Summary
Sphingolipids are essential molecules of the mammalian epidermis. Keratinocytes generate and secrete huge amounts of ceramide-precursors to the extracellular domain of the stratum corneum, where they are further metabolized to specific ceramide species. The arrangement of ceramides to well-organized lipid lamellae is essential to form the epidermal barrier. Besides their role as structural components sphingolipids are also critical molecules involved in the modulation of epidermal cells. Sphingosine-1-phosphate (S1P) has been identified as a prominent signaling molecule which regulates fundamental functions of keratinocytes and skin dendritic cells. Thus, S1P inhibits proliferation of keratinocytes and induces their differentiation. Moreover, antigen uptake, migration and cytokine production of dendritic cells are under the control of this sphingolipid. A dysregulation of the sphingolipid metabolism has been discussed in inflammatory skin disorders like atopic dermatitis. Animal models of contact dermatitis provide evidence that topical treatment with S1P is connected with an anti-inflammatory action suggesting a novel approach for the treatment of atopic dermatitis.
Keywords: Sphingolipids, sphingosine, 1-phosphate, keratinocytes, dendritic cells, atopic dermatitis
Cite this as Japtok L, Bäumer W, Kleuser B. Sphingosine-1-phosphate as a signaling molecule in the skin - Relevance in atopic dermatitis. Allergo J Int 2014; 23: 54–9
Sphingolipids as structural components of the skin barrier
In the year 1884 the German physician J. L. Thudichum was able to isolate an up to then unknown new class of lipids from the human brain through fractional crystallization. However, the elucidation of the structure of these novel lipids presented a great puzzle, so that he termed them sphingolipids in anology to the Greek sphinx. The sphingolipids are of particular significance in the development of the epidermal barrier. Skin cells, particularly keratinocytes, produce large amounts of glycosylceramides and sphingomyelin (structure see Fig. 1) that are secreted into the stratum granulosum and the stratum corneum [1, 2]. Here cleavage and further metabolism of the sphingolipids to various ceramide species occur [3]. These play a decisive role in the formation of the lamellar structures of skin lipids. Together with cholesterol derivatives and fatty acids they form a crystalline matrix surrounding the structural proteins and corneocytes of the skin. The significance of ceramides can be seen in the fact that they compose more than 50 % of the lipid fraction [4]. It is therefore not surprising that dysfunctions in sphingolipid metabolism are associated with a disturbed skin barrier. In fact, a reduced ceramide content of the skin is frequently found in patients with atopic dermatitis [5]. As a result there is a disturbed lipid barrier and an increased transepidermal water loss. Here it is of interest that the chain length of the N-acylated fatty acids in the ceramides of the skin displays a far greater variance than in ceramides in other tissues. Especially long-chain, polyunsaturated fatty acids (C26 or longer) are characteristic for the ceramides of the skin. This also appears to be of great significance for the formation of the lamellar skin barrier [6]. When this enzyme, termed ceramide synthase 3, is lacking, a disturbance of the skin barrier results [6].
Fig. 1.
