Abstract
Shortened telomere length is associated with increased cancer incidence and mortality. Populations experiencing chronic stress have accelerated telomere shortening. In this exploratory study, we examined associations between longitudinal changes in patient reported outcomes (PRO) of psychologic distress and peripheral blood mononuclear cell (PBMC) telomere length to test the hypothesis that modulation of the chronic stress response would also modulate telomere dynamics. Archived PBMC specimens (N = 22) were analyzed from a completed and reported randomized, longitudinal trial that showed a psychosocial telephone counseling intervention improved quality of life (QOL) and modulated stress-associated biomarkers in cervical cancer survivors. PROs and biospecimens were collected at baseline and 4 months postenrollment. Telomere length of archived PBMCs was evaluated using the flow-FISH assay. Longitudinal changes in psychologic distress, measured by the Brief Symptom Inventory-18, were significantly associated with increased telomere length within the CD14+ (monocyte) population (r = 0.46, P = 0.043); a similar trend was observed for the CD14− population. Longitudinal changes in telomere length of the CD14− subset, primarily T lymphocytes, were associated with longitudinal increases in the naive T-cell population (r = 0.49, P = 0.052). Alterations in the chronic stress response were associated with modulation of telomere length in PBMCs, with evidence for mobilization of “younger” cells from progenitor populations. These data provide preliminary support for the (i) capacity to modulate the chronic stress response and the associated accelerated telomere shortening, (ii) inclusion of telomere length in the biobehavioral paradigm, and (iii) potential link between the chronic stress response and biologic mechanisms responsible for genomic integrity and carcinogenesis.
Introduction
Telomeres are specialized repetitive DNA sequences, typically ranging from 5,000 to 15,000 bp in humans, which are the critical chromosome capping DNA sequences and play an important role in maintaining genomic integrity (1, 2). The loss of telomere repeats diminishes telomeric functional capacity and has been associated with the development of hematologic and solid tumor malignancies (1, 2). Recent studies have noted shortened telomeres in prema lignant or dysplastic tissues and in “normal” epithelium adjacent to tumors (field defect) supporting the contribution of telomeric shortening to early carcinogenesis (3–6). It is also well established that upon full malignant transformation, the majority of tumors have engaged mechanisms to abnormally lengthen telomeres. This variation in telo-mere dynamics over the course of carcinogenesis complicates the interpretation of studies on the role of telomeres in the transformation process and cancer progression. Nevertheless, there are recent reports associating shortened telo-mere length of peripheral blood cells with the incidence and mortality of epithelial and hematologic malignancies (7–9). Thus, it appears clear that defining factors that influence telomere dynamics is highly likely to identify targets for cancer prevention and control.
Chronic psychologic stress has been associated with accelerated telomere shortening in peripheral blood mononuclear cells (PBMC), beyond that expected with age, in a variety of clinical settings (10–15) and has been linked to disease progression in a number of chronic diseases, most notably cardiovascular disease (14–20). Although controversial, there is accumulating evidence that chronic psychologic stress is associated with increased cancer development, progression, and mortality in both humans (21–23) and recently in animal models (24–27). Several groups have begun to elucidate downstream biologic effects of the chronic stress response that may contribute to these associations (28–32). Taken together, this suggests another previously unrecognized element in the biobehavioral paradigm, telomeric shortening, which may be applicable to cancer populations and “at-risk” populations (33). It is provocative to consider the chronic stress–associated accelerated telomere loss as a possible mechanism for the potential association of psychologic stress with cancer development and progression. If true, this would be an avenue to mitigate cancer risk and improve outcomes (34, 35).
Because cervical cancer survivors often experience profound and protracted disruptions in quality of life (QOL) domains (36–41), it is reasonable to argue that the disease, treatment, and life circumstances merge to produce exceptional chronic stress, compromising optimal health (38, 39). The response to these chronic stressors can manifest as psychologic distress, with greater distress indicative of less effective coping mechanisms and an exacerbated chronic stress response including perturbations of the psychoneuro-immune axis (30). This population provides an opportunity to evaluate methods for reducing distress, improving QOL, and modulating the down stream chronic stress response–associated physiologic processes (30, 39). Results from our previous randomized study of a population of cervical cancer survivors showed that participation in a culturally sensitive psychosocial telephone counseling (PTC) intervention was associated with significantly improved QOL as well as modulation of stress-associated biomarkers and the psychoneuroimmune axis (30). This completed pilot study provided a longitudinal data set and archived biospecimens to explore changes in the chronic stress response and downstream modulation of PBMC telomere length along with potential involved mechanisms for any observed changes. Specifically, we examined whether (i) attenuation of the chronic stress response would be associated with modulations of telomere length of PBMCs and (ii) telomere length modulations would result from the introduction of naive cellular subsets into the PBMC population. PBMCs contain different cellular subsets including most prominently T lymphocytes (CD3+, CD14−) and monocytes (CD14+), with other minor subsets. Longitudinal changes in telomere length were determined by an assay combining flow cytometry and FISH (flow-FISH). Telomere length of monocytes and the CD14− cellular subsets were examined separately to assess changes in telomere dynamics among these 2 cellular subsets. In addition, longitudinal changes in the percentage of naive T lymphocytes within the CD14− population, characterized by expression of CD45RA, were determined by immunophenotyping. To our knowledge, this is the first longitudinal study examining the association between longitudinal changes in the chronic stress response and modulations of telomere length in a cancer population.
