Abstract
House dust mites, cats and dogs are amongst the most frequent sources of indoor allergens in Europe. The fact that the allergens of house dust mites cause allergic disease through inhalation of house dust was discovered in 1964. The diagnosis of mite allergy is regularly complicated by its often nonspecific symptoms, which frequently develop insidiously and by no means always include attacks of paroxysmal sneezing and itching. Antibody-based immunological detection methods can be used to measure exposure to mite allergens. The structure and function of more than 20 allergens from Dermatophagoides pteronyssinus and D. farina are known. Other relevant indoor allergens come from mammals kept in households. Here again, allergens have been described and diagnostic as well as exposure-measurement tools are available. It is important to remember indoor pests and other „unwelcome lodgers“ as a possible cause in the case of unexplained symptoms experienced indoors. This short overview summarizes the current key points on the subject of „mites and other indoor allergens“. The present article provides an overview of several articles published in a special issue of the German journal Allergologie [February 2015; 38(2)] on the subject of „Mites and other indoor allergens“.
Key words: allergen sources, single allergens, cats, dogs, moulds, pests
Introduction
Residing predominantly indoors is part of the western lifestyle, i. e. living and housing conditions associated with the rise in allergic diseases. In Europe, house dust mites, cats and dogs are amongst the most frequent sources of indoor allergens responsible for allergic reactions in the upper and lower airway [1]. Sensitization to moulds is far less frequent. Recent studies in Germany on the prevalence of sensitization to inhalant and food allergens, conducted on a population-based sample of 7,025 18- to 79-year-old adults by means of specific immunoglobulin E (IgE) detection, showed a prevalence of sensitization to the house dust mite Dermatophagoides pteronyssinus, an important indoor allergen, of 15.9 %, followed by dog dander and cat epithelium both at 7 % and the moulds Aspergillus fumigatus and Cladosporium herbarum at 2.3 % and 1.3 %, respectively. In the US, the two cockroaches, Blattella germanica and Periplaneta Americana, but also mouse and rat allergens, represent relevant sources of indoor allergens. However, other, mostly unwanted indoor „inhabitants“ can also represent — albeit rarely — allergen sources [3].
The current special issue of the journal Allergologie [February 2015; 38(2)] on the subject of „Mites and other indoor allergens“ includes six articles that describe relevant sources of indoor allergens [3, 4, 5, 6, 7, 8], with particular focus on the mite as an allergen source [4, 5, 6, 7]. Attention is also paid to cats, dogs and other fur-bearing animals [8], as well as storage and public health pests, in terms of their significance as indoor allergens [3]. The present article summarizes the key points discussed in the above-mentioned special issue.
The discovery of mites as an allergen source [4]
It has been known since at least the 17th century that the inhalation of house dust can cause asthma and rhinitis. It wasn’t until 1964, however, that the group working with Reindert Voorhorst and the married couple, Frits T. Spieksma and Marise I. Spieksma-Boezeman, demonstrated that the presence of house dust mites in dust samples taken from homes in the Juliana street in Leiden caused asthma symptoms. Spieksma-Boezeman proved not only that house dust mites are the main source of allergens in house dust, but also that there are greater numbers of house dust mites in damp houses than in dry [9]. The first extracts for diagnostic skin tests were described by Brown in 1968 [10] and Frankland in 1970 [11], and extracts were available for immunotherapy in 1971 [12].
Not all mites are alike
All mites (Acari) belong to the arthropods — and to the arachnid class (Arachnida) within this phylum. They are classified into numerous orders according in particular to the presence and position of the external openings of the respiratory system (stigmata): Astigmata (absent), Prostigmata (anterior), Cryptostigmata (hidden), Mesostigmata (mid), Metastigmata (posterior). In addition to house dust mites of the superfamily Pyroglyphoidea (with D. Pteronyssinus, D. Farina and Euroglyphus maynei), storage mites belonging to the Acaroidae and Glycyphagidae families also cause allergies. All mite species found in houses or apartments and that are capable of eliciting IgE-mediated sensitizations are referred to as „domestic mites“. The biology of approximately 40 storage mites in Germany is similar to the biology of house dust mites, but not identical [4].
