Abstract
Adoptive transfer of adult-seropositive, cytomegalovirus (CMV)-specific T-cells can effectively restore antiviral immunity after transplantation. Lack of CMV-specific memory T-cells in blood from CMV-seronegative adult and cord blood (CB) donors restricts the availability of donor-derived virus-specific T-cells for immunoprophylaxis. Here we demonstrate the feasibility of naïve-donor-derived CMV-specific T-cell therapy for transplant recipients. Primed naïve T-cells recognized only atypical epitopes and with a similar avidity to CMV-seropositive-derived T-cells recognizing typical epitopes, but T-cells from CMV-seropositive donors recognizing atypical epitopes had a lower avidity suggesting the loss of high-avidity T-cells over time. Clonotypic analysis revealed T-cells recognizing atypical CMVpp65 epitopes in the peripheral blood of recipients of CB grafts who did not develop CMV. T-cell receptors from atypical epitopes were most common in unmanipulated CB units explaining why these T-cells expanded. When infused to recipients, naïve donor-derived virus specific T-cells that recognized atypical epitopes were associated with prolonged periods of CMV-free survival and complete remission.
INTRODUCTION
Adoptive immunotherapy is emerging as an attractive alternative to chemotherapy for both viral infections (1–5) and relapse (6–8) developing after hematopoietic stem cell transplantation (HSCT). Most, if not all, antigen-specific T-cells adoptively transferred to humans are derived from memory T-cell populations (9); hence, virus-specific T-cells –although largely effective- have not been available for patients undergoing HSCT from virus naïve-donor sources including cytomegalovirus (CMV)-seronegative or umbilical cord blood (CB) donors (10, 11). Although preclinical data have been reported for human antigen-specific T-cells generated from naïve T-cells, with the exception of our previous study from CB (12), they are mostly restricted to Epstein-Barr virus (EBV)-specific T-cells, or mitogen-stimulated T-cells bearing exogenous T-cell receptors (TCRs) (13, 14), and therefore the naïve T-cell response to CMV, including epitope usage, avidity, and polyclonality, has not been addressed.
CMV-specific T-cells were first described as those able to recognize the immunodominant antigen immediate early-1 (IE-1)(15), but later reports emphasized the importance of T-cells that target a tegument phosphoprotein of 65 kDa (pp65)(16, 17). The original epitope identification studies focused on NLVPMVATV (hereafter NLV), a human leukocyte antigen (HLA)-A2-restricted epitope within pp65(16) that we define as a “typical” epitope because of its common detection in HLA A2-positive donors. Use of more advanced techniques, such as overlapping peptide pools, lentiviral vectors containing a chimeric CMV-pp65/IE-1 protein, and bioinformatics, have allowed the identification of other less-common (“atypical”) epitopes targeted by CMV-specific T-cells (18–20) and in murine models, subdominant epitopes have been shown to be protective (21). It should be stressed that all of these epitopes—both typical and atypical—have been identified in memory T-cells. Whether the memory T-cell repertoire mirrors that of naïve T-cells in vivo remains to be determined.
HIV-seronegative women who are resistant to infection recognize epitopes that differ from those recognized by HIV-seropositive woman who are not protected from HIV despite a CD8+ HIV-specific T-cell response (22), suggesting a disparity between the immunodominant, persisting epitopes and the initial, protective epitopes, presumably generated from naïve T-cells.
We have developed a protocol enabling the activation and expansion of multivirus (CMV, EBV, and adenovirus)-specific T-cells from CB, a source of naïve T-cells. (12, 23) In previous studies of T-cell responses to CMVpp65 from seropositive (CMVpos) donors, most HLA-A2-donors recognized the typical NLV epitope (24). By contrast, the HLA-A2-restricted pp65-specific T-cell lines generated from CB did not recognize NLV but only atypical epitopes of CMVpp65 (12). We hypothesized that naïve T-cells from CMV-seronegative (CMVneg) adult donors –would also recognize atypical epitopes of CMVpp65. If so, it might be possible to increase the availability of CMV-specific T-cells for patients at the greatest risk of CMV disease: CMVpos recipients receiving grafts from CMVneg donors. We therefore used CMV-seronegative donors as a way to compare the epitope specificity of T-cells expanded from the naïve T-cells of CMVneg donors and CB to CMVpos donors.
Here we demonstrate not only the feasibility of generating pp65-specific T-cells from CMVneg donors but also the ability of T-cells of naïve origin to recognize atypical epitopes of pp65, supporting our working hypothesis. We further show that virus-specific T-cells derived from a naïve T-cell population may be protective in vivo despite their atypical epitope repertoire.
RESULTS
CMVpp65-specific T-cells can be expanded from CMVneg donors
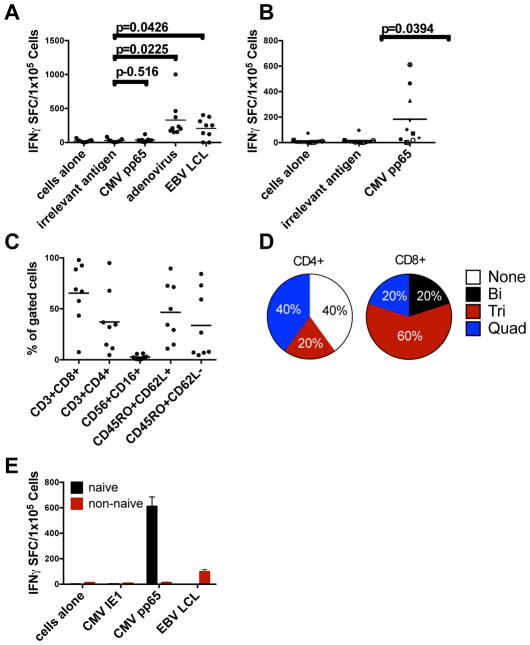
Using dendritic cells (DCs) and EBV-positive lymphoblastoid cell lines (EBV-LCLs) expressing CMVpp65 from an adenoviral vector (Ad5f35pp65) as sources of antigen and antigen presenting cells, we previously showed that we could expand CMV-, EBV-, and adenovirus-specific T-cells from the naïve T-cells of CB (12, 23). To test whether CMV-specific T-cells could be expanded from CMVneg donors, we used the same method of T-cell expansion. The resultant T-cells recognized EBV and adenovirus, but CMVpp65-specific T-cells were below the limits of detection (Figure 1A). To limit the expansion of memory T-cells specific for adenovirus and EBV, we first selected for CD45RA+ cells from the peripheral blood mononuclear cells (PBMCs), and as a source of antigen for the first stimulation we used DCs pulsed with an overlapping peptide library (Pepmix) spanning the entire amino acid sequence of CMVpp65. For the second and subsequent stimulations, we used Pepmix-pulsed EBV-LCLs. With this revised method together with the cytokines IL-7, IL-12, and IL-15 as before, we were able to expand CMVpp65-specific T-cells in a reproducible manner from 8 of 10 CMVneg adult donors as detected by IFN-γ ELISPOT assay. (Figure 1B) Most (mean 65%, range: 8–98%) of these T-cells were CD8+ and comprised both central and effector memory cells with only a minimal number of NK cells (CD3− CD16+ CD56+) (mean: 3%, range: 1–6%) (Figure 1C). Because recent studies suggest that polyfunctional antigen-specific T-cells have superior in vivo function (25, 26), we evaluated the polyfunctionality of five donor T-cell lines by staining for IFN-γ, GM-CSF, IL-2, TNF-α, and CD40L, and found that most of the CD8+ T-cells released at least three of the five cytokines or activation markers tested (Figure 1D, Figure S2). To demonstrate that the T-cells expanded from CMVneg donors were derived from the naïve population, we sorted naïve donor PBMCs for CD45RA+ CCR7+ or CD45RA-CCR7- by flow cytometry, followed by expansion with DC, EBV-LCL and CMV Pepmixes. As shown in Figure 1E, the CMVpp65-specific T-cells were indeed derived from the naïve population and not the memory (non-naïve) population.
Figure 1. CMV-specific T-cells expanded from CMV-seronegative donors.