Materials and Methods
Participants and study design
Participants (N = 22) were selected from the previously described study cohort (N = 50 enrolled, n = 36 completed study; ref. 30) based on the=availability of remaining PBMC biospecimens and complete patient reported outcome (PRO) data. This completed and previously reported randomized clinical study of cervical cancer survivors explored the methodologic feasibility of implementing a PTC intervention, determined its potential benefits, and investigated potential associations between intervention-induced longitudinal modulations in QOL measures and longitudinal modulations in chronic stress response–associated neuroendocrine and immune parameters (30). Patients with documented histologic diagnoses of squamous cell carcinoma of the uterine cervix pathologic stage I, II, or III; 9 to 24 months removed from diagnosis; and fluent in English or Spanish were identified from the regional cancer registries and randomized to receive PTC or usual care (UC) after providing informed consent. Demographic and disease data were obtained at baseline; PROs of distress [Brief Symptom Inventory (BSI)-18] and phlebotomy samples were collected at baseline and at 4 months after enrollment, approximately 1 month after completion of PTC for participants randomized to counseling.
Measure of distress
The BSI-18 (42) was used to measure psychologic distress in the completed trial. In this 18-item measure, each item is rated on a 5-point Likert scale from 0 (not at all) to 4 (always), with lower scores reflecting less distress. The BSI-18 contains 3 subscales that assess anxiety, depression, and somatization, as well as an overall total or global score. All BSI-18 data reported herein reflect the raw global scores. Normative data for oncology patients show good reliability (Cronbach α: 0.89; ref. 42).
Reagents and biospecimens
Archived cryopreserved PBMC biospecimens from the completed biobehavioral study (30) were examined for changes in telomere length and the quantification and contribution of naive T-cell subsets using the flow-FISH assay and immunophenotyping respectively (see below). For flow-FISH, biotinylated CD14 (clone: 3-C39; BD Biosciences) was used to delineate CD14+ (monocyte) subsets and CD14− subsets. Biotinylated IgG2a (eBioscience) was used as the isotype control. Biotinylated reagents were detected by Streptavidin-Cy5 (BioLegend). For evaluation of naive T-cell (CD3+CD45RA+CD45RO−) percentage, CD3 (clone: UCHT1; Immunotech/Beckman Coulter), CD45RA (clone: HI100; BioLegend), and CD45RO (clone: UCHL1; BioLegend) were used. As the process of hybridization in the flow-FISH assay can compromise antibody–antigen binding, a cross-linking agent [bis(sulfosuccinimidyl) suberate] BS3 (Thermo Fisher Scientific) was used to stabilize the binding of antibodies to their respective cell surface antigens. The fluorescein isothiocyanate (FITC)-labeled peptide nucleic acid (PNA) probe, complementary to the human telomere DNA repeat sequence (TTAGGG), was used to determine relative telomere length in the flow-FISH analyses (Panagene Inc.). A nucleic acid dye, LDS-751 (Exciton), was used to distinguish between PBMCs and the internal standard (see flow-FISH procedure below). Other miscellaneous chemical reagents were procured from Fisher Scientific.
Flow-FISH procedure
The Flow-FISH assay used in this study was modified for cryopreserved biospecimens from methods developed by Baerlocher and colleagues (43). This methodology includes an internal standard of non-human thymocytes. Although the original description of flow-FISH methodologies used bovine thymocytes, we used porcine thymocytes due to issues of access. Fresh porcine thymi were obtained, transported in media, and processed within 6 hours as described (43). The pooled single-cell thymocyte suspension was treated with 0.2% fresh formaldehyde at room temperature with agitation for 10 minutes. Subsequently, cells were centrifuged at 450 × g at 4°C and washed twice with 1 × PBS and then divided into aliquots of 2.5 × 106 to 5 × 106 cells and preserved in the vapor phase of liquid nitrogen identically to the archived PBMCs. For each assay, 5 × 106 to 10 × 106 cryopreserved PBMCs from each time point (T1 and T2) and one stored vial of porcine thymocytes containing 2.5 × 106 to 5 × 106 cells were thawed. PBMCs were initially resuspended in 90 μL of fluorescence-activated cell-sorting (FACS) buffer [1 × PBS with 1% bovine serum albumin (BSA), 0.1% sodium azide] and incubated with Fc receptor blocking reagent (10 μL/107 cells; Miltenyi Biotec Inc.) for 10 minutes on ice. Porcine thymocytes and PBMCs were then resuspended at 107 cells/mL in FACS buffer and 4 × 105 PBMCs and 1 × 105 porcine thymocytes were placed into individual 1.5-mL microfuge tubes (see PNA labeling procedure below). All conditions were run in duplicate for each time point.
Cellular subset analyses required labeling cells before hybridization. Biotinylated anti-CD14 or isotype control was added to respective tubes (according to manufacturer instructions) and incubated at 4°C in the dark for 25 minutes with periodic agitation. Samples were then washed 3 times with excess FACS buffer at 300 × g for 5 minutes at 4°C. Subsequently, Streptavidin-Cy5 was added to the tubes (according to manufacturer's instructions) and incubated at 4°C in the dark for 25 minutes. Samples were washed as above. The BS3 cross-linking agent, at 2 mmol/L final concentration, was added and incubated for 30 minutes in the dark at room temperature. The reaction was then quenched with a final concentration of 1 mmol/L Tris for 15 minutes. Samples were then washed with 5% dextrose, 10 mmol/L HEPES, and 0.1% sterile BSA and centrifuged at 300 × g for 5 minutes at 16°C. All but 100 μL of the supernatants were removed by aspiration, and cells were resuspended in this residual volume.
