Abstract
In this manuscript, a clinical case of a patient treated with adalimumab for Behcet’s disease develops lichen planopilaris. A variety of mucocutaneous lichenoid eruptions have recently been described in association with tumor necrosis factor alpha inhibitors. The authors briefly discuss the clinical and pathological presentation of lichen planopilaris as well as a potential pathogenesis of cutaneous adverse effects seen as the result of tumor necrosis factor alpha inhibitor therapy. They review all case reports of lichen planopilaris occurring on tumor necrosis factor alpha inhibitors and suggest its classification as a fourth recognized pattern on this therapy.
The systemic adverse effects of tumor necrosis factor alpha (TNF) inhibitors are well known. Recently, attention has been directed to the potential and often paradoxical cutaneous adverse effects seen in association with TNF alpha inhibitors including lichen planus-like eruptions, psoriasis, alopecia areata, and lupus-like syndromes.1,2 In addition, a variety of lichenoid reactions have been added to the emerging cutaneous adverse effects associated with TNF alpha inhibitors. Three patterns of lichenoid reactions have been reported—lichen planus (LP), maculopapular lichenoid reaction, and psoriasis-like with lichen planus histology. Lichen planopilaris (LPP) has been reported in few cases associated with infliximab and etanercept therapy.3
Of the TNF inhibitors, adalimumab is fully humanized and is purported to have a decreased risk for neutralizing antibody development. However, all of the TNF alpha inhibitors have been reported to stimulate an antibody response during therapy with no clear relationship to efficacy or adverse effects from developing antibodies while on these medications.4 Many of the adverse reactions associated with this group of agents have appeared in the rheumatoid arthritis population, which may reflect increased coexistence of diseases characteristic to the rheumatoid arthritis population or a longer time frame of market availability of biologies for this patient population.
Garcovich et al2 was the first to describe a patient who developed LPP after treatment with etanercept for psoriasis. Discontinuation of etanercept decreased the progression of LPP lesions; however, the patient’s psoriasis flared several months later. Upon restarting etanercept, the patient gradually developed new LPP lesions.2 Fernandez-Torres et al3 also reported a case of LPP induced by inflixamab in a patient treated for psoriasis. Herein, the authors contribute an additional case report of LPP associated with adalimumab therapy for Behcet’s disease and review the diversity of these lichenoid eruptions seen during TNF alpha inhibitor therapy (Table 1). They suggest LPP to be the fourth lichenoid reaction type that may develop during TNF alpha inhibitor therapy and caution clinicians to be aware of this eruption.
TABLE 1.
Overview of lichenoid reactions associated with TNF alpha inhibitors
| STUDY | UNDERLYING DISEASE | DRUG | REACTION TYPE | TIME TO REACTION | TIME TO RESOLUTION | CESSATION OF TNF | OUTCOME | THERAPY FOR REACTION |
|---|---|---|---|---|---|---|---|---|
| Vergara et al16 | Ankylosing spondylitis | Infliximab | LP | 3 weeks | NR | No | Recovery | Topical steroids |
| Bovenschen et al17 | Severe psoriasis | Etanercept | LP | 5 weeks | NR | Yes | Recovery | NR |
| Battistella et al18 | RA | Etanercept MTX | Linear LP | 4 months | 4 months | Yes | Recovery | Topical steroid continue MTX |
| Musumeci et al19 | Severe psoriasis | Etanercept | LP with pterygium | 8 months | 1 month | No | Improved | Topical steroid |
| Moss et al20 | Crohn’s disease | Infliximab Azathioprine | Oral LP | 3 weeks | 1 month | No | Improved | Topical tacrolimus |
| De Simone et al21 | Psoriasis and psoriatic arthritis | Adalimumab | Oral LP | 1 month | 1 month | No | Recovery | None |
| Asarch et al1 | Severe psoriasis | Infliximab | Oral LP Acral LP Perianal LP |
2 months | NR | Yes | Partial recovery | Cyclosporine, prednisone, topical triamcinolone |
| Asarch et al1 | Severe psoriasis | Adalimumab | Oral LP Acral LP |
5 months | NR | No | Recovery | Oral and topical steroid |
| Fernandez- Torres et al3 | Severe psoriasis | Infliximab plus MTX | LPP | 11 months | NR | No | Stabilization | Oral steroid |
| *Garcovich et al2 | Psoriasis with psoriatic arthritis | Etanercept | LPP | 8 months | 3 months | Yes | Recurred on rechallenge | NSAID, cyclosporine topical steroid |
| Abbasi et al22 | Severe psoriasis | Etanercept | LPP | NR | NR | NR | Persisted | Class I topical steroid, topical tacrolimus |
| Current study | Behcet’s Disease | Adalimumab | LPP | 12 months | 12 months | No | Stabilized | MTX |
| Seneschal et al23 | RA | Etanercept MTX | Psoriasis-like LP | 2 months | NR | NR | NR | NR |
