemicals were from Wako unless stated otherwise. Samples were centrifuged at 21,600 g for 5 min, and supernatants were used for analysis. Palmitoylation was performed as previously described, by incubating SP-C (final concentration 10 mM) and palmitoyl chloride (final concentration 200 mM, Sigma) in TFA at room temperature for 10 min, and then stopping the reaction with nine times volume of 80% ethanol or 80% methanol (14). For analysis of mouse samples, bronchoalveolar lavage fluid was obtained as previously described (15). The procedure was approved and performed in accordance with the guidelines of the animal experimentation committees of the University of Tokyo and the National Center for Global Health and Medicine. Bronchoalveolar lavage fluid was centrifuged at 40,000 g for 15 min to obtain large aggregate surfactant as a pellet (16). The pellet was dissolved in TFA and diluted 1:10 in methanol. For qualitative analysis of SP-C in surfactant drugs, SP-C was extracted by the method of Bligh and Dyer (17), the lower phase was dried by centrifugal evaporation, and the pellet was dissolved in TFA followed by 1:10 dilution with methanol. Depalmitoylation of SP-C from drugs was performed as follows. Drugs were incubated in a solution containing 5% octyl glucoside and 0.8 M hydroxylamine (pH 7.4) at 37°C for 1 h. Then, SP-C was extracted by the method of Bligh and Dyer (17). Lower phase extraction was performed totally three times to maximize the extraction efficiency.
Analysis of SP-C by LC-MS
LC was performed using Acquity ultra-performance LC (UPLC) system (Waters), and the column was an UPLC ethylene bridged hybrid (BEH)300 C4 column. The mass spectrometer was a triple stage quadrupole Vantage system (Thermo). Solvents A and B for chromatography were 0.05% formic acid/water and 0.05% formic acid/methanol, respectively. The gradient started at 50% solvent B and was linearly increased to 99% solvent B over 10 min. This concentration was maintained for 10 min and then the composition was shifted to the initial one for the next analysis. MS was performed in the positive ion mode, with the spray voltage at 3,000 V, capillary temperature at 220°C, and vaporizer temperature at 450°C. For MS2 analyses, the collision gas was argon at 0.8 mTorr and the collision energy was 20, 30, 40, or 50 V. The conditions for selected reaction monitoring (SRM) are provided as a supplementary table.
Analysis of ion suppression
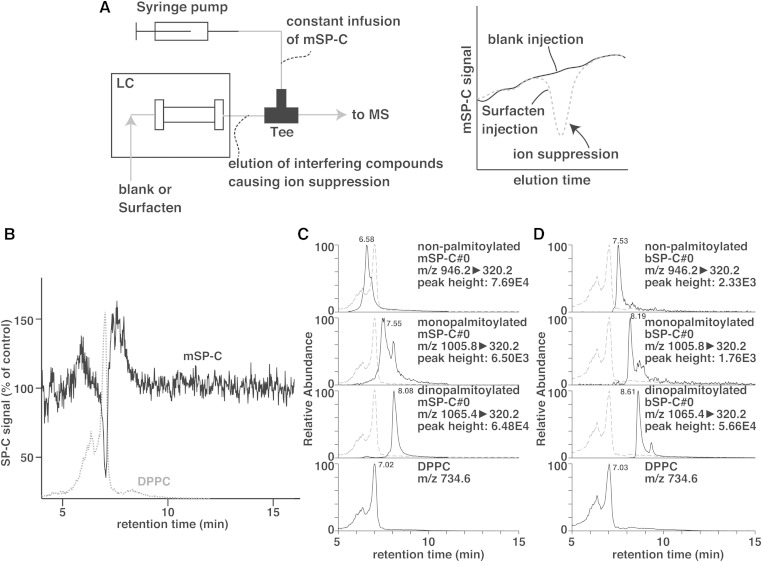
Ion suppression was analyzed by postcolumn infusion (Fig. 6A) (18). LC-MS was performed as described above. A 0.01 mg/ml solution of mouse SP-C was infused postcolumn via a tee connector using a syringe pump at a flow rate of 5 μl/min. The signals of infused mouse SP-C were analyzed under blank or Surfacten injection into the LC-MS, and the percentage of signals were calculated. In parallel, a precursor ion scan of m/z 184.1 was performed using a collision energy of 37 V to monitor phosphatidylcholine molecules that might have caused ion suppression.
Fig. 6.
Analysis of ion suppression by phosphatidylcholine. A: Ion suppression by components of Surfacten was analyzed by postcolumn infusion of mSP-C. Blank or Surfacten is injected into the LC while measuring signals of the infused mSP-C. Interfering compounds eluted from the column after Surfacten injection cause ion suppression, which can be detected by comparing the mSP-C signals with those of a blank injection. B: The signals are percent of those when a blank sample was injected into the column. Elution pattern of DPPC is overlaid in gray. C, D: Retention times of mSP-C (C) and bSP-C (D) with different palmitoylation status are compared with that of DPPC (overlaid in gray).