Relative telomere length was determined in duplicate samples in the presence or absence of the PNA FITC–labeled telomere probe to account for the increase in cellular auto-fluorescence intrinsic to the hybridization process. Thirty-five microliters of hybridization solution (70% deionized formamide, 20 mmol/L Tris, 20 mmol/L NaCl, and 1% BSA) alone or containing 0.26 μg/mL of PNA probe was added to each sample. Samples were denatured at 85°C for 2.5 minutes and then incubated at room temperature in the dark for 60 minutes. Subsequently, samples were washed 2 times with 5% dextrose, 10 mmol/L HEPES, 0.1% BSA, and 0.1% Tween-20 and centrifuged at 900 × g for 5 minutes at 16°C. Supernatants were removed by aspiration. Two hundred microliters of the LDS-751 nucleic acid stain solution (1 × PBS, 0.1% BSA, 1,000 U/mL RNase T1, and 10 ng/mL LDS-751) was added to the samples and incubated on ice for 20 minutes (43). Following this final incubation, samples were immediately run on the Accuri C6 flow cytometer and analyzed using FlowJo v. 9.3.3 for Mac. In our laboratory, the flow-FISH methodology has a 10% intra-assay variation (10 replicates of PBMCs from a single normal donor sample assayed on a single day) and a comparable nearly 10% interassay variation (the same normal donor PBMC sample assayed on 10 separate days).
Evaluation of naive T-cell percentage
Standard cell surface labeling and flow cytometric analyses were used. The percentage of naive T cells was evaluated by incubating PBMCs with fluorochrome-conjugated antibodies against CD3 (clone: UCHT1, Immunotech/Beckman Coulter), CD45RA (clone: HI100, BioLegend), CD45RO (clone: UCHL1, BioLegend), or appropriate iso-type control antibodies for 1 hour at 4°C in the dark. Cells were washed and resuspended in flow cytometric buffer as above and were immediately analyzed by flow cytometry. Analyses were conducted as above.
Statistical analyses
Descriptive statistics including means, SE, and ranges were computed for the BSI-18 global scores and biologic data at each time point. All data were normally distributed. Longitudinal data are presented as differences scores: T2 (4 months postenrollment) – T1 (baseline). Because telo-mere length is known to decrease with age and baseline telomere length has been strongly associated with longitudinal change in telomere length (44–47), associations with telomere length were estimated using partial correlations that adjusted for both age and baseline telomere length. All statistical analyses were 2-sided. Given the small sample size and the desire to explore associations between modulation of the chronic stress response and telomere length, irrespective of whether participants were randomized to receive PTC or UC, all analyses combined the PTC and UC groups.
Results
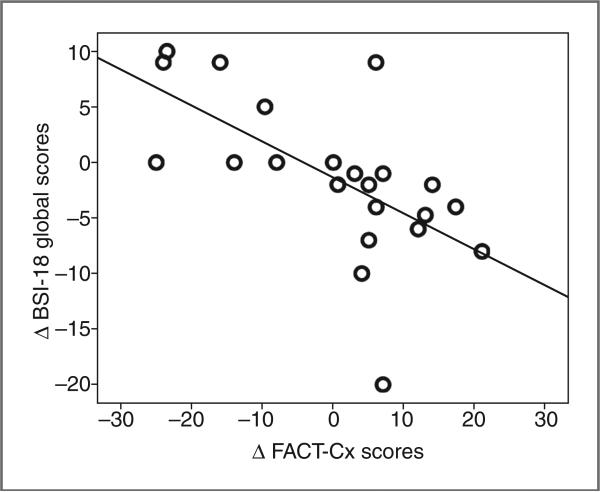
Participant medical and demographic information for the entire clinical study cohort have been described in detail previously (30). The demographic and diagnosis data for this subset (N = 22), summarized in Table 1, are broadly representative of the larger study cohort for whom stress-associated biomarkers were previously reported (30). The majority of participants were either Caucasian (46%) or Hispanic (32%), spoke English as their preferred language, had a stage I or II diagnosis, were partnered, and had some college education (Table 1). At the time of testing, all subjects were non-smokers, although 8 reported past use of cigarettes. Ten subjects participated in PTC whereas 12 were in the usual care arm. The PTC intervention, which consisted of 6 sessions, was specifically designed to help women cope with the stressful events and feelings of distress associated with cervical cancer. Sessions included a psychosocial interview, managing stress and emotions, enhancing health and wellness, addressing relational and sexual concerns, followed by a summary and 1-month booster session. A session review letter, generated by the counselor after each session, recapitulated the session's content and reinforced adaptive coping strategies. We reported previously that change in global QOL, as measured by the FACT-Cx, was significantly associated with changes in stress-associated biomarkers and immune profile (30). We observed a significant correlation between the global BSI-18 and QOL as measured by the FACT-Cx, (r = 0.47, P = 0.032; Fig. 1). This supports psychologic distress, as measured by the BSI-18, as a component of or contributing factor to the global QOL derangement. Not surprisingly, we observed that distress (BSI-18 scores) was a more sensitive measure and provided stronger associations with the longitudinal changes in telomere index than global measures of QOL (FACT-Cx).
Table 1.
Demographic and disease characteristics
| Participants (N = 22) | |
|---|---|
| Age, y | Mean = 45.6 (SE = 2.7) n (%) |
| Ethnic background | |
| Caucasian non-Hispanic | 10 (46) |
| Hispanic | 7 (32) |
| Asian/Pacific Islander | 3 (13) |
| African America | 2 (9) |
| Preferred language | |
| English | 19 (86) |
| Spanish | 2 (9) |
| Other | 1 (5) |
| Stage | |
| I | 11 (50) |
| II | 4 (18) |
| III | 2 (9) |
| Unknown | 5 (23) |
| Marital/partner status | |
| Married/partnered | 12 (55) |
| Single/divorced/separated/widowed | 10 (45) |
| Education | |
| High school or less | 6 (27) |
| Some college or more | 16 (73) |
Figure 1.
Longitudinal association of chronic stress response and QOL. Correlation plot of association between longitudinal changes in BSI-18 global scores and FACT-Cx scores (N = 22), showing that decreased distress (an improved stress response) is associated with improved QOL. Data points reflect longitudinal change from baseline to T2, Pearson correlation coefficient, r = −0.47, P = 0.032, 2-sided analysis; plot features least-squares regression line.