| Seneschal et al23 | Ankylosing spondylitis | Infliximab MTX | Psoriasis-like LP | 8 months | NR | NR | NR | NR |
| Seneschal et al23 | RA | Etanercept | Psoriasis-like LP | 18 months | NR | NR | NR | NR |
| Verea et a I24 | Crohn’s disease | Infliximab | Psoriasis-like LP | 6 weeks | NR | Yes | Stabilized | Topical steroids |
| Fendrie et al25 | RA | Etanercept | Maculopapular | 1.5 months | NR | Yes | Recovery | Topical and systemic steroids |
| Fendrie et al25 | RA | Adalimumab | Maculopapular | 3 weeks | NR | Yes | Recovery | NR |
| Fendrie et al25 | RA | Lenercept | Maculopapular | 2 months | NR | Yes | Recovery | Topical steroid |
| Beuthien et al4 | RA | Adalimumab | EM-like | 3 months | NR | Yes | Recovery | None |
| Vergara et al16 | RA | Infliximab MTX | EM-like | NR | NR | Switch to etanercept | NR | Switched etanercept; EM-like again |
| Vergara et al16 | RA | Infliximab azathioprine | EM-like | NR | NR | Yes | NR | Topical steroids |
| Vergara et al16 | RA | Infliximab | EM-like | NR | NR | Yes | NR | Topical steroids |
Patient restarted etanercept and LPP progressed; biologic was permanently discontinued.
NR=not reported, RA=rheumatoid arthritis, MTX=methotrexate
CASE REPORT
A 58-year-old woman with a nine-year history of Behcet’s disease was initiated on adalimumab after failing other systemic therapies, including oral corticosteroids, colchicine, dapsone, mycophenolate mofetil, pentoxifylline, cyclosporin rinse, refecoxib, infliximab, and etanercept. The patient was on etanercept for two years. The patient immediately started adalimumab after discontinuing etanercept. After approximately one year of weekly adalimumab injections, the patient presented with a two-month history of progressive, patchy hair loss. On physical examination, she had approximately 10 patches of alopecia in the occipital and parietal regions, representing five percent of the scalp surface. The patches exhibited perifollicular erythema, scale, crust, and scarring. Routine chemistries, complete blood count, C-reactive protein, hepatitis serologies, and fungal culture were all within normal limits.
Horizontal and vertical sections of a scalp punch biopsy revealed a brisk lymphocytic lichenoid infiltrate within the infundibular and isthmic portions of the hair follicles. Also apparent were necrotic keratinocytes and concentric fibrosis. Peribulbar inflammation was absent.
She was diagnosed with LPP and started on topical clobetasol foam and methotrexate 7.5mg/week with folic acid. She had a decrease in perifollicular erythema and stabilization of alopecia after one year. She wished to continue adalimumab despite the development of LPP due to sustained symptomatic control of Behcet’s disease achieved on the biologic.
DISCUSSION
Lichenoid eruptions in association with TNF alpha inhibitors were first reported by Vergara et al in 2002.16 Since then, numerous cases of lichenoid eruptions, including cutaneous and oral LP, maculopapular, LPP, and psoriasis-like LP, have been reported (Table 1). Lichen planopilaris is the most recent cutaneous lichenoid adverse reaction comprising a lymphocytic scarring alopecia that presents as hair loss and perifollicular erythema, which can be seen in association with cutaneous LP. Histopathologically, LPP is characterized by a lymphocytic, lichenoid interface dermatitis of the follicular infundibulum. Lichen planopilaris is a relatively rare cause of scarring alopecia in the United States accounting for approximately 1.15 to 7.59 percent of cases reported in four hair research centers.5,6 Lichen planopilaris is difficult to treat and must be managed appropriately to prevent advancement of the disease and permanent fibrosis to the hair follicles. This report of LPP developing in a patient on adalimumab therapy not only adds to the spectrum of lichenoid disease found in association with this drug family, but also emphasizes the potential for irreversible side effects in select patients. This case also underscores how little we know about the mechanism of action or predictability of lichenoid reactions as the patient described herein had no adverse effects while on etanercept for two years, but developed LPP within 10 months of starting adalimumab therapy. Currently, there is no way to predict these reactions, and paradoxically, TNF alpha inhibitor therapies have been regularly reported as efficacious in many forms of LP.7-9
Erythema multiforme-like (EM) reactions have also been reported to develop in patients on TNF alpha inhibitors. This reaction type deserves mention in this review due to the shared dermatopathologic pattern seen with EM and lichenoid reactions. Development of these patterns after treatment with the same drug class suggests a shared immunologic response.