Anatomy and habitat of mites
Whilst house dust mites (Fig.1, Fig.2) are between 0.1–0.4 mm long, storage mites can grow up to 0.6 mm in length; however, they are virtually invisible to the naked eye. One of their striking features is the reduced segmentation characteristic of arthropods (e. g. insects). Both house dust and storage mites communicate via pheromones [13].
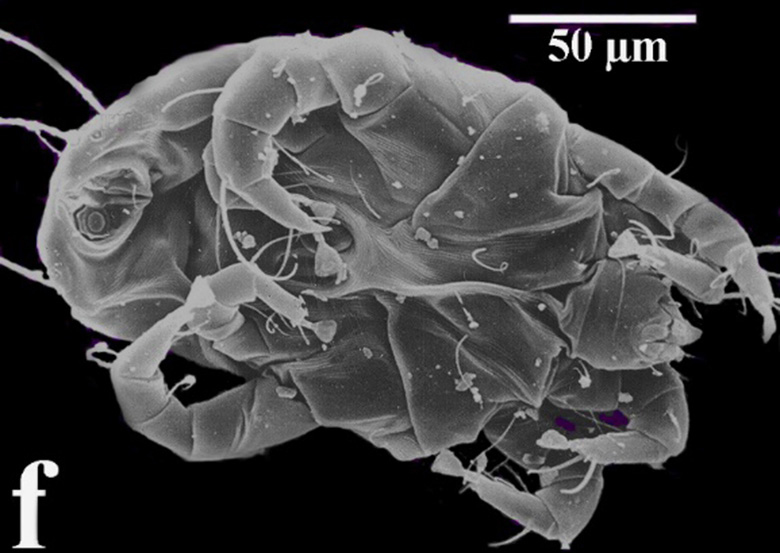
Fig. 1: Underside of a male specimen of Dermatophagoides pteronyssinus (image by J.-T. Franz; from [4])
© (2) Franz J.-T.

Fig. 2: Lateral opisthosomal gland of the Dermatophagoides pteronyssinus (image by J.-T. Franz; from [4])
© (2) Franz J.-T.
The reproduction and development of mites is crucially affected by the microclimate of a house. Relative ambient air humidity of 75 % at 15°C is ideal for their development. As a person sleeps, the temperature in their mattress rises to 25°C–30°C and relative air humidity increases due to body perspiration during sleep, making conditions on the whole optimal for mite development. The number of mites found in rugs and carpets fluctuates according to the seasons, rising in the summer months when heating is turned off and room humidity is at its highest. The mite population is small following the heating period at the beginning of the summer; it reaches its peak in the late summer and drops again to its minimum in the late autumn and winter [14, 15]. Studies in Germany have shown that unusually cold winters result in a reduction in mite numbers [16]. Comparable findings have been made for a number of health resorts at high altitudes. Large differences in temperature between summer and winter are also believed to be associated with a lower incidence of asthma [17]. However, if winters become milder and are associated with higher air humidity in the future, we may see an increase in and broadening of rhinitis and asthma symptoms.
Quantifying mite allergen exposure in the home [5]
Mite proteins (mite allergens) that originate from mite faeces or decaying mite remains and are bound to dust particles are responsible for sensitization and the onset of symptoms [18, 19]. Antibody-based immunological detection methods capable of identifying single or multiple mite allergens are available for the quantification of mite allergen exposure. The two-site immunoassays based on monoclonal antibodies against the major group 1 and 2 allergens of D. Pteronyssinus (Der p 1, Der p 2) and D. Farina (Der f 1, Der f 2) have been used since 1987 to estimate house dust mite exposure [20, 21]. Although mite allergen levels in dust samples taken from mattresses, furniture or floor surfaces can generally be measured using this immunoassay [22], allergen levels in samples of airborne dust are often too low to exceed the measurement method’s detection limit [23]. Detection methods based on polyclonal antibodies have proved helpful with samples of this kind, despite the fact that they are not always able to distinguish between mite species, but nevertheless have a high detection rate due to their simultaneous identification of several single allergens [24, 25, 26]. The study by Sander et al. [5] measures mite allergen exposure in households. To this end, samples were taken in living rooms and bedrooms in 36 households over a 14-day period using an electrostatic dust fall collector (EDC) and in 16 households during housework using personal pumps. Mite allergen levels in the sample extracts were determined using five different immunoassays (domestic mites [25], D. Pteronyssinus, Acarus siro, Tyrophagus putrescentiae and Lepidoglyphus destructor [27, 28]). In total, 94 % of EDC samples and 75 % of personally collected samples were positive with the domestic mite assay, which recognizes allergens in numerous mite species and can serve as a screening instrument for mite allergen exposure. The Tyrophagus assay was able to detect antigens in 53 % and 56 % of samples, respectively, and the D.-pteronyssinus assay in 50 % and 13 %, respectively. Acarus and Lepidoglyphus antigens were detected only rarely, but when they were, this was partially in high levels. As the studies showed, mite exposure could generally be measured in living areas and whilst performing housework. Of the storage mite antigens, Tyrophagus antigens were the most frequently detected, not however in the highest concentrations.
