Abstract
The continued high prevalence of allergic diseases in Western industrialized nations combined with the limited options for causal therapy make evidence-based primary prevention necessary. The recommendations last published in the S3-guideline on allergy prevention in 2009 have been revised and a consensus reached on the basis of an up-to-date systematic literature search.
Evidence was sought for the period between May 2008 and May 2013 in the Cochrane and MEDLINE electronic databases, as well as in the reference lists of recent review articles. In addition, experts were surveyed for their opinions. The relevance of retrieved literature was checked by means of two filter processes: firstly according to title and abstract, and secondly based on the full text of the articles. Included studies were given an evidence grade, and a bias potential (low/high) was specified for study quality. A formal consensus on the revised recommendations was reached by representatives of the relevant specialist societies and (self-help) organizations (nominal group process).
Of 3,284 hits, 165 studies (one meta-analysis, 15 systematic reviews, 31 randomized controlled trials, 65 cohort studies, 12 case-control studies and 41 cross-sectional studies) were included and evaluated. Recommendations on the following remain largely unaltered: full breastfeeding for 4 months as a means of allergy prevention (hypoallergenic infant formula in the case of infants at risk); avoidance of overweight; fish consumption (during pregnancy/lactation and in the introduction of solid foods for infants); vaccination according to the recommendations of the German Standing Committee on Vaccination (Ständige Impfkommission, STIKO); avoidance of air pollutants and tobacco exposure and avoidance of indoor conditions conducive to the development of mold. The assertion that a reduction in house-dust mite allergen content as a primary preventive measure is not recommended also remains unchanged. The introduction of solid foods into infant diet should not be delayed. In the case of children at risk cats should not be acquired as domestic pets. Keeping dogs is not associated with an increased risk of allergy. The updated guideline includes a new recommendation to consider the increased risk of asthma following delivery by cesarean section. Additional statements have been formulated on pre- and probiotic agents, psychosocial factors, medications, and various nutritional components.
Revising the guideline by using an extensive evidence base has resulted not only in an endorsement of the existing recommendations, but also in modifications and in the addition of new recommendations. The updated guideline enables evidence-based and up-to-date recommendations to be made on allergy prevention.
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s40629-014-0022-4 and is accessible for authorized users.
Key words: Allergy, evidence, S3-guidelines, primary prevention, revision
Introduction
The prevalence of allergic diseases such as allergic asthma, hay fever, and atopic dermatitis has continued to rise in Western industrialized nations in recent years [1]. The reasons for this development and increase remain largely unknown. Since causal therapy approaches are limited, prevention assumes a particularly important role in addressing this upward trend [2]. With the support of the German Federal Ministry for Health and Social Insurance and under the auspices of the German Action Alliance for Allergy Prevention (Aktionsbündnis Allergieprävention, abap), the first S3-guideline on allergy prevention was published in 2004 [3] and first updated 5 years later [4]. A second revision has now been undertaken according to the methodology of evidence based consensus guidelines. The current guideline and the methodology on which it is based is presented here.
Methods
The methodology behind the revision of this guideline complies with national and international standards on the development of evidence based consensus guidelines [5–7].
Objective
The primary objective of the guideline is the prevention of the main atopic diseases: atopic dermatitis, allergic rhinoconjunctivitis, and (allergic) asthma.
The guideline refers exclusively to measures of primary prevention and is based on the following modified definitions from the category of allergies of the abap):
Primary prevention includes the elimination or reduction of (partial) causes relevant to disease development, including modifications to causal or predisposing environmental and occupational factors on the one hand, whilst increasing individual tolerance on the other. Although primary prevention is particularly effective among at-risk groups (those with a genetic predisposition), it is directed in a limited form at the overall population and includes health promotion specifically with regard to allergies.
The target group of secondary prevention includes individuals with early signs of disease (e.g., bronchial or nasal hyperreactivity in the case of proven sensitization), as well as sensitized but as yet asymptomatic individuals. The aim of secondary prevention is to prevent disease manifestation and symptom progression. Measures to this end include the avoidance of clinically relevant allergens and toxic/irritant substances, counseling, and - in the case of early signs of disease - possibly also drug prophylaxis and specific immunotherapy (Desensitization).
In line with this definition, measures are subdivided in the recommendation algorithm according to genetically predisposed and non-predisposed individuals as appropriate. Studies on individuals already affected by disease, including those that had the prevention of a second disease as their goal, have not been considered for the purposes of the updated guidelines.
Target population
The target population comprises individuals, in particular children, both with and without genetic predisposition to atopic diseases. Genetically predisposed children (i.e., children at risk) are defined as having at least one parent or sibling affected by one of the atopic diseases mentioned here. Thus, in addition to the general population, also young families, couples planning a family, pregnant women, and individuals with a family history of allergy are included in the target population.
Area of health care
The guidelines are directed at medical and non-medical professionals who are involved in the treatment of individuals defined as the target population.
Target user group/addressees
Users and multipliers of the guideline include all medical and non-medical organizations and groups of individuals concerned with preventive measures and allergy prevention in particular. In addition to representatives of the relevant specialist, occupational, and patient organizations, physicians in all specialty groups, in particular pediatricians, dermatologists, otorhinolaryngologists, pneumologists, and allergists, as well as patients and self-help organizations, can be considered part of the target group.
Literature search for evidence
An electronic literature search was performed in the MEDLINE (Medical literature analysis and retrieval system online) and Cochrane databases for the time period between May 2008 and May 2013.
Three categories of key terms were defined for the search strategy:
The “disease“ group: asthma, allergy, allergic, atopic, hay fever, dermatitis, eczema, rhinitis
The „measures“ group: prevention, risk factor, epidemiology
The “study-type“ group: randomized controlled trials, clinical trials, controlled study, case control study, cohort study, systematic review, meta-analysis.
Terms within a group were linked using „or“ and between groups using “and“ (Tab. 1).
Table 1.
Evidence search strategy (S3-guidelines on allergy prevention)
| Database/source | Search step/search terms | Hits |
|---|---|---|
| MEDLINE | (((((((“Allergy and Immunology“[Mesh]) OR “Asthma“[Mesh]) OR „Rhinitis, Allergic, Seasonal“[Mesh]) OR „Dermatitis, Atopic“[Mesh])) OR (Asthma OR Allergy OR allergic OR atopic OR hay fever OR dermatitis OR eczema OR rhinitis))) AND (((prevention OR risk factor OR epidemiology))) AND ((Clinical Trial[ptyp] OR Controlled Clinical Trial[ptyp] OR Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp] OR systematic[sb]) AND (“2008/05/01“[PDat] : “2013/05/31“[PDat])) LIMITS: Human | 2,517 |
| Allergy AND Medi* AND Prevention Asthma AND Medi* AND Prevention |
329 | |
| Cochrane | Asthma | 165 in “Cochrane Reviews“ und 92 in „Other Reviews“ |
| Allergy | 37 in “Cochrane Reviews“ und 7 in “Other Reviews“ | |
| Surveys of experts | 70 | |
| References of overview articles | 67 |
RCT, randomized controlled study
Studies in humans published in German or English were included. Studies targeted at non-allergic diseases, as well as treatment and drug studies, were excluded.
Furthermore, the reference lists of recent overview articles were reviewed for relevant references and all members of the consensus group were requested to provide a list of relevant citations.
In a first screening step, the titles and abstracts of all citations retrieved were reviewed. Studies that did not have the specified atopic diseases as their primary objective were excluded, as were treatment and drug trials. In a second screening step, the remaining articles were obtained in full-text and their content reviewed for suitability.
Evidence analysis
In addition to assigning formal levels of evidence (1a–4) (Tab. 2), the studies were analyzed by means of critical appraisal of the applied methods according to pre-defined criteria (e.g., sample size, chronological sequence between exposure and disease, consideration of other influencing factors) and by completing the relevant extraction tables. This critical appraisal resulted in dichotomous estimations of each study‘s bias potential: high (-) or low (+).