A variety of mechanisms have been proposed to explain the development of cutaneous adverse effects observed in association with TNF alpha inhibitor therapy. The exact pathophysiology of LPP in association with TNF alpha inhibitors remains elusive. Type I interferon (IFN) is thought to play a role in the chronic inflammation of LP.10 Some authors suggest that the suppression of TNF may lead to the development of opposing inflammatory cytokines, such as IFN, which may activate T cells and dendritic cells.11
TNF has recently been described to have two receptor types with variable roles in the immune system. Faustman et al12 described two TNF receptor pathways: TNF receptor 1 and 2. TNF receptor 1 (TNFR1) is expressed on almost all cells of the body and plays a role in apoptosis while TNF receptor 2 (TNFR2) is confined to the immune system and helps control autoreactive CD4+ and CD8+ T cells. A possible pathway associated with LP may be one of imbalance. Suppression of TNF leaves IFN free to wreak havoc on the inflammatory process. Such an imbalance allows for increased levels of cytotoxic T cells.1 Nonselective TNF inhibition allows an increase in autoreactive, cytotoxic CD8+ T cell activity and may explain the potential for development of LP.2 Studies have examined the inflammatory infiltrate in case series of LP and observed a predominance of CD8+ T lymphocyte populations comprising the infiltrate. CD8+ cells have the ability to autoactivate against keratinocytes and therefore are thought to play a role in the development of LP.13
Recent microarray analysis and knock out mouse models for LPP demonstrate decreased expression of peroxisome proliferator-activated receptor gamma (PPARγ). PPARγ is a transcription factor responsible for lipid homeostasis and downregulation of the inflammatory pathway in many cell types including the pilosebaceous unit. Loss of function of PPARγ is proposed to occur from genetic predisposition and or environmental triggers. Proinflammatory cytokines, such as IFN and TNF, can diminish PPARγ expression.14 Therefore, unopposed IFN may lead to the downregulation of PPARγ and cause LPP to occur in patients on TNF inhibitors. PPARγ agonists, such as thiazolidinediones, may be a potential therapy for LPP. Several case reports utilizing this drug class have shown favorable results.5,15 However, use of these agents in TNF-associated LPP has not been reported.
Figure 1.
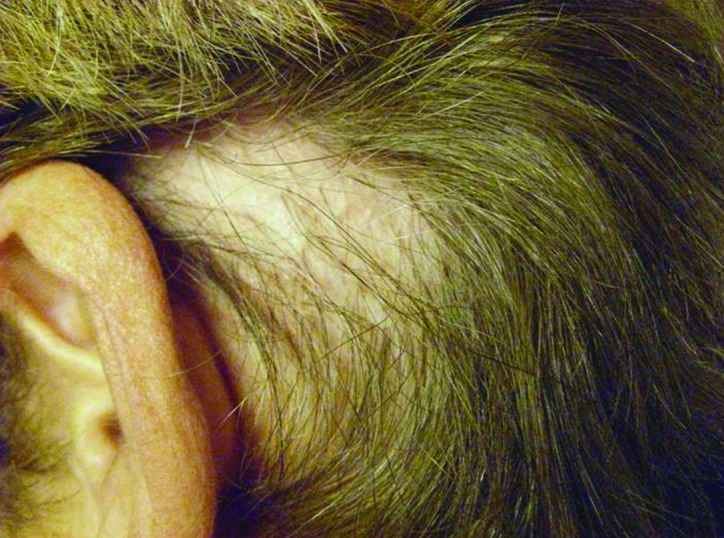
A patch of alopecia of the posterior auricular scalp demonstrating scale crust, scarring, and resolving perifollicular erythema.