Known single house dust mite allergens: structure, function und relevance [6]
In Europe, the two house dust mite species D. Pteronyssinus and D. Farina are regarded as the main triggers of mite allergy and have the highest sensitization rate among indoor allergens [25]. The prevalence of sensitization to house dust mites in Germany is 16 % in adults [2] and 22 % in children [29]. More than 20 allergens from D. Pteronyssinus and D. farinae have been identified and, moreover, accepted and listed by the World Health Organization and International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-Committee [30]. According to nomenclature rules, the allergens are referred to as Der p (from the mite species D. Pteronyssinus) or Der f (D. Farina). The allergens are classified into groups according to chronological order of purification and their homology to allergens already identified (according to the IUIS, groups 1–33 are known to date) [30, 31, 32, 33]. The respective allergens have also been described for other house dust and storage mites, such as D. microceras, Blomia tropicalis, Euroglyphus maynei and Lepidoglyphus destructor (Tab.1). Approximately 80 %–90 % of all mite allergy sufferers react with partially severe allergic symptoms to major group 1 and 2 allergens. Der p 23, which was first identified in 2013, is also classified as a major allergen, since it, too, is of considerable clinic importance given its sensitization rate of around 70 % [34%]. At present, only natural Der p 1, recombinant Der p 2 and Der p 10 are used in routine in vitro allergy diagnosis; Der p 23 is not currently available. There is discussion as to whether sensitization to major mite allergens is a precondition of effective specific immunotherapy (SIT) [35]. However, the extracts used for this have been verified at best for group 1 and 2 allergens (e. g. Der p 1 and 2), but not for other major allergens. Group 5, 7 and 21 allergens are detected in approximately 30 % of mite allergy sufferers and are associated with the onset of allergic asthma [36, 37]. In contrast to Der p 1, the proteases in groups 3, 6 and 9 play more of a minor role and demonstrate only very weak IgE binding. With an IgE reactivity of approximately 10 % in Europe, the tropomyosin Der p 10 is also only a minor allergen. However, due to its high sequence homology to other tropomyosins, it is an important cross allergen to foods of animal origin and is associated with sometimes severe reactions [38, 39]. Moreover, it is important to bear in mind that immunotherapy with house dust mite extracts can cause, among other things, clinically relevant sensitization to crustaceans [40]. It was recently discovered that although Der p 11, the mite paramyosin, plays more of a secondary role in patients with a respiratory form of house dust mite allergy, it is a major allergen in patients with atopic dermatitis [41].
Tab. 1.