RESULTS AND DISCUSSION
Quantification of SP-B and SP-C by Western blotting
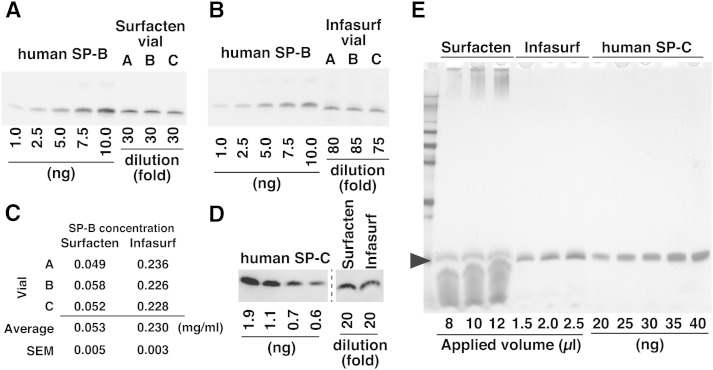
The initial purpose of our study was the quantification of SP-B and SP-C in a surfactant drug, Surfacten. We first attempted to quantify SP-B by Western blotting. SP-B of Surfacten could be detected by Western blot after SDS-PAGE under nonreducing conditions, and was compared with the signals from various amounts of purified human SP-B (Fig. 1A). For comparison, we performed a similar analysis using another surfactant drug, Infasurf, in which SP-B concentration is already reported (Fig. 1B) (5). We quantified SP-B in three vials each of Surfacten and Infasurf using standard curves of human SP-B, and obtained average concentrations of 0.053 mg/ml in Surfacten and 0.23 mg/ml in Infasurf (Fig. 1C). Our results are in good agreement with the reported concentration of SP-B in Infasurf, which is 0.26 mg/ml (5).
Fig. 1.
Analysis of SP-B and SP-C by Western blotting. A, B: Western blot analysis of SP-B in Surfacten (A) and Infasurf (B). Each sample lane contains 2.5 μl of diluted surfactant drug. C: Quantification of SP-B based on Western blotting. Data for each vial is calculated from three experiments. D: Western blot analysis of SP-C in Surfacten and Infasurf. Each sample lane contains 9 μl of diluted drug. E: Silver staining of gels after SDS-PAGE reveals that some materials comigrate with SP-C (arrowhead) in surfactant drugs. Proteins are also seen in the upper part of the gel in Surfacten lanes.
Using a similar approach, we tried to quantify SP-C. Although SP-C could be detected by Western blotting, the signals were very weak and quantification suggested concentrations of 0.001–0.002 mg/ml (Fig. 1D). This seemed to be unnatural given that SP-C concentration tends to be higher than that of SP-B in surfactant drugs (19). This discrepancy might be explained by factors in surfactant drugs that perturb the detection of SP-C. Silver staining of gels after SDS-PAGE of Surfacten revealed a large amount of some material (probably lipids) that could not be separated from SP-C (Fig. 1E). This material might have affected the transfer on the membrane and/or the detection of SP-C by antibodies. Because electrophoresis of SP-B was performed under nonreducing conditions, it was detected as a dimer, and the band could be resolved from the unknown material. This explains why only SP-C analysis using Western blotting was problematic. In addition, silver-stained gels revealed additional large molecular weight proteins in Surfacten (Fig. 1E), making amino acid compositional analysis meaningless without further processing.
Analysis of SP-C peptide by LC-MS
Due to the reasons described above, we wanted to establish a novel method to quantify SP-C. Because LC-MS is suitable for analysis of both peptides and lipids, we reasoned that the extremely hydrophobic SP-C should also be measurable. In addition, LC-MS should also be suitable for a qualitative analysis of the degree of palmitoylation. We established methods for the analysis of murine SP-C (mSP-C) and bovine SP-C (bSP-C), because the former would be useful for the analysis of multiple mouse models, and because the latter is the component in Surfacten and Infasurf. The results for mSP-C analysis are shown first.
We first performed preliminary experiments to test whether a synthetic nonpalmitoyolated SP-C peptide can be detected by LC-MS. These experiments revealed multiple precautions required for the handling and analysis of SP-C by LC-MS. First, mSP-C peptide is extremely hydrophobic and cannot be dissolved in most solvents. We needed to reconstitute the peptide in TFA, and then dilute it with methanol to obtain a solution. Even under this condition, we found that SP-C is, in part, insoluble, because pellets appeared after centrifugation of 1 mg/ml solutions. No pellet was visible when the concentration was less than 0.1 mg/ml, thus care should be paid not to operate the analyses using concentrations of SP-C that are too high. In addition, we found that SP-C is incompatible with acetonitrile, probably due to its insolubility in this solvent, because we never detected SP-C signal when 100% acetonitrile was chosen as solvent B for LC. Finally, we found that the extreme hydrophobicity of SP-C leads to a strong retention in the C18 columns often used for peptide analysis, which results in severe peak tailing and carryover. Therefore, in the following experiments we used SP-C in TFA/methanol (1:9) and the LC was operated using 0.05% formic acid/water and 0.05% formic acid/methanol as solvents, using a Waters UPLC BEH300 C4 column for separation.
