Summary
At an incidence of 1:500, celiac disease (formerly sprue) is an important differential diagnosis in patients with malabsorption, abdominal discomfort, diarrhea and food intolerances. Celiac disease can induce a broad spectrum of both gastrointestinal and extraintestinal symptoms, e.g. dermatitis herpetiformis (Duhring’s disease). A variety of oligo- and asymptomatic courses (e.g. anemia, osteoporosis, depression) through to refractory collagenic celiac disease are seen.
In HLA-DQ2 and -8 predisposed individuals, celiac disease is provoked by contact with wheat gliadin fractions through a predominantly Th1 immune response and an accompanying Th2 response, which can eventually lead to villous atrophy. Using appropriate serological tests (IgA antibodies against tissue-transglutaminase, endomysium and deamidated gliadin peptides) under sufficient gluten ingestion, the diagnosis can be made more reliably today than previously. The same IgG-based serological tests should be used in the case of IgA deficiency.
Diagnosis can either be made in children and adolescents with anti-transglutaminase titers exceeding ten times the standard for two of the above-mentioned serological markers and HLA conformity or it is made by endoscopy and histological Marsh classification in adults and in cases of inconclusive serology. If clinically tolerated, gluten challenges are indicated in patients that already have reduced gluten intake, in borderline serological results, discordance between serological and histological results or in suspected food allergy.
The diagnosis of celiac disease needs to be definitive and robust before establishing a gluten-free diet, since lifelong abstention from gluten (gliadin < 20 mg/kg foodstuffs), cereal products (wheat, rye, barley and spelt) as well as from preparations and beverages containing gluten, is necessary. With effective elimination of gluten, the prognosis regarding complete resolution of small bowel inflammation is good. Refractory courses are seen only in rare cases, accompanied by enteropathy-associated T-cell lymphoma.
Keywords: Celiac disease, sprue, enteropathy, malabsorption, cereal intolerance
Cite this as Hahn M, Hagel AH, Hirschmann S, Bechthold C, Konturek P, Neurath M, Raithel M. Modern diagnosis of celiac disease and relevant differential diagnoses in the case of cereal intolerance. Allergo J Int 2014; 23: 67–77
Introduction
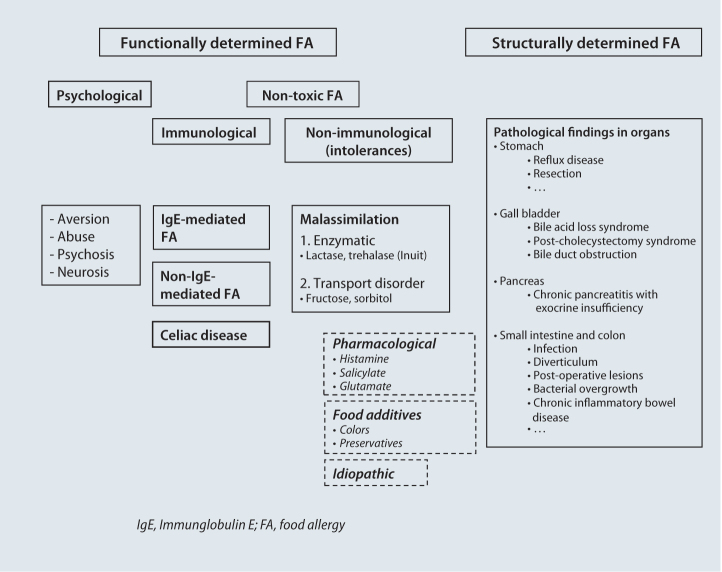
Celiac disease is a gluten-sensitive enteropathy previously referred to as “sprue” (ICD-10: K90.0). It represents a chronic immune-mediated disorder of the mucous membrane of the small intestine. Gluten peptides (alcohol-soluble gluten fractions, so-called gliadins) found in cereals (wheat, rye, barley and spelt) and related prolamins serve are triggers leading to celiac onset in genetically predisposed individuals [1, 2, 3]. Whilst the disorder often used to be referred to as celiac disease in children, the term “celiac sprue” is used for adults. According to the more recent nomenclature, the term celiac disease should be used for all age groups. In the broad spectrum of varying etiologies of food intolerance, celiac disease represents a distinct immune-mediated entity (Fig. 1). From an historical perspective, celiac disease was initially believed to be a malabsorption disorder, only later being interpreted as a hypersensitivity reaction in type-IV allergy to wheat or its constituents. Finally, after gaining a precise understanding of its pathogenesis on the basis of the characteristic production of transglutaminase (TG) antibodies, it is classified today as an autoimmune reaction [1, 2, 3].
Fig. 1.
Polyetiologic spectrum by food allergies
The prevalence of celiac disease is subject to wide geographical variation, reaching 1:500 in Germany, for example, whereby women are more commonly affected than men. At the same time, it is assumed that the disorder is diagnosed in only 10 %–20 % of affected individuals (the so-called iceberg phenomenon). Virtually all individuals suffering from celiac disease carry one of the two human leukocyte antigen (HLA) subtypes DQ2 or DQ8. A number of diseases frequently occur in association with celiac disease: autoimmune diseases such as autoimmune thyroiditis (10 %–20 %), lactose malabsorption (20 %–30 %, often also as a result of villous atrophy), type-1 diabetes mellitus (2 %–7 %), selective immunoglobulin (Ig)-A deficiency (3 %–10 %), Turner syndrome (8 %) and Down’s syndrome (7 %).