Single allergens of the house dust and storage mite that have been characterized. All allergens listed are officially listed in the WHO/IUIS allergen database (www.allergen.org)
| Allergen group | Allergen | Protein family | MW (kDa) | Sensitization (%)* |
|---|---|---|---|---|
| 1 | Der p 1 Der f 1 Der m 1 Blo t 1 Eur m 1 |
Cysteine protease | 24 27 25 39 - |
74–00 70–100 - - - |
| 2 | Der p 2 Der f 2 Blo t 2 Eur m 2 Lep d 2 Tyr p 2 Gly d 2 |
Lipid-binding protein | 15 15 - - 16 16 15 |
62–100 >90 - - 88 79 95 |
| 3 | Der p 3 Der f 3 Blo t 3 Eur m 3 Tyr p 3 |
Trypsin | 31 29 - - 26 |
9–97 16 51–100 - 58 |
| 4 | Der p 4 Blo t 4 Eur m 4 |
α-Amylase | 60 56 - |
25–74 4–28 - |
| 5 | Der p 5 Blo t 5 Lep d 5 |
Unknown | 14 14 - |
30–55 20–74 9 |
| 6 | Der p 6 Der f 6 Blo t 6 |
Chymotrypsin | 25 25 25 |
41–65 41 8 |
| 7 | Der p 7 Der f 7 Lep d 7 |
Unknown | 26, 30, 31 30–31 - |
31–53 46 62 |
| 8 | Der p 8 Der f 8 Blo t 8 |
Gluthation-S-Transferase | 27 32 27 |
10–40 - 25 |
| 9 | Der p 9 | Serin protease | 29 | 92 |
| 10 | Der p 10 Der f 10 Blo t 10 Tyr p 10 Lep d 10 |
Tropomyosin | 36 37 33 - - |
6–28 46–81 20–29 - 13 |
| 11 | Der p 11 Der f 11 Blo t 11 |
Paramyosin | 103 98 110 |
42–67 71–87 52 |
| 12 | Blo t 12 | Unknown | 14 | 50 |
| 13 | Der f 13 Blo t 13 Tyr p 13 Lep d 13 Aca s 13 |
Fatty acid-binding protein | - - 15 - 15 |
- 11 - 13 23 |
| 14 | Der p 14 Der f 14 Eur m 14 |
Lipid transfer protein | 177 177 177 |
- 66–84 |
| 15 | Der p 15 Der f 15 |
Chitinase | - 98, 109 |
70 70 |
| 16 | Der f 16 | Gelsolin | 53 | 47 |
| 17 | Der f 17 | Calcium-binding protein | 53 | 35 |
| 18 | Der p 18 Der f 18 |
Chitin-binding protein | - 60 |
63 54 |
| 19 | Blot 19 | Antimicrobial peptide | 7 | 10 |
| 20 | Der p 20 Der f 20 |
Arginine kinase | - 40 |
14–44 50 |
| 21 | Der p 21 Der f 21 Blo t 21 |
Unknown | - 14 13 |
26 - 58–95 |
| 22 | Der f 22 | Unknown | - | |
| 23 | Der p 23 | Peritrophin-like protein | 14 | 74 |
| 24 | Der f 24 Tyr p 24 |
Ubiquinol-cytochrome c reductase-binding protein |
13 18 |
- 11 |
| 25 | Der f 25 | Triosephosphate isomerase | 34 | 60–75 |
| 26 | Der f 26 | Myosin light chain | 18 | 29 |
| 27 | Der f 27 | Serpin | 48 | 35 |
| 28 | Der f 28 | Heat shock protein | 70 | 68–70 |
| 29 | Der f 29 | Cyclophylin | 16 | 70–85 |
| 30 | Der f 30 | Ferritin | 16 | 60–63 |
| 31 | Der f 31 | Cofilin | 15 | 31 |
| 32 | Der f 32 | Inorganic pyrophosphatase | 35 | 15 |
| 33 | Der f 33 | α-Tubulin | 52 | 25 |
MW, molecular weight; Der p, Dermatophagoides pteronyssinus; Der f, Dermatophagoides farinae; Der m, Dermatophagoides microceras; Blo t, Blomia tropicalis; Eur m, Euroglyphus maynei; Lep d, Lepidoglyphus destructor; Tyr p, Tyrophagus putrescentiae; Gly d, Glycyphagus domesticus; Aca s, Acarus siro; —, no information available
*Sensitization rates are based on a number of studies with different patient groups and test systems [enzyme linked immunosorbent assay (ELISA), Immunoblot, ImmunoCAP®, skin test]. Thus they represent merely a guide and not absolute figures.
Diagnosis and treatment of mite allergy [7]
After taking the patient history and recording clinical findings, skin testing and/or serological measurement of specific IgE to identify IgE-mediated sensitization are carried out in the case of suspected mite allergy. In the case of suspected mite allergy, Klimek et al. [7] recommend testing for house dust and storage mites, taking at least the following mite species into consideration:
Dermatophagoides farinae
Dermatophagoides pteronyssinus
Acarus siro
Lepidoglyphus destructor
Tyrophagus putrescentiae
In the case of perennial allergic rhinitis (AR) due to house dust mites, nasal provocation testing (NPT) is particularly indicated when the patient history provides inconclusive information.