(A) Specificity of EBV-, CMV-, and adenovirus-specific T-cells from CMV-seronegative donors over 16 to 23 days as shown by an IFN-γ ELISPOT assay. (B) CMV specificity of T-cells expanded by enriching for naïve T-cells and using overlapping peptides of CMVpp65. Lines were considered positive if they were >5 spots above the negative control with confirmatory individual peptide pools when possible. (C) Phenotype of cells shown in Panel B. (D) Polyfunctionality of five of the responding T-cell lines as determined by intracellular or surface staining. Positive cells were counted as >2% above background staining. The markers tested were IFN-γ, TNF-α, GM-CSF, CD40L and IL-2. (E) Derivation of CMVpp65-specific T-cells from the naïve population. Before stimulation, CD3+ T-cells were sorted for CD45RA+/CCR7+ cells (naïve) and CD45RA-/CCR7- (“non-naïve”) cells, and were then stimulated as indicated and tested for specificity to CMVpp65 and EBV-LCLs. T-cells derived from the naïve fraction are shown in black and the non-naïve fraction in grey. Error bars represent the standard deviation from the mean. *P<0.005 versus non-naïve by two-tailed t-test. In panels A–C, each symbol represents a T-cell line taken from 10 CMVneg donors and the grey bars indicate the mean.
CMVpp65-specific T-cells from the naïve T-cell population recognize atypical epitopes
We previously showed (12), that CMVpp65-specific T-cells from CB did not recognize the expected typical peptides such as the HLA-A2-restricted peptide NLVPMVATV. To determine whether this result was unique to NLV, we compared the peptide repertoires of pp65-specific T-cells from HLA-A2 adult CMVneg and CMVpos donors using CMV peptide pools. In this approach, the pp65 protein is dissected into 20mer peptides overlapping by 15 amino acids, which are then divided into 22 pools such that each peptide is uniquely present in two pools allowing us to map the specificities of the T-cell line. Using this method, T-cells from three of three CMVpos donors recognized pools 2 and 21 (Figure 2A), corresponding to the well-characterized epitope NLVPMVATV. By contrast, the pp65-specific T-cells from HLA-A2 CMVneg donors failed to respond to the 9mer NLVPMVATV; instead, many of these cells recognized alternative peptides such as those found in pools 7/8 and 13, corresponding to the 20mer DTPVLPHETRLLQTGIHVRV (Figure 2B). To fine map this epitope, we tested the reactive T-cells for their recognition of all 9mers contained within the 20mer DTPVLPHETRLLQTGIHVRV, showing that the 9mer recognized by the HLA-A2+ pp65-specific T-cells was LQTGIHVRV (hereafter LQT; Figure S1A). Besides LQT, pp65-specific T-cells from HLA-A2+ naive donors also recognized the atypical epitope MLNIPSINV, while those from an HLA- B35+ donor recognized the novel HLA-B35-restricted epitope DANDIYRIF as determined by fine mapping of the 20mer DANDIYRIFAELEGVWQPAA (Figure S1B). Epitope recognition by all of the T-cell lines derived from CMVneg donors is shown in Table S1.
Figure 2. Comparison of recognition of typical and atypical epitopes of CMVpp65 by CMV-seropositive versus seronegative donors.
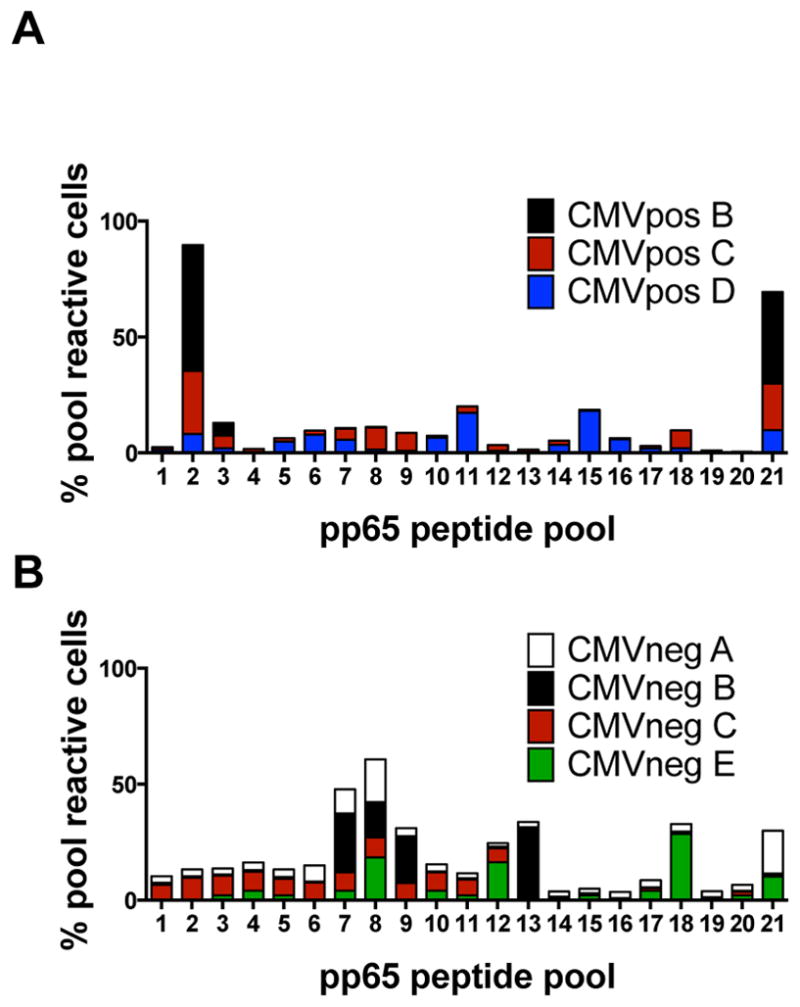
(A) Epitope specificity for the three CMV-seropositive HLA-A2+ donors, based on overlapping peptide pools of CMVpp65. The pp65 protein was divided into 20 amino acids overlapping by 11 amino acids. The peptides were then distributed into a total of 22 pools, with each peptide present in two pools. Pools 2 and 21 contain the 20mer peptide 98, harboring the typical HLA-A2-restricted epitope NLVPMVATV. The percentages of cells recognizing each pool from each of the three donors are shown. This was calculated by totaling the total number of spots across all pools and dividing by spots per pool (B) Peptide-pool recognition by four CMV-seronegative HLA-A2+ donors.
T-cells recognizing atypical epitopes are detectable in CMVpos donors
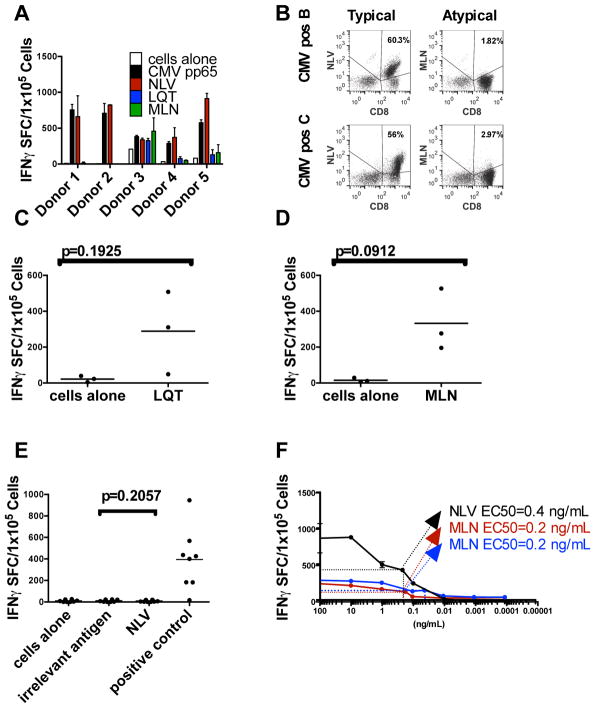
Given that the atypical epitopes we identified have not been identified previously or have not been commonly reported, we considered that T-cells specific for atypical epitopes are not naturally expanded in response to CMV infection. To examine this possibility, we expanded T-cells from seropositive donors by stimulation with the entire pp65 antigen using our standard T-cell expansion protocol (1, 24). As shown in Figure 3A, B, the response of T-cells from five CMVpos HLA-A2 donors was largely directed against NLV, but a small proportion of the population recognized MLN or LQT, suggesting that such circulating T-cells are infrequent and likely out-competed during the ex vivo expansion step by more frequent clones that recognize NLV. To determine if the T-cells recognizing the atypical epitopes from HLA-A2+ CMVpos donors could be amplified, we used DCs pulsed with only the atypical peptide, resulting in the expansion of MLN- and LQT-specific T-cells without the requirement for a naïve T-cell isolation step (Figure 3C, D). Knowing that we could force the expansion of atypical epitope-specific T-cells from CMVpos donors, we next asked whether we could force the expansion of NLV-specific T-cells from HLA A2+ CMV naïve sources. As shown in Figure 3E, we were unable to generate such cells from any of the eight CB and CMVneg donor lines.
Figure 3. Recognition of typical and atypical epitopes by CMV-seropositive donors.