Pathogenesis of celiac disease
Gluten peptides (gliadin fraction) are taken up via the mucous membrane of the small intestine and deamidated by tissue transglutaminase. The complexes formed by tissue transglutaminase and the modified gliadin are taken up by DQ2+ and DQ8+ antigen-processing cells and presented to CD4+ helper cells via the major histocompatibility complex (MHC) class-II receptor complex [1, 2, 3]. Following activation, these T-helper cells stimulate cytotoxic CD8+ lymphocytes (e.g., intraepithelial lymphocytes) by means of increasingly expressed Th1 cytokines (interleukin 2, 6 and γ-Interferon) on the one hand, and B-lymphocytes via Th2 cytokines (interleukin 4, 5, 10) on the other. This results in cytotoxic and antibody-mediated mechanisms as well as fibroblast activation (matrix metalloproteinase secretion), which together lead to epithelial damage, inflammatory cell infiltration of the lamina propria and ultimately to varying degrees of villous atrophy [2, 3, 4, 5]. Florid immune responses result in lesions characteristic of celiac disease, such as increased intraepithelial lymphocytes (> 25 lymphocytes/100 epithelial cells), crypt hyperplasia and partial or total villous atrophy [2].
Celiac disease-like symptoms and differential diagnosis
Independent of this clearly defined pathogenetic disease mechanism of celiac disease, there is currently evidence that gastrointestinal symptoms resulting from gluten exposure can also be provoked in patients who, for the most part, are not genetically predisposed by HLA-DQ2 and/or HLA-DQ8 (referred to as non-celiac disease gluten intolerance) [5]. In contrast to celiac disease, interleukin-6 expression levels in gastric tissue, which, among others things, amplify the mucosal inflammatory reaction in celiac disease, are not elevated in this setting. To what extent local intestinal IgE antibodies (local type-1 allergy), local cellular allergic responses to food (local type-IV allergy) or other non-immunological mechanisms play a role here in patients with symptoms of irritable bowel syndrome or food intolerance is currently unclear [4, 6, 7, 8, 9, 10] (Tab. 1).
| Immune-mediated mechanisms |
| • Celiac disease – prolamines (gliadin peptides) – transglutaminase-IgA antibodies (see Tab. 3) |
| - Systemic detection |
| - Local intestinal detection |
| • Food allergy to wheat or other cereal types |
| - Systemic detection |
| - Local intestinal detection |
| Type I – e.g. wheat flour, wheat pollen, gluten, gliadin (e.g. ω-5 gliadin), other allergens (differential diagnosis: gluten substitutes, e.g. lupin flour, corn) |
| Type IV – e.g. wheat flour, wheat pollen, other allergens |
| Non-immune-mediated mechanisms |
| • Non-celiac disease gluten intolerance – no glutaminase-IgA antibody production (gluten sensitivity without celiac disease or food allergy) |
| e.g. gluten ataxia, myopathy, irritable bowel syndrome |
| • Fructan-induced abdominal symptoms |
| - Fructooligosaccharide and fructopolysaccharide, e.g. irritable bowel syndrome |
| • Small intestinal bacterial overgrowth, carbohydrate malassimilation |
Immunologically active prolamins, which are only partially digestible by humans, are found in various cereal types. They include gliadin in wheat, secalin in rye, hordein in barley and avenin in oat, whereby immunological reactivity in celiac disease decreases according to the sequence given here from wheat through to oat and is determined by the patient’s sensitivity. Therefore, a gluten-free diet in celiac disease always includes at least wheat, spelt, rye and barley, whereas only wheat (gliadin) needs to be specifically avoided in wheat-mediated food allergy, but not rye and barley, assuming there is no crossreactivity with other cereal types.
In the case of gluten sensitivity (hitherto) classified as non-immunological, the specific IgA antibodies to transglutaminase and endomysium are negative, whereas approximately 50% of patients exhibit nonspecifically elevated antibodies to gliadin as well as occasional HLA-DQ2/DQ8 positivity. It is not yet known whether this form of gluten sensitivity is triggered by local immunological mechanisms after all or whether it is the result of a simple permeability disorder in the gastrointestinal tract (another disease?). IgA, Immunoglobulin A; HLA, human leukocyte antigen
Schuppan et al. discuss the involvement of α-amylase/trypsin inhibitors, components of wheat, which, by activating the Toll-like receptor-4 (TLR4), contribute to the mediation of inflammation in both celiac disease and non-celiac disease patients. Animal models have shown that mice in which the TLR4 gene has been switched off do not respond to oral provocation with α-amylase/trypsin inhibitors with a systemic inflammatory response [11].
In addition to systemic or locally detectable allergic responses to wheat allergens, a variety of non-immunological mecha-nisms also need to be considered in the differential diagnosis of patients with wheat or cereal intolerance. In this context, ruling out fructan-induced abdominal symptoms plays an important role in TG-IgA-negative patients, for example, since it is precisely wheat-based foodstuffs containing fructo-oligo- or polysaccharides that cannot be digested, are osmotically active and can cause symptoms of irritable bowel syndrome [10]. Small intestinal bacterial overgrowth, which leads to increased bacterial proliferation following food intake [8, 10, 12, 13], can also cause abdominal symptoms following cereal ingestion. Small intestinal bacterial overgrowth may be associated with intolerance to simple (e. g., fructose) and complex carbohydrates (e. g., starch, fructose polymers).
The term gluten sensitivity is sometimes used for those patients who, despite celiac disease-like symptoms, show no TG-IgA antibodies [14].
Clinical picture of celiac disease
Celiac disease was long considered a pediatric syndrome with the typical combination of symptoms comprising diarrhea, steatorrhea, malabsorption and failure to thrive. More recently, however, an increasing number of adults and elderly individuals have also been diagnosed with celiac disease, the clinical spectrum of the disease has expanded and the clinical picture has altered [2, 3, 4, 5, 8, 13, 15, 16]. It is assumed that only around 10 %–40 % of affected patients exhibit typical symptoms (see also “Classic celiac disease with typical symptoms”) [1, 2, 3, 12, 13, 15]. Today, more than 50 % of patients present with atypical symptoms such as anemia, iron deficiency without anemia, abdominal pain, psychiatric symptoms like mood swings, skin lesions, osteoporosis, loss of appetite and growth retardation. Dermatitis herpetiformis (Duhring’s disease), depression, gluten-sensitive ataxia or spontaneous abortion [12, 13, 15, 16] are seen more rarely.
The various forms of celiac disease
A distinction is made between seven clinical forms of celiac disease (formerly sprue) on the basis of phenotype and clinical course; these forms are discussed below.
