Abstract
OBJECTIVE
The purpose of this study was to evaluate the role of CT perfusion in monitoring response to neoadjuvant antiangiogenic and radiation therapy in resectable soft-tissue-sarcomas and correlate the findings with tumor size, circulating and tumor biomarkers, and gene expression.
SUBJECTS AND METHODS
This phase II clinical trial included 20 patients (13 men and 7 women; mean age, 55 years) with soft-tissue sarcomas who were undergoing treatment with the antiangiogenic drug bevacizumab followed by bevacizumab, radiation, and surgical resection. The patients underwent CT perfusion and diagnostic contrast-enhanced CT at baseline, at 2 weeks after bevacizumab therapy, and after completion of bevacizumab and radiation therapy. Multiple CT perfusion parameters (blood flow, blood volume, mean transit time, and permeability) were correlated with tumor size, circulating and tumor biomarkers, and gene expression.
RESULTS
Two weeks after bevacizumab therapy, there was substantial fall in blood volume (31.9% reduction, p = 0.01) with more pronounced reduction in blood flow, blood volume, and permeability after treatment completion (53–64% reduction in blood flow, blood volume, and permeability; p = 0.001), whereas tumor size showed no significant change (p = 0.34). Tumors with higher baseline blood volume and lower baseline tumor size showed superior response to bevacizumab and radiation (p = 0.05). There was also an increase in median plasma vascular endothelial growth factor and placental-derived growth factor concentration after bevacizumab therapy paralleled by a decrease in tumor perfusion depicted by CT perfusion, although this was not statistically significant (p = 0.4). The baseline tumor microvessel density (MVD) correlated with blood flow (p = 0.04). At least 20 different genes were differentially expressed in tumors with higher and lower baseline perfusion.
CONCLUSION
CT perfusion is more sensitive than tumor size for monitoring early and late response to bevacizumab and radiation therapy. CT perfusion parameters correlate with MVD, and the gene expression levels of baseline tumors could potentially predict treatment response.
Keywords: bevacizumab, CT perfusion, radiation therapy, soft-tissue sarcoma
Soft-tissue sarcomas arise in nearly 10,000 persons in the United States each year, striking individuals of all ages (median age, 50 years), with roughly 40% of patients dying of either locoregional recurrence or distant metastasis [1]. The treatment of primary tumors usually includes surgery and radiation and sometimes chemotherapy. Local recurrence after surgery alone can be as high as 33% for extremity tumors and 82% for retroperitoneal tumors [2, 3]. Several prospective and retrospective studies have shown the value of radiation in reducing local recurrence for extremity and retroperitoneal tumors [2, 4-6]. Despite aggressive surgery and radiation, sarcomas adjacent to vital structures (e.g., major vessels and nerves) and all retroperitoneal and pelvic tumors still have a significant risk of local recurrence. Although controversial, adjuvant chemotherapy is being considered to reduce local and distant recurrence [7]. Soft-tissue sarcomas express proangiogenic factor vascular endothelial growth factor A (VEGF-A), which modulates tumor angiogenesis. Expression of VEGF-A in soft-tissue sarcomas correlates with extent of disease and survival and anti-VEGF agents have been shown to suppress tumor angiogenesis in mouse sarcoma models [8-10]. Some recent clinical trials have examined the value of the VEGF inhibitor, bevacizumab in combination with radiation to improve treatment efficacy for primary soft-tissue sarcomas [11-18].
As with other solid cancers, monitoring of treatment response to local and systemic therapies in sarcomas has been performed by serial tumor size measurements using the most accepted Response Evaluation Criteria in Solid Tumors (RECIST) [19-23]. However, these conventional methods that rely on changes in tumor burden are ineffective in capturing changes in tumor microenvironment after antiangiogenic treatment because the vascular changes precede morphologic alterations [23, 24]. However, dynamic contrast-enhanced imaging techniques, such as perfusion CT and MRI are more robust methods to quantify changes in tumor vascularity after antiangiogenic therapy [17, 24-29]. In clinical trials performed in various malignancies, CT perfusion has been validated as an effective surrogate to monitor early antiangiogenic response to bevacizumab [12, 17, 26-28, 30]. Moreover, correlation has also been established between the CT perfusion parameters and microvessel density (MVD) count in pancreatic endocrine tumors and prostate cancer [31, 32]. Circulating levels of biomarkers of angiogenesis, such as VEGF, placental-derived growth factor, and basic fibroblast growth factor, show an increase after bevacizumab administration in rectal cancer and hepatocellular carcinomas due to receptor blockade [17, 30, 33, 34]. Preliminary studies have shown that bevacizumab-induced increase in plasma levels of circulating biomarkers parallels the decrease in tumor vascularity depicted by CT perfusion [17, 30]. In the past several years, there has been a growing interest in linking imaging parameters with tumor genetics to explore the use of imaging as a potential surrogate for determination of gene expression signatures [35]. Associating CT perfusion parameters with gene expression patterns of tumor perfusion could enhance the value of CT perfusion in monitoring response to targeted antiangiogenic therapies. Therefore, the purpose of our study was to investigate the role of CT perfusion as an imaging biomarker to monitor response to antiangiogenic therapy and to correlate it with RECIST-defined burden in soft-tissue sarcoma. Additionally, we compared the changes in CT-derived tumor perfusion parameters with changes in plasma and tissue biomarkers of angiogenesis and with gene expression.