(A) Virus-specific T-cells from five CMV-seropositive donors were expanded against the entire CMVpp65 antigen. After three stimulations, they were tested for recognition of atypical (LQT, MLN) and typical (NLV) epitopes as determined by the IFN-γ ELISPOT assay and (B) pentamer analysis. (C and D) T-cells from CMVpos donors were stimulated three times with DCs pulsed with the indicated peptide. The number of cells that secrete IFN-γ in response to stimulation with LQT (C) or MLN (D) peptide is shown on approximately day 21 of culture. Gray bars indicate mean n values. (E) Failure to generate NLV-specific T-cells from CMV-seronegative donors (grey diamonds, n=3) or CB (black diamonds, n=5). T-cells were stimulated with DCs pulsed with the peptide NLV in the absence of other peptides. After three stimulations, the resulting cells were tested for their ability to recognize NLV. Grey bars indicate mean values. SEB, Staphylococcal enterotoxin B. (F) T-cells from CMV-seropositive donors were stimulated as above with the peptide NLV and tested by limiting dilution for the mean one-half effective concentration (EC50). Error bars indicate the standard deviation from the mean of triplicate wells; a representative result is shown (see table 1 for the avidity of other lines). (G) T-cells from CMV-seropositive donors were stimulated with the atypical peptide MLN and tested by limiting dilution to determine the EC50. (H) T-cells from CB and CMV-seronegative donors were stimulated with the atypical peptide MLN and tested by limiting dilution to determine the EC50. Values shown in panels EG are the numbers of spots above background.
Because T-cells specific for the atypical epitopes MLN and LQT could be expanded ex vivo, we considered whether the avidities of these T-cell receptors for their respective peptide/MHC complexes might be lower than those recognizing the previously identified typical epitopes. To explore this idea, we used limiting dilutions of peptides in IFN-γ release assays. For NLV-specific T-cells from four CMVpos donors, the mean peptide concentration needed to induce a half-maximum γIFN response (EC50) was 0.23 ng/ml (range: 0.03–0.4 ng/mL) (Figure 3F and Table 1), as previously reported (27). By contrast, CMVpos donor-derived T-cells that recognized the atypical epitope MLN showed a substantially weaker avidity: mean of 4.8 ng/ml (range: 0.4–11 ng/ml) (Figure 3F and Table 1). The mean values for T-cells derived from CMVneg donors or CB specific for MLN were 0.45 (range: 0.3–0.6) and 0.52 ng/ml (range: 0.2–0.8 ng/mL) respectively (Figure 3F and Table 1), indicating a 30-fold stronger avidity than seen with CMVpos donor-derived T-cells (P=0.019679; paired t test on paired samples of log-transformed data) and only slightly weaker than observed for the typical epitope NLV, suggesting that T-cells recognizing atypical epitopes may indeed be protective but do not persist.
Table 1.
Avidity of CMVpos, CMVneg, and CB T-cells recognizing typical and atypical epitopes
| T-cell source | NLV (mean EC50 ng/ml) | MLN (mean EC50 ng/ml) |
|---|---|---|
| CMVPos A | 0.4 | 3.0 |
| CMVpos E | 0.08 | Not determined |
| CMVpos B | 0.03 | 0.4 |
| CMVpos C | 0.4 | 11 |
| Mean | 0.23* | 4.8* |
| CMVneg H | 0.3 | |
| CMVneg C | 0.6 | |
| Mean | 0.45 | |
| CB line 1 | 0.7 | |
| CB line 2 | 0.2 | |
| CB line 3 | 0.8 | |
| CB line 4 | 0.5 | |
| CB line 5 | 0.4 | |
| Mean | 0.52 |
indicates P=0.019679 of paired t test of log-transformed values from CMVpos A, B, C.
Live CMV does not stimulate T-cells specific for typical epitopes from CB or CMVneg donors
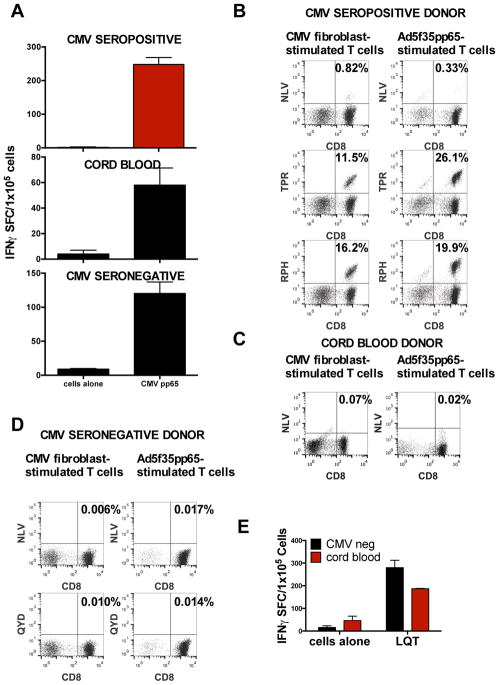
A possible cause of the expansion of T-cells recognizing atypical epitopes in this study is that the epitopes presented to naive T-cells under in vitro priming conditions (including overlapping peptide libraries or the Ad5f35pp65 vector-transduced DCs and LCLs) differ from those prevalent during in vivo priming by live CMV. Thus, to test whether CMV-infected antigen-presenting cells (APCs) would restore the typical pattern of epitope recognition, we infected HLA-A2+ allogeneic fibroblasts with CMV AD169 and used them together with DCs to stimulate the T-cells. As shown in Figure 4A, this modification resulted in pp65-specific T-cells from CB as well as CMVneg and CMVpos donors. The T-cells from CMVpos donors recognized typical epitopes of pp65 (Figure 4B), while stimulation with CMV-infected fibroblasts did not restore typical epitope recognition by T-cells from either CMVneg donors (Figure 4C) or CB (Figure 4D). Instead, these CMVpp65-specific T-cells continued to recognize atypical epitopes (Figure 4E).
Figure 4. Epitope recognition by T-cells stimulated with live CMV.
(A) Comparison of response to CMVpp65 by T-cells from HLA-A2+ CMV-seronegative donors, CMV-seropositive donors, and CB. The T-cells were stimulated with CMV-infected HLA-A2+ fibroblasts that were cocultured with DCs and then tested with an IFN-γ ELISPOT assay. Mean ± SD values from triplicate wells are shown. (B) Typical epitope (HLA-A2-restricted NLV, B7-restricted TPR and RPH, or A24-restricted QYD) recognition of cells from a CMV-seropositive donor line stimulated with CMV-infected fibroblasts or an adenoviral vector expressing CMVpp65 as described. (C) Typical epitope recognition by CB T-cells stimulated with CMV-infected fibroblasts or the adenoviral pp65 vector. (D) Typical epitope recognition by CMV-seronegative T-cells stimulated with CMV-infected fibroblasts or the adenoviral pp65 vector. (E) Atypical epitope recognition by CB-derived T-cells generated with CMV-infected fibroblasts. Pool 13 contained the atypical epitope LQT.
T-cells recognizing atypical epitopes are polyclonal and exhibit TCR diversity
The marked disparity in the T-cell avidity for atypical epitopes between naïve and CMVpos donors (Figure 3) suggested a difference in clonality. We therefore sorted T-cells from CMVpos donors that were specific for NLV and MLN, as well as those specific for MLN from CB and CMVneg donors based on pentamer positivity, extracted RNA from the sorted cells, and sequenced the TCRs. As shown in Table S2, CMVpos donor-derived T-cells that recognized NLV were oligoclonal, while T-cells specific for MLN, regardless of donor origin, were polyclonal (P=0.02, Fisher’s Exact Test), suggesting a possible difference when priming naïve T-cells.
Since CB-derived T-cells represent an immature population of T-cells that have not undergone clonal expansion in the presence of antigen, and hence are representative of the starting naïve T-cell population in vivo, we also considered that the TCR repertoire in CB and the frequency of atypical epitope recognition might be increased as compared to TCRs identified in mature T-cells that recognize NLV. To pursue this hypothesis, we isolated HLA-A2+ cells from five unmanipulated HLA-A2+ CB units, extracted the DNA from these units, and then compared the TCR sequences with those identified in Table S2 that represent T-cells able to recognize MLN, LQT, or NLV. Five of the five CB units had detectable typical and/or atypical TCRs in the unmanipulated CB unit (Table S3); however, of the 22 TCRs specific for NLV, only 1 was found in any of the CB units sequenced, whereas five different TCRs specific for MLN or LQT were identified in the HLA-A2+ CB units. These results, which underscore the very low frequency of TCRs for the NLV epitope, help to explain the difficulty of generating T-cells specific for canonical CMVpp65 epitopes from naïve sources.