Classic celiac disease with typical symptoms
Classic symptoms include diarrhea, weight loss, malabsorption disorders, primarily in infants or small children (aged 1–5 years), often associated with failure to thrive, growth retardation, and endocrine disorders [1, 2, 3, 6, 12]. This form becomes less common with increasing age.
Atypical celiac disease (oligo- or monosymptomatic)
Gastrointestinal symptoms are completely absent in approximately 40 % of affected individuals (adults) [6, 15, 16]. Relevant extraintestinal manifestations include, among many others:
Anemia (iron deficiency)
Osteoporosis
Chronically elevated transaminases
Atrophic and erythematous tongue
Arthritis
Psychiatric and neurological disorders (depressive episodes)
Chronic fatigue
The erythemas, plaques and herpetiform blisters characteristic of dermatitis herpetiformis and sometimes mistaken for skin manifestations of food allergy occur in 5%–10% of all celiac disease patients as an extraintestinal symptom. Efflorescences are most commonly found on the extensor sides of the extremities.
Asymptomatic celiac disease (silent celiac disease)
Positive antibody findings, HLA-DQ2 and/or -DQ8 positivity and pathological small intestine biopsy in the absence of dis-ease symptoms is referred to as asymptomatic celiac disease [6, 12, 16, 17, 18, 19]. This particular form is often diagnosed incidentally and, from a histological perspective, responds well and promptly to gluten abstention. However, the symptom-free patient often primarily lacks the understanding needed to adhere to a diet and requires information and training. Abstention from gluten nevertheless has the potential to prevent the long-term complications of celiac disease (e.g., enteropathy-associated T-cell lymphoma).
Latent celiac disease
Here again, the patient is symptom-free – or virtually so – at the time of examination. In contrast to the above-mentioned asymptomatic or silent disease, however, the patient history suggests manifest celiac disease in the past (differential diag-nosis: other diseases), the typical HLA-DQ2 or -DQ8 positivity-related manifestations of which have (evidently) regressed over the further disease course [6, 12, 16, 19]. Serological changes in latent celiac disease are often borderline or conflicting (negative), whilst histology is normal.
As in asymptomatic celiac disease, it remains unclear whether a conscious or unconscious modification in diet or other associated immune changes are responsible for the absence of disease manifestation or its regression.
Potential celiac disease
Potential celiac disease is diagnosed in patients with a positive antibody test and typical HLA constellation but negative small intestine biopsy. Despite normal histology, patients may have asymptomatic or oligosymptomatic disease and do not always develop histologically detectable celiac disease in the long term; nevertheless, this patient group generally responds well to a gluten-free diet [6, 12, 15, 16].
Forms of celiac disease refractory to therapy
Celiac disease refractory to therapy is predominantly seen in the classic and atypical forms, where no sufficient response to a gluten-free diet can be achieved despite positive serology and also usually characteristic histology. In fact, in rare cases, dis-ease progression is observed in spite of diet and is accompanied by increased inflammatory activity, the formation of subepithelial collagen bands beneath the intestinal epithelium (collagen celiac disease) and increased malabsorption [3, 15, 16, 20]. It is unclear in this context whether the disease, in the presence of a normal T-cell phenotype, is further triggered by incomplete gluten abstention (compliance), whether other infectious antigens are having a pathogenetic effect, whether related gastrointestinal allergies are present (e.g. gluten substitutes, lupin flour, corn) or whether, in the presence of aberrant T-cell populations (possibly malignant mutations), an autonomous dysfunction of the intestinal immune system already moving in the direction of lymphoma development has occurred [1, 16, 17, 18, 19, 20, 21]. At all events, in the case of celiac disease refractory to therapy or collagen celiac disease, classic dietary interventions are inadequate, making it necessary to take recourse to immunosuppressive approaches, such as systemic or local glucocorticoids, azathioprine or, in particularly refractory cases, anti-tumor necrosis factor (TNF) antibodies. In the literature, therapy-refractory celiac disease is divided into two subtypes according to the intraepithelial lymphocyte phenotype: subtype II is made distinct from subtype I by the loss of normal surface markers (CD3, CD4, CD8), as well as T-cell receptor chain rearrangement. The likelihood is greater in subtype I that, by intensifying treatment as described above, symptoms will improve. The prognosis for subtype II, however, remains poor [22].
Early-onset celiac disease, seronegative form or presence of a potential differential diagnosis
Early-onset or seronegative disease is assumed in patients exhibiting typical symptoms of celiac disease and a prompt re-sponse to a gluten-free diet. However, it is possible that serology is initially negative with this disease variant, since the disease process is at an early stage (e.g., onset following a viral or bacterial infection), making repeat serological tests over a 3-month period and HLA typing advisable [4, 6, 16]. From a differential diagnostic perspective, a seronegative local form of celiac disease should be considered (local transglutaminase-IgA immune response) and needs to be sought using special diagnostic procedures or clinical provocation with an adequate volume of gluten [17, 18, 19].
Other relevant differential diagnoses of this early form of celiac disease, as shown in Tab. 1 [5, 7, 9, 18], can imitate celiac disease clinically and often represent a significant diagnostic challenge in routine clinical practice: although patients report recurrent symptoms following the ingestion of bread, wheat products or products containing cereals, the disease cannot be confirmed by celiac disease diagnosis. It is important here to identify in particular those patients with wheat protein-mediated food allergy who exhibit specific IgE antibodies to wheat pollen/flour, gliadin, gluten or exhibit other cereal types systemically (seropositive) or locally (seronegative) and who can develop the same clinical picture as celiac disease [4, 6, 7, 17, 19].