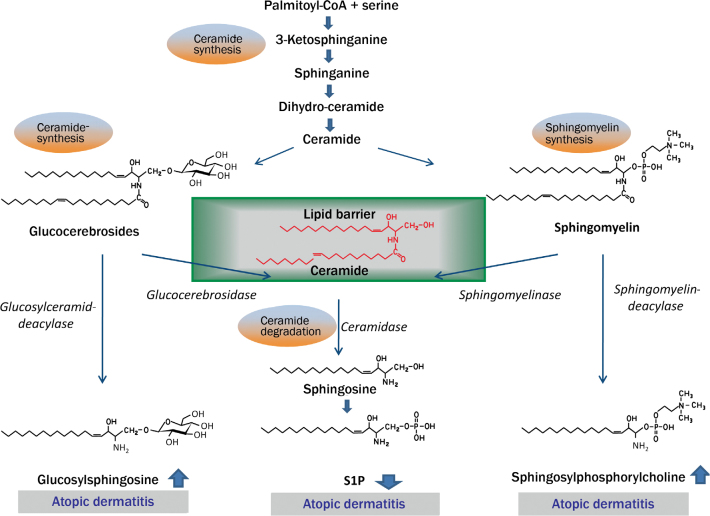
Sphingolipid rheostat in atopic dermatitis. Keratinocytes produce sphingolipids via the de novo pathway from palmitoyl-CoA and serine in the form of glucocerebrosides and sphingomyelin. These are secreted into the stratum corneum where they are transformed into ceramides and are available for the skin barrier. In atopic dermatitis the increased activity of the two enzymes glucosylceramide and sphingomyelin-deacylase result in reduced ceramide levels and in a disturbance of the epidermal barrier. An increased S1P lyase activity is also discussed, so that S1P concentrations are reduced leading to a reduction in function as a biological signaling molecule.
© B. Kleuser
Further, it has become clear in recent years that sphingolipids not only represent essential structural components. Rather it appears that specific sphingolipids derivatives act as central signal molecules that modulate the properties of the skin barrier, but more importantly the biological function of keratinocytes and immune cells of the skin. Here the sphingolipid sphingosine-1-phosphate (S1P) plays a leading role.
Sphingosine-1-posphate as biologically active signaling molecule
The role of specific sphingolipid derivatives as signaling molecules became evident in 1998, after the first G-protein-coupled cell membrane receptor for S1P was identified [7]. S1P is an intermediate of sphingolipid metabolism that can be formed during the degradation of ceramides (Fig. 2). Ceramidases catalyze the cleavage of ceramides to sphingosine that in a further step is phorphorylated by sphingosine kinases to active S1P. The degradation of S1P can take two different pathways. In a reversible process phosphatases can hydrolyze S1P back to sphingosine. An irreversible degradation of S1P occurs through S1P lyase that cleaves the sphingolipid into phosphoethanolamine and hexadecenal [8]. The inhibition of S1P lyase in vivo is associated with elevated S1P concentrations in tissue and lymph [9]. The altered S1P levels are linked to an immunosuppressive effect. Under physiological conditions an S1P concentration gradient exists between blood, lymph and lymphatic tissue. The cause is the secretion of S1P by erythrocytes leading to higher plasma levels of S1P [10]. In lymph and particularly in the lymphatic tissue the S1P concentrations are much lower; here only the endothelial cells are involved in the production [11]. S1P mediates a major share of its effects by binding on five S1P receptor subtypes that are termed S1P1–S1P5 [12]. In recent years it has been found that S1P is a central molecule regulating the circulation of T lymphocytes between lymph, plasma and tissue. Of particular significance here is the function of the S1P1 receptor subtype of the T cells. Stimulation of S1P1 receptor is a chemotactic stimulus for the migration of T lymphocyctes out of lymphatic tissue; the concentration gradient of S1P is decisive for this [13]. When this receptor is blocked, the T lymphocytes remain in the lymphatic tissues. In face of high concentrations of S1P in the blood, the S1P1 receptor is internalized. Only in the secondary lymphatic tissues is the S1P1 receptor reexpressed due to the low level of S1P, so that once again outward migration according to the concentration gradient can occur. Different possibilities exist to influence this finely regulated migration behavior of T lymphocytes. The pharmacological inhibition of the S1P lyase also results in increased S1P content in the lymphatic tissues; this results in a disturbance of the concentration gradient. The lyase inhibitor LX2931 is a substance undergoing clinical trials for the treatment of rheumatoid arthritis [9]. Fingolimod (FTY720), in contrast, is already licensed for multiple sclerosis. The metabolically active form of fingolimod attacks all S1P receptors with the exception of S1P2 receptor and in contrast to S1P leads to a long-lasting internalization of the S1P1 receptor in T lymphocytes. The lymphatic tissues therefore do no longer react to the chemotactic S1P stimulus and the lymphocytes remain in the lymph nodes [12]. The immunomodulatory effect of S1P is, however, not limited to T cells. Rather, other immune and tissue cells are also affected by the sphingolipid. In the skin these are particularly keratinocytes and dendritic cells.