Subjects and Methods
Subjects
This prospective study was part of a phase II clinical trial in compliance with HIPAA regulations and was approved by the institutional review board [33]. All patients were required to give written informed consent. The inclusion criteria for the study were patients 18 years old or older with histologically proven resectable soft-tissue sarcomas measuring at least 5 cm in size, intermediate- or high-grade sarcomas (on basis of histopathologic evaluation from prior biopsy or surgery), no metastatic disease, and adequate renal function (serum creatinine level ≤ 1.4 mg/dL) [33]. Tumors were considered resectable if they did not invade vital organs and structures. All tumors included in the study were stage II or stage III. The following patients were excluded: those with clinically significant cardiovascular disease precluding surgical intervention, recent thromboembolic events, and hypercoagulable disorder (factor V Leiden deficiency, protein C deficiency, and protein S deficiency). Small tumors (< 5 cm) were excluded because these tumors often do not require radiation therapy before surgical resection. Between 2006 and 2009, 20 consecutive patients, including 13 men and seven women (mean age, 55 years; age range, 26–75 years) participated in this study. Preoperative local tumor staging was performed in all the patients on MRI (n = 15) or CT (n = 11).
Study Design
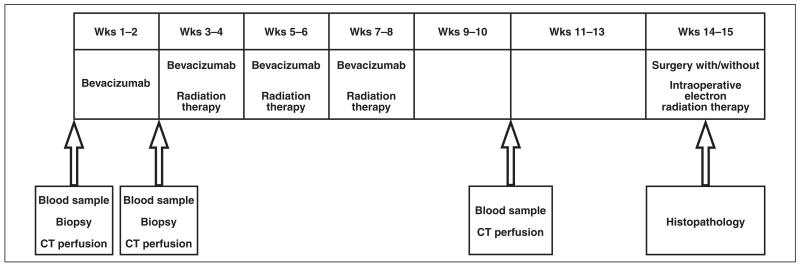
The preoperative treatment protocol consisted of administration of a single dose of bevacizumab (5 mg/kg IV) followed 2 weeks later by treatment with the combination of bevacizumab and radiation therapy for 6 weeks [33] (Fig. 1). After 6–7 weeks from the completion of therapy, all patients underwent surgical resection and, detailed pathologic analysis was undertaken of the specimen. All the patients underwent CT perfusion before treatment, 2 weeks after start of treatment, and 2 weeks after treatment completion (week 10). Blood samples were obtained in all patients before treatment and at weeks 2, 6, and 10 after the start of treatment. The tumor samples were obtained by image-guided percutaneous biopsy before treatment, at week 2, and from the resected specimen at the time of surgery.
Fig. 1.
Flowchart shows study design. All patients received dose of bevacizumab followed by combination of bevacizumab and radiation for 6 weeks. Tumors were resected surgically 6–7 weeks after completion of treatment. CT perfusion scans and blood samples were obtained before treatment, at week 2, and at week 10. Blood samples were obtained before treatment and at weeks 2, 6, and 10. Tumor samples were obtained before treatment, at week 2, and at time of surgery.
Pathologic and Clinical Endpoints of Response
On the basis of the percentage of tumor necrosis seen on surgical pathology, a good response was considered when necrosis of ≥ 80% was confirmed. The patients were followed using contrast-enhanced CT every 3 months until recurrence or death for assessment of progression-free and overall survival. Mean follow-up was 36 months (range, 15–60 months) and 18 of 20 patients had a follow-up of greater than 24 months.
CT Perfusion
Technique
All the CT perfusion examinations were performed on 16-MDCT or 64-MDCT scanners (Lightspeed or Discovery CT750 HD, GE Healthcare). An initial unenhanced CT examination with 5-mm slice thickness was performed to localize the tumor site, and a 2- to 4-cm ROI was selected for dynamic perfusion imaging by a radiology fellow with at least 7 years of experience in cross-sectional imaging. Given the large size of most of the tumors and limited tumor tissue that could be sampled during dynamic scanning, the target area for dynamic scanning was selected to include the largest tumor mass and avoid frank necrosis or calcification. The dynamic CT perfusion protocol consisted of a cine acquisition in the selected region of the tumor at a static table position for 40–45 seconds after IV injection of 50–70 mL of iopamidol (Isovue 370, Bracco Diagnostics) followed by a 30 mL saline chaser at an injection rate of 5–7 mL/s. About 7 to 12 seconds elapsed from the start of injection before initiating scanning on the basis of tumor location in the abdomen or the extremity. The CT parameters for the cine acquisition included a 1-second gantry rotation time, reconstruction interval of 0.5 second, slice thickness of 5 mm, 80–100 kVp, and 140–200 mA. The cine acquisition was followed by a delayed phase acquisition, which included 16 sets of CT data acquisitions of the tumor every 13 seconds.
CT Perfusion
Data processing and data analysis
After completion of the study, the CT perfusion data were transferred to a processing workstation (Advantage Windows 4.0, GE Healthcare) and analyzed using perfusion software (CT perfusion 3.0, GE Healthcare). This software relies on the deconvolution method for perfusion analysis. The ROI on the tumor (area range, 210–887 mm2) was manually drawn to include the entire tumor for analysis. The arterial ROI was drawn on the closest major artery, which included the aorta, iliac arteries, and femoral arteries. The software generated the following perfusion parameters: blood flow (BF, in mL/100 g of wet tissue/min), blood volume (BV, in mL/100 g of wet tissue), mean transit time (MTT, in seconds), and permeability-surface area product (PS, in mL/100 g of wet tissue/min). The perfusion parameters obtained in four to eight consecutive sections (5 mm) were then averaged.
Tumor Size
Tumor size measurements were performed on a PACS workstation (Version 4.0, Agfa). The longest tumor dimension was measured on axial scans as defined by RECIST criteria [23]. The absolute and percentage changes in the tumor size were computed for each patient, and the baseline measurements were compared with other time points at 2 weeks and after treatment completion.