Table 2.
Levels of evidence (Oxford Centre for Evidence-based Medicine, March 2009, www.cebm.net)
| 1a | Systematic review of RCT |
| 1b | Individual RCT |
| 1c | (All or none) |
| 2a | Systematic review of cohort studies |
| 2b | Individual cohort studies and poor-quality RCT |
| 2c | ("Outcomes" research; ecological studies) |
| 3a | Systematic review of case-control studies |
| 3b | Individual case-control studies |
| 4 | Case series (and poor-quality case-control or cohort studies) |
RCT, randomized controlled study
The overall body of evidence was put in tabular form according to subject areas, number of studies, study types, levels of evidence, and recommendation classes. In addition, evidence tables listing the number of studies retrieved and evaluated according to study type, main outcome (protective, no effect, risk factor), and methodological quality (+ high, - low) were created for each subject area (not shown here).
Draft guidelines
Based on the articles retrieved and evaluated, a draft proposal for the revised prevention guideline was drawn up at a preparatory meeting attended by C. Muche-Borowski (AWMF, evidence basing), M. Kopp (DGKJ), I. Reese (German Task force on Dietetics in Allergology), T. Werfel (DGAKI), and T. Schäfer (coordinator) and circulated among the guideline group. Suggestions on additions and revisions were discussed and, where appropriate, included.
Consensus
The consensus group consisted of all those persons who had collaborated on the preparation of and consensus process for the previous edition of the guideline. In addition, representatives of other specialist societies were proposed for inclusion in the consensus group.
The recommendations were approved by the consensus group formed in this way. Each organization was allowed a maximum of two delegates with a joint voting right. The nominal group technique was chosen as the consensus process and requires all parties involved to be present at a meeting. The Process is strictly structured and can be broken down into the following steps:
Presentation of the statements on which consensus is sought.
-
2.
Each participant writes comments and desired points for discussion on the given statements.
-
3.
The facilitator invites each participant in turn to share their comments, and similar comments are recorded on a flip chart.
-
4.
A vote is taken on each recorded point as to who wishes to discuss this particular point.
-
5.
Topics are then ranked according to these votes.
-
6.
Each member of the group comments in turn on the individual discussion points.
-
7.
After several rounds, the participants finally agree - either by voting or using a ranking system - on a particular formulation.
-
8.
Steps 1-6 are repeated for each statement under discussion.
The consensus meeting took place in January 2014 in Marburg/Lahn and Mr. PD Dr. H. Sitter (University of Marburg and AWMF) acted as facilitator.
Evidence levels for the consensus-based recommendations are expressed with the terms “proof“ and “indications“. This terminology is in line with the methods formulated by the German Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG). Moreover, the “General methods 3.0“ state, for example, the following: “As a rule, if the conclusion is drawn that ‘proof‘ is available, it is required that a meta-analysis of studies shows a corresponding statistically significant effect (with minor outcome-related uncertainty of results). If a meta-analysis is not feasible, at least 2 studies conducted independently of one another should be available that show minor outcome-related uncertainty of results and a statistically significant effect, and whose results are not questioned by further comparable studies with sufficient outcome-related certainty of results (‘consistency of results‘). The two studies conducted independently of one another need not necessarily be of exactly identical design. Which deviations in design between studies are acceptable depends on the research question posed. Despite showing statistically significant effects, as a rule a meta-analysis of studies with high outcome-related uncertainty of results or results from individual studies can consequently at most provide indications of the effects of an intervention. If, in exceptional cases, proof of the benefit of an intervention is inferred from one individual study, then specific requirements apply to this study and its results“.
The consensus group assigned each recommendation with a recommendation class (A, B or 0), which is given in parentheses after the recommendation in question. Recommendation classes were assigned in a formalized manner on the basis of levels of evidence (Fig. 1). However, the consensus process made provision for assigning alternative recommendation classes where justified. Subject areas for which no recommendations on prevention could be formulated were assigned an evidence level only.
Fig. 1.
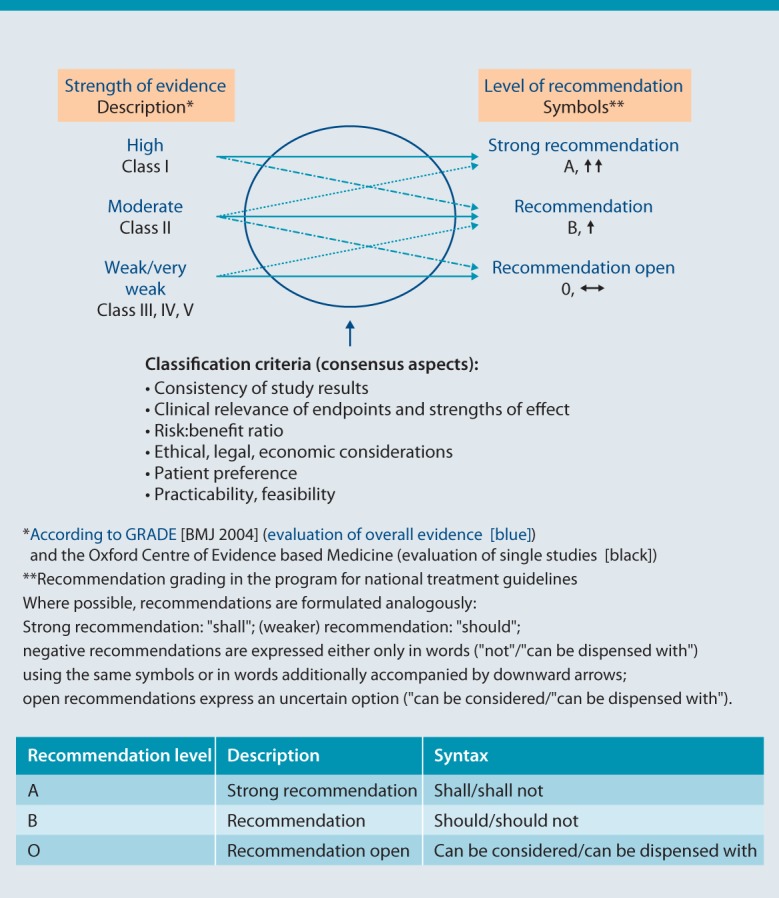
Strength of evidence, level of recommendation, and syntax (from [5])
Results
Using the search strategy described above, it was possible to retrieve 3,284 hits in the MEDLINE and Cochrane databases. In addition, articles found in the reference lists of review articles and recommended by members of the consensus group were included. By means of a two-step selection process, the first according to title and abstract, the second according to full-text versions, 173 original articles were ultimately evaluated, of which 165 were included in the analysis. These comprised one meta-analysis (MA), 15 systematic reviews (SR), 31 randomized controlled trials (RCT), 65 cohort studies (CS), 12 case-control studies (CC) and 41 cross-sectional studies (Fig. 2).
Fig. 2.
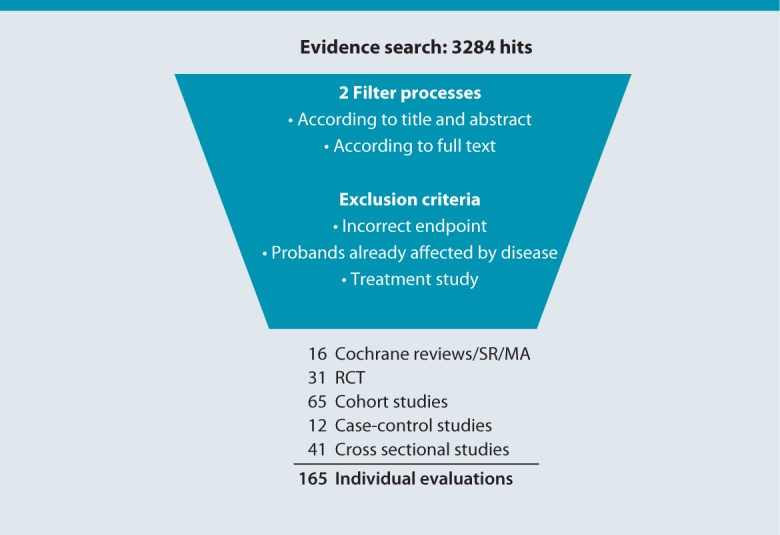
Results of the literature search
The overall body of evidence is shown in Tab. 3 according to subject area, number of studies, study types, levels of evidence, and recommendation classes.