In the flow-FISH assay, telomere length is indexed by the intensity of the FITC fluorescence, reflecting the number of PNA probes that bind the repeating sequence of the telomeres. We gated on CD14+ (monocyte) and CD14− populations for each patient sample and calculated a telomere mean fluorescence intensity (MFI) index for each of these subpopulations. The telomere MFI index was defined as the shift (i.e., difference) in MFI from PNA probe–negative samples to PNA probe–positive samples normalized to the difference in PNA probe–negative and PNA probe–positive MFI of the internal standard (i.e., control porcine thymocyte populations). Thus, the telomere index and the flow-FISH methodology provide a measure of relative telomere length, which is well suited to longitudinal analyses where limited biologic specimens are available.
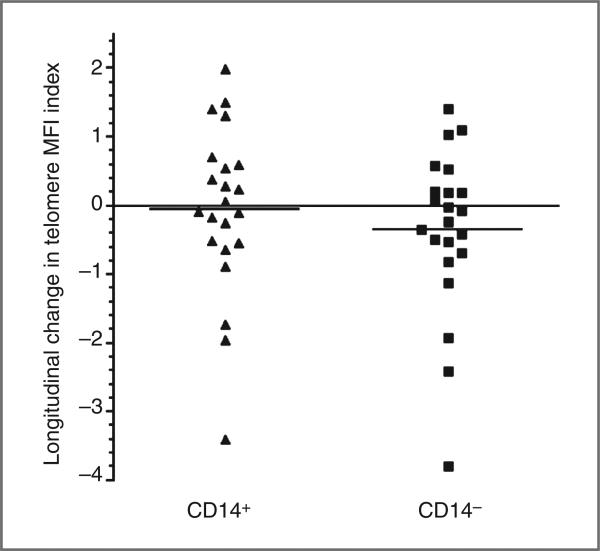
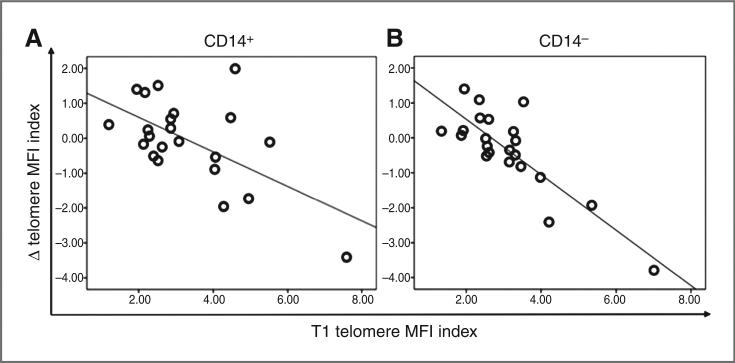
Telomere MFI indices were determined for CD14+ (monocyte) and CD14− subsets for baseline (T1) and T2 time points (N = 22). The mean change in raw telomere MFI index for CD14− subsets was 0.35 and ranging from −3.79 to 1.40, which corresponds to a 54% decrease to a 72% increase, respectively. In CD14+ subsets, the mean change in raw telomere MFI index was 0.06 and ranging from −3.41 to 1.99, which corresponds to a 45% decrease to a 43% increase, respectively. Thus, there was a wide range of change in telomere length from baseline to T2 in both subpopulations, with a substantial proportion of participants showing an increase in telomere length (Fig. 2). In addition, we observed that baseline telomere length MFI index was predictive of change in telomere MFI index in the CD14− subpopulation (r = −0.85, P < 0.001) and CD14+ subpopulation (r = −0.62, P = 0.003), as has been reported previously (Fig. 3; refs. 44, 46, 47). Not surprisingly, changes in CD14− and CD14+ telomere MFI index were associated as well (r = 0.82, P < 0.001).
Figure 2.
Longitudinal change in telomere length MFI index of CD14+ and CD14− subsets. Data points were obtained from subtracting the baseline telomere length MFI index from the T2 telomere length MFI index of CD14+ and CD14− subsets.
Figure 3.
Baseline and longitudinal change in telomere length are associated in CD14+ and CD14− subsets. A, correlation plot of baseline and longitudinal change in telomere length MFI Index of CD14+ subsets (N = 22), showing that shorter telomeres at baseline were more likely to increase at T2, Pearson correlation coefficient, r = −0.62, P = 0.003, 2-sided analysis; plot features least-squares regression line. B, correlation plot of baseline and longitudinal change in telomere length MFI index of CD14− subsets (N = 22), showing that shorter telomeres at baseline were more likely to increase at T2, Pearson correlation coefficient, r = −0.85, P < 0.001, 2-sided analysis; plot features least-squares regression line.
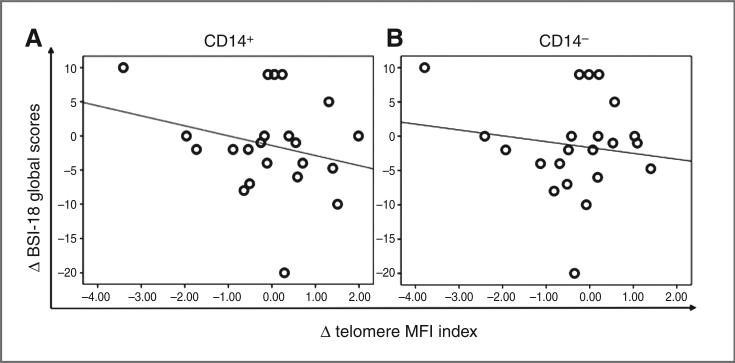
An association was observed between a decrease in distress (BSI-18 Global scores) and an increase in telomere MFI index in the CD14+ (monocyte) subpopulation (r = −0.46, P = 0.043; Fig. 4A). A similar trend was observed for CD14− subsets but, did not reach statistical significance (r= −0.36, P = 0.100; Fig. 4B). The fact that these analyses were conducted controlling for both age and baseline telomere index supports the significance of the associations despite the limited sample size and statistical power. Improved global QOL was positively associated with increased telo-mere MFI index of CD14+ (r = 0.44, P = 0.053) and CD14− (r = 0.39, P = 0.094) subsets as well, although the correlations did not reach statistical significance (Supplementary Fig. S1).