Circulating Biomarker Analysis
The analysis of circulating biomarkers in this trial has been previously reported [33]. For circulating biomarkers, blood samples were collected in ethylenediaminetetraacetic acid containing Vacutainer tubes (Becton Dickinson) for measurements of plasma levels of VEGF, soluble VEGF receptors 1 (sVEGFR-1), placental-derived growth factor (PIGF), basic fibroblast growth factor (bFGF), interleukin-6 (IL-6), IL-8, and tumor necrosis factor α (TNF α). ELISA Kits (R&D Systems) were used for stromal cell–derived factor 1 α (SDF-1α), soluble c-KIT, sVEGFR-2, and sVEGFR-3 [33].
Tumor Biomarker Analysis
For assessment of tumor biomarkers, tissue samples were obtained by image-guided percutaneous needle (14- to 18-gauge) biopsy before the start of therapy and at 2 weeks after the start of bevacizumab. The surgical specimen obtained after tumor resection was used for evaluating changes after the completion of bevacizumab and radiation therapy. Tissue CD31, proliferation cell nuclear antigen (PCNA) immunohistochemistry, and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) immunofluorescence were performed as previously described to assess for tumor MVD, proliferation, and apoptosis, respectively [33].
Genetic Microarray Analysis
A 24-gene signature for treatment response was identified to differentiate tumors with good histologic response (≥ 80% necrosis) from those with poor response (< 80% necrosis) [33]. On the basis of the median blood flow of 47 mL/100 g/min, the patient cohort was divided into two groups using a baseline blood flow cutoff of 50 mL/100 g/min. Group 1 constituted those patients with low baseline tumor perfusion (blood flow value of < 50 mL/100 g/min) and group 2 included patients with high baseline tumor perfusion (blood flow value of ≥ 50 mL/100 g/min). Satisfactory specimens for DNA analysis were found in 16 of 20 patients, and four samples were excluded because on histologic examination a significant amount of nontumor tissue was present or RNA isolation was inadequate. The deficiency in these samples did not affect other data points. All statistical analyses were conducted using statistical software (R, R Project for Statistical Computing). Hierarchic clustering was performed as previously described. To identify genes with expression associated with baseline tumor perfusion, we used the Student t test to rank the genes and used the top M genes in the k-nearest neighbors class prediction method [36]. This genetic expression profile was obtained before treatment (baseline). Leave-one-out cross-validation testing was performed using GenePattern (Broad Institute) with accuracy of 82.3% (13 true predictions of 16 leave-one-out tests) [37]. We report 20 genes that appeared 10 times or more in the 16 tests in a predictor size of M = 50.
Statistical Analysis
Statistical analysis was performed using SAS software, system release 8.2 and Microsoft Excel 2003. The tumor CT perfusion values, RECIST measurements, and tumor density were expressed as mean ± SD. The baseline tumor CT perfusion and RECIST measurements were compared with those obtained at 2 weeks after bevacizumab therapy and after completion of bevacizumab and radiation therapy using the Student t test. For correlation with treatment outcome, we categorized patients as good histologic responders (≥ 80% necrosis) and poor responders (< 80% necrosis) on the basis of the percentage of tumor necrosis present on surgical pathology. We also categorized the patients as those with favorable and unfavorable clinical outcomes on the basis of long-term follow-up results. The baseline values and percentage changes in tumor measurements (CT perfusion values and tumor size) between the various groups were compared using the Wilcoxon rank sum test; p values were used for comparison, and a value of < 0.05 was considered to indicate a statistically significant difference. The Spearman correlation coefficient was used to establish correlation between different variables. The r values closer to 1 indicated a positive relationship and a value closer to −1 indicated a negative relationship.
Results
Patient and Tumor Characteristics
Of the 20 patients with soft-tissue sarcomas (mean size, 8.25 cm; range, 5.0–20.2 cm), 14 had tumors located in the extremity (mean size, 7.3 cm; range, 5–15 cm) and six in the retroperitoneum (mean size, 15.2 cm; range, 7.8–20.2 cm). The histologic subtypes of soft-tissue sarcomas were, fibroblastic sarcoma (n = 8), liposarcoma (n = 6), leiomyosarcoma (n = 4), fibroblastic osteosarcoma (n = 1), and undifferentiated sarcoma (n = 1).
Pathologic Response
On the surgical specimen, nine tumors (45%) showed ≥ 80% pathologic necrosis and were categorized as good responders. Three of these nine tumors showed complete pathologic response with 100% necrosis. In the remaining 11 (55%) tumors, necrosis was present (mean, 49.5%; range, 20–65%) but did not meet the defined criteria of good response (≥ 80% necrosis).
Comparison of Imaging Biomarkers at 2 Weeks After Bevacizumab Therapy
At 2 weeks after bevacizumab therapy, substantial reduction in tumor blood volume (mean change: 31.9%, 3.95 ± 3.1 vs 2.7 ± 2.1 mL/100 g, p = 0.01) and a measurable reduction in blood flow (58.1 ± 50.0 vs 46.0 ± 50.0 mL/100 g/min, p = 0.1) and permeability (12.2 ± 11.4 vs 9.4 ± 7.7 mL/100 g/min, p = 0.1) was recorded (Table 1). Tumor size did not show substantial change (8.1 ± 4.4 vs 8 ± 4.6 cm, p = 0.34).