Table 3.
Complete overview of evaluated studies according to number and study type, the evidence levels derived, and consensus-based levels of recommendation according to topic
| Area | Number and type of study | Evidence levels | Recommendation levels |
|---|---|---|---|
| Breastfeeding | 0 MA, 1 RCT, 7 CS, 0 CC, 3 CSS | 1x1b, 7x2b | A |
| Maternal diet during pregnancy and/or lactation | 0 MA, 5 RCT, 15 CS, 0 CC, 0 CSS | 5x1b, 15x2b | A and B |
| Breast-milk substitutes in at-risk infants | 0 MA, 2 RCT, 2 CS, 0 CC, 0 CSS | 2x1b, 2x2b | A |
| Introduction of solid foods and infant diet up to the age of 1 year | 1 MA, 2 RCT, 8 CS, 3 CC, 5 CSS | 2x1b, 8x2b, 3x3b | A and B |
| Diet after the age of 1 year and body weight | 2 SR, 0 RCT, 4 CS, 2 CC, 7 CSS | 4x2b, 2x3b | A |
| General diet and vitamin D: during pregnancy/lactation and up to the age of 1 year | 2 SR, 1 RCT, 4 CS, 3 CC, 4 CSS | 1x1b, 2x2b, 3x3b | - |
| Effects of probiotics and prebiotics | 3 SR, 15 RCT, 1 CS, 1 CC, 0 CSS | 15x1b, 1x2b | - |
| Keeping pets | 2 SR, 0 RCT, 3 CS, 0 CC, 1 CSS | 3x2b | B |
| House dust mites | 0 MA, 0 RCT, 3 CS, 1 CC, 2 CSS | 3x2b, 1x3b | A |
| Mold and dampness | 3 MA, 0 RCT, 3 CS, 1 CC, 0 CSS | 3x2a, 3x2b, 2x3b | B |
| Exposure to tobacco smoke | 0 MA, 0 RCT, 2 CS, 0 CC, 1 CSS | 2x2b | A |
| Vaccination | 0 MA, 1 RCT, 1 CS, 0 CC, 2 CSS | 1x1b, 1x2b | A |
| Motor vehicle emissions | 0 MA, 1 RCT, 0 CS, 0 CC, 3 CSS | 4x2b, 2x3b | B |
| Non-specific immune modulation | 1 SR, 0 RCT, 4 CS, 0 CC, 5 CSS | 4x2b | - |
| Pharmaceutical drug use | 1 SR, 1 RCT, 4 CS, 1 CC, 5 CSS | 1x1b, 4x2b, 1x3b | - |
| Psychological factors | 0 SR, 0 RCT, 1 CS, 0 CC, 1 CSS | 1x2b | - |
| Childbirth | 1 SR, 0 RCT, 2 CS, 0 CC, 1 CSS | 2x2b | - |
| In total | 12 SR, 4 MA, 29 RCT, 64 CS, 12 CC, 4 CSS | 28x1b, 3x2a, 66x2b, 14x3b | - |
CC, case-control study; CS, cohort study; MA, meta-analysis; RCT, randomized controlled study; SR systematic review; CSS, cross-sectional study
The consensus-based recommendations on the primary prevention of asthma, hay fever, and atopic dermatitis apply to at-risk and not-at-risk individuals, unless explicitly stated or indicated otherwise, and are as follows:
Recommendations
With regard to nutrition, the consensus group unanimously supports the recommendations of the specialist societies and organizations (www.fke-do.de, www.dge.de, www.dgkj.de) on the primary prevention of asthma, hay fever, and atopic dermatitis in terms of a balanced and varied diet in infants and young children, as well as in pregnant and breastfeeding women.
Breastfeeding
Breastfeeding has many benefits, both for mother and child. Current data support the recommendation that infants should be predominantly breastfed up to the age of 4 months. (A)
Maternal nutrition during pregnancy and/or lactation
A balanced and varied diet is recommended during pregnancy and lactation.
Dietary restrictions (avoiding potent food allergens) as a means of primary prevention should not be made during pregnancy or lactation. (A)
There is evidence that including fish in the maternal diet during pregnancy and/or lactation has a protective effect against the development of atopic diseases in children. Fish should form part of the maternal diet during pregnancy and lactation. (B)
Breast-milk substitutes in at-risk children
Not, or only partly breastfed at-risk children should receive hydrolyzed infant formula. Current data support this recommendation up to the age of 4 months. (A)
Soy-based infant formulas as a means of allergy prevention are not recommended. (A)
The introduction of solid foods and infant nutrition during the first year of life
The current recommendation in Germany to introduce solid foods to infants over the age of 4-months is reasonable given increasing nutritional requirements.
The introduction of solid foods should not be delayed as a means of allergy prevention. (A)
There is no evidence to suggest that dietary restriction in the form of avoiding potent food allergens in the first year of life has a preventive effect. Such a measure is therefore not recommended. (B)
There is currently no reliable evidence that the introduction of potent food allergens during the first 4 months of life has a preventive effect.
There is evidence that a child‘s consumption of fish during the first year of life has a protective effect against the development of atopic diseases. Fish should be introduced in solid foods. (B)
Body weight
There is evidence that an increased body mass index (BMI) is positively associated with asthma. Excess weight/obesity in children should be avoided in order to better promote asthma prevention. (A)
Keeping pets
Restrictions on the keeping of pets are unnecessary among individuals not at increased risk of allergy.
In the case of at-risk children, the following applies:
Families at increased risk of allergy should avoid acquiring cats.
Keeping dogs is not associated with an increased risk of allergy. (B)
Housedust mites
Specific measures, e.g., dust mite allergen-proof mattress covers (encasings) to reduce exposure to house dust mites are not recommended as a means of primary prevention. (B)
Mold and dampness
Indoor conditions that promote the growth of mold (high humidity, insufficient ventilation) should be avoided. (B)
Exposure to tobacco smoke
Active and passive exposure to tobacco smoke increases the risk of allergy (in particular the risk of asthma) and should be avoided. This recommendation applies as early on as during pregnancy. (A)
Indoor air pollutants
There is evidence that indoor air pollutants can increase the risk for atopic diseases, most notably asthma (volatile organic compounds, e.g., formaldehyde, can be emitted in particular by new furniture or during painting and renovation works).
Exposure to indoor air pollutants should be kept to a minimum. (B)
Motor vehicle emissions
Exposure to nitrogen oxides and particulate matter (PM 2.5) is associated with an increased risk of allergy, particularly asthma.
Exposure to motor vehicle emissions should be kept to a minimum. (B)
Vaccinations
There is no evidence that vaccinations increase the risk of allergy, but rather that they reduce allergy risk.
Vaccination according to STIKO recommendations is recommended for all children, including at-risk children. (A)
Caesarean section
There is evidence that infants delivered by caesarean section have an increased risk of allergy.
This should be taken into consideration when selecting the mode of birth, provided caesarean section is not medically indicated. (B)
Statements
Whilst statements were adopted for the following subject areas (evidence levels given in parentheses), no recommendations were made.
Effects of probiotics: As yet, probiotics have only been shown to have a preventive effect on atopic dermatitis. Due to the heterogeneity of bacterial strains and study designs, it is not possible to make recommendations on specific preparations, forms of application, or duration and time of use (1a–2b).