Figure 4.
Longitudinal association of chronic stress response and telomere index of CD14+ and CD14− subsets. A, correlation plot of longitudinal changes in BSI-18 global raw scores and telomere length MFI index of CD14+ subsets (N = 22), showing that decreased distress was associated with increased telomere length. Data points reflect longitudinal change from baseline to T2, Pearson correlation coefficient, r = −0.46, P 0.043, 2-sided analysis; plot features least-squares regression line. B, correlation plot of longitudinal changes in BSI-18 global raw scores and telomere length MFI index of CD14− subsets (N = 22), showing that decreased distress was associated with increased telomere length. Data points reflect longitudinal change from baseline to T2, Pearson correlation coefficient, r = 0.36, P = 0.1, 2-sided analysis; plot features least-squares regression line. These analyses were controlled for age and baseline telomere length.
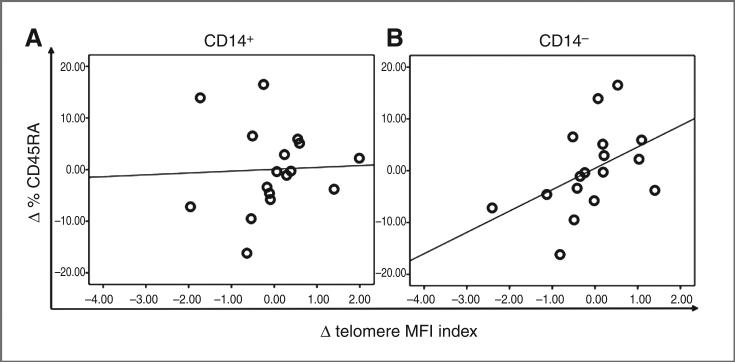
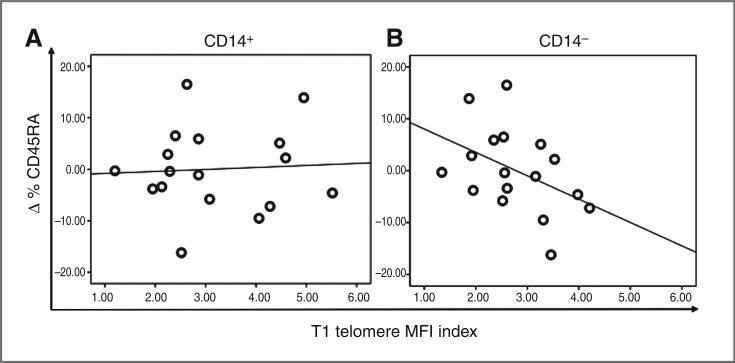
Given the previously described shift in immunologic stance associated with improved QOL (30), we hypothesized that one potential mechanism for the observation of the association between decreased distress, improved chronic stress response, and increased telomere length could be mobilization of “younger” leukocytes from progenitor pools. As there are no established cellular markers for “younger” monocytes, we examined the lymphocyte population for longitudinal changes in the naive T-cell population (CD45RA+) in PBMCs, even though the associations were less robust for the predominant lymphocyte (CD14−) population. Naive T cells have not yet undergone activation and proliferation, thus they represent a younger population relative to non-naive T-cell populations (CD45RO+), which include long-lived memory T cells. Longitudinal changes in telomere index in the CD14− subsets were associated with a increase in percentage of CD45RA+ T-cell subsets (r = 0.49, P = 0.052; Fig. 5B). As expected, there was no association between longitudinal change in telomere index in the monocyte (CD14+) subset and the change in percentage of CD45RA+ T cells (r = 0.09, P = 0.731; Fig. 5A). Given that baseline telomere length was associated with longitudinal changes in telomere length, we examined whether baseline telomere length was also associated with longitudinal changes in the percentage of CD45RA+ T cells, given the data supporting a role for mobilization of naive T cells as a potential mechanism for the observed increase in telomere index. We observed that baseline telomere MFI index of CD14− populations was inversely associated with a longitudinal change in percentage of CD45RA+ T-cell subsets (r = −0.44, P = 0.088; Fig. 6B), suggesting that shorter telomere length at baseline was associated with a larger increase in the percentage of CD45RA+ T cells at T2. This association was not observed in monocyte (CD14+) subsets (r = 0.07, P = 0.811; Fig. 6A).
Figure 5.
Longitudinal association of telomere index of CD14+ and CD14− subsets and percentage change in CD45RA+ naive T cells. A, correlation plot of longitudinal changes in telomere length MFI Index of CD14+ subsets and percentage change of CD45RA+ naive T cells (N = 17), showing that increased telomere length in CD14+ subsets was not associated with increased percentage of CD45RA+ naive T cells. Data points reflect longitudinal change from baseline to T2, Pearson correlation coefficient, r = 0.09, P = 0.731, 2-sided analysis; plot features least-squares regression line. B, plot of longitudinal changes in telomere length correlation MFI index of CD14− subsets and percentage change of CD45RA+ naive T cells (N = 17), showing that increased telomere length in CD14− subsets was associated with increased percentage of CD45RA+ naive T cells. Data points reflect longitudinal change from baseline to T2, Pearson correlation coefficient, r = 0.49, P = 0.052, 2-sided analysis; plot features least-squares regression line. These analyses were controlled for age and baseline telomere length.
Figure 6.