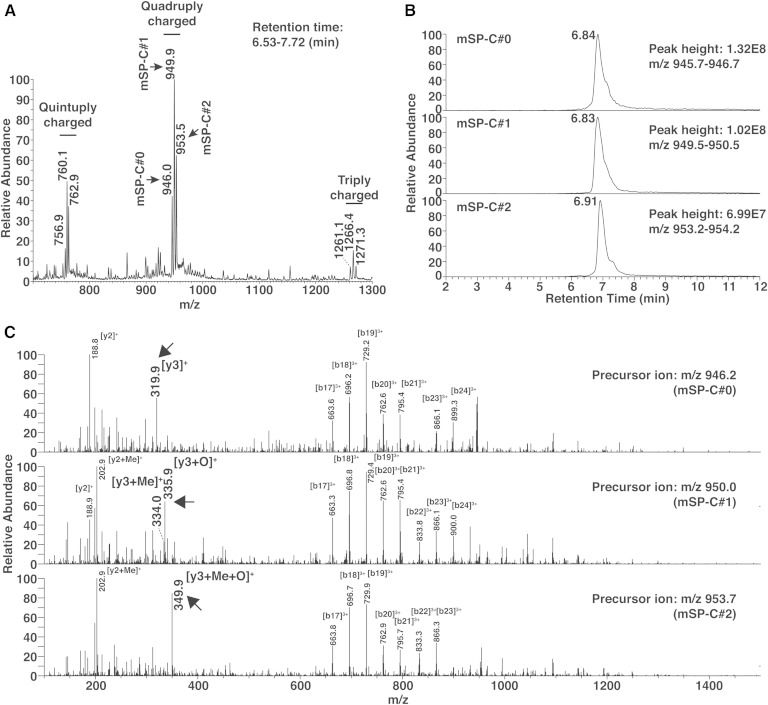
We first performed full scans of mSP-C in the positive ion mode to characterize the signals from this peptide. mSP-C was detected as triply, quadruply, and quintuply charged ions, and the quadruply charged ions gave the strongest signals (Fig. 2A). In agreement with the theoretical average m/z (946.2) of quadruply charged mSP-C, we detected a peak cluster with an average m/z of 946.0. In addition, we detected two additional quadruply charged peak clusters with m/z values that were 3.9 and 7.5 larger (Fig. 2A). Previous studies have shown that SP-C peptide can be oxidized at its methionine (the third residue from the C terminus) and methylated at its C terminus during the handling and/or storage as experimental artifacts (10). Methylation might occur when the peptide is handled in methanol (20). The m/z 3.9 change is consistent with a mixture of mSP-C that received either modification, because methylation and oxidation should result in m/z 3.5 and 4.0 increases, respectively. The m/z 7.5 increase is consistent with mSP-C that received the two modifications. To discriminate SP-C forms with different artificial modifications, we will illustrate SP-C without modification as SP-C#0, SP-C with one modification (methylation or oxidation) as SP-C#1, and SP-C with two modifications as SP-C#2, when required. Because these modifications are artifacts that occur in vitro during the handling and are probably difficult to prevent completely (10), it was important to establish methods to detect mSP-C#0, mSP-C#1, and mSP-C#2 individually. Chromatograms of the three forms showed that modifications in mSP-C do not largely affect its retention time (Fig. 2B). We performed MS2 analyses to detect fragment ions that can discriminate each mSP-C form. Consistent with the expected modification sites of mSP-C, the b-series fragments (related to the N terminus) were common in each form of mSP-C, while the C terminus-related y-series fragments differed (Fig. 2C, arrows). The y3 fragment ions of mSP-C#1 were consistent with a mixture of methylated and oxidized mSP-C. The y3 fragment ions of mSP-C#2 showed that both methylation and oxidation had occurred. These characteristic y3 ions could be used for further SRM analyses.
Fig. 2.
Analysis of synthetic mSP-C peptide by LC-MS. A, B: A full scan mass spectrum (A) of mSP-C by LC-MS and extracted chromatograms (B) of the quadruply charged ions. C: MS2 analysis of mSP-C with different artificial modifications. Arrows indicate y3 ions that are characteristic for each modification.