Effects of prebiotics: As yet, prebiotics have only been shown to have a preventive effect on atopic dermatitis. Due to the small number and heterogeneity of studies, no recommendations can be made. (1b–2b)
Nutrition in general and vitamin D: There is evidence that the consumption of fruit and vegetables (a so-called Mediterranean diet), Ω-3-fatty acids (FA) (or a good Ω3:Ω6 ratio), and milk fat has a preventive effect on atopic diseases.
Recent studies have produced conflicting results with regard to the relevance of vitamin D in the development of allergic diseases.
On the whole, there is currently insufficient data to permit the formulation of recommendations. (1b–3b)
Non-specific immune modulation: There is evidence that early non-specific immune stimulation has a protective effect against allergic diseases. Examples include growing up on a farm, attending a nursery before the age of 2 years, and having a large number of older siblings. (2b–3b)
Pharmaceutical drugs: The link described between the use of antibiotics, paracetamol or acetaminophen and atopic diseases cannot be reliably interpreted due to potential confounding factors. As yet, no causal link has been found between the use of these pharmaceutical drugs and the development of atopic diseases. (2a–3b)
Psychosocial factors: There is evidence that adverse psychosocial factors (e.g., stressful life events) during pregnancy and childhood can contribute to the onset of atopic disease. (2b)
Discussion
The updated S3-guideline on allergy prevention continues to endorse many of the existing recommendations, revises other recommendations, and adopts a number of new recommendations and statements.
Recent studies provide further support for the recommendations on breastfeeding, the keeping of pets, mold and dampness, as well as on exposure to toxic substances.
Modified or newly adopted recommendations are discussed below.
In light of the current literature, intensive discussion took place with regard to dietary recommendations. These exist in Germany in the form of the general recommendations of the DGKJ [8] and the “Young Families‘ Network“ (Netzwerk Junge Familie) [9]. According to these recommendations, breastfeeding represents the preferred and natural form of nutrition for infants. Furthermore, these guidelines recommend exclusive breastfeeding (i.e., no supplementary feeding) until the age of 4–6 months and also specify that breastfeeding can and should be continued even after the introduction of solid foods. The preventive effects of breastfeeding on allergic diseases continue to be reported. Overall, however, these effects tend to wane. There is no evidence base to support the notion that long-term, and in particular exclusive, breastfeeding strengthens these preventive effects in terms of allergy prevention [10, 11]. Numerous studies indicate a link between the introduction of solid foods from the age of 4 months and better tolerance development. Correspondingly, there is evidence that extended exclusive breastfeeding may be associated with an increased risk of allergies [12, 13]. Naturally, results on breastfeeding are derived from observational studies. Methodological bias, e.g., due to reverse causality, requires scrutiny here. In the future, parental predisposition will need to be considered in a more differentiated light, since German investigations indicate that extended breastfeeding increases a child‘s risk of allergy particularly in those cases where the mother is herself affected by allergies. However, current data continue to support the recommendation that breastfeeding should be continued up to the age of 4 months, in line with the World Health Organization‘s (WHO) definition of “predominant breastfeeding.“ Emphasis was also put on the fact that breastfeeding is beneficial for both mother and child in general [14].
The use of hydrolysate formula as a substitute for breast milk in cases where at-risk infants are not or only partly breastfed, continues to be recommended up to the age of 4 months. It should be noted here that a number of the infant formulas tested in studies are no longer available on the German market [15]. The body of evidence and extent of reported effects vary for the preparations tested in Germany: Beba HA (Nestle, Vevey, Switzerland), Hipp HA (Hipp, Pfaffenhofen, Germany), Nutramigen (Mead Johnson, Diezenbach, Germany) and Nutrilon Premium (Nutricia/Numico, Zoetermeer, The Netherlands). Evidence of a preventive effect conferred by soy-based infant formulas is still lacking. Moreover, health concerns exist in this regard [16, 17] and have recently been the subject of discussion [18]. The recommendation that soy-based infant formula is not suitable as a means of allergy prevention remains unchanged.
As previously, the guideline continues to recommend a balanced and varied diet during pregnancy and lactation. A statement takes into account the observations that the consumption of fruit and vegetables (a so-called Mediterranean diet), long-chain Ω3 FA, a good ratio of Ω-3 to Ω-6 FA, and milk fat is associated with a lower prevalence of allergy [19–22]. The consumption of fruit and vegetables is considered beneficial due to the intake of antioxidants as well as prebiotic nutritional components.
These latter possibly play a beneficial role in the development of complex intestinal microflora, which in turn has a positive effect on the development of oral tolerance [23]. The intake of Ω-3 FA, in particular long-chain Ω-3 polyunsaturated fatty acids [PUFA: eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA)] apparently causes an altered immune response associated with a protective effect against allergies [24, 25]. The trans-fatty acids typical of ruminants are considered primarily responsible for this protective effect of milk fat [26–28]. There are numerous health concerns with regard to the trans-fatty acid esters formed during the industrial hydrogenation process and a threshold value for babyfood and olive oil has been set on an EU level [29]. Whilst data from individual controlled interventional studies supports the consumption of Ω-3 FA, the positive effects of fruit, vegetable, and milk fat have only been reported in observational studies. No recommendation was made on this topic.
The existing recommendation that no prophylactic dietary restrictions (i.e., avoidance of potent food allergens) should be undertaken, but that fish should be incorporated in the maternal diet during pregnancy and lactation as a means of allergy prevention has been retained on the basis of additional evidence supporting both statements [30, 31]. Naturally, recommendations on fish consumption do not apply to individuals with known or suspected fish intolerance.
From a physiological and nutritional perspective, the current German recommendation to introduce solid foods between the age of 4 and 6 months is reasonable on the basis of rising nutritional requirements [32]. In this context, the reader is additionally referred to the recommendations of the DGKJ (www.dgkj.de ) and the German Young Families‘ Network (www.gesund-ins-leben.de ). Delaying the introduction of solid foods beyond the age of 4 months as a means of allergy prevention confers no added benefit. There is no evidence that prophylactic avoidance of potent food allergens during the first year of life has a preventive effect. However, there is also no reliable evidence that the deliberate introduction of potent food allergens before the age of 4 months has a protective effect [33].
Since there is yet further evidence that early fish consumption has a protective effect [34-36], the recommendation to introduce fish in solid foods has been retained in the guidelines.
Evidence points to a lower prevalence of allergies among infants and young children receiving a Mediterranean diet containing Ω-3 FA, a good ratio of Ω-3/Ω-6 FA, and milk fat respectively in their diet [22, 37].
Study results on vitamin D levels or vitamin D supplementation and allergic diseases are conflicting. Indeed, one German investigation showed a higher prevalence of dermatitis in the case of high vitamin D levels [38]. Therefore, the current data were considered insufficient to adopt any recommendations.
The use of probiotics as a means of allergy prevention remains a controversial topic in Germany. For this reason, only a statement has been adopted on this topic. Although recent meta-analyses show a significant reduction of 21% in the risk of dermatitis [39, 40], considerable differences were seen between the preparations/bacterial strains used. The most recent studies in particular show a consistent preventive effect. This considerable preventive effect is limited to atopic dermatitis. However, this also applies to, e.g., the use of hydrolyzed foods and can most likely be explained by the fact that only dermatitis reaches a sufficient prevalence in this age group for effects to be regarded as significant. Indeed, it has not been possible as yet to reproduce this effect in Germany. The fact that study designs in terms of bacterial strains used, the quantities administered, and the time point and duration of use vary makes the formulation of a concrete recommendation challenging. Stratified analyses suggest that use during pregnancy has a greater effect compared with postnatally, but also that duration, quantity, and number or type of bacterial strains produce no differences in effect.
The current Cochrane review reports that prebiotics produce a significant reduction of 32 % in the risk of atopic dermatitis [41]. However, with only four studies evaluated, the evidence base is relatively weak and the results of single studies are heterogenous. Therefore, whilst this observation has been included in a statement, no recommendation has been adopted.