Baseline telomere length of CD14+ and CD14− subsets and longitudinal percentage change in CD45RA+ naive T cells. A, correlation plot of baseline telomere index of CD14+ subsets and longitudinal percentage change in CD45RA+ naive T cells (N = 17), showing that telomere length of CD14+ subsets at baseline were not associated with percentage of CD45RA+ naive T cells at T2. Pearson correlation coefficient, r = 0.07, P = 0.811, 2-sided analysis; plot features least-squares regression line. B, correlation plot of baseline telomere index of CD14− subsets and longitudinal percentage change in CD45RA+ naive T cells (N = 17), showing that shorter telomere length of CD14− subsets at baseline was associated with a larger percentage of CD45RA+ naive T cells at T2. Pearson correlation coefficient, r = −0.44, P = 0.088, 2-sided analysis; plot features least-squares regression line.
Discussion
To our knowledge, this is the first study to report longitudinal associations between cancer patient–reported distress and telomere length. Importantly, these data support the capacity to modulate the accelerated telomere shortening documented in chronically stressed populations when levels of distress are decreased (i.e., when there is an improvement in the chronic stress response). Interestingly, longitudinal reductions in distress were significantly associated with increased telomere length in CD14+ (monocyte) subsets and also in CD14− subsets, albeit to a lesser extent. These findings are consistent with those recently presented by Lin and colleagues (48), showing a similar longitudinal association between decreased perceived stress and increased PBMC telomere length, although their study did not examine PBMC subsets. Together these results suggest that telomere length is another downstream chronic stress–associated biologic effect that is subject to modulation and is not restricted to the adaptive immune system.
Telomere length is known to decrease with age and exposure to chronic stress has been associated with accelerated telomere shortening (10–15). Thus, one might predict that modulating or decreasing the chronic stress response would simply decrease the rate of telomere shortening over time. Our findings of an association between longitudinal reductions in distress (i.e., chronic stress response) and increased telomere length were unexpected. However, there is precedence for increased telomere length in leukocytes over time (44–46, 48), although the time frame for these longitudinal studies is longer than the current report and the clinical situations somewhat different. The increase in telomere length associated with a decrease in distress could develop in 1 of 2 ways: (i) activation of telomere lengthening mechanisms in circulating leukocytes or (ii) mobilization of younger cells with longer telomeres from progenitor pools.
We explored the latter by examining changes in the percentage of naive T cells among PBMCs over time. Longitudinal increases in telomere length among CD14− subsets, the majority of which are composed of T cells (>60%), were associated with an increased percentage of CD45RA+ CD45RO− naive T cells, suggesting that mobilization of “younger” cells leads to an increase in the proportion of naive cells relative to mature and/or senescent cells (49–51), thereby contributing to the observed increase in CD14− PBMC telomere length. Consistent with previous reports describing an inverse association between baseline telomere length and longitudinal increase in telomere length, we observed that baseline telomere length of CD14− subsets was also inversely associated with a longitudinal increase in the percentage of CD45RA+ naive T cells. There is no comparable cell marker for “young” monocytes. But the consistency of findings reported herein between the monocyte and CD14− PBMC populations support generalization of this mobilization of younger cells to other PBMC compartments. Because of the limitations of archived biospecimens (limited PBMCs), it was not possible to isolate CD45RA+ T cells to determine whether their telomere length could reasonably account for the observed longitudinal increases. However, naive T cells have been reported to have telomeres that are 1.5 to 2.4 kb longer than memory T cells suggesting this is likely the case (52). Confirmation awaits carefully designed prospective clinical studies.
Telomerase or alternative telomere lengthening mechanisms could also potentially be engaged in circulating PBMCs. Although activation of T cells has been associated with an acute induction of telomerase activity, we do not have evidence for global activation of T cells in these subjects. However, elevated levels of well-established “stress hormones” such as cortisol and catecholamines (e.g., epinephrine) have been linked with low levels of telomerase (13, 14, 53). It is possible that the persistently elevated levels of cortisol associated with chronic psychologic stress prevent telomerase from acting on and maintaining telomere length. Thus, a reduced chronic stress response with its associated decrease in systemic exposure to cortisol might yield increased telomerase activity.
Longitudinal changes in telomere length have been challenged as being primarily due to assay variability and technical reproducibility (54). This report examines a “normal” population in a longitudinal manner. They conclude that approximately 24 bp of telomere length is lost per year, although others postulate a greater normal rate of loss (1) and although over several years (~6), a small proportion of individuals had lengthening of their leukocyte telomeres; over a more substantial time frame, >12 years, there was essentially no telomere lengthening observed. However, this was not a stressed population in which individuals would have been expected to have accelerated telomere lengthening, in some cases proposed to be 5 to 10 times the normal rate of shortening. In addition, over this extended time frame, any fluctuations due to psychosocial parameters may be masked by the age-associated changes or may represent stability of their psychologic status within this “normal” population. Thus, it is difficult to generalize results from this population and these time frames, as reported by Chen and colleagues, to this study and study population (54).
It is provocative to consider the ramifications of this association between reduced chronic stress response (decreased distress) and increase in telomere length in other tissues besides PBMCs. The most common malignancies arise from epithelium with stem cell pools, which regenerate on a periodic basis, and that may be subject to this same process. Given the recognition that shortened telomeres have been documented in premalignant and dysplastic cells as well as in “normal” cells adjacent to primary malignancies, the potential for restoring normal telomere dynamics, even partially, regardless of involved mechanism(s), could make it less likely that an additional genetic insult would trigger malignant transformation and thus decrease the rate of carcinogenesis. Testing this hypothesis will require carefully designed future clinical studies in which prospective, longitudinal, relevant biospecimens can be obtained.