Virus-specific T-cells derived from a naïve source are safe and may be protective in vivo
To determine whether T-cells recognizing atypical epitopes are protective, we first showed that T-cells specific for either MLN or NLV could prevent the dissemination of CMV from human fibroblasts (Table S4). We next evaluated MLN and LQT-specific T-cells in PBMC samples obtained at 3, 6, and 12 months after CB Transplantation (CBT) from 22 HLA-A02+ patients. As shown in Table 2, in 7 of these patients, T-cell receptors recognizing either MLN or LQT epitopes were detected. Five patients demonstrated TCRs recognizing the NLV epitope, while three patients recognized both typical and atypical epitopes. One transplant recipient whose T-cells recognized MLN did not develop CMV reactivation despite the absence of detectable T-cells recognizing typical epitopes, which are generally considered protective in this setting (28).
Table 2.
Identification of TCR sequences specific for typical and atypical epitopes in Cord Blood Transplant Recipients
| CB Transplant Recipients (% TCRs) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CMVneg | CMVpos | |||||||||
| 1 | 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| CMV reactivation? | No | Yes | No | No | No | No | Yes | No | Yes | |
| Peptide | CDR3 | |||||||||
| NLV | CASSLDRVTGELFF | .00578 | ||||||||
| CASSPGTGREQFF | 0.00536* | |||||||||
| CASSLTNEQFF | 0.00486 | 0.01544 | ||||||||
| CASSLTSEQFF | 0.04603* | 0.00448 | ||||||||
| CASSLAERSYEQYF | ||||||||||
| CASSSVNEQFF | 0.33467# | |||||||||
| CASSLAPGATNEKLFF | 0.00026 | |||||||||
| MLN | CASSFGVNTEAFF | 0.0053 | 0.00614 | |||||||
| CASSLGLNYEQYF | 0.00027 | |||||||||
| CSARDRDRGYEQYF | 0.00055 | |||||||||
| CASRAVSTDTQYF | ||||||||||
| CATSPTANTEAFF | ||||||||||
| CASSPSGYNEQFF | 0.00921# | 0.00382 | ||||||||
| CASSLDLGASTDTQYF | 0.00263 | |||||||||
| CASSFRGDTEAFF | 0.0015 | |||||||||
| LQT | CASSPPGGSGNTIYF | 0.00127 | 0.00216 | |||||||
| Time points Observed | 12 mo | 12 mo | *3mo #12 mo |
12 mo | *3+12 mo #3+6+12 mos |
6 mo | 12 mo | 12 mo | 12 mo | |
| Time points Not Observed | 3 mo | Only time pt | 6 mos | 3 or 6 mo | 3 mo | 3 mo | 3 mo | 3 or 6 mo | 6 mo | |
CMVneg, CMV-seronegative recipient. CMVpos, CMV-seropositive recipient. CDR3, complimentarity determining region 3.
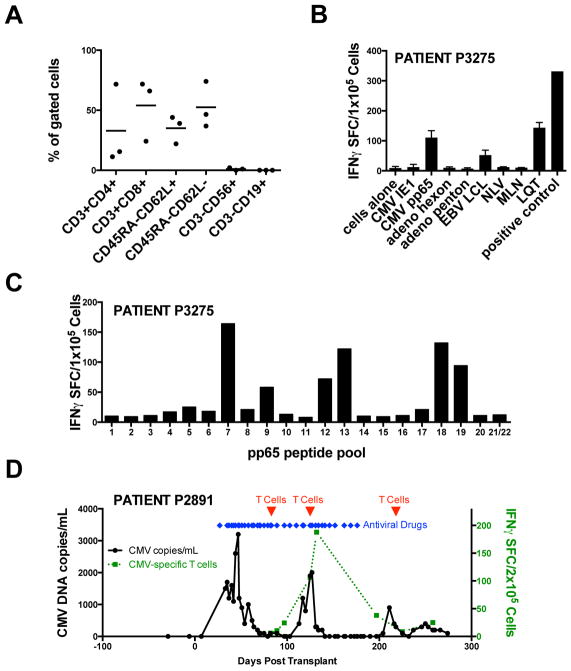
In more rigorous testing we transferred multivirus-specific T-cells that had been generated from the 20% fraction of CB units into a different set of patients that had been transplanted with the 80% fraction. The infused T-cell lines had a predominantly effector memory phenotype; ELISPOT and/or pentamer staining of T-cells from HLA A2+ donor P3275 confirmed specificity for the atypical CMVpp65 epitope LQT but not the typical epitope NLV (Figures 5A–C). Three consecutive patients (aged 1–5 years) received CB-derived multivirus-specific T cells between 63 and 85 days post-transplantation at doses ranging from 5×106/m2 to 1×107/m2. Patients received CB grafts from 5/6 or 6/6 donors for Fanconi Anemia, Severe Combined Immunodeficiency (SCID), or Acute Lymphoblastic Leukemia (ALL) after receiving myeloblative (n=1) or submyeloablative (n=2) conditioning regimens (Table 3). After T cell infusion, there were no episodes of infusion-related toxicity or graft versus host disease (GvHD) (Table 3). Two of these patients, including one whose multivirus-specific T-cell line comprised T-cells specific for the atypical CMVpp65 epitope LQT (Figure 5B, C), remained free of CMV as well as EBV and adenovirus at more than 2 years post CBT following infusion of CB-derived T-cells. The third patient was on Foscarnet at the time of T-cell infusion and was transiently positive for CMV in the blood shortly after infusion #1. Foscarnet was discontinued 4 weeks post T-cells but the CMV reactivated soon after and he was treated with a second dose of CTL and of antiviral pharmacotherapy was reinitiated (Foscarnet followed by Ganciclovir). As shown in Figure 5D, the rise in CMV-specific T-cells in the peripheral blood began around the time of the first T-cell infusion and was observed at the time when the viral load reduced from 1900 to zero copies/ml. Despite clearance of the CMV viremia within 16 days of T-cell therapy, antiviral pharmacotherapy was continued for an additional 4 weeks followed by a subsequent reactivation after it was stopped. After administering a third dose of CTL, the virus cleared without requiring additional pharmacotherapy. Three months after the first T-cell infusion, this patient was also antigen positive for adenovirus in his stool associated with diarrhea, which resolved spontaneously without additional antiviral therapy. Patient PBMCs were also analyzed for pp65 specificity by peptide pool analysis and for the detection of T-cells recognizing atypical CMV epitopes when possible. However, the CMV response was barely above the limits of detection of the ELISPOT assay in recipients who did not have CMV-reactivation. Unfortunately, patient P2891, who did reactivate CMV, received a CB-derived T-cell line recognizing unknown CMV epitopes (Figure S3). However, TCR sequencing was performed on the infused T-cell line and the patient PBMCs obtained pre infusion and at 6 months post T-cell infusion. Unique TCRs which were detected in the infused T-cell product but not in patient PBMCs obtained pre infusion were detected in patient PBMCs collected 6 months post infusion as shown in Figure S4.
Figure 5. CB-derived T-cells for clinical use.
(A) Phenotype of three CB-derived T-cell lines manufactured from the 20% fraction of a clinical CB unit. (B) Reactivity of the CB line infused in to patient P3275 to viral antigens and epitopes of pp65 including the typical epitope NLV and the atypical epitope LQT. The response to LQT was also confirmed by pentamer analysis. (D) CMV load of P2891 (left) in relation to the number of reactive cells from the peripheral blood (right). The timing of T-cell infusions is indicated by arrows.
Table 3.