TABLE 1. Early and Late Tumor Response as Depicted by CT Perfusion.
| CT Perfusion Parameter | Pretreatment | At Week 2a | Percentage Change(p) |
Posttreatmentb | Percentage Change (p) |
|---|---|---|---|---|---|
|
| |||||
| Blood flow (mL/100 g/min) | 58.1 ± 50 | 46.4 ± 50.0 | −20.2 (0.1) | 27.7 ± 33.0 | −53.2 (0.0005) |
| Blood volume (mL/100 g) | 3.95 ± 3.1 | 2.7 ± 2.1 | −31.9 (0.01) | 1.4 ± 1.2 | −63.2 (0.001) |
| MTT (s) | 10.0± 4.1 | 9.5 ± 3.5 | −6.0 (0.47) | 9.4 ± 3.9 | −7.0 (0.5) |
| Permeability surface area product (mL/100 g/min) | 12.2 ± 11.4 | 9.4 ± 7.7 | −22.7 (0.1) | 4.3 ± 3.9 | −64.4 (0.005) |
| Tumor size (cm) | 8.1 ± 4.4 | 8.0 ± 4.6 | −3.0 (0.3) | 7.7 ± 4.4 | −4.0 (0.2) |
Note—Except where indicated otherwise, data are mean ± SD. A p value of < 0.05 indicates a significant difference between baseline and postantiangiogenic treatment values. MTT = mean transit time.
Refers to 2 weeks after initiation of bevacizumab therapy.
Refers to after completion of bevacizumab and radiation therapy.
Comparison of Imaging Biomarkers After the Completion of Bevacizumab and Radiation Therapy
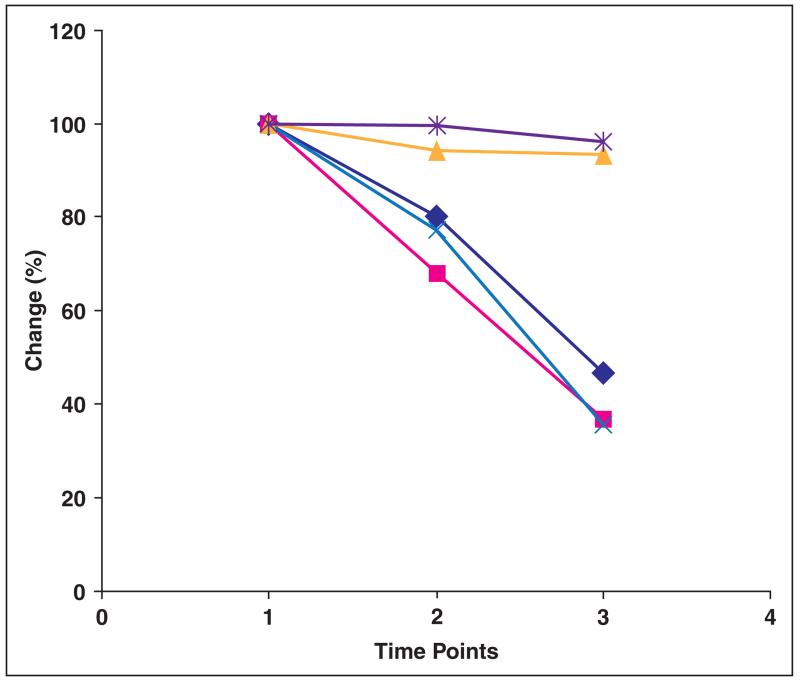
After treatment completion (at 10 weeks), further reduction (> 50%) in tumor CT perfusion parameters was noted: blood flow (58.1 ± 50.0 vs 27.7 ± 33.0 mL/100 g/min, p = 0.0005), blood volume (3.95 ± 3.1 vs 1.4 ± 1.2 mL/100 g, p = 0.001), and permeability (12.2 ± 11.4 vs 4.3 ± 3.9 mL/100 g/min, p = 0.005) (Table 1 and Figs. 2 and 3). However, the RECIST-defined tumor measurement showed insignificant change (8.1 ± 4.4 vs 7.7 ± 4.4 cm, p = 0.23).
Fig. 2.
Chart shows change in perfusion parameters and tumor size from baseline (time point 1) to 2 weeks after start of bevacizumab therapy (time point 2) and after completion of treatment (time point 3). Chart shows percentage change in each of parameters compared with baseline. Dark blue line indicates blood flow, pink line indicates blood volume, yellow line indicates mean transit time, light blue line indicates permeability surface area product, and purple line indicates RECIST (Response Evaluation Criteria in Solid Tumors).
Fig. 3.
Serial CT perfusion scans in 62-year-old man with malignant fibrous histiocytoma of left thigh.
A and B, Axial contrast-enhanced CT image (A) and corresponding color blood flow map (B) show high tissue perfusion (blood flow = 160 mL/100 g/min) before start of bevacizumab therapy. Tumor size is 5.6 cm.
C and D, CT perfusion scan (C) obtained 2 weeks after start of bevacizumab therapy shows decrease in tumor perfusion (blood flow = 100 mL/100 g/min) on corresponding color blood flow map (D). Tumor size is essentially unchanged at 5.5 cm (C).
E and F, CT perfusion scan (E) obtained after treatment completion shows significant reduction in tumor perfusion (blood flow = 60 mL/100 g/min) on color blood flow map (F), whereas tumor size was unchanged at 5.6 cm.