The following constitute absolute contraindications to NPT:
Acute inflammatory diseases of the nose or paranasal sinuses
Procedures in the nasal cavity or paranasal sinuses that lie less than 8 weeks in the past
Since numerous medications interfere with NPT results, a period of abstention from relevant substances should be observed.
From a therapeutic perspective, avoidance is recommended in many cases. The following procedure is recommended:
Detection of significant mite/mite allergen exposure
Elimination of existing mites
Cleaning the premises to remove mite allergens
Preventing contact with mite allergens
Creating unfavourable living conditions for mites
Drug treatment of mite-induced AR consists primarily of administering mast cell stabilisers, antihistamines (AH), glucocorticosteroids (GCS), leukotriene receptor antagonists (LRA) and decongestants. Particular attention should be paid here to ensuring good anti-inflammatory efficacy. At present, the administration of topical GCS is the most effective form of pharmacological treatment in mite-induced AR and therefore represents, together with non-sedating AH, the treatment of choice. A new mechanism of action (MP29-02) combines the nasal administration of GCS and AH and reduces nasal symptoms more effectively compared with the current standard therapeutic agents.
As with inhalation allergies in general, allergen-specific immunotherapy (AIT) is the only causal treatment form available also for mite-induced AR, besides abstention. In addition to established subcutaneously administered forms of AIT, new studies using sublingual preparations that will make easier and more patient-friendly AIT possible in the future by using „mite tablets“ were recently published. The general rule of thumb is that immunotherapy can be recommended when symptoms have already been present for at least 2 years and allergen avoidance is either impossible or insufficient. Naturally, parallel to AIT, refurbishment measures aimed at reducing indoor mite levels, as well as drug treatment, are beneficial and, as the case may be, necessary. The efficacy of omalizumab — a monoclonal anti-IgE antibody that has been approved since 2005 for the treatment of severe bronchial asthma in patients aged from 12 years under the trade name Xolair® — has now been adequately proven for the treatment of moderate to severe, therapy-resistant, uncontrolled allergic asthma, in particular also in mite allergy [42, 43, 44].
Dogs, cats and Co.: domestic pets as indoor allergen sources [8]
Domestic pets represent a source of a variety of animal allergens that adhere to animal hair and dander and are thus dispersed in indoor areas. Dogs and cats are the most popular domestic pets, followed by rabbits, guinea pigs and hamsters. The prevalence of sensitization to animal allergens is subject to stark variation and depends on the region and collective studied (e. g. exposed or atopic individuals, asthma sufferers). A multi-centre European GA2LEN study, in which skin tests with various outdoor and indoor allergens were carried out in over 3,000 patients, found a mean prevalence of sensitization of 26.3 % to cats and 27.2 % to dogs for all of Europe [1], with considerable regional differences (16.1 %–56 % for cats and 16.8 %–49.3 % for dogs). The highest sensitization rates were found in Scandinavian countries. The frequency of sensitizations in the general population is markedly lower compared with patient collectives [2]. In 1991, Fel d 1, the major cat allergen, was the first animal hair allergen to be identified [45]. It quickly became clear that many animal allergens belong to particular protein families: the serum albumins and the lipocalins (Tab.2). Lipocalins make up a group of proteins that occur ubiquitously in nature and have a molecular weight (MW) of 16–22 kDa. Despite their similar three-dimensional structure, they exhibit widely differing amino acid sequences. Amino acid identities are often only 20 %. Many lipocalins play a role in social behaviour in that they transport pheromones. The precise function of allergenic lipocalins is largely unknown.
Tab. 2.