Characteristics of CB transplant recipients.
| P2891 | P3010 | P3275 | |
|---|---|---|---|
| Sex | M | M | M |
| Age | 5 years | 2 years | 1 year |
| Disease | Fanconi Anemia | ALL | SCID |
| Donor HLA | A*23:01,30:01; B*58:01,57:01; C*07:18,07:01; DRB1*15:03,15:02; DQB1*08:02,06:01 | A*02:AGA,33:01; B*07:ANVB,14:02; C*05:01,08:02; DRB1*03:01,15:01; DQB1*02:01,06:02 | A*01:BMMP,02:01; B*08:01:01,39:05; C*07:WTR,07:WCP; DRB1: 03:01,04:07:01; DQB1: 02:01:01,03:02:01 |
| HLA match | 5/6 | 5/6 | 6/6 |
| Conditioning Regimen | Flu/Cy/TBI | Flu/Cy/TBI | Bu/Cy/Flu |
| GvHD prophylaxis | CSA, MMF | CSA | Pred, MMF |
| Immune suppression at time of T cell infusion | CSA | CSA | Pred, MMF |
| Recipient CMV serostatus | Positive | Positive | Not Determined but PCR negative |
| Viral Infections | CMV, Adenovirus | None | None |
| Cell Dose | 5×106/m2 | 5×106/m2 | 1×107/m2 |
| Concurrent antiviral drugs | Foscarnet → ganciclovir | None | None |
| Viral Outcome | CMV reactivation and Adenovirus infection resolved | No viral reactivations | No viral reactivations |
| Cell dose | 5×106 cells/m2 | 5×106 cells/m2 | 1×107 cells/m2 |
| Duration of cell culture | 57 days | 50 days | 55 days |
SCID, Severe combined immunodeficiency; ALL, Acute Lymphoblastic Leukemia; CSA, Cyclosporin A; MMF, mycophenolate mofetil, Pred, Prednisone; Bu, Busulfan; Cy, Cytoxan; Flu, Fludarabine; TBI, Total Body Irradiation
DISCUSSION
The objective of this study was to test the ability of T-cells derived from naïve populations to recognize atypical epitopes of CMVpp65. After expanding CMV-specific T cells from two naïve sources (CB and CMVneg donors), we established their suitability for clinical use in a series of experiments designed to elucidate the epitope-specific acitivity, clonality, and TCR diversity of these novel cells. The safety and efficacy of these naïve-derived T-cells was assessed in three consecutive patients who had undergone CB transplantation and were at high risk for CMV infection. One caveat with this approach is that the efficacy of the naïve-derived T-cells cannot be irrefutably determined with such a small sample size. However, the study was designed as a phase 1 pilot study to evaluate the safety of the CB-derived T-cells and was not designed to test efficacy. An additional limitation of our approach is the relatively limited number of unmanipulated HLA-A2+ cord blood samples that could be used to detect atypical and typical epitopes. Due to the high cost of TCR sequencing, we were limited to only 5 CB units and hence the results were too small to determine the significance.
Here we show that CMV-specific T-cells can be generated from the naïve T-cell populations of CMVneg donors in a good manufacturing practices (GMP)-compliant manner. The presence of memory T-cells specific for other viral antigens, such as adenovirus, limited the expansion and detection of CMVpp65-specific T-cells from the naïve population in adult CMVneg donors, but removing the adenoviral antigens and isolating the naïve T-cells allowed the specific expansion of pp65-specific T-cells. When tested for epitope specificity, the naïve T-cells from CMVneg donors, like those from CB recognized only atypical epitopes of pp65, while T-cells from each of three HLA-A2+ CMVpos donors recognized the typical HLA-A2-restricted epitope NLV. Of the atypical epitopes identified in this study, at least two (DAN and LQT) have not previously been reported while MLN,(29) though reported previously, is nonetheless atypical. Moreover, the T-cells recognizing atypical CMV epitopes appear to protect against CMV in vivo as demonstrated by (i) the recovery of T-cells specific for atypical CMVpp65 epitopes in patients who do not have CMV reactivation and (ii) the successful transfer of naïve cord blood or CMVneg donor-derived CMV-specific T-cells to prevent or treat infections in patients undergoing CBT. T-cells recognizing atypical CMV epitopes are detectable in vivo in CBT recipients in a similar fraction of patients and at similar frequencies as T-cells recognizing typical epitopes and persist up to a year after transplant.
Aside from our previous work with cord blood T-cells, the only other report describing the reactivation of CMV-specific T-cells from naïve populations in adult donors was described by Falkenburg et al (30), who showed that primary CMV-specific responses can be elicited from the naïve donor T-cell repertoire, although the extensive manipulation required makes translation of this strategy to the clinic problematic. Further, in this study CD45RO cells were depleted and non-depleted T-cells were stimulated with single peptides (including NLV), followed by an enrichment of activated cells after the second stimulation using CD137 immunomagnetic selection. In some cases, cells were further enriched after subsequent stimulations using immunomagnetic tetramer sorting. Given the extensive manipulation needed to expand these cells, the Falkenburg study corroborates our finding that NLV-specific T-cells are at a low frequency prior to exposure to CMV, and in our system the naïve T-cells were generated in competition with all pp65 peptides, and could be generated in the presence of naturally infected APCs. It remains to be tested whether T-cells targeting atypical epitopes would require such extensive manipulations in the system tested above.
A pivotal question at the outset of our study was whether T-cells specific for atypical epitopes circulate in the peripheral blood of CMVpos donors. Even after T-cells from CMVpos donors were expanded with the entire CMVpp65 antigen, the frequency of such T-cells remained low. Yet, by using only a single atypical peptide, such as LQT, we were able to expand atypical epitope-specific T-cells from both the naïve and memory T-cell populations. This result stands in marked contrast to our unsuccessful attempts to expand NLV-specific T-cells from HLA A2+ cord blood or CMVneg donors (31).
What could account for the different epitope recognition patterns of naïve versus memory T-cells? (i) Fibroblasts or epithelial cells (not DCs) are typically the first cells infected by CMV (32) and therefore would be expected to present different antigens than do DCs, (ii) CMV expresses at least 15 proteins that modulate the immune response to the virus and the peptides presented by the APC (33, 34), so that in the absence of these immunomodulatory factors, naïve T-cells might recognize different epitopes of CMVpp65, and (iii) T-cells recognizing atypical epitopes are more abundant at the time of primary infection but are replaced by T-cells recognizing typical epitopes. To pursue possibilities (i) and (ii) we generated CMV-specific T-cells using live CMV-infected fibroblasts cocultured with DCs as APCs. This modification did not lead to the preferential activation of typical epitopes by naïve T-cells and these APCs were also able to activate T-cells specific for atypical epitopes indicating that these epitopes are naturally processed and presented during infection. To address the third possibility, we searched for known NLV, MLN, and LQT TCRs in 5 HLA-A2 cord blood units. Here we showed for the first time that TCRs recognizing atypical epitopes are approximately 27-fold more common than NLV-specific clones in unmanipulated cord blood units. This finding suggests that the initial naïve T-cell response to CMV is a stochastic response where T-cells of similar avidities but different precursor frequencies compete. Soon after, these T-cells are overwhelmed by the typical epitope responses, like those from NLV. Alternatively, high avidity T-cells initially responding to atypical epitopes may undergo exhaustion, allowing for the expansion and detection of typical epitopes.
In other disease models, notably those driven by simian immunodeficiency virus (SIV), EBV and HIV, naïve T-cells have been reported to recognize different epitopes (and even antigens) than do memory T-cells (22, 35, 36). The HLA-A*02-restricted HIV peptide SLYNTVATL is found in chronically infected individuals but not in those with primary infection (22). In an analysis of EBV infection, T-cells recognizing lytic epitopes were always present and dominant during primary infection but decreased over time, whereas T-cells recognizing the latent peptides were at a low frequency during the primary infection but then increased at later times (37, 38). Indeed, clonotypic analysis of these cells showed that the original T-cells were replaced by different T-cells over time, leading us to believe that this is possible during CMV infection as well. Finally, our results add clinical perspective to a recent report (39) showing that in an SIV model, Rhesus (Rh) CMV vectors expressing the simian immunodeficiency viral gene elicit SIV-specific CD8+ T-cells that do not recognize canonical epitopes and instead target unusual, diverse and highly promiscuous epitopes, but only when the RhCMV gene Rh189 (US11) is present, suggested that CMV may be responsible for the atypical epitope recognition. Although we were unable to detect typical epitopes in vitro when using APCs infected with CMV, this study corroborates our previous report of atypical epitope specificity in cord blood and suggests that the T-cell response to CMV is more complicated than initially believed. Testing the evolution of CMVpp65 epitope recognition in patients undergoing primary infection with CMV might help understand the mechanism for the eventual dominance of the response to typical epitopes.
Much recent emphasis has been placed on identifying the optimal T-cell population for use in adoptive immunotherapy. Studies reported over the last few years have been largely restricted to comparisons of different memory subsets (40), with only scant attention paid to T-cells from naïve sources. Based on the data presented here, we argue that despite the unusual epitope repertoire, it is possible to utilize naïve-derived T-cells to expand CMV-specific T-cells for at-risk patients and also begs the question whether T-cells recognizing epitopes identified for other viral and tumor antigens undergo the same selection process. Therefore, the optimal phenotype (14, 41) for adoptive transfer and also the ideal epitope targets remain to be determined (22). This study, however, establishes the proof of concept for utilizing antigen-specific T-cells from naïve donors. However, the lengthy procedure for T cell generation and the use of the B95-8 EBV virus required for to manufacture EBV-LCL make this therapy difficult to translate beyond small center studies. Therefore, for this approach to become broadly applicable there is a need to eliminate the use of autologous EBV LCL, perhaps by using artificial antigen presenting cells, and to reduce the culture time from naïve donors. Another approach would be to isolate T-cells secreting IFN-γ shortly after they are stimulated with viral peptides, as utilized with T-cells derived from CMV-seropositive donors (4), or to use mulitmers targeting atypical epitopes to select out the virus-specific T-cell fraction that could be transferred to the recipient (42, 43). Whether enough cells could be isolated using either of these techniques remains unanswered.