Imaging Biomarkers and Their Correlation With Pathologic Response
At surgical pathology after the completion of treatment, nine tumors showed a good response with ≥ 80% necrosis (> 100% necrosis in three). In the remaining 11, the pathologic response was suboptimal (< 80%). There was a trend toward higher baseline tumor perfusion in good responders versus poor responders, with higher mean blood volume at baseline in the good responders that almost reached statistical significance (p = 0.05) (Table 2). The baseline tumor size was lower in good responders. Comparison of other CT perfusion parameters (BF, MTT, and PS) at baseline did not show any statistically significant differences between good responders and poor responders. In addition, the changes in CT perfusion values and tumor size at 2 weeks after bevacizumab therapy and completion of treatment did not show statistically significant differences between good responders and poor responders.
TABLE 2. CT Perfusion and Tumor Size Measurements Among Good Responders and Poor Responders at Baseline.
| CT Perfusion Parameter | Good Responders (n = 9) |
Poor Responders (n = 11) |
p a |
|---|---|---|---|
|
| |||
| Blood flow (mL/100 g/min) | 72.7 ± 39.4 | 46.1 ± 57.5 | 0.2 |
| Blood volume (mL/100 g) | 5.4 ± 3.4 | 2.8 ± 2.4 | 0.05 |
| MTT (s) | 10.2 ± 4.4 | 9.8 ± 4.1 | 0.9 |
| Permeability surface area product (mL/100 g/min) | 15.7 ± 13.2 | 9.3 ± 9.2 | 0.2 |
| Tumor size (cm) | 5.9 ± 2 | 9.8 ± 5.2 | 0.05 |
Note—Except for p, data are mean ± SD. MTT = mean transit time. A p value of < 0.05 indicates a significant difference in the baseline and values between good responders and poor responders.
Imaging Biomarkers and Their Correlation With Long-Term Clinical Outcome
The mean follow-up period was 36 months (range, 15–60 months). Eight (40%) patients had unfavorable clinical outcome with development of distant metastases (n = 8). The median time to distant recurrence was 7 months (range, 2–36 months). There were no significant differences in the baseline CT perfusion values and tumor size between patients with favorable (n = 12) and unfavorable (n = 8) outcomes. On comparison of percentage changes in CT perfusion values at 2 weeks after start of bevacizumab therapy and after completion of treatment, there was no significant difference between the two groups.
CT Perfusion and Circulating Biomarkers
Median plasma VEGF concentration rose sixfold to sevenfold at 2 weeks after bevacizumab therapy and after completion of bevacizumab and radiation therapy (p < 0.0001). Similarly, placental-derived growth factor concentration increased twofold throughout neoadjuvant treatment (p < 0.0001). These changes paralleled the decrease in tumor perfusion values depicted on CT perfusion (20–64% decrease, r = −0.2); however, there was no significant correlation (p = 0.4). The changes in circulating levels of basic fibroblast growth factor, sVEGFR-1, sVEGFR-2, sVEGFR-3, IL-6, IL-8, TNF-α, SDF-1α, soluble c-KIT, circulating progenitor cells, and VEGFR-2-positive monocytes did not correlate with the changes in tumor perfusion seen on CT perfusion.
CT Perfusion and Tumor Biomarkers
The CT perfusion parameters of liposarcomas (n = 6): blood flow, 18.9 ± 3.0 mL/100 g/min; blood volume, 2.6 ± 4.7 mL/100 g; MTT, 13.1 ± 2.7 seconds; and permeability, 9.5 ± 17.7 mL/100 g/min was grossly different from leiomyosarcomas (n = 4): blood flow, 89.3 ± 57.5 mL/100 g/min; blood volume, 4.7 ± 2.6 mL/100 g; MTT, 7.4 ± 2.5 seconds; and permeability, 16.3 ± 8.8 mL/100 g/min and fibrosarcomas (n = 8): blood flow, 70.8 ± 38.0 mL/100 g/min; blood volume, 4.6 ± 2.1 mL/100 g; MTT, 9.9 ± 4.8 seconds, and permeability, 12.4 ± 8.1 mL/100 g/min, with the former showing lower perfusion values (p, 0.01–0.03).
CT perfusion characteristic comparison of the retroperitoneal and extremity tumors revealed that extremity tumors had significantly higher baseline tumor perfusion (n = 14): blood flow, 80 ± 49 mL/100 g/min, blood volume, 5.3 ± 3.0 mL/100 g; MTT, 9.5 ± 4.5 seconds, and permeability, 16 ± 12.2 mL/100 g/min compared with retroperitoneal tumors (n = 6): blood flow, 17.4 ± 15.0 mL/100 g/min (p = 0.004); blood volume, 1.4 ± 1.0 mL/100 g (p = 0.003); MTT, 11.0 ± 3.4 seconds (p = 0.4); and permeability, 5 ± 4 mL/100 g/min (p = 0.03). The CT perfusion values were compared with tumor MVD, proliferation, and apoptosis obtained using immunohistochemical methods for CD31, PCNA, and TUNEL, respectively. The baseline tumor MVD, proliferation, and apoptosis were variable in different histologic types, which was similar to the variation in CT perfusion values. There was significant correlation between the baseline MVD and baseline blood flow (r = 0.5, p = 0.04). However, the baseline blood volume, MTT, and permeability did not correlate with the baseline tumor MVD (r = −0.4 to 0.4, p > 0.05). The baseline CT perfusion values also did not correlate with the baseline tumor cell proliferation or apoptosis values. The percentage changes in CT perfusion values during and after treatment did not correlate with the changes in tumor MVD, proliferation, or apoptosis.