Analysis of palmitoylated mSP-C
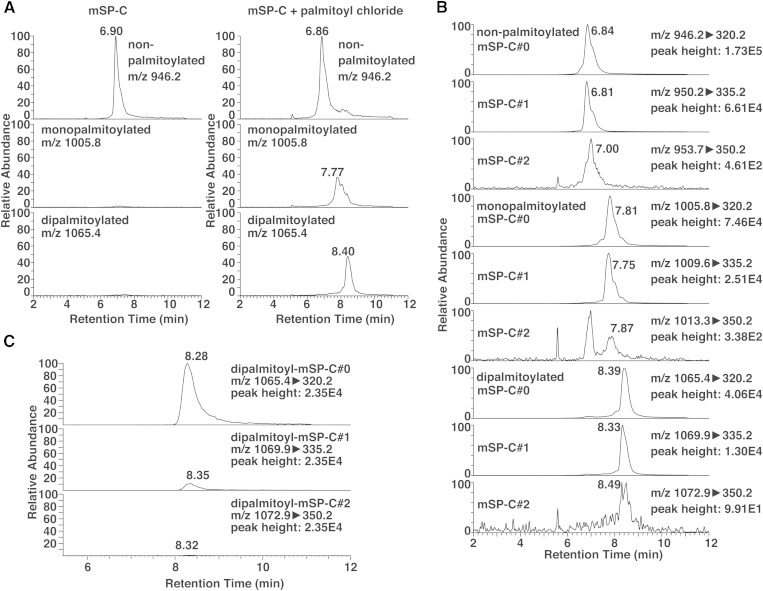
Because mature SP-C is palmitoylated in vivo (4) and we did not know whether this palmitoylation is preserved in drugs, we had to establish a method to detect the palmitoylation status of SP-C. It has been reported that incubation of nonpalmitoylated SP-C peptide with palmitoyl chloride in TFA leads to palmitoylation at the cysteine residues (14). Indeed, when we performed full scans of mSP-C after incubation with palmitoyl chloride, additional peaks appeared with m/z values consistent with monopalmitoyl-mSP-C and dipalmitoyl-mSP-C. The retention time of mSP-C increased together with the palmitoylation status (Fig. 3A). These peaks were not detected when mSP-C was not incubated with palmitoyl chloride (Fig. 3A). We performed MS2 analyses of mono- and dipalmitoyl-mSP-C for each of the mSP-C#0, mSP-C#1, and mSP-C#2 forms that were analyzed (supplementary Fig. 1). Although the quality of the MS2 data varied between different mSP-C forms, the b-series ions were consistent with palmitoylation at cysteines, and the y3 ions were consistent with the artificial modifications, similarly with Fig. 2C. Using these y3 characteristic ions, we could establish SRM channels to detect mSP-C#0, mSP-C#1, and mSP-C#2 separately, with different channels based on the palmitoylation status (Fig. 3B). Because SRM is highly sensitive, detection of all mSP-C forms was possible, even for those that provided low quality MS2 signals. The collision energies for each mSP-C form were optimized to give the strongest signals.
Fig. 3.
Analysis of palmitoylated mSP-C by LC-MS. A: Chromatograms extracted from full scans of mSP-C with different palmitoylation status. B: Chromatograms of nine SRM channels that discriminate both the artificial modifications and the palmitoylation status of mSP-C incubated with palmitoyl chloride. See supplementary Fig. 1 for MS2 analyses of each mSP-C form. C: Detection of dipalmitoyl-mSP-C from a bronchoalveolar lavage fluid sample. See supplementary Fig. 2 for chromatograms of all SRM channels.
Using this method, we tested whether mSP-C can be measured in biological samples. We obtained large aggregate surfactants from mouse bronchoalveolar lavage fluid, and analyzed them by the established LC-MS method. We found that mSP-C can be measured using this method, and the signal was predominantly related to dipalmitoyl-mSP-C#0 (Fig. 3C; supplementary Fig. 2). For the analysis, an equivalent of only 5 μl of bronchoalveolar lavage fluid was injected (from a total of 4 ml obtained per mouse). This shows the feasibility and high sensitivity of our method for analysis of biological samples. Also, because mSP-C#1 and mSP-C#2 signals were very weak, the modifications seemed to be, indeed, in vitro artifacts, and suggest that some biological systems prevent the oxidation of SP-C in vivo. The absence of non- or monopalmitoyl-mSP-C shows that SP-C processing and palmitoylation are a very sophisticated process.
Differences in signal responses between SP-C#0, SP-C#1, and SP-C#2
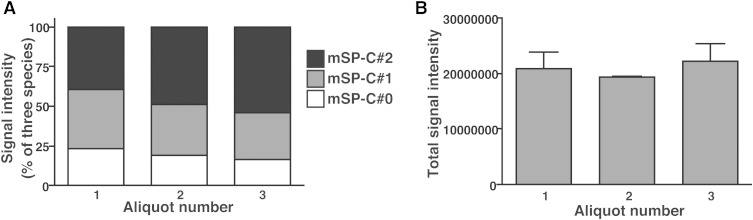
It would be ideal that mSP-C#0, mSP-C#1, and mSP-C#2 have similar signal responses, otherwise their conversions during handling and storage might strongly affect the results. To investigate this issue, we prepared three aliquots of mSP-C that were handled separately (which would lead to different patterns of artificial modifications) and analyzed them consecutively to compare the signals of the different forms. As expected, the signal ratio between mSP-C#0, mSP-C#1, and mSP-C#2 differed in different aliquots (Fig. 4A). Importantly, the sums of the three forms were similar between aliquots (Fig. 4B), showing that the artificial modifications do not largely affect the signal response of SP-C. This suggests that the sum of signals from the three forms provides a reliable measure of SP-C level.
Fig. 4.
Comparison of signal responses between mSP-C with different artificial modifications. A: Comparison of relative signal intensity between nonpalmitoylated mSP-C#0, mSP-C#1, and mSP-C#2. The sum of the three forms is 100%. Three aliquots that were handled separately were analyzed. B: The sums of signals from the three forms analyzed in (A) are compared between aliquots.