Inhalant mammalian allergens that have been characterized (modified according to [8]). All allergens listed are also officially listed in the WHO/IUIS allergen database (www.allergen.org)
| Animal species | Allergen | Protein family | MW (kDa) | Allergen source | Sensitization (%)* |
|---|---|---|---|---|---|
| Cat (Felis domesticus) | Fel d 1 Fel d 2 Fel d 3 Fel d 4 Fel d 5 Fel d 6 Fel d 7 Fel d 8 |
Secretoglobin Serum albumin Cystatin Lipocalin IgA IgM Lipocalin Latherin |
18 69 11 22 400 800–1000 18 24 |
Saliva, dander Serum, skin Dander Saliva Saliva, serum Serum Saliva |
60–100 14–23 10 63 38 - 38 19 |
| Dog (Canis familiaris) | Can f 1 Can f 2 Can f 3 Can f 4 Can f 5 Can f 6 |
Lipocalin Lipocalin Serum albumin Lipocalin Kallikrein Lipocalin |
23–25 19 69 18 28 27–29 |
Saliva, dander Saliva, dander Serum, skin, saliva Saliva, dander Urine Saliva, dander |
50–75 22–30 25–35 35 70 61 |
| Horse (Equus caballus) | Equ c 1 Equ c 2 Equ c 3 Equ c 4 |
Lipocalin Lipocalin Serum albumin Latherin |
22 17 67 17–19 |
Dander, saliva Dander Serum, skin Dander, saliva |
76 50 18–50 77 |
| Cow (Bos domesticus) | Bos d 2 Bos d 3 Bos d 6 |
Lipocalin Calcium-binding protein Serum albumin |
20 11 67 |
Hair, dander Hair, dander Serum, Haut |
>90 43 - |
| Rabbit (Oryctolagus cuniculus) | Ory c 1 Ory c 3 Ory c 4 |
Lipocalin Secretoglobin Lipocalin |
17–18 19–21 24 |
Saliva, dander Saliva, dander Saliva |
- 77 46 |
| Rat (Rattus norvegicus) | Rat n 1 | Lipocalin | 17 | Urine | 73–87 |
| Mouse (Mus musculus) | Mus m 1 | Lipocalin | 17 | Urine | 66 |
| Guinea pig (Cavia porcellus) | Cav p 1 Cav p 2 Cav p 3 Cav p 4 Cav p 6 |
Lipocalin Lipocalin Lipocalin Serum albumin Lipocalin |
20 17 18 66 18 |
Hair, urine Saliva, hair Saliva, hair Serum Saliva |
70 65 54 52 - |
MW, molecular weight; —, no information available
* Sensitization rates are based on a number of studies with different patient groups and test systems (enzyme linked immunosorbent assay [ELISA], Immunoblot, ImmunoCAP®, skin test). Thus they represent merely a guide and not absolute figures.
Serum albumins are the main protein in plasma; they regulate colloid-osmotic pressure and transport fatty acids, hormones, bilirubin and other substances thanks to high protein binding. They are large globular proteins with an MW of 66 kDa and high amino acid identity (80 % on average) between various mammals [46]. Serum albumins are responsible for IgE cross-reactivity in in vitro diagnosis with mammalian epithelium extracts.
Test extracts for all animals, except the dwarf hamster, are available for in vitro IgE diagnosis. Individual components are currently only available for the dog, the cat and the mouse. Due to cross-reactive molecules like those of serum albumin and various lipocalins, determining primary sensitization unequivocally is often challenging, with the result that the clinical history assumes central importance. Skin testing solutions are also available for all domestic pets. However, for the diagnosis of hamster allergy, there is only one skin test solution for the golden or field hamster, not for the Roborovski and Djungarian dwarf hamsters. This can lead to false-negative results in the case of sensitization to the dwarf hamster. The occurrence and distribution of some indoor animal allergens have been the subject of intensive investigation in recent decades. An important prerequisite of assessing allergen exposure is the availability of a reliable quantification test. Although a diversity of animals are known to be allergen sources, sensitive and specific immunoassays have been validated for only dog, cat, horse, cow, mouse and rat allergens to date. „Sandwich ELISAs“ (ELISA, enzyme linked immunosorbent assay) for major allergens of these species are commercially available from Indoor Biotechnologies (Charlottesville, USA). Studies on exposure to animal allergens showed that animal allergens are ubiquitous, independent of the presence of animals. Thus, for example, cat and dog allergens were often detected in households with no domestic pets, as well as in schools, nursery schools, hospitals, offices and on public transport. This ubiquitous distribution in the environment is strongly related to the common characteristics of animal allergens. Firstly, they are efficiently distributed in the environment through the loss of hair and dander, as well as the secretion of body fluids from the animals themselves. Secondly, allergens tend to bind to small dust particles (< 10 μm) that scarcely sediment. Benefitting from good floating properties, they can be easily transferred to previously unexposed areas, where they accumulate in textiles such as carpets, upholstered furniture and mattresses. Clothing and human hair are considered main allergen carriers in this process [47. 48]. Mice (Mus musculus) and rats (Rattus norvegius) are only rarely kept as domestic pets; however, rodent infestation can cause high indoor allergen exposure. This appears to be a relevant problem in large cities in the US. Elevated Mus m 1 and Rat n 1 levels were found in the households of individuals coming into contact with laboratory animals in an occupational context [49]. The transfer of allergens from the workplace to the home was also demonstrated using bovine allergens, which are found in high levels in the homes of cattle farmers, as an example [50]. Although numerous studies show similar effects, it is not always possible to compare the measured values directly with one another. Results are strongly affected by differences in study design (choice of dust collection method, type of quantification assay, data analysis, calculation of results) [51].