CT Perfusion and Genetic Analysis
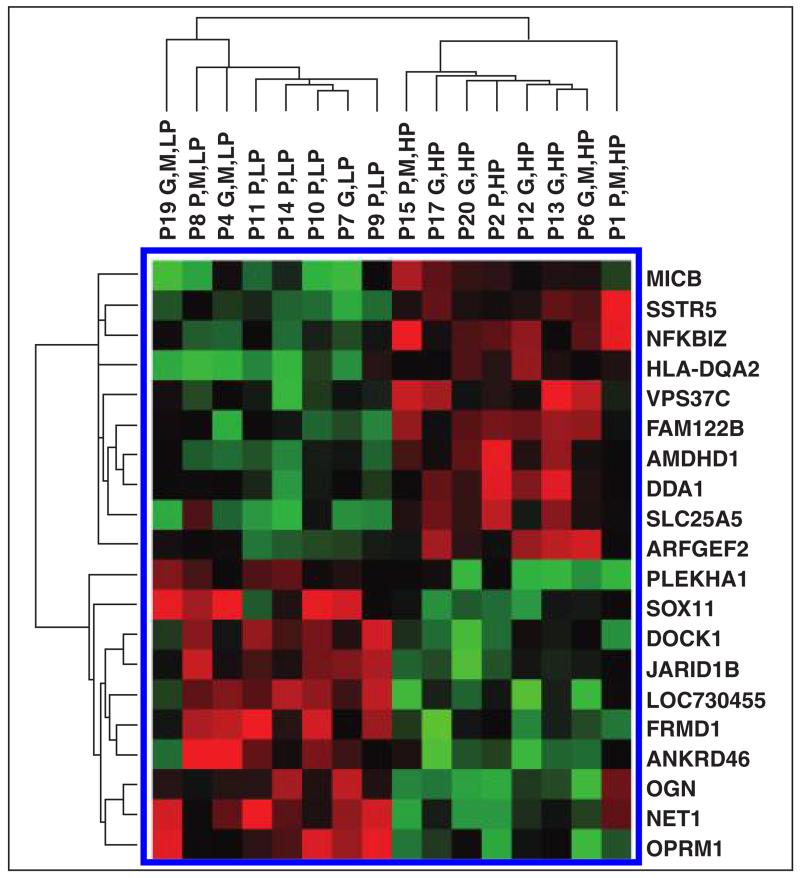
Tumors with higher baseline blood flow (≥ 50 mL/100 g/min) showed better histologic response to bevacizumab and radiation therapy compared with those with lower blood flow (< 50 mL/100 g/min) (67% vs 27%) (Table 3). However, the percentage changes in tumor CT perfusion values 2 weeks after the start of bevacizumab therapy and after treatment completion (bevacizumab and radiation) were not significantly different between the two groups. The expression of 20 marker genes identified from the k-nearest neighbors-leave-one-out cross-validation method clearly segregated the patients with high- and low-perfusion tumors (Fig. 4).
TABLE 3. Baseline CT Perfusion Values in High and Low Perfusion Group Patients.
| CT Perfusion Parameter | High Perfusiona (n = 9) |
Low Perfusion (n= 11) |
p |
|---|---|---|---|
|
| |||
| Blood flow (mL/100 g/min) | 104.1 ± 38.8 | 20.7 ± 14.7 | < 0.001 |
| Blood volume (mL/100 g) | 6.7 ± 2.3 | 1.6 ± 1.2 | < 0.001 |
| MTT (s) | 8 ± 3.8 | 11.7 ± 3.7 | 0.04 |
| Permeability surface area product (mL/100 g/min) | 19.4 ± 12.5 | 6.3 ± 6 | 0.006 |
Note—Except for p, data are mean ± SD. MTT = mean transit time. A p value of < 0.05 indicates a significant difference between high and low perfusion group patients.
The patient cohort was divided into two groups using a baseline blood flow cutoff of 50 mL/100 g/min on the basis of the median blood flow of 47 mL/100 g/min. High baseline tumor perfusion group was blood flow value of ≥ 50 mL/100 g/min and low baseline tumor perfusion group was < 50 mL/100 g/min.
Fig. 4.
Gene expression microarray image shows that expression of 20 gene classifiers identified from k-nearest neighbors–leave-one-out cross-validation test could segregate patients with high baseline tumor perfusion and low baseline perfusion. Patient number is given for each sample. Color bar shows extent of normalized expression intensities in heat map, Red and green indicate high and low expression, respectively.
Discussion
With the introduction of targeted therapies for various solid tumors, limitations of conventional imaging approaches in monitoring treatment effectiveness have been highlighted [28, 30, 38-43]. There has been increasing effort in developing novel image biomarkers as effective imaging surrogates of treatment response. In this phase II clinical trial of patients with intermediate- or high-grade soft-tissue sarcomas undergoing bevacizumab and radiation therapy followed by surgical resection, we found that CT-derived tumor perfusion was a more sensitive biomarker than the established RECIST criteria in monitoring early antiangiogenic effects and response to bevacizumab and radiation therapy. The tumors with higher baseline blood volume showed good pathologic response. The treatment-induced changes in tumor perfusion depicted by CT paralleled the changes observed in the circulating biomarkers.
There is increasing evidence supporting the role of CT perfusion for measuring tumor microvascular changes and monitoring response to targeted therapies for various solid cancers [28, 29, 31, 44-46]. However, its role has not been reported in soft-tissue sarcomas, and our study validates the role of CT perfusion in soft-tissue sarcomas treated with the bevacizumab and radiation therapy regimen. At 2 weeks after the start of bevacizumab therapy, there was a significant drop in blood volume along with a measurable decrease in blood flow and permeability, whereas after completion of therapy, a substantial drop was seen in blood flow, blood volume, and permeability. Similar results have been shown by various researchers who have investigated the antiangiogenic effect of bevacizumab in different tumors [28, 29, 31, 44-46]. In contrast to the changes in CT perfusion, we did not observe any significant change in tumor size during or after completion of treatment. These findings highlight the limited value of tumor size estimation in monitoring both early and late antiangiogenic activity following bevacizumab in soft-tissue sarcoma. Therefore, CT perfusion is a more sensitive marker for measuring changes in tumor vascularity after antiangiogenic therapy.