Analysis of bSP-C in surfactant drugs
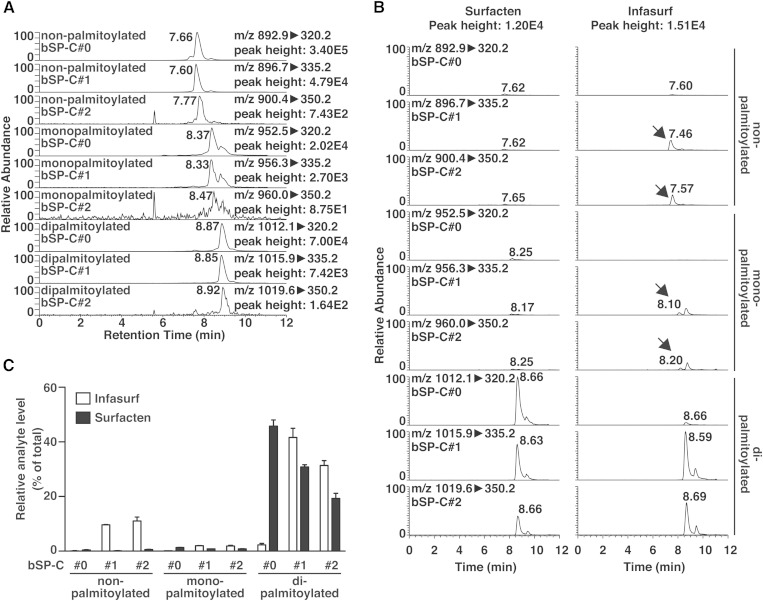
We performed similar experiments to establish a method to detect bSP-C. After optimization, bSP-C could be detected by SRM with discrimination of the palmitoylation status, as well as the artificial modifications, similarly to mSP-C (Fig. 5A). Using this method, we performed qualitative analysis of bSP-C in Surfacten and Infasurf. It is important to note that Surfacten was obtained as a powder and dissolved freshly before analysis, while Infasurf was imported to our laboratory as a solution in saline. Therefore, the characteristics that will appear in the following results may derive from differences in both drugs and storage conditions. Because the major purpose of this study is to show that LC-MS can be used for the analysis of SP-C, we will not discuss the biological relevance of the differences between the two drugs. We successfully detected bSP-C from both drugs with some differences (Fig. 5B). While bSP-C in Surfacten was almost fully dipalmitoylated, one part of bSP-C in Infasurf was nonpalmitoylated or monopalmitoylated. In addition, most of bSP-C signal in Infasurf was detected as bSP-C#1 or bSP-C#2, while bSP-C#0 signal could be seen in Surfacten. The proportion of signal from each analyte is illustrated in Fig. 5C. We speculate that the lack of dipalmitoyl-bSP-C#0 in Infasurf is due to the storage conditions. Our analyses show that SP-C in drugs can be qualitatively analyzed by LC-MS for quality control and/or comparison between drugs.
Fig. 5.
Qualitative analysis of bSP-C by LC-MS. A: Chromatograms of nine SRM channels that discriminate both the artificial modifications and the palmitoylation status of bSP-C incubated with palmitoyl chloride. B: Analysis of bSP-C in Surfacten and Infasurf using the nine SRM channels. Signals of bSP-C with reduced palmitoylation are detected in Infasurf (arrows). C: Peak areas from (B) are illustrated as the percentage in each drug. Error bars are SEM of three vials.
Analysis of ion suppression
As stated above, the initial purpose of this study was to quantify bSP-C in Surfacten. To obtain quantitative information of SP-C levels, it was important to analyze the degree of ion suppression, because the high levels of phosphatidylcholine present in surfactant might affect the ionization efficiency. To analyze whether the components of surfactant drugs affect SP-C ionization, we monitored the signal of mSP-C that was constantly infused postcolumn (Fig. 6A) (18). We compared the signal of mSP-C when blank or Surfacten was injected in the column (Fig. 6B). We found that mSP-C signal is suppressed only in a narrow time range, which coincided with the elution of the major surfactant phosphatidylcholine, DPPC. Therefore, if SP-C elution is simultaneous with DPPC, ion suppression will occur. We compared the retention time of mSP-C and bSP-C with different palmitoylation status, and found that they were separated from DPPC by LC (Fig. 6C, D). Therefore, in the case of SP-C from these two species, ion suppression will not affect the results, but it will be important to analyze the retention time and compare it to DPPC when analyzing SP-C from other species.