In summary, we show that CMV-specific T-cells can be generated from CB and blood from CMV seronegative adult donors, and that these naïve-derived cells recognize atypical epitopes of pp65 because their precursor frequency is higher than that of T-cells recognizing typical epitopes. When transferred to cord blood transplant recipients, these cells are safe and were efficacious in one patient experiencing viral reactivation. Our findings have important implications for the transplantation field, in which increasing emphasis is being placed on the use of third party “off-the-shelf” virus specific T-cell therapies for all patients undergoing HSCT or solid organ transplantation, irrespective of their HLA type (44–47). The use of a T-cell product that can recognize unusual and diverse epitopes may be useful for avoiding immune escape when the T-cells are not completely HLA matched between donor and recipient.
MATERIALS AND METHODS
Study Design
The objective of this study was to test the ability of T-cells from naïve populations to recognize atypical epitopes of CMVpp65 and in immunocompromised recipients of transplants from virus-naïve donors. After expanding CMV-specific T-cells from two naïve sources (CB and CMVneg donors), we established their suitability for clinical use in a series of experiments designed to elucidate the epitope-specific activity, clonality, and TCR diversity of these novel cells. The safety and efficacy of such T-cells was assessed in three consecutive patients who had undergone CB transplantation and were at high risk for CMV infection.
Patients
The clinical trial (#151992 at Baylor College of Medicine) was open to candidates for CB Transplantation from 5/6 or 6/6 HLA antigen-matched or mismatched CB donors. Patients with a life expectancy <6 weeks or severe kidney or liver disease were excluded, and graft-vs-host disease (GvHD) (grade 3 or 4) was a contraindication for the infusion of T-cells. The investigation was reviewed by the Food and Drug Administration, the Recombinant DNA Advisory Committee, the Institutional Review Boards of Baylor College of Medicine and the National Marrow Donor Program. All participants or their guardians gave informed consent upon enrollment. The characteristics of the three patients who were studied are reported in Table 3.
Generation of Multivirus-specific T-cell Cultures
Virus-specific T-cells were generated from fresh or frozen CB units obtained from the M.D. Anderson Cancer Center Cord Blood Bank or from mothers who consented to the protocol approved by the Baylor College of Medicine (BCM) Institutional Review Board (IRB). For patients enrolled on clinical trial 151992, the 80% fraction of the clinical CB unit was infused in the patient as specified by the protocol. The 20% fraction of the unit was used to manufacture virus-specific T-cells according to the investigational new drug application with the FDA, and as detailed below. Subjects enrolled signed informed consent on the protocols approved by the FDA and the IRB. In addition, CMV-specific T-cells were derived from healthy human donors who had consented to a protocol approved by the Institutional Review Board of Baylor College of Medicine. The donors were HLA typed by the HLA laboratory of Houston Methodist Hospital and tested for CMV-seropositivity by the Gulf Coast Regional Blood Center.
Generation of dendritic cells from PBMC or CB mononuclear cells
CB was thawed and then purified by Ficoll (Lymphoprep; Nycomed) gradient separation. Peripheral blood (PB) mononuclear cells (MC) from healthy CMV-seronegative donors were isolated using Ficoll. Both CB and PB MNCs were washed twice and resuspended in CellGenix media (CellGenix USA) and plated at 1×107 cells per well in DC medium (CellGenix media plus 2 mM L-glutamine) (GlutaMAX, Invitrogen) in a 6-well plate (Costar, Corning, NY) for 1 hr at 37°C in a humidified CO2 incubator. Nonadherent cells were removed by rinsing with 1X PBS (Sigma, St. Louis, MO, USA), and frozen. Cells adherent after 1 hour were cultured in DC media with 800 U/ml GM-CSF (Sargramostim Leukine; Immunex) and 500 U/ml IL-4 (R&D Systems) for 5 days. On day 5, the cells were harvested for maturation.
Maturation of DCs and transduction with Ad5f35-pp65 vector
CB-derived DCs as well as adult CMVpos and CMVneg DCs were transduced and matured using a cytokine cocktail consisting of GM-CSF, IL-4, IL-1β, TNF-α, IL-6, (R&D Systems) and PGE2 (Sigma) (“cytokine cocktail”) for 1 day. On day 6, the DCs were harvested and used to stimulate T-cells. CB-derived DCs were also transduced at an MOI of 1000 vp on day 5 while PBMC-derived DCs were pulsed with 200 ng pp65 Pepmix per 1×106 DCs for 1 hour and then washed on day 6.
Generation and pulsing of EBV-transformed B cell lines from PBMCs
As the source of APCs for the second and third stimulations, 5 × 106 peripheral blood mononuclear cells (PBMC) were infected with concentrated supernatants from a B95-8 working cell bank as previously described (1) and subsequently used to establish EBV-LCLs. The day of the second and third T-cell stimulation, LCLs were pulsed for one hour with 200 ng of pp65 Pepmix (JPT Peptide Technologies) per ~5×106 LCLs at 37°C. The cells were irradiated at 40 Gy, washed, resuspended at 5 × 105 cells/ml of complete media (RPMI (Hyclone) plus human serum and GlutaMAX), and then used as stimulators at a ratio of 1 stimulator to 4 effectors in a 24-well plate or 5 stimulators to 1 effector in a GRex10 gas permeable culture device as published (23).
Generation of CMVpp65-specific cultures derived from CMVnegs
PBMCs were isolated using Ficoll as described above. Adherent cells were used for DC generation, and T-cell-containing nonadherent cells were frozen for use on day 7. After thawing, nonadherent cells were labeled for immunomagnetic selection of CD45RA+ cells, washed, and then selected by MACS®. The CD45RA+ cells were then resuspended in 45% RPMI (Hyclone) and 45% CLICKS (Irvine Scientific) with 10% Human Serum plus GlutaMAX™ (T-cell medium). Cells were resuspended at 2×106/ml and cocultured with autologous, Pepmix-pulsed DCs at a ratio of 20 PBMCs to 1 DC in the presence of the cytokines 10 ng/ml IL-7 and IL-12, (R&D Systems) and 5 ng/mL IL-15 (CellGenix). Cultures were restimulated on days 10 and 17 with irradiated (40 Gy), pp65 Pepmix-pulsed autologous LCLs at a responder-to-stimulator ratio of 4:1 plus IL-15 (5 ng/ml) on day 10 and 50 U/mL IL-2 (Proleukin) on days 17 and day 20. To confirm the origin of the pp65-specific T-cell populations we sorted CD45RA/CCR7 double positive and double negative T-cell populations by flow cytometry and stimulated them with pp65-Pepmix-pulsed DCs followed by pp65-pepmix-pulsed LCLs as described above.
Introducing CMV antigens with CMV-AD169-infected fibroblasts
CMV AD169-infected irradiated (40 Gray) allogeneic HLA-A2+ foreskin fibroblasts were passaged 3 days after the initiation of DC culture. On day 5, CMV-infected fibroblasts were harvested, counted, and plated with DCs at a ratio of 1 fibroblast to 4 DCs in media containing the cytokine cocktail. On day 6 or 7, the DCs were used to stimulate T-cells.
Enzyme-linked immunospot (ELISPOT) assay
ELISPOT analysis was used to determine the frequency and function of T-cells in the T-cell lines secreting IFN-γ when stimulated with Pepmixes for CMV-pp65, Ad-hexon, Ad-penton, and CMV-IE-1 as previously described (24). Spot-forming cells (SFC) were enumerated by Zellnet Consulting and compared with input cell numbers to obtain the frequency of virus-reactive T-cells.
Immunophenotyping, multimer analysis and intracellular cytokine staining
T-cell lines were analyzed with monoclonal antibodies to: CD3, CD4, CD8, CD56/16, CD45RA, CD62L (Becton Dickinson). To detect CMV-pp65- and CMV-IE-1-specific T-cells in the T-cell lines, we used the soluble CMV-pp65 pentamers HLA-A*02-NLV, HLA-A*02-LQT, HLA-A*02-MLN, HLA-A*24-QYD, HLA-B*7-TPR, HLA-B*7-RPH, and HLA-A*01-YSE (prepared by Proimmune Inc). Tetramers for HLA-A*02-LQT, HLA-A*02-MLN, and HLA-B*35-DAN were first created by the Protein Core Facility of Baylor College of Medicine and the Dan L. Duncan Cancer Center. Functionality of the T-cells was assessed by the flow cytometric detection of intracellular accumulation of IFN-γ, granulocyte-macrophage colony stimulating factor (GM-CSF), interleukin 2 (IL-2), tumor necrosis factor-α (TNF-α), and CD40 ligand (CD40L) (all from BD Biosciences) as previously described. (48, 49) Briefly, T-cells were stimulated with 1 μg/mL final concentration of each Pepmix; medium alone was used as the negative control. After incubation for 1 hour at 37°C, 10 μg/mL of Brefeldin A (Sigma) was added for an addition 5 hours. Cells were then washed, fixed, and analyzed using a FACSAria or LSRII flow cytometer (BD Biosciences). When possible at least 200,000 live events were acquired per tube. Data analysis was performed using FlowJo.