Complications seen in the various celiac disease forms
In addition to the macronutrient loss caused by malassimilation (maldigestion and malabsorption), unidentified celiac dis-ease can cause weight loss, endocrine disorders (amenorrhea, osteoporosis, etc.), micronutrient loss accompanied by deficits due to iron, zinc, magnesium and selenium loss, etc. or vitamin deficiency (e. g., folic acid, vitamin B12, vitamin E) [6, 16]. This leads to further disease symptoms and complications, which may prompt patients to seek advice from specialized medical fields other than gastroenterology (e.g., endocrinology, neurology), such that relevant laboratory values and related symptoms should give rise to a careful consideration of celiac disease in the differential diagnosis. Rare complications include the development of gastrointestinal ulcers, which in turn increase the risk of hemorrhage, perforation or structure formation. In addition, patients with various forms of celiac disease exhibit an increased risk for the development of enteropathy-associated T-cell lymphoma of the small intestine [1, 2, 6, 20], which has a considerable effect on the prognosis of this otherwise effectively treatable disease. However, there are a number of other rare complications seen in undetected celiac disease, such as the development of “brown bowel” syndrome due to severe vitamin E deficiency [3, 16, 21], and dysregulation of epithelial DNA repair genes depending on the duration and degree of intestinal inflammation [23], which in turn bears the further risk of causing epithelial neoplasms.
Rational diagnosis of celiac disease
Bearing in mind the high percentage of undiagnosed celiac disease patients (the “iceberg” phenomenon) and the broad spectrum of clinical manifestations, including oligosymptomatic or atypical disease forms, it becomes clear that following the appropriate and reliable diagnostic steps is essential in order to reach the diagnosis of celiac disease and improve its long-term prognosis.
As a general rule, laboratory investigations (serology and HLA diagnosis), transabdominal ultrasound, endoscopy and histology are principally used in addition to compiling a thorough patient history. Characteristic abnormalities are presented in Tab. 2, whereby in general, transglutaminase serology and/or histological classification (the gold standard) according to the Marsh criteria are used to confirm the diagnosis in the majority of patients today [1, 2, 3, 24, 25, 26]. Further techniques to examine the 4- to 6-m long small intestine are only necessary in the case of very particular diagnostic questions (see below: capsule endoscopy, balloon enteroscopy, magnetic resonance imaging [MRI] according to Sellink, etc).
| Method | Potential abnormalities |
|---|---|
| Laboratory |
Specific disease indicators
_ Detection of IgA antibodies to transglutaminase > endomysium > gliadin _ HLA-subtype classification positive for DQ2/DQ8 Nonspecific signs of malassimilation _ Protein, nutrient and vitamin deficiency _ Positive D-xylose test _ Positive hydrogen breath tests (lactose, fructose etc.) |
| Ultrasound |
Nonspecific findings
_ Fluid-filled intestinal loops and wall thickening _ To and fro peristalsis (“washing machine phenomenon”) |
| Endoscopy |
Nonspecific findings
_ Loss of Kerckring’s folds, increased or absent mucosal vascular markings, mosaic structure, fissure-like lesions, fine nodular mucosa, partial or total villous atrophy |
| Histology |
Diagnostic criteria according to the Marsh classification
_ Increased intraepithelial lymphocytes (> 40/100 epithelial cells), crypt hyperplasia and villous atrophy (partial, subtotal, complete) |
The D-xylose test (25 g D-xylose administered orally with fluids), which is no longer used routinely in primary diagnosis, generally only helps identify malabsorption in the jejunum if less than 4 g of the xylose administered is measured in urine after a 5-h test period, assuming kidney function is normal. This test has low specificity and is not suited to establishing a celiac disease-specific diagnosis. However, it can be used as a quantitative measure of the jejunum’s absorptive capacity during disease follow-up in individual patients. The 13C sorbitol breath test, which has shown superior sensitivity compared to the H 2-sorbitol breath test during disease follow-up, can also be used to this end in future [33]. H2, hydrogen; HLA, human leukocyte antigen
It is important to establish from the patient history whether the individual has already undertaken self-initiated wheat or gluten abstention or reduction. This significantly influences the sensitivity of the available tests [1, 2, 3, 16, 24, 25, 26]. Therefore, where symptoms permit, the German Coeliac Society (Deutsche Gesellschaft für Zöliakie, DGZ) recommends exposure with at least 20 g gluten (two to four pieces of bread per day) for at least 1 month in order to identify the clinical picture conclusively [1, 2, 3, 8, 13, 15]. Failure to reach this exposure volume produces the risk of false-negative results; thus, in equivocal cases, a standardized gluten exposure test is sometimes necessary in order to document immune phenomena for the purposes of confirming the diagnosis. Since gluten provocation is not possible – or desired – in patients with severe disease, a variety of other diagnostic strategies are often used in clinical routine; these, however, offer lower diagnostic accuracy. In such cases, testing endoscopic biopsies with the in vitro gliadin challenge may offer a significant advantage to previous diagnostic approaches in the future, as evidenced by Tortora et al. who were able to show high diagnostic accuracy for the detection or exclusion of celiac disease by determining HLA-DR in biopsies [18, 19].
According to the criteria of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), and in addition to the above-mentioned criteria, serological diagnosis (primarily transglutaminase, the most sensitive and specific test available today) and/or histological confirmation of the diagnosis as well as remission under a gluten-free diet are also required [24].
Serology
Given the availability of more specific serological methods to detect celiac disease-induced antibodies (Tab. 3), the necessity for endoscopy with histological confirmation of the diagnosis has increasingly come under critical scrutiny in the various disease groups (children < 2 years, children > 2 years, adults; symptomatic/asymptomatic patients, positive or negative tissue transglutaminase antibodies, etc.) [15, 19, 24]. Thus, due to the costs of endoscopy and histology, as well as their comparatively high invasiveness (particularly in children), there is controversy regarding the need for biopsy to confirm the diagnosis of celiac disease. For this reason, a different approach is taken in children compared with adults, in part since the increased risk of malignancy with age in the latter age group in particular supports the need for endoscopic screening.