Estimation of bSP-C concentration in surfactant drugs
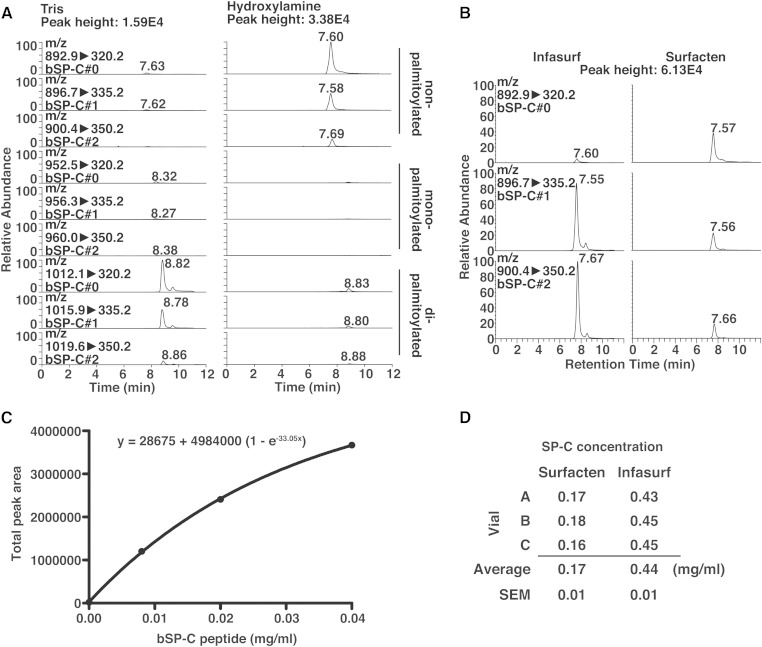
Using the established LC-MS method, it should be possible to quantify SP-C if standards for every form are available. However, it was too laborious and difficult to obtain standards of mono- and dipalmitoyl-SP-C with high purity, while completely avoiding oxidization and methylation. Therefore, we chose to remove the palmitoyl-moieties from bSP-C in drugs to estimate the total bSP-C concentrations. Hydroxylamine is often used to remove palmitoylation from peptides (21). Indeed, incubation of Surfacten with hydroxylamine resulted in an almost complete disappearance of mono- and dipalmitoyl-bSP-C levels, as well as increases in nonpalmitoylated bSP-C levels (Fig. 7A). This was not observed when Surfacten was incubated with Tris. Therefore, removal of palmitoylation by hydroxylamine was successful, making comparison with standard curves of nonpalmitoylated SP-C possible. We measured nonpalmitoylated bSP-C levels after hydroxylamine treatment of Surfacten and Infasurf (Fig. 7B). Similarly with the trends of SP-B concentration, bSP-C signals were weaker in Surfacten than in Infasurf. In addition, bSP-C#0 was almost undetectable in Infasurf, consistently with the qualitative analysis of Fig. 5B, C. Because the sum of SP-C#0, SP-C#1, and SP-C#2 provides a stable value that is not affected by the proportion among each form (Fig. 4A, B), the sums could be compared with a standard curve. We analyzed signals from synthetic nonpalmitoylated bSP-C at various concentrations to obtain a standard curve (Fig. 7C). We found that the signals were not linear. Although the reason of this nonlinearity is unknown, we speculate that this is due to the partial insolubility of SP-C as described above, and that even if no pellet is visible after centrifugation, one fraction of SP-C is not dissolved. Whatever the cause of the nonlinearity, the signal from bSP-C could be fitted using a nonlinear regression, making estimation of bSP-C concentrations possible (Fig. 7C). The estimated concentrations of total bSP-C in Surfacten and Infasurf are shown in Fig. 7D. It is known that Infasurf contains 0.7 mg/ml protein, in which the SP-B concentration was 0.23 mg/ml in this study (Fig. 1C) (5). The silver staining result (Fig. 1D) suggests that the majority of proteins in Infasurf are SP-B and SP-C. Therefore, the estimated concentration of SP-C (0.44 mg/ml) was highly consistent with the expected one (0.47 mg/ml based on our SP-B concentration data, and 0.44 mg/ml based on the SP-B concentration from the literature), showing the feasibility of our LC-MS-based approach to estimate total SP-C concentration in samples.
Fig. 7.
Estimation of bSP-C concentration in surfactant drugs. A: Chromatograms of SRM channels as in Fig. 5B, using Surfacten with or without depalmitoylation procedures. B: Chromatograms of SRM channels for detection of nonpalmitoylated bSP-C, after depalmitoylation of surfactant drugs. C: Standard curve of synthetic bSP-C with nonlinear regression. The peak areas are the sums of the three channels as in (B). D: Estimation of total SP-C concentration in surfactant drugs.
Advantages and limitations of the new method
In this report, we described a novel method to analyze and perform relative quantification of SP-C using LC-MS. As shown by the results, this method is more reliable than Western blotting. In addition, the use of MS leads to less background than in traditional LC methods (22, 23). Also, we can analyze SP-C in impure preparations, making it a superior method to amino acid compositional analysis (9). In addition, we can estimate not only SP-C amount, but also its quality. Therefore, this method is a superior alternative for routine quality control of surfactant drugs to evaluate the processes of drug preparation and storage.