Fig. 2.
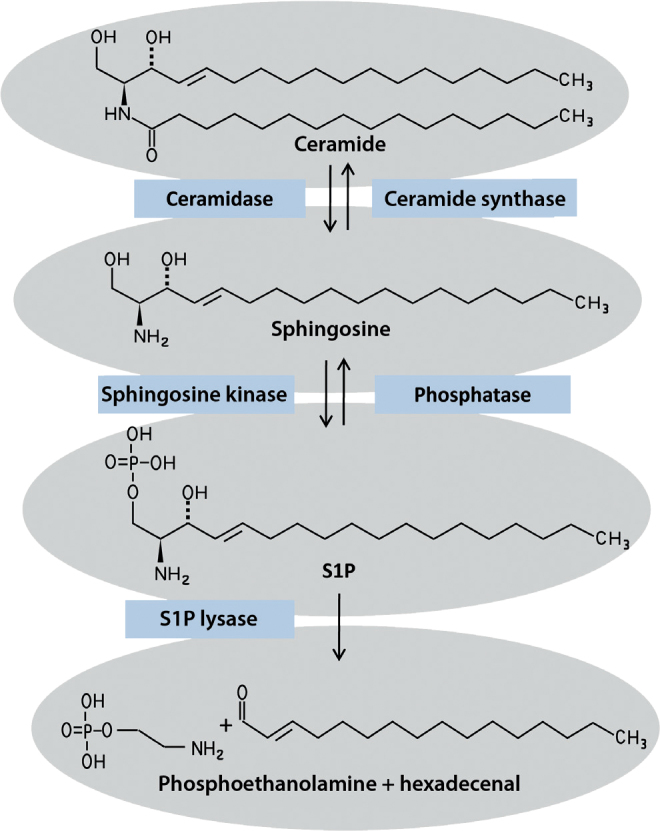
Metabolism of sphingosine-1-phosphate. S1P can be produced from ceramides via intermediates from sphingosine. Besides this reversible pathway, S1P can also be irreversibly cleaved to phosphoethanolamine and hexadecenal by S1P lyase.
© B. Kleuser
Sphingosine-1-phosphate as biologically active signaling molecule of the skin
Keratinocytes are the most important skin cells for the formation of the epidermal lipid barrier. However, they are not only involved in the biosynthesis of sphingolipids, rather numerous functions of the epidermal cells are also regulated by biologically active sphingolipid molecules. Of particular importance here, too, is once again the sphingolipid S1P. Even though S1P possesses a proliferation-promoting effect in numerous cells, in keratinocytes after stimulation with S1P there is an inhibition of cell division. This is not due to a cytotoxic effect, but is a result of the cells entering the G0 phase of the cell cycle [14]. Not only is there an inhibition of proliferation, but also a strong elevation of the intracellular calcium content by S1P [15]. For keratinocytes this is the most important signal for the differentiation process, so that after S1P stimulations keratinocytes are transformed into corneocytes. This effect is also utilized in the treatment of psoriasis with calcitriol or calcipotriol. Here it could be shown that the antiproliferative and differentiation-promoting effect of active vitamin D3 as well as its analog calcipotriol are mediated via the production of S1P [14]. The receptor subtype for the antiproliferative effect of S1P could also be identified in keratinocytes. After stimulation of the S1P2 receptor subtype by S1P, there is an inhibition of the Akt signaling pathway essential for cell proliferation in keratinocytes. It is therefore of significance that the already mentioned fingolimod is not capable of stimulating exactly this receptor subtype and therefore also has no proliferation-inhibiting effect [16].
Besides the keratinocytes, numerous immunocompetent cells are found in the skin. The antigen-presenting cells play a central role here.