Current data continue to support the recommendation that overweight/obesity should be avoided in children as a means of allergy prevention. Negative effects are described primarily in relation to asthma, and a recent meta-analysis describes asthma risk as being higher among overweight boys compared with girls [42]. Avoiding overweight even in early childhood is of crucial importance.
Current data on the keeping of household pets support existing recommendations to a large extent. As such, no restrictions are recommended for children not at risk. Results on the keeping of dogs and cats differ. According to recent meta-analyses, keeping dogs is associated with a significant reduction of 28% in the risk of atopic dermatitis and a non-significant reduction of 23% in the risk of asthma [43, 44]. According to the same meta-analyses - in the case of heterogeneous single studies - keeping cats is not associated with either an increased or a reduced risk of atopic diseases. However, individual studies indicate that keeping cats produces a significantly increased risk of dermatitis among at-risk children with, e.g., a filaggrin loss-of-function mutation [45]. A restrictive recommendation for at-risk children has been retained in the guidelines accordingly, whereby the recommendations are formulated in a more concrete and user-oriented manner. Thus, the guidelines recommend that cats should not be acquired in the case of at-risk children. However, since study results are on the whole conflicting, the guidelines make no recommendation to remove cats already kept in homes. This decision should be taken on a case-by-case basis.
Little has changed in the study data on reducing house dust mite allergen levels as an individual measure of primary prevention. According to a 2009 Cochrane review summarizing three interventional cohort studies, this measure confers no preventive effect [46]. Accordingly, the guidelines state that primary prevention of this kind cannot be recommended. This does not apply to measures of secondary and tertiary prevention, for which there is good evidence of efficacy.
Current data continue to support the existing recommendations relating to the effects of in- and outdoor air pollutants, including exposure to tobacco smoke, [47, 48]. The recommendations have merely been modified to comply with AWMF language use in recommendations.
The guideline also retains the existing recommendation on vaccination.
The statement on the beneficial effects of non-specific immune stimulation in early childhood has also been largely retained. Any reference to the parasitic worm infections also associated with a lower prevalence of allergy has been eliminated on the grounds of poor practicability. A recent meta-analysis confirms a significant reduction of around 30% in the risk of asthma symptoms as a result of exposure to a farming environment in childhood [49]. According to the results of the Protection against allergy: study in rural environments (PASTURE) study, the risk of childhood dermatitis sinks as the number of animal types with which the mother had contact during pregnancy rises [50]. A study on the prophylactic use of bacterial lysates showed no effect on the primary endpoint. However, a significant reduction in dermatitis risk was seen in the subgroup of children with one atopic parent [51].
A new recommendation on caesarean section has been adopted in the guidelines. It takes into account the body of evidence indicating an increased risk particularly of asthma in children delivered by caesarean section [52, 53]. The lack of immune system stimulation caused by exposure to bacteria in the natural birth canal, for example, is discussed as a possible causal mechanism. Other immunological phenotypes have been observed in line with this in children delivered by caesarean section [54]. Changes in lung and liver function, as well as in stress behavior in these children have been described. Given that currently approximately every third infant in Germany is delivered by caesarean section, this fact should be considered when selecting a mode of birth.
Numerous studies suggest a link between pharmaceutical drug use (in particular antibiotics and paracetamol) and atopic diseases. However, these results should be interpreted with caution due to potential reverse causality. Subgroup analysis of studies that were able to minimize reverse causality indicates that a significant link was no longer visible in those particular studies [55]. Accordingly, the statement points out that evidence of a causal link between the use of the above-mentioned medications and the development of atopic disease is lacking to date.
A new statement on psychosocial factors has been adopted. A growing number of studies indicate that experiencing stressful life events (e.g., parental separation, death of a parent, etc.), either during pregnancy or in early childhood, increases the risk of subsequent atopic diseases [56]. Early therapeutic counseling could represent a preventive approach in these children.
With 165 individual publications considered and evaluated, the body of evidence supporting the present guideline revision can be considered extensive. At the same time, guidelines on prevention possess particular methodological features that make them distinct from treatment guidelines in particular. Not only are multiple objective criteria - such as asthma, allergic rhinitis, and atopic dermatitis - investigated, but also multiple influencing factors need to be considered. Restricting the evidence base to one particular study type (e.g., RCT) is impossible, since many of the prevention measures being investigated are not suited to investigation in a randomized trial (e.g., breastfeeding, smoking). Therefore, cohorts and case-control studies need to be included and recommendations on prevention indirectly derived from the associations described there.
S3 guidelines (guidelines at the highest level of development) must satisfy, among other things, the following five requirements:
Logic: “Clinical practice guidelines,“ by means of logical analysis, should reflect the decision-making and action-taking processes that result in the resolution of a specific problem (clinical algorithm) [57]. Although this requirement is aimed more at treatment guidelines, recommendations on prevention have been implemented in an algorithm differentiated on the basis of at-risk and not-at-risk children (Fig. 3).
-
2.
Consensus: Formal consensus procedures should avoid any lack of transparency or distortion of the adopted recommendations as a result of group dynamics or differences between participants in terms of status, personality, political beliefs, or financial interests. Therefore, the involvement of all potential users of the guidelines is crucial to their feasibility (acceptance and practical application) [58]. A formal consensus process (nominal group process) took place involving the members of the consensus group together with representatives from patient organizations and an external facilitator. The advantages of a nominal group process compared with, e.g., the Delphi method include more group dynamics, better group interaction, a greater sense of ownership, and more opportunities for clarification. Disadvantages include the fact that contributions are less anonymous, the process can be very time-consuming, and participants generally have only one opportunity to offer feedback.
-
3.
“Evidence-based medicine“ addresses the problem of methodological perspective versus clinical relevance. The basis for the adopted recommendations was formed according to the criteria of evidence-based medicine using a systematic literature search and methodological critical analysis.
-
4.
Decision analysis is the analysis of the expected benefit in relation to the costs involved. It is a quantitative comparison of the alternative procedures available [59]. Also from an economic perspective, it is to be assumed that the expected benefit of the recommended measures is always greater than the costs produced. In individual cases, e.g., smoking cessation, compliance with the prevention recommendation results in a win-win situation (cost of smoking saved plus cost of treatment saved). The use of hypoallergenic infant formula produces one-off costs of around € 400 in a 6-month period; in contrast, asthma treatment in Germany costs on average more than € 650/year per patient. A cost-effectiveness analysis used in the German Infant Nutritional Intervention (GINI) study on infant formulas classified all preparations as cost-effective [60]. With an average saving of € 478, the extensively hydrolyzed casein-based formula performed best in „intention to treat“ analyses for atopic dermatitis. Partially and extensively hydrolyzed whey formulas also proved cost-effective with savings of € 430 and € 42, respectively.
-
5.
Outcome analysis describes the appraisal of the overall outcome achieved by a combination of diagnostic and therapeutic steps. This includes health status as objectively recorded by the physician (e.g., mortality, complication rates) and quality of life as assessed by the patient. Qualitatively empirical processes are required to determine which objective criteria are truly suited to proving the effectiveness of a procedure in clinical routine (analysis of clinical relevance from the perspective of the physician and the patient) [59]. The outcome analysis of the present prevention guideline describes different concepts to those mentioned above. Outcomes represent, e.g., the access and knowledge of the existence and content of the guideline that can be determined by surveying members of the specialist societies. The now-completed initial survey on the level of knowledge on allergies in the general population forms part of the outcome analysis. Following implementation, it will be possible to measure corresponding modifications in the level of knowledge. The clinically relevant objective criterion lies in the expected reduction in incidence.
Fig. 3.