Some limitations also exist for this method. First, our standard SP-C peptides did not have 100% purity. Because the purity of bSP-C peptide used in Fig. 7C was >70%, the calculation of the concentrations was done under the assumption that the peptide was 80% pure. If more accurate values are required, standards with higher purity should be used. Second, we could not control the degree of the artificial modifications (methylation and oxidation), and the ratio of signals from SP-C#0, SP-C#1, and SP-C#2 varied among samples. Therefore, we had to use the total of signals from the three forms for the calculation of bSP-C concentration in surfactant drugs. Although the results of Fig. 4A, B suggest that the signal response from each form is not largely different, this limitation might cause some inaccuracy. Third, we found that when we spike synthetic SP-C (dissolved in TFA) in a surfactant drug sample, the recovery of the spiked peptide after extraction is limited compared with SP-C in the drug. The reason for this is unknown, but we speculate that the differences in physical states of SP-C in TFA and in the surfactant environment lead to an uneven mixing, and that the spiked sample could not be extracted. Therefore, we could not include some internal standard (for example deuterated SP-C) into the samples and the extraction efficiencies could not be taken into account. To reduce inaccuracy from this limitation, we performed re-extraction several times to obtain a high recovery. Therefore, our method remains semi-quantitative. Although these limitations existed, it is important to note that the estimated concentrations were consistent with the expected values in Infasurf (5); thus, we think that the results were not largely affected. Investigation of better solvents, as well as antioxidants, to prevent in vitro artificial modifications and establishment of extraction methods that are not affected by the physical state of SP-C will improve the accuracy of the method.
Relevance of the new method and future perspectives
The various properties of SP-C that we described in this report show that this peptide behaves like a lipid, and we speculate that mature SP-C is not identified during most of conventional proteomics studies. It is therefore important to have a specific method for the measurement of SP-C as in this study. We have already used this method to show that mSP-C levels are not affected in a mouse model of DPPC insufficiency (15). In addition, the approaches to optimize SP-C measurement in this study might serve as a prototype for the analysis of other peptides that have similar hydrophobic properties and/or extensive lipid modifications. Such peptides might appear when analyzing tryptic digests of transmembrane proteins, and their analyses will improve the coverage of proteome analyses.
In this study, we provided two strategies (qualitative and semi-quantitative) for the analyses of SP-C. The qualitative analysis is relevant when comparing samples that might have altered SP-C modifications. Oxidation might occur during preparation of surfactant drugs, and possibly during oxidative stress in vivo. We speculate that methionine oxidation does not largely affect the properties of SP-C. Indeed, although bSP-C#0 was very low in Infasurf, it is established as a surfactant drug with high efficacy (5). On the other hand, because it has been reported that extensive oxidation might affect the properties of SP-C (24), it will also be interesting to establish methods to detect other amino acid targets of oxidation in the future. Methylation is rather an experimental artifact, but might also appear if methanol is used during surfactant drug preparation. Palmitoylation is important for full SP-C function, and might be affected during various diseases, such as has been reported in pulmonary alveolar proteinosis (25). By combining biophysical assays and the qualitative information from this method, it will be possible to analyze how these chemical modifications affect the functions of SP-C.
The semi-quantitative method would be important to compare SP-C amounts between samples. This might be used to compare different surfactant drugs or different batches of each drug. It will also enable analyzing SP-C levels during diseases. For example, some familial interstitial lung disease cases with SP-C absence were reported (26). Therefore, by performing the qualitative and semi-quantitative analyses in parallel, we can obtain different types of information that compensate each other. As discussed above, this information is important for drug quality control, drug development, and disease biology.
Finally, because analysis of SP-C from different species should be easily performed following the same procedures as in this report, it will probably be possible to extend this method for clinical studies to analyze SP-C from human subjects, and to investigate whether the quantity and quality of SP-C is affected during various diseases.
Supplementary Material
Acknowledgments
The authors are grateful to K. Waku, M. Yamada, and all members of the Shimizu laboratory for valuable suggestions and to D. Hishikawa for providing BALF samples.
Footnotes
Abbreviations:
- bSP-C
- bovine surfactant protein C
- DPPC
- dipalmitoylphosphatidylcholine
- mSP-C
- murine surfactant protein C
- SP
- surfactant protein
- SRM
- selected reaction monitoring
- TFA
- trifluoroacetic acid
- UPLC
- ultra-performance LC
This work was supported by JSPS KAKENHI Grants 24229003 (T.S.), 25116707 (Y.K.), 23790324 (H.S.), 24790805 (T.H.), and the grants for National Center for Global Health and Medicine 24-001 and 25-201 (T.S.). Materials and fees for this research were provided, in part, by Mitsubishi Tanabe Pharma Corporation. The Department of Lipidomics, Graduate School of Medicine, University of Tokyo is financially supported by Shimadzu Corporation and Ono Pharmaceutical Company, LTD.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and one table.