Peptide pools to evaluate the breadth of the virus-specific response
Panels of 20mer peptides (overlapping by 15 amino acids and covering the entire amino acid sequence of CMVpp65 from the CMV AD169 strain) were synthesized (Proimmune). For CMV-pp65, 22 peptide pools comprising 2 to 12 20mer peptides were prepared, so that each 20mer peptide was represented in two pools. The 20mer peptides overlap by 15 amino acids, such that the minimal epitope likely overlaps multiple sequential peptides. These CMV-pp65 peptide libraries were designed to identify HLA class I and HLA class II restricted epitopes.
Cytotoxicity assay
T-cells were tested for specific cytotoxicity against autologous LCLs or PHA blasts and autologous LCLs (or PHA blasts) pulsed with CMV-IE-1, CMV-pp65, Ad-hexon and Ad-penton Pepmixes. 51Cr-labeled target cells were mixed with effector cells at doubling dilutions to produce the specified effector: target (E:T) ratios. Target cells incubated in complete medium or 5% Triton X-100 (Sigma) were used to determine spontaneous and maximal 51Cr release, respectively. After 4 hours supernatants were collected and radioactivity was measured on a gamma counter. The mean percentage of specific lysis of triplicate wells was calculated as 100 × (experimental release - spontaneous release)/(maximal release - spontaneous release). The ability of effector cells to lyse CMV-infected targets was assessed by using HLA-A*0201+ fibroblasts infected with the AD169 laboratory strain of CMV.
Viral dissemination Assay
Human HLA-A02+ fibroblasts were infected with CMV AD169 at various dilutions in the presence of 10 ng/ml of IFN-γ. After ~4 hours, the virus was removed and NLV-specific T-cells, MLN-specific T-cells, or no T-cells were added to the culture together with IL-2. After 3–5 days the supernatant or cell lysate was added to fresh human fibroblasts. After 8 days the fibroblasts were scored for their cytopathic effect (CPE) (Supplementary Table S3).
Evaluating TCR precursor frequency
HLA-A2+ CB units were stained and selected for CD3+ cells using MACS selection (Miltenyi Biotec). These cells were then sent to Adaptive Biotechnologies for DNA extraction and TCR sequencing.
Identifying Atypical Epitopes
The 20mer peptide was identified as above using overlapping peptide pools. The 20mer peptide was then divided into all possible 9mer peptides and these peptides were synthesized and tested for specificity with the T-cell line by IFN-γ.
TCR sequencing
Frozen peripheral blood samples were obtained from recipients of double CB transplants who had been consented to a protocol approved by the Institutional Review Board of Harvard Cancer Center/Dana-Farber Cancer Institute/Massachusetts General Hospital. PBMCs from 10 mL of whole blood were isolated using Ficoll as described above and incubated with fluorophore-conjugated monoclonal antibodies: anti-CD3 V450 (clone UCHT1, BD Biosciences), anti-CD4 APC-H7 (clone RPA-T4, BD Biosciences), anti-CD8 Pacific Orange (clone 3B5, Invitrogen), anti-CD25 PE-Cy7 (clone M-A251, BD Biosciences), anti-CD127 PE-Cy5 (clone eBioRDR5, eBioscience). Cell analysis was performed with the FACSCanto II system (BD Biosciences) and FACSDiva software (BD Biosciences). Genomic DNA was extracted from mixed PBMCs using the QIAamp DNA Blood Mini kit as per manufacturer’s instructions (Qiagen). A median of 96 ng of genomic DNA (range 0.8–400ng) for mixed PBMCs was sent to Adaptive Biotechnologies (Seattle, WA) for TCRβ sequencing on their ImmunoSeq platform, based on multiplex PCR amplification with 45Vβ primers and 13 Jβ primers followed by sequencing of TCRβ CDR3 regions on the Illumina Genome Analyzer Cluster Station. Analysis and compilation of resulting sequences was performed by Adaptive Biotechnologies. HLA typing was performed by the HLA Laboratory of Brigham and Women’s Hospital (Boston, MA), Massachusetts General Hospital (Boston, MA), or were sent to the American Red Cross and tested for CMV-seropositivity by the Brigham and Women’s Hospital Virology Lab, the Massachusetts General Hospital Virology Lab, or the Rhode Island Blood Center.
Recipient PBMC samples thawed and analyzed were originally obtained at 3, 6, and 12 months after transplantation from recipients of double CB grafts who received reduced-intensity conditioning consisting of fludarabine, melphalan, and anti-thymocyte globulin either on clinical trials or standard treatment protocols between the years 2005 and 2012 at DFCI or MGH. Graft-versus-Host disease prophylaxis was Tacrolimus and Rapamycin. Samples were selected for analysis if recipients demonstrated >80% donor T-cell chimerism and were not experiencing disease relapse. There were no restrictions based on duration of survival, infections, or GvHD history. The earliest samples were analyzed from 3 months after transplant. Sample availability in the tissue bank was occasionally a limiting factor.
TCRβ sequencing results from 43 PBMC samples representing 22 HLA-A02+ cord recipients were searched for TCRβ sequences corresponding to those described as recognizing atypical CMV epitopes described in this manuscript or typical epitopes as compiled after an extensive literature search. Six recipients, reflecting 13 samples, received an umbilical cord unit which was ex vivo stimulated (50). Time points from which CMV-specific sequences were identified are indicated on Table 2.
Statistical analysis
Student’s t test (two-tailed) was used to test for significance in selected sets of values, assuming equal variance. A p value <0.05 was considered significant. When applicable, values were log-transformed to account for variability between donors. Other statistical tests are stated in the text. Unless otherwise stated, all values are reported as means ± standard deviation.
Supplementary Material
Figure S1A, B. Identifying Atypical epitopes from CMVpp65.
Figure S2. Polyfunctionality of pp65-specific T cells derived from naïve T-cells.
Figure S3. Atypical epitope recognition in patient receiving cord blood-derived virus-specific T- cells.
Figure S4. Shared T-cell receptors in the CB-derived virus-specific T-cells and Patient P2891 6 months post-T-cell infusion.
Table S1. Epitope Recognition by CMV-seronegative donors
Table S2. Polyclonality of T-cells from CMVnegs and CMVpos donors that recognize typical and atypical epitopes
Table S3. Precursor frequencies of TCRs recognizing NLV and MLN from CB
Table S4. Inhibition of CMV dissemination by T-cells recognizing typical and atypical epitopes. Source Data
Acknowledgments
The authors would like to thank Jill Williams, Cindy De Los Santos, Stephanie Ku, Yu-Feng Lin, and Yasmin Hazrat for their assistance with this study.
Funding: This work was supported by a postdoctoral fellowship, PF-13e046e01-LIB, from the American Cancer Society awarded to PJH, and CPRIT RO1 RP100469 (to CMB) and NCI PO1 CA148600e02 (CMB and EJS) awards.
Footnotes
Author contributions: PJH, JJM, SN, PS, JWB, and CRC performed experiments and HL performed the statistical analyses; EJS contributed reagents; and PJH, GDH, HL, RAK, CAM, and KSL, HEH, CMR, EJS, JAB, JRR, and CMB designed experiments and contributed to writing the manuscript.