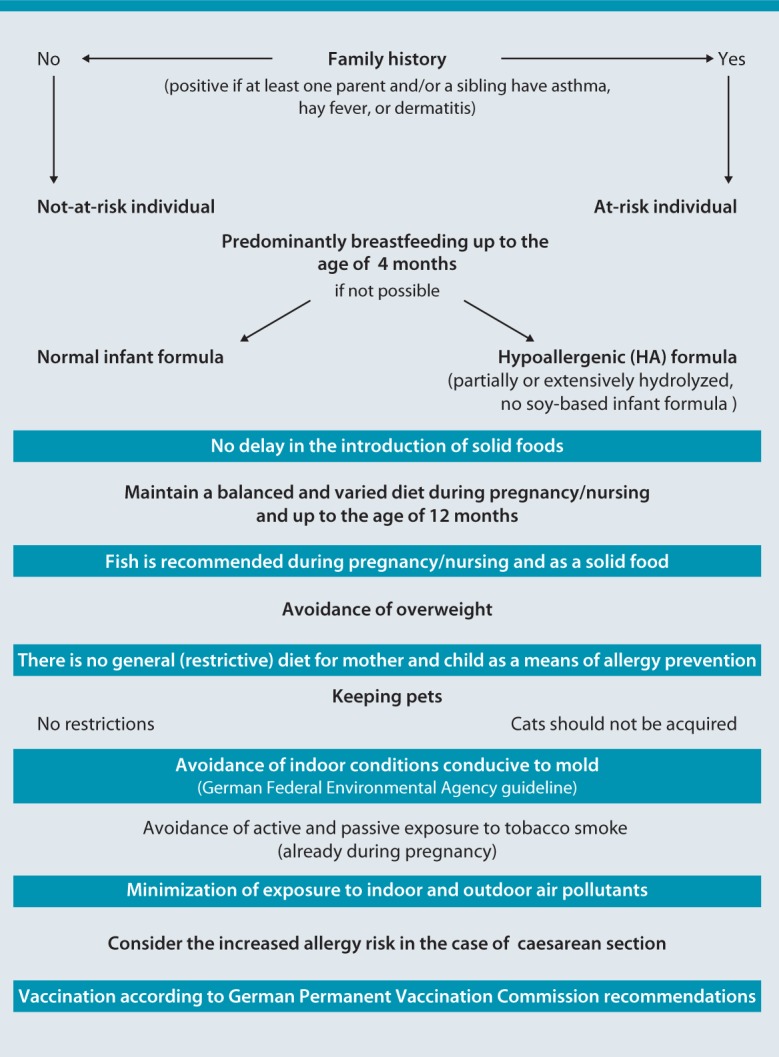
Algorithm for the primary prevention of asthma, hay fever, and atopic dermatitis in at-risk and not-at-risk individuals
Dissemination and implementation
The guidelines will be published in the national specialist journals of participating societies and organizations, as well as on the Internet. Information leaflets will also be available for laypersons and medical professionals.
Participating specialist societies
German Medical Association of Allergologists (Ärzteverband Deutscher Allergologen, AeDA)
German Working Group on Dermatological Prevention (Arbeitsgemeinschaft Dermatologische Prävention, ADP)
German Task force on Dietetics in Allergology (Arbeitskreis Diätetik in der Allergologie)
Professional Association of German Dermatologists (Berufsverband der Deutschen Dermatologen, BVDD)
German Professional Association of ENT Physicians (Berufsverband der HNO-Ärzte, BVHNO)
German Professional Association of Pediatricians (Berufsverband der Kinder- und Jugendärzte, BVKJ)
German Allergy and Asthma Association (Deutscher Allergie- und Asthmabund, DAAB)
German Dermatological Society (Deutsche Dermatologische Gesellschaft, DDG)
German Society for Oto-Rhino-Laryngology, Head and Neck Surgery (Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie, DGHNOKHC)
German Society for Pneumology (Deutsche Gesellschaft für Pneumologie, DGP)
German Society for Psychosomatic Medicine (Deutsche Gesellschaft für Psychosomatische Medizin, DGPM)
German Society for Pediatric Allergology and Environmental Medicine (Gesellschaft für Pädiatrische Allergologie und Umweltmedizin, GPA)
German Society for Pediatric Gastroenterology and Nutrition (Gesellschaft für pädiatrische Gastroenterologie und Ernährung, GPGE)
Kinderumwelt GmbH (a non-profit company with limited liability, fully owned by the German Academy of Pediatrics)
German Allergy/Asthma Prevention and Information Network (Präventions- und Informationsnetzwerk Allergie/Asthma, PINA)
Therapie Schwelmer Modell GmbH
Consensus meeting participants
ADP: Dr. Andreas Kleinheinz, Jennifer Vagts, Dermatological Center, Elbe Clinics Stade/Buxtehude, Buxtehude
German Task force on Dietetics in Allergology: Dr. Imke Reese, Nutrition Counseling and Therapy with Special Focus on Allergology, Munich
BVDD: Dr. Klaus Strömer, Monchengladbach
DDG: Prof. Dr. Margitta Worm, Charité Allergy Center, Clinic for Dermatology, Venereology, and Allergology, Charité University Hospital, Charité Campus Mitte, Berlin
DGAKI: Prof. Dr. Thomas Werfel, Clinic and Polyclinic for Dermatology and Venereology, Hannover Medical School; Prof. Eckard Hamelmann (DGAKI Pediatrics Section), Clinic for Pediatric Medicine, Ruhr University Bochum; Prof. Dr. Torsten Schäfer (coordination), Dermatological Practice, Immenstadt Bochum
DGKJ: Prof. Dr. Matthias Kopp, Clinic for Pediatric Medicine, Schleswig-Holstein University Clinic, Lübeck Campus; Prof. Dr. Albrecht Bufe, Department of Experimental Pneumology, Ruhr University Bochum
DGP: Dr. Horst Müsken, Specialist Medical Practice for Allergology and Pneumology, Bad Lippspringe
DGPM: Prof. Dr. Uwe Gieler, Gießen and Marburg University Clinic, Gießen site, Dermatological Clinic
GPA: PD Dr. Christian Vogelberg, Clinic and Polyclinic for Pediatric Medicine, Carl Gustav Carus University Clinic, Dresden; Prof. Dr. Carl-Peter Bauer, Gaißach Specialist Clinic
GPGE: Prof. Dr. Sybille Koletzko, Dr. von Haunersches Children‘s Hospital at the LMU, Pediatric Clinic and Pediatric Polyclinic of the Ludwig Maximilian University of Munich, Munich
Kinderumwelt GmbH: Dr. Sabine Schmidt, Kinderumwelt GmbH, Osnabrück
PINA: Prof. Dr. Ulrich Wahn, Prof. Dr. Susanne Lau, Charité Allergy Center, Clinic for Pediatrics with Special Focus on Pneumology and Immunology, Charité University Hospital, Berlin
Schwelmer Modell: Mechthild Hellermann, Therapie Schwelmer Modell GmbH, Schwelm
AWMF: PD Dr. Helmut Sitter (facilitator), Institute for Theoretical Surgery, Marburg University; Dr. Cathleen Muche-Borowski (evidence basing), AWMF, Marburg, and Institute for General Medicine, Hamburg-Eppendorf University Clinic
Written consent
German Professional Association of ENT Physicians: Dr. Gerald Gronke, Practice for ENT Medicine, Blankenfelde
BVKJ: Dr. Frank Friedrichs, Practice for Pediatric Medicine, Laurensberg
DAAB: Sabine Schnadt, German Allergy and Asthma Association, Monchengladbach
DGAKI, Pediatrics Section: Prof. Dr. Kirsten Beyer, Charité Allergy Center, Clinic for Pediatrics with Special Focus on Pneumology and Immunology, Charité University Hospital, Berlin
AeDA, DGHNOKHC: Prof. Dr. Ludger Klimek, Center for Rhinology and Allergology, Wiesbaden
Acknowledgements
The revision of this guideline was supported by the German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Pediatric and Adolescent Medicine (DGKJ).
Coordinator
Prof. Dr. Torsten Schäfer
Electronic supplementary material
Supplementary material, approximately 222 KB.