We recognize that the present data represent a small sample, which has limited statistical power for correlation analyses. Specifically, we acknowledge that the limited numbers of subjects in each arm of the randomized trial preclude establishing significant associations between PTC and UC with longitudinal changes in telomere length. We also appreciate that a longitudinal time frame of 4 months may not be optimal for studies of the associations between longitudinal changes in stress response and telomere length. However, it is reassuring, as noted above, that others (48) have observed similar longitudinal changes in telo-mere length associated with longitudinal decreases in perceived stress. We are aware of a previous study in which the authors have shown that 3 months of intensive lifestyle changes increased telomerase activity in PBMCs and conclude that at least 1 year may be needed to detect telomere length difference (55). Although the authors of this study report examination of telomerase, they do not report data on telomere length in their study population. In our study, the CD14°Cell population is more heterogeneous than the monocyte (CD14+) population. Because T cells are known to have a shorter life span and higher proliferation rate than B cells, it is possible that the non–T-cell contribution to the CD14− PBMC population may have blunted the capacity to detect changes in telomere length expected within this time frame in the T lymphocyte subset of the CD14− PBMC population (51). Studies with longer follow-up periods may reveal shifts in other PBMC subsets and would be helpful in characterizing the stability of these modulations, both in the stress response and in stress-associated biomarkers.
Although the flow-FISH assay is advantageous when evaluating longitudinal change in clinical samples because it can be conducted on limited biospecimens readily collected by routine phlebotomy, it is not able to evaluate the specific telomere length of individual chromosomes within a cell, nor does it provide a direct measure of number of telomere base pairs or telomeric repeats. Studies of telomere length must balance the biospecimen requirements with the desired endpoint to address specific hypotheses, as no single methodology for assaying telomere length will be optimal for all applications. Nevertheless, the flow-FISH assay provides reliable data indexing changes in telomere length within individual PBMC subsets as shown in this study and others.
Finally, it is challenging to identify the optimal patient-reported measure of chronic stress. For the purpose of showing association between a PRO and telomere length, we selected the validated BSI-18 instrument (42) due to its wide acceptance as a measure of distress in cancer populations and because we believe it to be a more direct measure of how individuals with cancer may respond to chronic stressors. Nevertheless, there is no clear consensus for the most appropriate PRO measure to evaluate an individual's chronic stress responses.
Although measures of chronic stress vary, they include a common construct that assesses stressful life circumstances, stress appraisals and distress, each of which can serve as an index of the chronic stress response. We submit that, particularly among cervical cancer survivors, the disease and long-term sequelae add to the stressful life circumstances already experienced by a majority of this survivor population (30). The significant correlation between the BSI-18 and the QOL FACT-Cx scores shows that higher levels of distress were generally associated with poorer QOL, supporting patient-reported distress as a contributory component of disruption of QOL in this study population and providing an additional avenue for future investigations.
The association between decreased distress and increased telomere length in PBMCs provides evidence for another component of the expanding biobehavioral paradigm. These data, if confirmed, taken with the epidemiologic data associating shorter telomeres with poorer cancer clinical outcomes (7–9, 21–23) could provide a potential target for decreasing cancer incidence and progression. These findings have important implications for the ability to “intercept” cancer across the entire disease trajectory, as recently described by Blackburn (56). These data underscore the need for novel cancer control measures that have the capacity to modulate precipitating and mediating biobehavioral factors that may contribute to cancer development and growth.
Acknowledgments
The authors thank Paige McDonald, PhD (Basic and Behavioral Research Branch, Cancer Control and Population Sciences, NCI) for guidance and support and Susie Hsieh, PhD, for contributions to the conduct of the study and manuscript preparation.
Grant Support
This work was supported, in part, by National Cancer Institute at the NIH (NCI R21CA098794, NCI RO1CA11836, NCI P30CA062203, and UC Irvine Chao Family Comprehensive Cancer Center to L.B. Wenzel and E.L. Nelson). K.A. Biegler was supported by UC Irvine Institutional Cancer Biology Training grant (NCI 5T32 CA09054-32) and travel/presentation support was provided by Accuri Cytometers Inc.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Authors’ Contributions
Conception and design: A.K.L. Anderson, L.B. Wenzel, E.L. Nelson
Development of methodology: K.A. Biegler, E.L. Nelson
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): E.L. Nelson
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): K.A. Biegler, A.K.L. Anderson, K. Osann, E.L. Nelson
Writing, review, and/or revision of the manuscript: K.A. Biegler, A.K.L. Anderson, L.B. Wenzel, K. Osann, E.L. Nelson
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K.A. Biegler, E.L. Nelson
Study supervision: A.K.L. Anderson, L.B. Wenzel, E.L. Nelson
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Oeseburg H, de Boer RA, van Gilst WH, van der Harst P. Telomere biology in healthy aging and disease. Pflugers Arch. 2010;459:259–68. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–21. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calado RT, Cooper JN, Padilla-Nash HM, Sloand EM, Wu CO, Scheinberg P, et al. Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia. 2012;26:700–7. doi: 10.1038/leu.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SM, Heaphy CM, Shi C, Eo SH, Cho H, Meeker AK, et al. Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias. Mod Pathol. 2011;24:256–66. doi: 10.1038/modpathol.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn E, Meeker A, Wang TL, Sehdev AS, Kurman RJ, Shih Ie M. Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol. 2010;34:829–36. doi: 10.1097/PAS.0b013e3181dcede7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lantuejoul S, Raynaud C, Salameire D, Gazzeri S, Moro-Sibilot D, Soria JC, et al. Telomere maintenance and DNA damage responses during lung carcinogenesis. Clin Cancer Res. 2010;16:2979–88. doi: 10.1158/1078-0432.CCR-10-0142. [DOI] [PubMed] [Google Scholar]