Competing interests: The authors declare no competing interests. PJH and CMB have filed a provisional patent application (Patrick J. Hanley and Catherine M. Bollard, Expansion of CMV-specific T cells from CMV-seronegative donors, #61896296)
REFERENCES AND NOTES
- 1.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature medicine. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 2.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, Sartor MM, Bradstock KF, Gottlieb DJ. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 4.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, Pang K, Mackinnon S, Lowdell MW. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 5.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. The New England journal of medicine. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 6.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S, Shpall EJ, Krance RA, Kamble RT, Carrum G, Hosing CM, Gee AP, Mei Z, Grilley BJ, Heslop HE, Rooney CM, Brenner MK, Bollard CM, Dotti G. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, Mato AR, Hickstein DD, Gea-Banacloche JC, Pavletic SZ, Sportes C, Maric I, Feldman SA, Hansen BG, Wilder JS, Blacklock-Schuver B, Jena B, Bishop MR, Gress RE, Rosenberg SA. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner MK, Heslop HE. Adoptive T cell therapy of cancer. Current opinion in immunology. 2010;22:251–257. doi: 10.1016/j.coi.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pergam SA, Xie H, Sandhu R, Pollack M, Smith J, Stevens-Ayers T, Ilieva V, Kimball LE, Huang ML, Hayes TS, Corey L, Boeckh MJ. Efficiency and risk factors for CMV transmission in seronegative hematopoietic stem cell recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1391–1400. doi: 10.1016/j.bbmt.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley PJ, Cruz CR, Shpall EJ, Bollard CM. Improving clinical outcomes using adoptively transferred immune cells from umbilical cord blood. Cytotherapy. 2010;12:713–720. doi: 10.3109/14653249.2010.517518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, Decker W, Molldrem JJ, Liu H, Gee AP, Rooney CM, Heslop HE, Dotti G, Brenner MK, Shpall EJ, Bollard CM. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savoldo B, Cubbage ML, Durett AG, Goss J, Huls MH, Liu Z, Teresita L, Gee AP, Ling PD, Brenner MK, Heslop HE, Rooney CM. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002;168:909–918. doi: 10.4049/jimmunol.168.2.909. [DOI] [PubMed] [Google Scholar]
- 14.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borysiewicz LK, Hickling JK, Graham S, Sinclair J, Cranage MP, Smith GL, Sissons JG. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. The Journal of experimental medicine. 1988;168:919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. Journal of virology. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin-Taylor E, Pande H, Forman SJ, Tanamachi B, Li CR, Zaia JA, Greenberg PD, Riddell SR. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. Journal of medical virology. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 18.Kern F, Faulhaber N, Frommel C, Khatamzas E, Prosch S, Schonemann C, Kretzschmar I, Volkmer-Engert R, Volk HD, Reinke P. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. European journal of immunology. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Elkington R, Walker S, Crough T, Menzies M, Tellam J, Bharadwaj M, Khanna R. Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. Journal of virology. 2003;77:5226–5240. doi: 10.1128/JVI.77.9.5226-5240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of experimental medicine. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtappels R, Simon CO, Munks MW, Thomas D, Deegen P, Kuhnapfel B, Daubner T, Emde SF, Podlech J, Grzimek NK, Oehrlein-Karpi SA, Hill AB, Reddehase MJ. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. Journal of virology. 2008;82:5781–5796. doi: 10.1128/JVI.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaul R, Dong T, Plummer FA, Kimani J, Rostron T, Kiama P, Njagi E, Irungu E, Farah B, Oyugi J, Chakraborty R, MacDonald KS, Bwayo JJ, McMichael A, Rowland-Jones SL. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. The Journal of clinical investigation. 2001;107:1303–1310. doi: 10.1172/JCI12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley PJ, Lam S, Shpall EJ, Bollard CM. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. Journal of visualized experiments : JoVE. 2012:e3627. doi: 10.3791/3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanley PJ, Shaffer DR, Cruz CR, Ku S, Tzou B, Liu H, Demmler-Harrison G, Heslop HE, Rooney CM, Gottschalk S, Bollard CM. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein-Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. 2011;13:976–986. doi: 10.3109/14653249.2011.575356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. Journal of virology. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. Journal of virology. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, Perna SK, Ennamuri S, Gottschalk S, Brenner MK, Heslop HE, Rooney CM, Leen AM. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micklethwaite K, Hansen A, Foster A, Snape E, Antonenas V, Sartor M, Shaw P, Bradstock K, Gottlieb D. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:707–714. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Aubert G, Hassan-Walker AF, Madrigal JA, Emery VC, Morte C, Grace S, Koh MB, Potter M, Prentice HG, Dodi IA, Travers PJ. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. The Journal of infectious diseases. 2001;184:955–963. doi: 10.1086/323354. [DOI] [PubMed] [Google Scholar]
- 30.Jedema I, van de Meent M, Pots J, Kester MG, van der Beek MT, Falkenburg JH. Successful generation of primary virus-specific and anti-tumor T-cell responses from the naive donor T-cell repertoire is determined by the balance between antigen-specific precursor T cells and regulatory T cells. Haematologica. 2011;96:1204–1212. doi: 10.3324/haematol.2010.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL, Cornelissen JJ. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98:1358–1364. doi: 10.1182/blood.v98.5.1358. [DOI] [PubMed] [Google Scholar]
- 32.Revello MG, Gerna G. Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications. Reviews in medical virology. 2010;20:136–155. doi: 10.1002/rmv.645. [DOI] [PubMed] [Google Scholar]
- 33.Loewendorf A, Benedict CA. Modulation of host innate and adaptive immune defenses by cytomegalovirus: timing is everything. Journal of internal medicine. 2010;267:483–501. doi: 10.1111/j.1365-2796.2010.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180:4836–4847. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, Sax PE, Boswell S, Kahn JO, Brander C, Goulder PJ, Levy JA, Mullins JI, Walker BD. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. The Journal of experimental medicine. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goulder PJ, Altfeld MA, Rosenberg ES, Nguyen T, Tang Y, Eldridge RL, Addo MM, He S, Mukherjee JS, Phillips MN, Bunce M, Kalams SA, Sekaly RP, Walker BD, Brander C. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. The Journal of experimental medicine. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. The Journal of experimental medicine. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharadwaj M, Burrows SR, Burrows JM, Moss DJ, Catalina M, Khanna R. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein-Barr virus infection. Blood. 2001;98:2588–2589. doi: 10.1182/blood.v98.8.2588. [DOI] [PubMed] [Google Scholar]
- 39.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. The Journal of clinical investigation. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, Olavarria E, Goldman J, Chakraverty R, Mahendra P, Craddock C, Moss PA. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. The Journal of experimental medicine. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saglio F, Hanley PJ, Bollard CM. The time is now: moving toward virus-specific T cells after allogeneic hematopoietic stem cell transplantation as the standard of care. Cytotherapy. 2014;16:149–159. doi: 10.1016/j.jcyt.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, Kapoor N, Pai SY, Rowley SD, Kebriaei P, Dey BR, Grilley BJ, Gee AP, Brenner MK, Rooney CM, Heslop HE. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M, Prockop SE, Koehne G, O’Reilly RJ. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer immunology, immunotherapy : CII. 2010;59:473–477. doi: 10.1007/s00262-009-0789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qasim W, Derniame S, Gilmour K, Chiesa R, Weber M, Adams S, Rao K, Amrolia P, Goulden N, Veys P, Gaspar H. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. British journal of haematology. 2011;154:150–153. doi: 10.1111/j.1365-2141.2011.08579.x. [DOI] [PubMed] [Google Scholar]
- 48.Sloand EM, Melenhorst JJ, Tucker ZC, Pfannes L, Brenchley JM, Yong A, Visconte V, Wu C, Gostick E, Scheinberg P, Olnes MJ, Douek DC, Price DA, Barrett AJ, Young NS. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011;117:2691–2699. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melenhorst JJ, Castillo P, Hanley PJ, Keller MD, Krance RA, Margolin J, Leen AM, Heslop HE, Barrett AJ, Rooney CM, Bollard CM. Graft Versus Leukemia Response Without Graft-versus-host Disease Elicited By Adoptively Transferred Multivirus-specific T-cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23:179–183. doi: 10.1038/mt.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, Pelus LM, Desponts C, Chen YB, Rezner B, Armand P, Koreth J, Glotzbecker B, Ho VT, Alyea E, Isom M, Kao G, Armant M, Silberstein L, Hu P, Soiffer RJ, Scadden DT, Ritz J, Goessling W, North TE, Mendlein J, Ballen K, Zon LI, Antin JH, Shoemaker DD. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1A, B. Identifying Atypical epitopes from CMVpp65.
Figure S2. Polyfunctionality of pp65-specific T cells derived from naïve T-cells.
Figure S3. Atypical epitope recognition in patient receiving cord blood-derived virus-specific T- cells.
Figure S4. Shared T-cell receptors in the CB-derived virus-specific T-cells and Patient P2891 6 months post-T-cell infusion.
Table S1. Epitope Recognition by CMV-seronegative donors
Table S2. Polyclonality of T-cells from CMVnegs and CMVpos donors that recognize typical and atypical epitopes
Table S3. Precursor frequencies of TCRs recognizing NLV and MLN from CB
Table S4. Inhibition of CMV dissemination by T-cells recognizing typical and atypical epitopes. Source Data