Sphingolipid S1P centrally impacts the homeostasis of dendritic cells by modulating several functions from antigen uptake up to T cell interaction. In the presence of S1P the endocytotic capacity of the dendritic cells is reduced. In the mouse model the topical application of S1P reduces the ability for antigen uptake by epidermal dendritic cells by more than 40 % [17]. The cause for this reduced antigen uptake is that similar to keratinocytes the Akt signaling pathway is also inhibited in dendritic cells. In these cells the activation of this signaling cascade is, however, associated with a reconstruction of the cytoskeleton that in a special form is of decisive importance for the regulation of macropinocytosis. Here, too, the inhibition of macropinocytosis by S1P results from a stimulation of the S1P2 receptor subtype. After uptake of the antigens there is migration of dendritic cells that is also strongly modulated by S1P. Interestingly, further S1P receptor subtypes are responsible for the modulation of the targeted cell migration. The S1P1 and S1P3 receptor subtypes impact the chemotactic response of the antigen-presenting cells. These receptors are responsible for the fact that the dendritic cells migrate in the direction of an S1P gradient [18]. When this gradient is disturbed by topical application of S1P, migration from the epidermis in direction of the lymph nodes is inhibited. It is there that the interaction of the activated and mature dendritic cells with the naïve T cells occurs. Here, too, a great impact by the sphingolipid is observed. When dendritic cells are treated with S1P, the cytokine pattern is specifically altered. Especially interleukin(IL)-12 secretion of activated dendritic cells is inhibited by S1P [19]. This is associated with an antiinflammatory action. The differentiation of the naïve T cells to the cellular Th1 immune response is reduced. It must, however, be mentioned critically that this may be associated with a shift of the Th1/Th2 balance.
Antigen uptake, migration and interaction of dendritic cells with T lymphocytes are essential steps also in the pathogenesis of atopic dermatitis. A dysregulation of the sphingolipid metabolism may therefore play an important role.
Sphingolipid dysfunction in atopic dermatitis
Many gene mutations that can have an effect on the epidermal barrier have been reported in atopic dermatitis. The most prominent example are mutations in the filaggrin gene: As a structure-bound component the resulting protein is essential for the skin barrier [20]. However, the lipid composition is also altered in atopic dermatitis, so that no lipid structures with a lamellar organization can be formed. Especially ceramides are qualitatively and quantitatively altered [5]. The reason for this altered ceramide content appears to lie in the fact that in patients with atopic dermatitis the ceramide precursors are degraded in another fashion than in healthy skin. A detailed depiction of the altered sphingolipid metabolism is seen in Fig. 1. This disturbed barrier function is associated with an easier penetration of allergens and thus with increased contact to the dendritic cells (Fig. 3).
Fig. 3.
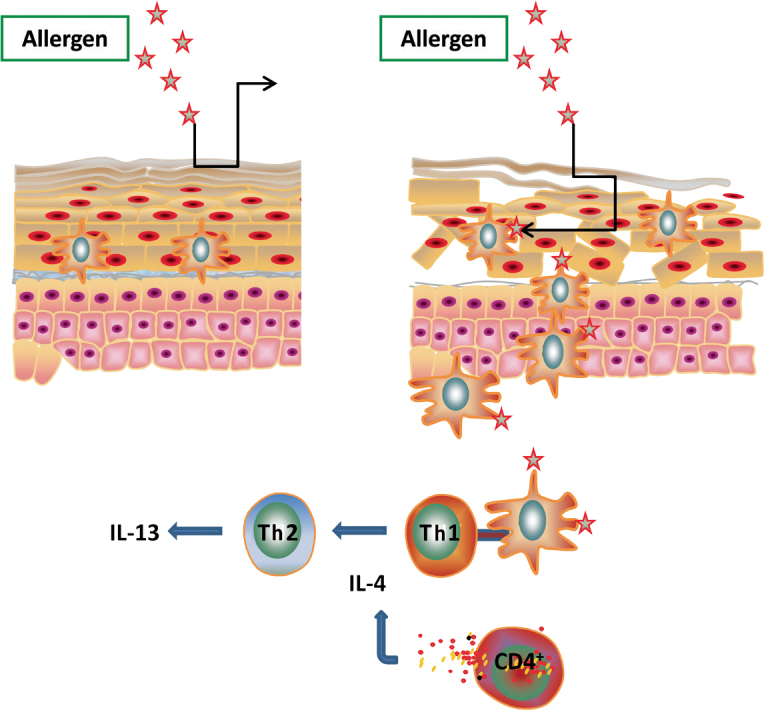
Sphingolipid dysregulation and atopic dermatitis. In the presence of a disturbed skin barrier, allergens have easier access to the antigen-presenting cells. These take the allergen up and migrate to the next lymph nodes. There the interaction with the naïve T cells occurs. S1P regulates antigen uptake, migration and cytokine secretion of dendritic cells.