Besides their role in monitoring antiangiogenic changes after bevacizumab therapy, there is growing interest in exploring the ability of CT perfusion to predict treatment response and patient selection for more personalized therapeutic options. Appropriate patient selection for antiangiogenic drugs permits optimal use of resources and also helps avoid unnecessary complications resulting from use of antiangiogenic drugs. The good responders in this study showed higher baseline tumor blood volume as compared to poor responders, which almost reached statistical significance. The good responders also showed a trend toward elevated blood flow and permeability values, although this was not statistically significant. These results are promising; however, further confirmation in studies with larger patient cohorts are necessary to establish the ability of CT perfusion to predict treatment response. Although we know of no similar studies on soft-tissue sarcomas, other authors have reported similar findings in patients with head and neck cancer [29].
The baseline CT perfusion parameters of the soft-tissue sarcomas in our study showed significant variability that can be accounted for by the inherent wide heterogeneity in this group of tumors [33]. The variability of the baseline CT perfusion values corresponded with the widespread heterogeneity in tumor MVD, tumor proliferation, and apoptosis. Willett et al. [30, 47] showed that CT perfusion parameters correlated with MVD, validating the use of this technique to quantify tumor angiogenesis. We found that the tumor MVD at baseline correlated with baseline tumor blood flow, validating the use of CT perfusion to quantify tumor angiogenesis in soft-tissue sarcoma. After treatment with bevacizumab and radiation, there was reduced tumor MVD, decreased tumor proliferation, and increased apoptosis. Although there was a significant parallel drop in tumor perfusion, we did not find significant correlation between the percentage changes in tumor perfusion and tumor biomarkers.
Bevacizumab raises the circulating levels of VEGF and placental-derived growth factor; therefore, these circulating biomarkers have been proposed as pharmacodynamic biomarker candidates for bevacizumab treatment. We have observed a twofold to sevenfold increase in these biomarkers in our study, which we previously reported [33]. However, the elevation in the plasma levels of VEGF and placental-derived growth factor did not show significant correlation with the decrease in CT perfusion parameters. Additional studies involving larger patient cohorts might be needed to further explore the relationship of these circulating biomarkers with CT perfusion values.
To our knowledge, there are no published studies investigating the gene expression of tumor angiogenesis and its correlation with CT perfusion values in soft-tissue sarcomas. Tumors with high baseline perfusion values often showed desirable treatment histologic response to bevacizumab and radiation therapy compared with the group with lower perfusion. Gene microarray analysis found that a 20-gene classifier could be used to differentiate patients with low- and high-perfusion tumors along with CT perfusion. The genetic basis for the occurrence of phenotypically high baseline perfusion in responders further consolidates the position of CT perfusion as a potential predictive biomarker of treatment outcome.
Our study has several limitations. The small and heterogeneous sample size limits the power of the treatment outcome analysis. CT perfusion analysis was limited to 2–4 cm of tumor tissue, and thus the analyzed tumor portion is not necessarily representative of the entire tumor and could be prone to sampling error in large tumors or multifocal disease. Additionally, the response criteria on CT perfusion are not defined, and the distinction between tumors with high and low perfusion was based on the median CT perfusion values. Another limitation was that the study included different histologic types of soft-tissue sarcomas in the analysis given the considerable rarity of these tumors. Finally, we acknowledge that the findings of this study are specific to the studied tumors and the software and analytic techniques used.
Notwithstanding these limitations, our study supports the role of CT perfusion as a potential biomarker for monitoring early antiangiogenic changes and late treatment effects in soft-tissue sarcoma over the conventional RECIST approach. The baseline CT perfusion parameter of blood flow correlated with the tissue biomarker MVD, suggesting that CT perfusion potentially can provide an indirect measure of tumor biology and angiogenesis. Our study also showed the potential prognostic value of baseline tumor CT perfusion in predicting pathologic tumor response because tumors with higher perfusion values are likely to have a better response after treatment completion. However, further corroboration in larger additional studies is required to confirm the value of CT perfusion in predicting treatment response. Analysis of gene expression microarray identified a 20-gene signature differentiating patients with high and low baseline tumor perfusion.