| Disease entity | Serum IgA antibodies a,b | Serum IgG antibodies |
|---|---|---|
| 1. Tissue transglutaminase 2 (anti-TG2 IgA) | 1. Tissue transglutaminase 2 (anti-TG2-IgG)c | |
| 2. Anti-endomysium (EMA IgA) | 2. Anti-endomysium (EMA IgG) | |
| 3. Deamidated antigliadin (anti-DGP IgA) | 3. Deamidated antigliadin (anti-DGP IgG) | |
| 4. Conventional antigliadin (AGA)d | 4. Conventional antigliadin (AGA)d | |
| Celiac disease (classic, oligo- and/or monosymptomatic) | 95–100 % | 90–97 % |
| Silent celiac disease | 90 % | 90 % |
| Latent celiac disease | Borderline – negative | 30–60 % |
| Food allergy | (Borderline) – negative | Partially detectable 15 %–40 % |
| Irritable bowel | (Borderline) – negative | Partially detectable 30 %–36 % |
| Normal population | Negativ | 25%–30% |
a IgA diagnosis is only sensitive in IgA immunocompetent individuals. Therefore, determining serum IgA levels is necessary to exclude IgA deficiency or identify low IgA values (total IgA < 20 mg/dl).
b IgA diagnosis is superior to IgG diagnosis in IgA immunocompetent individuals. IgG-based diagnosis should only be used in the case of IgA deficiency (total IgA < 20 mg/dl).
c Primary diagnosis for the serological detection of celiac disease should be performed according to the sequence of antibody tests given here, starting with anti-TG2 IgA antibodies (anti-TG2-IgG in the case of IgA deficiency). Determining EMA or DGP complements the reliability of TG detection only when this latter is positive.
d Conventional AGA should no longer be used today, since deamidated gliadin peptides (DGP-IgG antibodies) are significantly more sensitive in the <2-year age group. In the presence of other gastrointestinal diseases, intestinal barrier disorder or following infection, conventional AGA are often nonspecifically elevated and can cause problems in terms of differential diagnosis (e. g. irritable bowel syndrome, food allergy, chronic inflammatory bowel disease) [36].
AGA, anti-gliadin antibodies; DGP, deaminated gliadin peptides; EMA, endomysium antibodies; Ig, immunoglobulin; TG, transglutaminase
Several authors, however, recommend in general a risk-adapted approach tailored to the individual situation, age group, symptoms and diet. Performing screening in all asymptomatic patients offers less promise and fewer cost benefits than initiating primary diagnosis using serological tests in the case of even slight clinical suspicion or the presence or a celiac disease-related disorder (Tab. 3).
Appropriate serological tests include the detection of IgA antibodies to:
Anti-tissue transglutaminase type-2 IgA (anti-TG2-IgA) antibodies; the autoantigen of the antiendomysial antibodies)
Endomysium (antiendomysium-IgA antibodies, EMA-IgA)
Gliadin
Having said that, antibodies to deamidated gliadin peptide (DGP) are considered to be significantly more reliable today than the conventional antibodies to anti-gliadin antibodies (AGA) often tested in the past.
With the anti-TG2-IgA antibodies to be used first, a primary sensitivity and specificity of 95 %–98 % is achieved in IgA-immunocompetent patients where adequate gluten intake and a two- to three-fold increase in the antibody titer compared to normal values are present and comorbidities are absent [12, 15, 24]. Since there are frequent variations from the above-mentioned status in clinical practice and false-low anti-TG titers may occur (e.g., viral infections, immune suppression), a combination of the first two antibody fractions mentioned in Tab. 3 is recommended in order to increase sensitivity and specificity [4, 12, 19, 24].
Thus, in the case of reasonable suspicion, in individuals reporting cereal intolerance or in the presence of a related underlying disease, diagnostic measures to identify celiac disease can be initiated in IgA-immunocompetent individuals. High TG2 antibody titers are predictive of damage to the small intestine mucosa that can be reliably evidenced histologically [24, 26, 27]. However, false-positive TG2 antibodies are also found in Down’s syndrome, other autoimmune diseases or neoplasia, liver disease, psoriasis or following Epstein-Barr virus (EBV) infection. Therefore, a second, confirmatory test involving EMA detection should be performed in the case of positive TG titers.
The same recommendation regarding antibody diagnosis is made when abnormal small intestine histology showing signs of the Marsh classification is incidentally found on endoscopy/histology in individuals lacking typical symptoms. However, it should be borne in mind here that all Marsh stages, ranging from elevated intraepithelial lymphocytes to complete villous atrophy, can all be seen in other diseases (e.g., gastrointestinal allergies, autoimmune enteropathy, infections such as Giardia lamblia and in recipients of allogenic stem-cell transplantation) and exhibit negative serology for anti-TG2-IgA, EMA-IgA or DGP-IgG antibodies [1, 3, 20, 24, 26, 27]. The term autoimmune enteropathy is applied to those cases in which histological findings falling within the Marsh classification are caused by antibodies against enterocytes. These heterogenous manifestations, seen more commonly in children than in adults, have not been overly accessible to systematic research to date [28].
It is of elementary importance to consider the patient’s IgA status when performing serology, since the above-mentioned IgA-based antibody tests can be negative in the case of IgA deficiency (5 %–10 % of celiac disease patients) [1, 4, 12, 19, 24]. In such cases, the same antibody tests for IgG should be used (Tab. 3), although these do not have quite the same sensitivity and specificity as IgA-based tests.
Determining HLA class as part of the diagnosis of celiac disease should be carried out secondary to the above-mentioned antibody testing using anti-TG2 and/or EMA antibodies, since it is not necessary in the case of typical symptoms and positive serology and histology, but rather only when the latter two are equivocal. Genetic testing for HLA-DQ2 or -DQ8 is only useful in the case of normal small intestine histology but positive or borderline antibody findings, since virtually all celiac disease patients test positive for one of these two markers [3, 5, 16, 24]. Even if IgA- and/or IgG-based celiac disease serology is negative, HLA typing can yield valuable information on disease predisposition: in rare cases, combined IgA and IgG antibody defects, intestinal protein loss with reduced antibody levels or other variables that intervene with serology can make serological diagnosis challenging.