© B. Kleuser
Further, the S1P content appears to be of great significance for the homeostasis of the skin. In atopic dermatitis there are increasing indications that the S1P concentration is reduced. Reduced S1P concentrations are then linked with an increased endocytosis capacity of dendritic cells. In addition, the proliferation of keratinocytes is increased and their differentiation disturbed. In skin biopsies on the messenger-RNA (mRNA) level, an increased expression of S1P lyase that irreversibly cleaves the lipid in phosphoethanolamine and hexadecenal has been identified [21]. The S1P levels in the skin in patients with atopic dermatitis have not yet, however, been measured. Nonetheless, a reduced S1P level is found in skin lesions of atopic dogs [22]. In this respect it must be mentioned that there are great similarities between human and canine skin diseases. It will therefore be highly interesting to see if a reduced S1P content can also be detected in humans. Finally, a reduced S1P level would be associated with increased antigen uptake, an altered migration behavior as well as an increased T cell interaction.
Topical application of S1P in the model of inflammation
Not only the course of atopic dermatitis, but also allergic contact dermatitis is affected by S1P. In a murine contact dermatitis model, the topical application of S1P was studied. TDI (Toluene-2,4-diisocyanate) was employed as hapten. The immunomodulatory effect of S1P was examined both in the sensitization as well as in the challenge phase. In the sensitization phase S1P reduces the weight and the cell number in the regional lymph nodes (auricular lymph nodes). In fact, the number of dendritic cells migrating from the skin into the lymph node was reduced after S1P application. Also the cytokine pattern in the regional lymph nodes was affected by topical treatment with S1P [23]. The lymph node cells demonstrated a significantly reduced secretion of the cytokines IL-6 and interferon(IFN)-γ. Corresponding to the reduced number of dendritic cells in the lymph node, immunohistochemical studies revealed that antigen-presenting cells remained in the epidermis. In the elicitation phase of contact dermatitis S1P likewise had an antiinflammatory effect. Topical S1P application led to a reduced accumulation of T cells in the skin. The following effects appear to be responsible for the altered immune behavior: Immunohistochemichal studies show that antigen uptake by the dendritic cells is reduced on the one hand. On the other hand, the migration behavior of these antigen-presenting cells is altered, so that they increasingly remain in the skin and are not available for interaction with the T cells in the lymph node. The altered chemotactic behavior of the cells can be explained by the fact that topically applied S1P results in an S1P1 receptor internalization. In this manner, the migration to the next lymph nodes is disturbed. On the other hand, the S1P gradient between tissue and lymph is also affected, so that the targeted migration is reduced.
These studies suggest that topically applied S1P may represent a novel option in the treatment of inflammatory skin diseases.
Abbreviations
- CoA
Coenzyme A
- IFN
Interferon
- IL
Interleukin
- mRNA
messenger RNA
- RNA
Ribonucleic acid
- S1P
Sphingosine-1-phosphate
- TDI
Toluene-2,4-diisocyanate
- Th
T helper cells
Footnotes
Conflicts of interest
The authors declare that no conflicts of interests exist.