Figure 2.
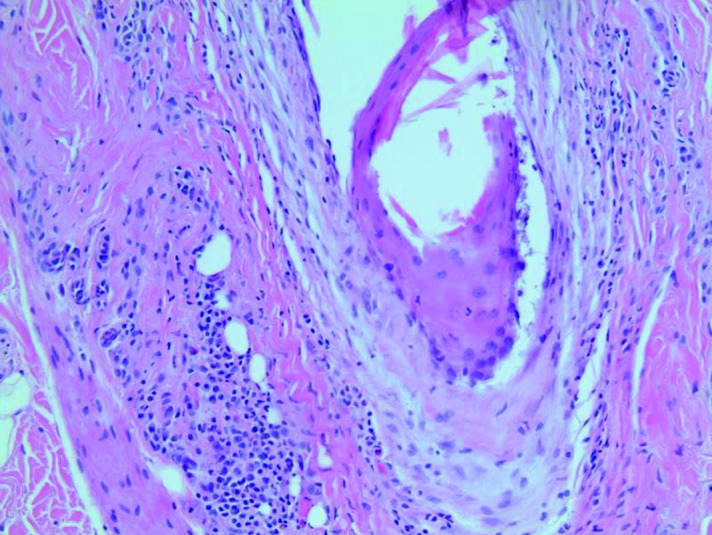
There is concentric fibrosis surrounding the upper follicle with an associated lichenoid infiltrate composed predominantly of lymphocytes.
CONCLUSION
This report of LPP developing in a patient undergoing treatment with adalimumab not only adds to the spectrum of lichenoid diseases found in association with this drug family, but also emphasizes the potential for irreversible side effects in select patients of permanent scarring alopecia. Currently, there is no way to predict these reactions, though TNF inhibitor therapies have been regularly reported as efficacious in many forms of LP. This reaction type may be more likely to occur in both genetically predisposed individuals and in those subjected to unknown environmental exposures. Recent consistent reporting of these reaction types suggests a possible new direction and understanding of lichenoid reactions. The next step in biologic therapy is to design pharmaceuticals with precise targets to prevent adverse reactions. Creation of highly specific binding TNF alpha inhibitors to TNFR1 would allow TNFR2 to regenerate oligodendrocytes and decrease the cytotoxic CD8+ profile, essentially preventing side effects, such as lichenoid reactions and demyelination of the central nervous system.12 Until such a time, understanding the etiology and presentation of these lesions will allow physicians to better educate patients regarding potential side effects and perhaps unfold the pathogenesis of lichenoid reactions.
Footnotes
DISCLOSURE:Drs. McCarty, Basile, and Bair have no conflicts of interest to declare. Dr. Fivenson serves on the advisory board of Amgen, Centrocor, Abbott, Warner Chilcott, Galderma, Novartis, Genentech, and Stiefel/GSK; is a consultant for Biolife; is an investigator for Dermik Amgen, Centrocor, Abbott, Allergan, Ferndale, Graceway, Biolife, Merck, Pfizer, Galderma, Aspreva, Astellas, Novartis, Genentech, Perrigo, Organogenesis, Ligand, Connetics, Clay-Park Labs, Jacobus, Smith & Nephew, Dow Pharma, Therakos, Seragen, ConvaTec, Leo Pharma, and Novum; and is a speaker for Amgen, Abbott, Warner Chilcott, Merck, Astellas, Genentech, and Stiefel/GSK.