REFERENCES
- 1.Agassandian M., Mallampalli R. K. 2013. Surfactant phospholipid metabolism. Biochim. Biophys. Acta. 1831: 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobe A. H., Ikegami M. 2001. Biology of surfactant. Clin. Perinatol. 28: 655–669. [DOI] [PubMed] [Google Scholar]
- 3.Whitsett J. A., Wert S. E., Weaver T. E. 2010. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu. Rev. Med. 61: 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson J. 1998. Structure and properties of surfactant protein C. Biochim. Biophys. Acta. 1408: 161–172. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan R., Paz P., Biniwale M. 2013. Non-invasive ventilation and surfactant therapy. J. Pulm. Respir. Med. S13 10.4172/2161-105X.S13-006. [Google Scholar]
- 6.Speer C. P., Sweet D. G., Halliday H. L. 2013. Surfactant therapy: past, present and future. Early Hum. Dev. 89: S22–S24. [DOI] [PubMed] [Google Scholar]
- 7.Lin S., Phillips K. S., Wilder M. R., Weaver T. E. 1996. Structural requirements for intracellular transport of pulmonary surfactant protein B (SP-B). Biochim. Biophys. Acta. 1312: 177–185. [DOI] [PubMed] [Google Scholar]
- 8.Ross G. F., Ikegami M., Steinhilber W., Jobe A. H. 1999. Surfactant protein C in fetal and ventilated preterm rabbit lungs. Am. J. Physiol. 277: L1104–L1108. [DOI] [PubMed] [Google Scholar]
- 9.Stark M., Wang Y., Danielsson O., Jörnvall H., Johansson J. 1998. Determination of proteins, phosphatidylethanolamine, and phosphatidylserine in organic solvent extracts of tissue material by analysis of phenylthiocarbamyl derivatives. Anal. Biochem. 265: 97–102. [DOI] [PubMed] [Google Scholar]
- 10.Liu S., Zhao L., Manzanares D., Doherty-Kirby A., Zhang C., Possmayer F., Lajoie G. A. 2008. Characterization of bovine surfactant proteins B and C by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 22: 197–203. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson M., Curstedt T., Jörnvall H., Johansson J. 1997. Reverse-phase HPLC of the hydrophobic pulmonary surfactant proteins: detection of a surfactant protein C isoform containing Nepsilon-palmitoyl-lysine. Biochem. J. 326: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conkright J. J., Apsley K. S., Martin E. P., Ridsdale R., Rice W. R., Na C. L., Yang B., Weaver T. E. 2010. Nedd4–2-mediated ubiquitination facilitates processing of surfactant protein-C. Am. J. Respir. Cell Mol. Biol. 42: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki Y., Fujita Y., Kogishi K. 1989. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am. Rev. Respir. Dis. 140: 75–81. [DOI] [PubMed] [Google Scholar]
- 14.Yousefi-Salakdeh E., Johansson J., Strömberg R. 1999. A method for S- and O-palmitoylation of peptides: synthesis of pulmonary surfactant protein-C models. Biochem. J. 343: 557–562. [PMC free article] [PubMed] [Google Scholar]
- 15.Harayama T., Eto M., Shindou H., Kita Y., Otsubo E., Hishikawa D., Ishii S., Sakimura K., Mishina M., Shimizu T. 2014. Lysophospholipid acyltransferases mediate phosphatidylcholine diversification to achieve the physical properties required in vivo. Cell Metab. 20: 295–305. [DOI] [PubMed] [Google Scholar]
- 16.Veldhuizen R. A., Inchley K., Hearn S. A., Lewis J. F., Possmayer F. 1993. Degradation of surfactant-associated protein B (SP-B) during in vitro conversion of large to small surfactant aggregates. Biochem. J. 295: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 18.Annesley T. M. 2003. Ion suppression in mass spectrometry. Clin. Chem. 49: 1041–1044. [DOI] [PubMed] [Google Scholar]
- 19.Bernhard W., Mottaghian J., Gebert A., Rau G. A., Von der Hardt H., Poets C. F. 2000. Commercial versus native surfactants: Surface activity, molecular components, and the effect of calcium. Am. J. Respir. Crit. Care Med. 162: 1524–1533. [DOI] [PubMed] [Google Scholar]
- 20.Jung S. Y., Li Y., Wang Y., Chen Y., Zhao Y., Qin J. 2008. Complications in the assignment of 14 and 28 Da mass shift detected by mass spectrometry as in vivo methylation from endogenous proteins. Anal. Chem. 80: 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan J., Roth A. F., Bailey A. O., Davis N. G. 2007. Palmitoylated proteins: purification and identification. Nat. Protoc. 2: 1573–1584. [DOI] [PubMed] [Google Scholar]
- 22.Bünger H., Kaufner L., Pison U. 2000. Quantitative analysis of hydrophobic pulmonary surfactant proteins by high-performance liquid chromatography with light-scattering detection. J. Chromatogr. A. 870: 363–369. [DOI] [PubMed] [Google Scholar]
- 23.van Eijk M., De Haas C. G., Haagsman H. P. 1995. Quantitative analysis of pulmonary surfactant proteins B and C. Anal. Biochem. 232: 231–237. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Capote K., Manzanares D., Haines T., Possmayer F. 2006. Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys. J. 90: 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voss T., Schäfer K. P., Nielsen P. F., Schäfer A., Maier C., Hannappel E., Maassen J., Landis B., Klemm K., Przybylski M. 1992. Primary structure differences of human surfactant-associated proteins isolated from normal and proteinosis lung. Biochim. Biophys. Acta. 1138: 261–267. [DOI] [PubMed] [Google Scholar]
- 26.Amin R. S., Wert S. E., Baughman R. P., Tomashefski J. F., Jr, Nogee L. M., Brody A. S., Hull W. M., Whitsett J. A. 2001. Surfactant protein deficiency in familial interstitial lung disease. J. Pediatr. 139: 85–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.