Abbreviations
- abap
German Action Alliance for Allergy Prevention (Aktionsbündnis Allergieprävention)
- BMI
Body mass index
- CC
Case-control study
- CS
Cohort study
- CSS
Cross-sectional studies
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FA
Fatty acids
- FKE
Research Institute on child nutrition
- GINI
German infant nutritional intervention
- IQWiG
Institute for Quality and Effciency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen)
- MA
Metaanalysis
- MEDLINE
Medical literature analysis and retrieval system online
- PASTURE
Protection against allergy: study in rural environments
- PM
Particulate matter
- PUFA
Polyunsaturated fatty acids
- RCT
Randomized controlled study
- STIKO
German Standing Commitee on Vaccination (Ständige Impfkommission)
- SR
Systematic review
- WHO
World Health Organization
References
- 1.Asher M., Montefort S., Björkstén B., Lai C.K., Strachan D., Weiland S., et al. Group IPTS. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Hamelmann E., Beyer K., Gruber C., Lau S., Matricardi P., Nickel R., et al. Primary prevention of allergy: avoiding risk or providing protection? Clin Exp Allergy. 2008;38:233–45. doi: 10.1111/j.1365-2222.2007.02901.x. [DOI] [PubMed] [Google Scholar]
- 3.Schäfer T., Borowski C., Diepgen T.L., Hellermann M., Piechotowski I., Reese I., et al. Konsensusgruppe des Aktionsbündnisses Allergieprävention. Evidenz-basierte und konsentierte Leitlinie „Allergieprävention“. Allergo J. 2004;13:252–60. doi: 10.1046/j.1439-0353.2004.04533.x. [DOI] [PubMed] [Google Scholar]
- 4.Muche-Borowski C, Kopp M, Reese I, Sitter H, Werfel T, Schäfer T et al. S3-Leitlinie Allergieprävention - Update 2009. Allergo J 2009;18:332–41
- 5.Muche-Borowski C, Selbmann HK, Nothacker M, Müller W, Kopp I; Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) - Ständige Kom-mission Leitlinen, eds. AWMF-Regelwerk „Leitlinien“. 1. Aufl. 2012
- 6.Grimshaw J., Eccles M., Russell I. Developing clinically valid practice guidelines. J Eval Clin Pract. 1995;1:37–48. doi: 10.1111/j.1365-2753.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 7.Sackett D, Rosenberg W, Gray J, Haynes R. Evidencebased medicine. How to practice and teach EbM. New York: Churchill Livingstone, 1997
- 8.Ernährungskommission der Deutschen Gesellschaft für Kinder- und Jugendmedizin. Empfehlungen zur Ernährung gesunder Säuglinge. Monatsschr Kinderheilk 2014; im Druck
- 9.Koletzko B., Bauer C.P., Brönstrup A., Cremer M., Flothkötter M., Hellmers C., et al. Säuglingsernährung und Ernährung der stillenden Mutter. Aktualisierte Handlungsempfehlungen des Netzwerks Gesund ins Leben - Netzwerk Junge Familie, ein Projekt von IN FORM. Monatsschr Kinderheilkd. 2013;161:237–46. doi: 10.1007/s00112-013-2870-2. [DOI] [Google Scholar]
- 10.Kramer M.S. Breastfeeding and allergy: the evidence. Ann Nutr Metab. 2011;59(1):20–6. doi: 10.1159/000334148. [DOI] [PubMed] [Google Scholar]
- 11.Morales E., García-Esteban R., Guxens M., Guerra S., Mendez M., Moltó-Puigmartí C., et al. Effects of prolonged breastfeeding and colostrum fatty acids on allergic manifestations and infections in infancy. Clin Exp Allergy. 2012;42:918–28. doi: 10.1111/j.1365-2222.2012.03969.x. [DOI] [PubMed] [Google Scholar]
- 12.Giwercman C., Halkjaer L.B., Jensen S.M., Bønnelykke K., Lauritzen L., Bisgaard H. Increased risk of eczema but reduced risk of early wheezy disorder from exclusive breast-feeding in high-risk infants. J Allergy Clin Immunol. 2010;125:866–71. doi: 10.1016/j.jaci.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Pohlabeln H., Mühlenbruch K., Jacobs S., Böhmann H. Frequency of allergic diseases in 2-year-old children in relationship to parental history of allergy and breastfeeding. J Investig Allergol Clin Immunol. 2010;20:195–200. [PubMed] [Google Scholar]
- 14.Schack-Nielsen L., Michaelsen K.F. Advances in our understanding of the biology of human milk and its effects on the offspring. J Nutr. 2007;137:503S–510S. doi: 10.1093/jn/137.2.503S. [DOI] [PubMed] [Google Scholar]
- 15.von Berg A., Filipiak-Pittroff B., Krämer U., Hoffmann B., Link E., Beckmann C., et al. for the GINIplus study group. Allergies in high-risk schoolchildren after early intervention with cow‘s milk protein hydrolysates: 10-year results from the German Infant Nutritional Intervention (GINI) study. J Allergy Clin Immunol. 2013;131:1565–73. doi: 10.1016/j.jaci.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Agostoni C., Axelsson I., Goulet O., Koletzko B., Michaelsen K.F., Puntis J., et al. ESPGHAN Committee on Nutrition. Stellungnahme zur Verwendung von Säuglingsnahrungen auf Sojaeiweißbasis. Monatsschr Kinderheilkd. 2006;154:913–6. doi: 10.1007/s00112-006-1409-1. [DOI] [Google Scholar]
- 17.Westmark CJ. Soy infant formula and seizures in children with autism: a retrospective study. PLoS One 2014;9:e80488. doi: 10.1371/journal.pone.0080488. eCollection 2014 [DOI] [PMC free article] [PubMed]
- 18.Vandenplas Y., Castrellon P.G., Rivas R., Jimenez Gutiérrez C., Diaz Garcia L., Estevez Jimenez J., et al. Safety of soyabased infant formulas in children. Br J Nutr. 2014;10:1–21. doi: 10.1017/S0007114513003942. [DOI] [PubMed] [Google Scholar]
- 19.Waser M., Michels K.B., Bieli C., Flöistrup H., Pershagen G., von Mutius E., et al. PARSIFAL Study team. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37:661–70. doi: 10.1111/j.1365-2222.2006.02640.x. [DOI] [PubMed] [Google Scholar]
- 20.Arvaniti F., Priftis K.N., Papadimitriou A., Papadopoulos M., Roma E., Kapsokefalou M., et al. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10-12 years old children: the PANACEA study. Pediatr Allergy and Immunol. 2011;22:283–9. doi: 10.1111/j.1399-3038.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 21.Chatzi L., Garcia R., Roumeliotaki T., Basterrechea M., Begiristain H., Iñiguez C., et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) motherchild cohort studies. Br J Nutr. 2013;17:1–11. doi: 10.1017/S0007114513001426. [DOI] [PubMed] [Google Scholar]
- 22.Saadeh D., Salameh P., Baldi I., Raherison C. Diet and diseases among population ages 0-18 years: myth or reality? Nutrients. 2013;5:3399–423. doi: 10.3390/nu5093399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hörmannsperger G., Clavel T., Haller D. Gut matters: microbe- host interactions in allergic diseases. J Allergy Clin Immunol. 2012;129:1452–9. doi: 10.1016/j.jaci.2011.12.993. [DOI] [PubMed] [Google Scholar]
- 24.Harbige L.S. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–41. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- 25.Calder P.C., Kremmyda L.S., Vlachava M., Noakes P.S., Miles E.A. Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc. 2010;69:373–80. doi: 10.1017/S0029665110001552. [DOI] [PubMed] [Google Scholar]
- 26.Jaudszus A., Krokowski M., Möckel P., Darcan Y., Avagyan A., Matricardi P., et al. Cis-9,trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a PPARgamma-related mechanism in mice. J Nutr. 2008;138:1336–42. doi: 10.1093/jn/138.7.1336. [DOI] [PubMed] [Google Scholar]
- 27.Thijs C., Müller A., Rist L., Kummeling I., Snijders B.E., Huber M., et al. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy. 2011;66:58–67. doi: 10.1111/j.1398-9995.2010.02445.x. [DOI] [PubMed] [Google Scholar]
- 28.Wijga A.H., van Houwelingen A.C., Kerkhof M., Tabak C., de Jongste J.C., Gerritsen J., et al. Breast milk fatty acids and allergic disease in preschool children: the Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol. 2006;117:440–7. doi: 10.1016/j.jaci.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Bundesamt für Risikobewertung, ed. Stellungnahme Nr. 015/2006. „Trans-Fettsäuren sind in der Ernährung unerwünscht - zu viel Fett auch“. 30. Januar 2006