REFERENCES
- 1.Asarch A, Gottlieb A, Lee J, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. . J Am Acad, Dermatol. 2009;61:104–111. doi: 10.1016/j.jaad.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Garcovich S, Manco S, Zampetti A, et al. Onset of lichen planopilaris during treatment with etanercept. Br J Dermatol. 2008;158:1161–1163. doi: 10.1111/j.1365-2133.2008.08529.x. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Torres R, Paradela S, Valbuena L, et al. Infliximab- induced lichen planopilaris. Ann Pharmacother. 2010;44:1501–1503. doi: 10.1345/aph.1P079. [DOI] [PubMed] [Google Scholar]
- 4.Beuthien W, Mellinghoff H, von Kempis J. Skin reaction to adalimumab. Arthritis Rheu. 2004;50:1690–1692. doi: 10.1002/art.20155. [DOI] [PubMed] [Google Scholar]
- 5.Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch Dermatol. 2009;145:1363–1366. doi: 10.1001/archdermatol.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochoa B, King L, Jr, Price V. Lichen planopilaris: Annual incidence in four hair referral centers in the United States. J Am Acad Dermatol. 2008;58:352–353. doi: 10.1016/j.jaad.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hollo P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. doi: 10.2340/00015555-1249. 2011 Nov 21. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121. doi: 10.2165/00128071-200708020-00010. [DOI] [PubMed] [Google Scholar]
- 9.Chao T. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325–328. [PubMed] [Google Scholar]
- 10.WenzelJ, Scheler M, Proelss J, et al. Type I interferon-associated cytotoxic inflammation in lichen planus. J Cutaneous Pathol. 2006;33:672–678. doi: 10.1111/j.1600-0560.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 11.Seneschal J, Milpied B, Vergier B, et al. Cytokine imbalance with increased production of interferon-alpha in psoriasiform eruptions associated with antitumour necrosis factor-alpha treatments. Br J Dermatol. 2009;161:1081–1088. doi: 10.1111/j.1365-2133.2009.09329.x. [DOI] [PubMed] [Google Scholar]
- 12.Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nature. 2010;9:482–493. doi: 10.1038/nrd3030. [DOI] [PubMed] [Google Scholar]
- 13.Sugerman P, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142:449–456. doi: 10.1046/j.1365-2133.2000.03355.x. [DOI] [PubMed] [Google Scholar]
- 14.Szeles L, Torocsik D, Nagy L. PPAR gamma in immunity and inflammation: cell types and diseases. Biochim Biophys Acta. 2007;1771:1014–1030. doi: 10.1016/j.bbalip.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Karnik P, Tekeste Z, McCormick T, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergara G, Silvestre J, Betlloch I, et al. Cutaneous drug eruption to infliximab: report of 4 cases with an interface dermatitis pattern. Arch Dermatol. 2002;138:1258–1259. doi: 10.1001/archderm.138.9.1258. [DOI] [PubMed] [Google Scholar]
- 17.Bovenschen H, Kop E, Van De Kerkhof P, Seyger M. Etanercept-induced lichenoid reaction pattern in psoriasis. JDermato Treat. 2006;17:381–383. doi: 10.1080/09546630600967174. [DOI] [PubMed] [Google Scholar]
- 18.Battistella M, Rivet J, Bachelez H, Liote F. Lichen planus associated with etanercept. Br J Dermatol. 2008;158:188–190. doi: 10.1111/j.1365-2133.2007.08258.x. [DOI] [PubMed] [Google Scholar]
- 19.Musumeci M, Lacarrubba F, Micali G. Onset of lichen planus during treatment with etanercept. Am J Clin Dermatol. 2010;11(Suppl l):55–56. doi: 10.2165/1153428-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Moss A, Treister N, Marsee D, et al. Clinical challenges and images in GI Oral lichenoid reaction in a patient with Crohn’s disease receiving infliximab. Gastroenterology. 2007;132:488,829. doi: 10.1053/j.gastro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 21.De Simone C, Caldarola G, D’Agostino M, et al. Lichenoid reaction induced by adalimumab. J Eur Acad Dermatol Venereol. 2008;22:626–627. doi: 10.1111/j.1468-3083.2007.02413.x. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi N, Orlow S. Lichen planopilaris noted during etanercept therapy in a child with severe psoriasis. Pediatr Dermatol. 2009;26:118. doi: 10.1111/j.1525-1470.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 23.Seneschal J, Lepreux S, Bouyssou-Gauthier M, et al. Psoriasiform drug eruptions under anti-TNF treatment of arthritis are not true psoriasis. Acta Derm Venereol. 2007;87:77–80. doi: 10.2340/00015555-0193. [DOI] [PubMed] [Google Scholar]
- 24.Verea MM, Del Pozo J, Yebra-Pimentel M, et al. Psoriasiform eruption induced by infliximab. Ann Pharmacother. 2004;38:54–57. doi: 10.1345/aph.1C477. [DOI] [PubMed] [Google Scholar]
- 25.Flendrie M, Vissers W, Creemers M, et al. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2005;7:R666–676. doi: 10.1186/ar1724. [DOI] [PMC free article] [PubMed] [Google Scholar]