References
- 1.Hamanaka S, Hara M, Nishio H, Otsuka F, Suzuki A, Uchida Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J Invest Dermatol. 2002;119:416–23. doi: 10.1046/j.1523-1747.2002.01836.x. [DOI] [PubMed] [Google Scholar]
- 2.Jensen JM, Fölster-Holst R, Baranowsky A, Schunck M, Winoto-Morbach S, Neumann C, et al. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J Invest Dermatol. 2004;122:1423–31. doi: 10.1111/j.0022-202X.2004.22621.x. [DOI] [PubMed] [Google Scholar]
- 3.Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, et al. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49:1466–76. doi: 10.1194/jlr.M800014-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–66. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–6. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 6.Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- 7.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–5. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 8.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–6. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 9.Bagdanoff JT, Donoviel MS, Nouraldeen A, Carlsen M, Jessop TC, Tarver J, et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(Isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–62. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]
- 10.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–7. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 11.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–76. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 14.Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schäfer-Korting M, Kleuser B. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–9. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 15.Lichte K, Rossi R, Danneberg K, ter Braak M, Kürschner U, Jakobs KH, et al. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J Invest Dermatol. 2008;128:1487–98. doi: 10.1038/sj.jid.5701207. [DOI] [PubMed] [Google Scholar]
- 16.Schüppel M, Kürschner U, Kleuser U, Schäfer-Korting M, Kleuser B. Sphingosine 1-phosphate restrains insulin-mediated keratinocyte proliferation via inhibition of Akt through the S1P2 receptor subtype. J Invest Dermatol. 2008;128:1747–56. doi: 10.1038/sj.jid.5701259. [DOI] [PubMed] [Google Scholar]
- 17.Japtok L, Schaper K, Bäumer W, Radeke HH, Jeong SK, Kleuser B. Sphingosine 1-phosphate modulates antigen capture by murine Langerhans cells via the S1P(2) receptor subtype. PLoS One. 2012;7:e49427. doi: 10.1371/journal.pone.0049427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radeke HH, von Wenckstern H, Stoidtner K, Sauer B, Hammer S, Kleuser B. Overlapping signaling pathways of sphingosine 1-phosphate and TGF-beta in the murine Langerhans cell line XS52. J Immunol. 2005;174:2778–86. doi: 10.4049/jimmunol.174.5.2778. [DOI] [PubMed] [Google Scholar]
- 19.Schröder M, Richter C, Juan MH, Maltusch K, Giegold O, Quintini G, et al. The sphingosine kinase 1 and S1P1 axis specifically counteracts LPS-induced IL-12p70 production in immune cells of the spleen. Mol Immunol. 2011;48:1139–48. doi: 10.1016/j.molimm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012;122:440–7. doi: 10.1172/JCI57416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo EY, Park GT, Lee KM, Kim JA, Lee JH, Yang JM. Identification of the target genes of atopic dermatitis by real-time PCR. J Invest Dermatol. 2006;126:1187–9. doi: 10.1038/sj.jid.5700234. [DOI] [PubMed] [Google Scholar]
- 22.Bäumer W, Rossbach K, Mischke R, Reines I, Langbein-Detsch I, Lüth A, Kleuser B. Decreased concentration and enhanced metabolism of sphingosine-1-phosphate in lesional skin of dogs with atopic dermatitis: disturbed sphingosine-1-phosphate homeostasis in atopic dermatitis. J Invest Dermatol. 2011;131:266–8. doi: 10.1038/jid.2010.252. [DOI] [PubMed] [Google Scholar]
- 23.Reines I, Kietzmann M, Mischke R, Tschernig T, Lüth A, Kleuser B, Bäumer W. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol. 2009;129:1954–62. doi: 10.1038/jid.2008.454. [DOI] [PubMed] [Google Scholar]