The ESPGHAN recently indicated that endoscopic histology can be dispensed with in children and adolescents due to the highly sensitive anti-TG2 antibodies in the presence of highly significantly raised anti-TG2 antibody levels (> 10 times the normal level), HLA-DQ2 or DQ8 positivity and/or confirmatory EMA or DGP [24]. Since TG2 antibody production is subject to variation in children under 2 years, the detection of IgG antibodies to deamidated gliadin peptide, a test that supports diagnosis in this age group, should be used instead.
Whilst the advantage of celiac disease-based antibody testing lies in its low invasiveness, it should always been borne in mind along the diagnostic pathway that gluten abstention should only be prescribed following completion of diagnosis, and that diagnosis in children and adolescents based on serology and HLA determination can only be is established through significantly elevated antibody titers combined with other conclusive findings (HLA positivity, second positive antibody find-ings). In all other cases, as well as in adults, endoscopic histology remains an integral part of the diagnostic procedure. Moreover, other gastroenterological diseases (e. g., gastritis, enteritis, ulcers, neoplasia) need to be ruled out most particularly in symptomatic adults.
Endoscopy and histology (Marsh criteria)
Endoscopic examination, primarily by means of esophagogastroduodenoscopy, combined with small intestine biopsy and the above-mentioned serological criteria represent the gold standard for diagnosis in adults. Biopsies are evaluated according to the Marsh criteria (intraepithelial lymphocyte infiltration, crypt hyperplasia, villous atrophy) (Tab. 4). Histological diagnosis shows poorer interobserver variation than an overall consideration of serology, symptoms, remission on abstention and biopsy together [7, 24]. Histological classification of a Marsch-1 lesion is insufficient to support the diagnosis of celiac disease, since bacterial or viral infections, gastrointestinal allergies as well as other diseases may also be present in this setting. In equivocal cases, particularly where Marsch-1 or -2 classifications are established, it is sometimes helpful to consult a second pathologist in order to broaden the spectrum of histological analysis, by means of special immunohistochemistry to quantify eosinophils and mast cells or to characterize lymphocytes, in order to review the differential diagnoses discussed above and identify other diseases [7, 9, 18, 25].
| Marsh criteria | Villi | Crypts | Intraepithelial lymphocytes (number/100 intestinal epithelial cells) |
|---|---|---|---|
| Marsh 0 | Normal | Normal | < 40 |
| Marsh 1 | Normal | Normal | > 40 |
| Marsh 2 | Normal | Hyperplastic | > 40 |
| Marsh 3a | Partial atrophy | Hyperplastic | > 40 |
| Marsh 3b | Subtotal atrophy | Hyperplastic | > 40 |
| Marsh 3c | Complete atrophy | Hyperplastic | > 40 |
Deep small-intestine endoscopy using balloon enteroscopy or capsule endoscopy is used in particularly challenging cases, whereby balloon enteroscopy offers the added advantage of obtaining tissue specimens from the deep small intestine for histology or functional biopsy testing and of performing endoscopically-guided segmental lavage to detect intestinal IgE antibodies [16, 17, 18, 19, 27, 29]. In this way, it is possible to localize and identify rarer differential diagnoses such as intestinal mastocytosis, eosinophilic gastroenteritis, areas of extensive lymphofollicular hyperplasia and lymphoma disease.
Capsule endoscopy should only be used when enteroscopy is not possible or when distal segments of the intestine inaccessible with endoscopy need to be evaluated. At present, its diagnostic sensitivity remains limited by its lack of controllability and inability to obtain biopsy specimens; as such, it is only suited to visualizing the small intestinal mucosa, typical celiac disease lesions and other lesions or complications, as well as documenting the localization of affected intestinal segments [12, 30]. In particular, capsule endoscopy – whilst less invasive than balloon enteroscopy – nevertheless appears to provide a good visual overview of the length of the affected mucosal segment due to its ability to visualize the entire intestinal tract. However, since capsule endoscopy as a purely diagnostic procedure lacks conclusive histology, its macroscopic evaluation is prone to misinterpretation, technical artefacts in image transmission, as well as motion and impurity artefacts, etc., which explains the lack of correlation between the extent of lesions seen on capsule endoscopy and clinical presentation [31].
Transabdominal ultrasound
Transabdominal ultrasound’s relevance in primary medical diagnosis is based on its non-invasive ability under good so-nographic conditions to rapidly visualize important abdominal organs and identify pathological organ changes in symptomatic patients (e. g., space-occupying lesions, chronic pancreatitis). However, abdominal ultrasound does not play a confirmatory role in the primary diagnosis of celiac disease, since clinically manifest celiac disease generally exhibits nonspecific findings, such as intestinal wall thickening (e.g., hypoechoic), fluid-filled intestinal loops and thickened wall, perfusion changes as well as to and fro peristalsis (“washing-machine phenomenon”). Moreover, there is high interobserver variability and numerous other differential diagnoses may produce a similar clinical picture (e. g., infectious gastroenteritis, Crohn’s disease).
Once the diagnosis of celiac disease has been established, ultrasound – much like other non-invasive tests (e. g., b-D-xylose or 13C sorbitol breath tests) – can be used for follow-up investigations [16, 20, 32, 33], since the parameters mentioned above can be quantified over time and a response to treatment recognized in the form of, e.g., reduced intestinal wall thickening.
Basic principles of the therapeutic approaches in celiac disease
At present, strict lifelong abstention from gluten represents the only definitive therapy for celiac disease [1, 2, 3, 4, 5, 29]. This is particularly valid for patients with classic oligosymptomatic and atypical forms of the disease. Results for the other celiac disease forms [e.g., asymptomatic (silent) or latent] are inconsistent. Nevertheless, in equivocal cases, it is preferable to aim for or discuss lifelong gluten abstention with patients, since latent inflammatory changes in the gastrointestinal tract can persist even in an asymptomatic course, possibly leading to complications only after a number of years [20, 21, 23, 25].