Acknowledgments
The phase II clinical trial was supported by National Institutes of Health/National Cancer Institute grants 1R21 CA117128-01 and 1R01 CA158301-01 (S. S. Yoon), Society for Surgical Oncology Clinical Investigator Award (S. S. Yoon), and Ira J. Spiro Translational Research Award (D. G. Duda).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Pisters PW, O’Sullivan B. Retroperitoneal sarcomas: combined modality treatment approaches. Curr Opin Oncol. 2002;14:400–405. doi: 10.1097/00001622-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50:127–131. doi: 10.1016/s0360-3016(00)01589-3. [DOI] [PubMed] [Google Scholar]
- 5.Petersen IA, Haddock MG, Donohue JH, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52:469–475. doi: 10.1016/s0360-3016(01)02595-0. [DOI] [PubMed] [Google Scholar]
- 6.Pisters PW, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol. 1994;12:1150–1155. doi: 10.1200/JCO.1994.12.6.1150. [DOI] [PubMed] [Google Scholar]
- 7.Schuetze SM, Patel S. Should patients with high-risk soft tissue sarcoma receive adjuvant chemotherapy? Oncologist. 2009;14:1003–1012. doi: 10.1634/theoncologist.2009-0007. [DOI] [PubMed] [Google Scholar]
- 8.Detwiller KY, Fernando NT, Segal NH, Ryeom SW, D’Amore PA, Yoon SS. Analysis of hypoxia-related gene expression in sarcomas and effect of hypoxia on RNA interference of vascular endothelial cell growth factor A. Cancer Res. 2005;65:5881–5889. doi: 10.1158/0008-5472.CAN-04-4078. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann AC, Danenberg KD, Taubert H, Danenberg PV, Wuerl P. A three-gene signature for outcome in soft tissue sarcoma. Clin Cancer Res. 2009;15:5191–5198. doi: 10.1158/1078-0432.CCR-08-2534. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SS, Stangenberg L, Lee YJ, et al. Efficacy of sunitinib and radiotherapy in genetically engineered mouse model of soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2009;74:1207–1216. doi: 10.1016/j.ijrobp.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Chen HX. Expanding the clinical development of bevacizumab. Oncologist. 2004;9(suppl 1):27–35. doi: 10.1634/theoncologist.9-suppl_1-27. [DOI] [PubMed] [Google Scholar]
- 12.Crane CH, Ellis LM, Abbruzzese JL, et al. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145–1151. doi: 10.1200/JCO.2005.03.6780. [DOI] [PubMed] [Google Scholar]
- 13.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS. Therapeutic options to target angiogenesis in human malignancies. Expert Opin Emerg Drugs. 2006;11:635–650. doi: 10.1517/14728214.11.4.635. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 17.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Nishino M, Jackman DM, Hatabu H, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR. 2010;195:W221–W228. doi: 10.2214/AJR.09.3928. [web] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–267. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. RadioGraphics. 2008;28:329–344. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- 24.Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47:161–178. doi: 10.1016/j.rcl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Sahani DV, Holalkere NS, Kambadakone A, Matthes K, Mino-Kenudson M, Brugge WR. Role of computed tomography perfusion in the evaluation of pancreatic necrosis and pancreatitis after endoscopic ultrasound-guided ablation of the pancreas in a porcine model. Pancreas. 2009;38:775–781. doi: 10.1097/MPA.0b013e3181a66fa6. [DOI] [PubMed] [Google Scholar]
- 26.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue—initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 27.Sahani DV, Kalva SP, Hamberg LM, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005;234:785–792. doi: 10.1148/radiol.2343040286. [DOI] [PubMed] [Google Scholar]
- 28.Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–125. doi: 10.1634/theoncologist.2007-0174. [DOI] [PubMed] [Google Scholar]
- 29.Zima A, Carlos R, Gandhi D, Case I, Teknos T, Mukherji SK. Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? AJNR. 2007;28:328–334. [PMC free article] [PubMed] [Google Scholar]
- 30.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.d’Assignies G, Couvelard A, Bahrami S, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407–416. doi: 10.1148/radiol.2501080291. [DOI] [PubMed] [Google Scholar]
- 32.Osimani M, Bellini D, Di Cristofano C, et al. Perfusion MDCT of prostate cancer: correlation of perfusion CT parameters and immunohistochemical markers of angiogenesis. AJR. 2012;199:1042–1048. doi: 10.2214/AJR.11.8267. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;15:1081–1090. doi: 10.1016/j.ijrobp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boige V, Malka D, Bourredjem A, et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063–1072. doi: 10.1634/theoncologist.2011-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol. 2009;70:232–241. doi: 10.1016/j.ejrad.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 36.Golub TR, Slonim DK, Tamayo P, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 37.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 38.Ng CS, Charnsangavej C, Wei W, Yao JC. Perfusion CT findings in patients with metastatic carcinoid tumors undergoing bevacizumab and interferon therapy. AJR. 2011;196:569–576. doi: 10.2214/AJR.10.4455. [DOI] [PubMed] [Google Scholar]
- 39.Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661–673. doi: 10.1148/radiol.2343031362. [DOI] [PubMed] [Google Scholar]
- 40.Park MS, Klotz E, Kim MJ, et al. Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology. 2009;250:110–117. doi: 10.1148/radiol.2493080226. [DOI] [PubMed] [Google Scholar]
- 41.Stacchiotti S, Collini P, Messina A, et al. High-grade soft-tissue sarcomas: tumor response assessment—pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447–456. doi: 10.1148/radiol.2512081403. [DOI] [PubMed] [Google Scholar]
- 42.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957–1971. [PubMed] [Google Scholar]
- 43.Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxy-glucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48–54. doi: 10.1016/S1470-2045(09)70333-X. [DOI] [PubMed] [Google Scholar]
- 44.Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Dushyant SV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST) Invest Radiol. 2012;47:11–17. doi: 10.1097/RLI.0b013e3182199bb5. [DOI] [PubMed] [Google Scholar]
- 45.Nitzl D, Ohlerth S, Mueller-Schwandt F, Angst A, Roos M, Kaser-Hotz B. Dynamic computed tomography to measure tissue perfusion in spontaneous canine tumors. Vet Radiol Ultrasound. 2009;50:347–352. doi: 10.1111/j.1740-8261.2009.01548.x. [DOI] [PubMed] [Google Scholar]
- 46.Squillaci E, Manenti G, Ciccio C, et al. Perfusion-CT monitoring of cryo-ablated renal cells tumors. J Exp Clin Cancer Res. 2009;28:138. doi: 10.1186/1756-9966-28-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willett CG, Kozin SV, Duda DG, et al. Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin Oncol. 2006;33(5 suppl 10):S35–S40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]