- 7.Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, et al. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6:e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:1238–50. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willeit P, Willeit J, Kloss-Brandstatter A, Kronenberg F, Kiechl S. Fifteen-year follow-up of association between telomere length and incident cancer and cancer mortality. JAMA. 2011;306:42–4. doi: 10.1001/jama.2011.901. [DOI] [PubMed] [Google Scholar]
- 10.Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere shortening in formerly abused and never abused women. Biol Res Nurs. 2012;14:115–23. doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–71. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Ann Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 13.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–87. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Huzen J, van der Harst P, de Boer RA, Lesman-Leegte I, Voors AA, van Gilst WH, et al. Telomere length and psychological well-being in patients with chronic heart failure. Age Ageing. 2010;39:223–7. doi: 10.1093/ageing/afp256. [DOI] [PubMed] [Google Scholar]
- 16.Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30:1649–56. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava S, Boyer JL. Psychological stress is associated with relapse in type 1 autoimmune hepatitis. Liver Int. 2010;30:1439–47. doi: 10.1111/j.1478-3231.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170:1884–91. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitton A, Dobkin PL, Edwardes MD, Sewitch MJ, Meddings JB, Rawal S, et al. Predicting relapse in Crohn's disease: a biopsychosocial model. Gut. 2008;57:1386–92. doi: 10.1136/gut.2007.134817. [DOI] [PubMed] [Google Scholar]
- 20.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–45. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 21.Costanzo ES, Sood AK, Lutgendorf SK. Biobehavioral influences on cancer progression. Immunol Allergy Clin North Am. 2011;31:109–32. doi: 10.1016/j.iac.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanzo M, Colucci R, Arunachalam M, Berti S, Moretti S. Stress as a possible mechanism in melanoma progression. Dermatol Res Pract. 2010;2010:483–93. doi: 10.1155/2010/483493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 24.Frick LR, Arcos ML, Rapanelli M, Zappia MP, Brocco M, Mongini C, et al. Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress. 2009;12:134–43. doi: 10.1080/10253890802137437. [DOI] [PubMed] [Google Scholar]
- 25.Hasen NS, O'Leary KA, Auger AP, Schuler LA. Social isolation reduces mammary development, tumor incidence, and expression of epigenetic regulators in wild-type and p53-heterozygotic mice. Cancer Prev Res. 2010;3:620–9. doi: 10.1158/1940-6207.CAPR-09-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci U S A. 2009;106:22393–8. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–7. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15:251–9. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson EL, Wenzel LB, Osann K, Dogan-Ates A, Chantana N, Reina-Patton A, et al. Stress, immunity, and cervical cancer: biobehavioral outcomes of a randomized clinical trial [corrected]. Clin Cancer Res. 2008;14:2111–8. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaker PH, Sood AK. Neuroendocrine influences on cancer biology. Semin Cancer Biol. 2008;18:164–70. doi: 10.1016/j.semcancer.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackburn EH, Tlsty TD, Lippman SM. Unprecedented opportunities and promise for cancer prevention research. Cancer Prev Res. 2010;3:394–402. doi: 10.1158/1940-6207.CAPR-10-0051. [DOI] [PubMed] [Google Scholar]
- 34.Andersen BL, Yang HC, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450–8. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegel D. Mind matters in cancer survival. JAMA. 2011;305:502–3. doi: 10.1001/jama.2011.69. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser NC, Hartoonian N, Owen JE. Toward a cancer-specific model of psychological distress: population data from the 2003–2005 National Health Interview Surveys. J Cancer Survivorship. 2010;4:291–302. doi: 10.1007/s11764-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 37.Herzog TJ, Wright JD. The impact of cervical cancer on quality of life–the components and means for management. Gynecol Oncol. 2007;107:572–7. doi: 10.1016/j.ygyno.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel L, DeAlba I, Habbal R, Kluhsman BC, Fairclough D, Krebs LU, et al. Quality of life in long-term cervical cancer survivors. Gynecol Oncol. 2005;97:310–7. doi: 10.1016/j.ygyno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Ashing-Giwa KT, Lim JW, Tang J. Surviving cervical cancer: does health-related quality of life influence survival? Gynecol Oncol. 2010;118:35–42. doi: 10.1016/j.ygyno.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 40.Biegler K, Wenzel L, Osann K, McClure L, Monk B, Nelson E, editors. Assessing quality of life and biomarkers in cervical cancer survivors. International Society of Quality of Life; New Orleans, LA: 2009. [Google Scholar]
- 41.Andersen BL. Stress and quality of life following cervical cancer. J Natl Cancer Inst Monogr. 1996:65–70. [PubMed] [Google Scholar]
- 42.Zabora J, BrintzenhofeSzoc K, Jacobsen P, Curbow B, Piantadosi S, Hooker C, et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics. 2001;42:241–6. doi: 10.1176/appi.psy.42.3.241. [DOI] [PubMed] [Google Scholar]
- 43.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc. 2006;1:2365–76. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 44.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1:81–8. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–9. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38:1725–34. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 48.Lin J, Puterman E, O'Donovan A, Epel E, Blackburn E, editors. Psychological stress and its relationship to telomere length maintenance [abstract].. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; Orlando, FL. Philadelphia (PA). 2011 Apr 2–6; AACR; 2011. [Google Scholar]
- 49.Aladdin H, Katzenstein T, Dreves AM, Ryder L, Gerstoft J, Skinhoj P, et al. T-cell receptor excisional circles, telomere length, proliferation and apoptosis in peripheral blood mononuclear cells of human immunodeficiency virus-infected individuals after 18 months of treatment induced viral suppression. Scand J Immunol. 2003;57:485–92. doi: 10.1046/j.1365-3083.2003.01258.x. [DOI] [PubMed] [Google Scholar]
- 50.Allsopp RC, Cheshier S, Weissman IL. Telomerase activation and rejuvenation of telomere length in stimulated T cells derived from serially transplanted hematopoietic stem cells. J Exp Med. 2002;196:1427–33. doi: 10.1084/jem.20021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol. 2008;59(Suppl 9):169–86. [PubMed] [Google Scholar]
- 52.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–7. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 53.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–5. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66:312–9. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–57. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn EH. Walking the walk from genes through telomere maintenance to cancer risk. Cancer Prev Res. 2011;4:473–5. doi: 10.1158/1940-6207.CAPR-11-0066. [DOI] [PubMed] [Google Scholar]