Successful gluten abstention depends on adequate patient training aimed at instilling an understanding of the recurrence of celiac disease following dietary mistakes, checking food ingredients and using gluten-free food substitutes. Spelt, while com-monly considered an alternative in known celiac disease, also contains gluten and, as such, does not represent an alternative foodstuff. The most important aspects of gluten-free nutrition are listed in Tab. 5. The DZG also provides lists of suitable foodstuffs (www.dzg-online.de). Gluten-free foodstuffs are defined as nutritional substances that contain less than 20 mg gluten per kilogram, whilst low-gluten foodstuffs can contain 20–100 mg/kg. Celiac disease patients often perceive their health and sense of well-being as significantly improved upon gluten abstention, whereas the rationale and purpose of gluten abstention in asymptomatic patients is frequently questioned and inconclusively established [6, 16, 34].
| Permitted | Prohibited |
|---|---|
| Corn, rice, millet Buckwheat, oats (up to 2 g/kg BW) Lupin flour Pulses (e. g. lentils, soy) Vegetables (e.g. lettuce, cucumber, tomatoes) Meat Poultry Fish |
Wheat, rye, barley Candied products Malt coffee, beer, drinks made from the above types of cereal Chocolates, malt confectionary, desserts, marzipan, potato chips, ready-to-eat potato products, vegetable stock Fruit preparations, fruit concentrates, baking ingredients Ketchup, mustard |
| Check ready meals with additives carefully | |
| e.g. cheese and sausage products, milk products, sauces, fillings |
In the case of manifest malabsorption resulting from symptomatic celiac disease, other aspects of nutrition often need to be discussed with the patient in the first instance, since mineral, electrolyte, iron, and vitamin deficiencies may appear due to damage to the small intestine and require targeted substitution [9, 16, 21]. In addition, other secondary food intolerances, such as lactase deficiency, fructose malabsorption or intolerance of histamine-rich foods, need to be considered when compiling a dietary plan. In cases of severe malabsorption or therapy-refractory disease, pancreas enzymes and the administration of glucocorticoids or immunosuppressants, such as azathioprine or cyclosporin, are occasionally necessary alongside gluten abstention and nutritional therapy [16, 20, 32, 34].
Novel strategies could potentially simplify the management of celiac disease in the future. These include the use of bacterial endopeptidases to breakdown ingested gluten. Genetically modified and hence gluten-free cereal types as well as other immunomodulatory measures to strengthen the anti-inflammatory immune response may gain in importance in the future [3, 32].
To what extent probiotic preparations – by modulating regulatory T cells, changing the intestinal humoral immune response to attenuate anti-TG2 or anti-DGP-IgA antibody secretion or stimulate tolerance mechanisms or protective cytokines and, lastly, by using the capacity of certain bacterial strains to hydrolyze gliadin polypeptides – represent a relevant therapy option in celiac disease is currently the subject of intense research [1, 3, 32, 35, 36].
Abbreviations
- AGA
Anti-gliadin antibodies
- Ab
Antibodies
- BA
Bile acids
- CCE
Cholecystectomy
- DD
Differential diagnosis
- DGP
Deamidated gliadin peptides
- DGZ
Deutsche Gesellschaft für Zöliakie (German Coeliac Society)
- EMA
Endomysium antibodies
- EBV
Epstein-Barr virus
- ESPGHAN
European Society for Paediatric Gastroenterology, Hepatology and Nutrition
- FA
Food allergy
- FI
Food intolerances
- H2
Hydrogen
- HLA
Human leukocyte antigen
- Ig
Immunoglobulin
- MHC
Major histocompatibility complex
- MRI
Magnetic resonance imaging
- TG
Transglutaminase
- TLR4
Toll-like receptor 4
- TNF
Tumor necrosis factor
Footnotes
Conflicts of interest
The authors state that there are no conflicts of interest.
References
- 1.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Hahn EG. Biomedicine. Gluten and the gut–lessons for immune regulation. Science. 2002;297:2218–20. doi: 10.1126/science.1077572. [DOI] [PubMed] [Google Scholar]
- 3.Tack GJ, Verbeek WH, Schreurs MW, Mulder CJ. The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol. 2010;7:204–13. doi: 10.1038/nrgastro.2010.23. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Lebwohl B, Green PH. Screening for celiac disease in average-risk and high-risk populations. Therap Adv Gastroenterol. 2012;5:37–47. doi: 10.1177/1756283X11417038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–14. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 6.Botero-Lopez JE, Araya M, Parada A, Mendez MA, Pizarro F, Espinosa N, Canales P, Alarcon T. Micronutrient deficiencies in patients with typical and atypical celiac disease. J Pediatr Gastroenterol Nutr. 2011;53:265–70. doi: 10.1097/MPG.0b013e3181f988fc. [DOI] [PubMed] [Google Scholar]
- 7.Raithel M, Hahn EG, Baenkler H-W. Klinik und Diagnostik von Nahrungsmittelallergien: Gastrointestinal vermittelte Allergien Grad I bis IV. Dtsch Arztebl. 2002;99:A780–6. [Google Scholar]
- 8.Reese I. Glutenbelastung als Voraussetzung für die Zöliakiediagnostik: Konsequenzen für diätetische Interventionen. Allergo J. 2011;20:389–92. [Google Scholar]