- 30.Maslova E., Granstrom C., Hansen S., Petersen S.B., Strøm M., Willett W.C., Olsen S.F. Peanut and tree nut consumption during pregnancy and allergic disease in children - should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J Allergy Clin Immunol. 2012;130:724–32. doi: 10.1016/j.jaci.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Maslova E., Strøm M., Oken E., Campos H., Lange C., Gold D., Olsen S.F. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis - longitudinal evidence from the Danish National Birth Cohort. Br J Nutr. 2013;110:1313–25. doi: 10.1017/S000711451300038X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexy U, Bartsch S, Ellrott T. Kinderernährung aktuell: Schwerpunkte für Gesundheitsförderung und Prävention. Wiesbaden: Umschau; 2009
- 33.Sausenthaler S., Heinrich J., Koletzko S. for the GINIplus and LISAplus Study Groups. Early diet and the risk of allergy: what can we learn from the prospective birth cohort studies GINIplus and LISAplus? Am J Clin Nutr. 2011;94(6Suppl):2012S–7S. doi: 10.3945/ajcn.110.001180. [DOI] [PubMed] [Google Scholar]
- 34.Alm B., Aberg N., Erdes L., Möllborg P., Pettersson R., Norvenius S.G. Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child. 2009;94:11–5. doi: 10.1136/adc.2008.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goksör E., Alm B., Pettersson R., Möllborg P., Erdes L., Aberg N., Wennergren G. Early fish introduction and neonatal antibiotics affect the risk of asthma into school age. Pediatr Allergy Immunol. 2013;24:339–44. doi: 10.1111/pai.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnusson J., Kull I., Rosenlund H., Håkansson N., Wolk A., Melén E., et al. Fish consumption in infancy and development of allergic disease up to age 12 y. Am J Clin Nutr. 2013;97:1324–30. doi: 10.3945/ajcn.112.045377. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Marcos L., Castro-Rodriguez J.A., Weinmayr G., Panagiotakos D.B., Priftis K.N., Nagel G. Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24:330–8. doi: 10.1111/pai.12071. [DOI] [PubMed] [Google Scholar]
- 38.Heimbeck I., Wjst M., Apfelbacher C.J. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy. 2013;68:906–10. doi: 10.1111/all.12167. [DOI] [PubMed] [Google Scholar]
- 39.Tang M.L., Lahtinen S.J., Boyle R.J. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626–34. doi: 10.1097/MOP.0b013e32833d9728. [DOI] [PubMed] [Google Scholar]
- 40.Pelucchi C., Chatenoud L., Turati F., Galeone C., Moja L., Bach J.F., La Vecchia C. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology. 2012;23:402–14. doi: 10.1097/EDE.0b013e31824d5da2. [DOI] [PubMed] [Google Scholar]
- 41.Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev 2013 Mar 28;3:CD006474 [DOI] [PubMed]
- 42.Chen Y.C., Dong G.H., Lin K.C., Lee Y.L. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14:222–31. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 43.Lødrup Carlsen K.C., Roll S., Carlsen K.H., Mowinckel P., Wijga A.H., Brunekreef B., et al. GA2LEN WP 1.5 ‘Birth Cohorts‘ working group. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PloS One. 2012;7:e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelucchi C., Galeone C., Bach J.F., La Vecchia C., Chatenoud L. Pet exposure and risk of atopic dermatitis at the pediatric age: A meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2013;132:616–22. doi: 10.1016/j.jaci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Bisgaard H., Simpson A., Palmer C.N., Bønnelykke K., McLean I., Mukhopadhyay S., et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;24(5):e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maas T., Kaper J., Sheikh A., Knottnerus J.A., Wesseling G., Dompeling E., et al. Mono and multifaceted inhalant and/ or food allergen reduction interventions for preventing asthma in children at high risk of developing asthma. Cochrane Database Syst Rev. 2009;8(3):CD006480. doi: 10.1002/14651858.CD006480.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell E.A., Beasley R., Keil U., Montefort S., Odhiambo J. ISAAC Phase Three Study Group. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from Phase Three of the ISAAC programme. Thorax. 2012;67:941–9. doi: 10.1136/thoraxjnl-2011-200901. [DOI] [PubMed] [Google Scholar]
- 48.Carlsten C., Dybuncio A., Becker A., Chan-Yeung M., Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68:291e295. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- 49.Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with metaanalysis. Pediatr Allergy Immunol. 2012;23:509–18. doi: 10.1111/j.1399-3038.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 50.Roduit C., Wohlgensinger J., Frei R., Bitter S., Bieli C., Loeliger S., et al. PASTURE Study Group. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol. 2011;127:179–85. doi: 10.1016/j.jaci.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Lau S., Gerhold K., Zimmermann K., Ockeloen C.W., Rossberg S., Wagner P., et al. Oral application of bacterial lysate in infancy decreases the risk of atopic dermatitis in children with 1 atopic parent in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:1040–7. doi: 10.1016/j.jaci.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Thavagnanam S., Fleming J., Bromley A., Shields M.D., Cardwell C.R. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629–33. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 53.Roduit C., Scholtens S., de Jongste J.C., Wijga A.H., Gerritsen J., Postma D.S., et al. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009;64:107–13. doi: 10.1136/thx.2008.100875. [DOI] [PubMed] [Google Scholar]
- 54.Hyde M.J., Mostyn A., Modi N., Kemp P.R. The health implications of birth by Caesarean section. Biol Rev Camb Philos Soc. 2012;87:229–43. doi: 10.1111/j.1469-185X.2011.00195.x. [DOI] [PubMed] [Google Scholar]
- 55.Penders J., Kummeling I., Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and metaanalysis. Eur Respir J. 2011;38:295–302. doi: 10.1183/09031936.00105010. [DOI] [PubMed] [Google Scholar]
- 56.de Marco R., Pesce G., Girardi P., Marchetti P., Rava M., Ricci P., Marcon A. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatr Allergy Immunol. 2012;23:724–9. doi: 10.1111/j.1399-3038.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- 57.Schoenbaum S., Gottlieb L. Algorithm based improvement of clinical quality. BMJ. 1990;301:1374–6. doi: 10.1136/bmj.301.6765.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black C., Peterson S., Mansfield J., Thliveris M. Using population based data to enhance clinical practice guideline development. Med Care. 1999;37(6Suppl):254–63. doi: 10.1097/00005650-199906001-00019. [DOI] [PubMed] [Google Scholar]
- 59.Lorenz W., Troidl H., Solomkin J., Nies C., Sitter H., Koller M., et al. Second step: testing-outcome measurements. World J Surg. 1999;23:768–80. doi: 10.1007/s002689900578. [DOI] [PubMed] [Google Scholar]
- 60.Mertens J., Stock S., Lüngen M., von Berg A., Krämer U., Filipiak-Pittroff B., et al. Is prevention of atopic eczema with hydrolyzed formulas cost-effective? A health economic evaluation from Germany. Pediatr Allergy Immunol. 2012;23:597–604. doi: 10.1111/j.1399-3038.2012.01304.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 222 KB.