- 9.Schwab D, Raithel M, Klein P, Winterkamp S, Weidenhiller M, Radespiel-Troeger M, Hochberger J, Hahn EG. Immunoglobulin E and eosinophilic cationic protein in segmental lavage fluid of the small and large bowel identify patients with food allergy. Am J Gastroenterol. 2001;96:508–14. doi: 10.1111/j.1572-0241.2001.03467.x. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–9. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Junker Y, Zeissig S, Kim SJ, Barisani D, Wieser H, Leffler DA, Zevallos V, Libermann TA, Dillon S, Freitag TL, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–2408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651–58. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 13.Riepe SP, Goldstein J, Alpers DH. Effect of secreted Bacteroides proteases on human intestinal brush border hydrolases. J Clin Invest. 1980;66:314–322. doi: 10.1172/JCI109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troncone R, Jabri B. Coeliac disease and gluten sensitivity. J Intern Med. 2011;269:582–90. doi: 10.1111/j.1365-2796.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 15.Rozenberg O, Lerner A, Pacht A, Grinberg M, Reginashvili D, Henig C, Barak M. A novel algorithm for the diagnosis of celiac disease and a compre-hensive review of celiac disease diagnostics. Clin Rev Allergy Immunol. 2012;42:331–41. doi: 10.1007/s12016-010-8250-y. [DOI] [PubMed] [Google Scholar]
- 16.Stein J, Kist M, Raithel M. Erkrankungen durch Nahrungsmittel. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2011. [Google Scholar]
- 17.Compilato D, Campisi G, Pastore L, Carroccio A. The production of the oral mucosa of antiendomysial and anti-tissue-transglutaminase antibodies in patients with celiac disease: a review. Scientific World Journal. 2010;10:2385–94. doi: 10.1100/tsw.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paajanen L, Vaarala O, Karttunen R, Tuure T, Korpela R, Kokkonen J. Increased IFN-gamma secretion from duodenal biopsy samples in delayed-type cow’s milk allergy. Pediatr Allergy Immunol. 2005;16:439–44. doi: 10.1111/j.1399-3038.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 19.Tortora R, Russo I, De Palma GD, Luciani A, Rispo A, Zingone F, Iovino P, Capone P, Ciacci C. In vitro gliadin challenge: diagnostic accuracy and utility for the difficult diagnosis of celiac disease. Am J Gastroenterol. 2011;107:111–7. doi: 10.1038/ajg.2011.311. [DOI] [PubMed] [Google Scholar]
- 20.Schuppan D, Kelly CP, Krauss N. Monitoring non-responsive patients with celiac disease. Gastrointest Endosc Clin N Am. 2006;16:593–603. doi: 10.1016/j.giec.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Reynaert H, Debeuckelaere S, De Waele B, Meysman M, Goossens A, Devis G. The brown bowel syndrome and gastrointestinal adenocarcinoma. Two complications of vitamin E deficiency in celiac sprue and chronic pancreatitis? J Clin Gastroenterol. 1993;16:48–51. doi: 10.1097/00004836-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Rubio-Tapia A, Murray JA. Classification and management of refractory coeliac disease. Gut. 2010;59:547–557. doi: 10.1136/gut.2009.195131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergmann F, Singh S, Michel S, Kahlert C, Schirmacher P, Helmke B, von Knebel Doeberitz M, Kloor M, Blaker H. Small bowel adenocarcinomas in celiac disease follow the CIM-MSI pathway. Oncol Rep. 2010;24:1535–39. doi: 10.3892/or_00001015. [DOI] [PubMed] [Google Scholar]
- 24.Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 25.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (“celiac sprue”) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 26.Mubarak A, Nikkels P, Houwen R, Ten Kate F. Reproducibility of the histological diagnosis of celiac disease. Scand J Gastroenterol. 2011;46:1065–73. doi: 10.3109/00365521.2011.589471. [DOI] [PubMed] [Google Scholar]
- 27.Krauss E, Konturek P, Maiss J, Kressel J, Schulz U, Hahn EG, Neurath MF, Raithel M. Clinical significance of lymphoid hyperplasia of the lower gastroin-testinal tract. Endoscopy. 2010;42:334–7. doi: 10.1055/s-0029-1243936. [DOI] [PubMed] [Google Scholar]
- 28.Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep. 2012;14:380–5. doi: 10.1007/s11894-012-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raithel M, Diebel H, Maiss J, Braun S, Nägel A, Paurevic D, Kraus F, Hahn EG. Praxis der Doppelballon-Enteroskopie (DBE): Indikationen, praktische Durchführung, Vor- und Nachbereitung des Enteroskops sowie erste klinische Ergebnisse. Endo-Praxis. 2006;22:6–18. doi: 10.1055/s-2007-982011. [DOI] [Google Scholar]
- 30.Hagel AF, de Rossi TM, Zopf Y, Lindner AS, Dauth W, Neurath MF, Raithel M. Small-bowel capsule endoscopy in patients with gastrointestinal food allergy. Allergy. 2011;67:286–92. doi: 10.1111/j.1398-9995.2011.02738.x. [DOI] [PubMed] [Google Scholar]
- 31.Murray JA, Rubio-Tapia A, Van Dyke CT, Brogan DL, Knipschield MA, Lahr B, Rumalla A, Zinsmeister AR, Gostout CJ. Mucosal atrophy in celiac disease: extent of involvement, correlation with clinical presentation, and response to treatment. Clin Gastroenterol Hepatol. 2008;6:186–93. doi: 10.1016/j.cgh.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly SC, Ellis HJ, Ciclitira PJ. Pharmacotherapy and management strategies for coeliac disease. Expert Opin Pharmacother. 2011;12:1731–44. doi: 10.1517/14656566.2011.592140. [DOI] [PubMed] [Google Scholar]
- 33.Tveito K, Hetta AK, Askedal M, Brunborg C, Sandvik L, Loberg EM, Skar V. Follow-up of coeliac disease with the novel one-hour 13C-sorbitol breath test versus the H2-sorbitol breath test. Scand J Gastroenterol. 2011;46:837–43. doi: 10.3109/00365521.2011.575175. [DOI] [PubMed] [Google Scholar]
- 34.Ukkola A, Maki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, Kaukinen K. Diet improves perception of health and well-being in symptomatic, but not asymptomatic, patients with celiac disease. Clin Gastroenterol Hepatol. 2010;9:118–23. doi: 10.1016/j.cgh.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 35.De Angelis M, Rizzello CG, Fasano A, Clemente MG, De Simone C, Silano M, De Vincenzi M, Losito I, Gobbetti M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue. Biochim Biophys Acta. 2006;1762:80–93. doi: 10.1016/j.bbadis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Marietta EV, Rashtak S, Murray JA. Correlation analysis of celiac sprue tissue transglutaminase and deamidated gliadin IgG/IgA. World J Gastroenterol. 2009;15:845–8. doi: 10.3748/wjg.15.845. [DOI] [PMC free article] [PubMed] [Google Scholar]