Other “unwanted” indoor lodgers are also potential allergen sources [3]
Other arthropods besides mites also belong to the rarer sources of indoor allergens, which can be grouped into the category of storage, material and public health or hygiene pests [52, 53]. These are usually „unwanted lodgers“ (Tab.3).
Tab. 3.
Examples of public health and storage pests that are indoor allergen sources (modified from [3])
| Allergen source | Distribution | Allergen | Symptoms and prevalence of sensitization |
|---|---|---|---|
| Bed bug (Cimex lectularius) | Worldwide | 32 kDa-Protein cNP (Cimex-lectularius-Nitrophorin) |
At times, immediate-type reactions; 57% of patients with bed bug bites had specific IgE against C.-lectularius -extracts, 30% specific IgE against cNP |
| Book louse (Liposcelis bostrichophila) | Damp homes, libraries, cellars; tatami mat infestation in Japan | Lip b 1 a (26 kDa; function unknown) | Respiratory symptoms; 22% of 185 Japanese individuals with allergic asthma had booklouse-specific IgE |
| Indian meal moth (Plodia interpunctella) | Favours plant material, grain products | Plo i 1a (arginine kinase; 40 kDa) Plo i 2a (thioredoxine) | Respiratory symptoms; 51% sensitization rate in 100 allergy patients with symptoms indoors |
| Flea/cat flea (Ctenocephalides felis) | Worldwide | Cte f 1a (18 kDa from the saliva of the cat flea) Cte f 2a, Cte f 3a | Flea allergy dermatitis (FAD), most frequent dermatological disease in cats and dogs; immediate and late-phase reactions |
| Common house spider (Tegenaria domestica) | Prevalent in homes | Teg do 7b
Teg do Hemocyaninb |
Respiratory symptoms; no systematic studies; isolated case |
| Cellar spider (Holocnemus plucei) | Cellars/homes | Arginine kinase (17 kDa) | Respiratory symptoms; only an isolated case |
| Head louse (Pediculus humanus capitis) | Worldwide; however, regional differences in head lice infestation | 20-kDa-Protein Ped h 7b (Tropomyosin) | Itching, bilateral nasal obstruction, runny nose, respiratory symptoms; no systematic studies; isolated case |
| Cockroaches (Blattodea)
German cockroach (Blattella germanica) American cockroach (Periplaneta americana) |
Primarily the tropics and subtropics | Bla g 1 bis Bla g 8; Bla g 11 (21–78,9 kDa) Per a 1, 3, 6, 7, 9, 10 (17–72 kDa) | In the US: risk factor for increased asthma morbidity („inner-city asthma problem“); allergic immediate-type reactions, e.g. rhinoconjunctivitis, allergic asthma, e.g.: 36.8% of 476 asthmatic children in the US had cockroach sensitization |
| Silverfish (Lepisma saccharina) | In human dwellings | Lep s 1a (Tropomyosin) | Respiratory symptoms; 30% of Dutch house dust mite allergy sufferers investigated had a specific reaction to silverfish |
| Housefly/common housefly (Musca domestica) | Incidence generally associated with humans | Mus do 7b (Tropomyosin) | Respiratory symptoms; several isolated cases |
| Pigeon tick (Argas reflexus) | Central and southern Europe (together with domestic pigeon) | Arg r 1a 18–19 kDa, im Immunoblot 22 kDa | From local inflammation after bite to anaphylactic systemic reactions; specific IgE: 8% of 148 with Argas bites; positive skin test: 16% of 148 with Argas bites |
a These allergens are officially listed in the IUIS allergen database (www.allergen.org); ; b information can be found at www.allergome.org.
Members of the Blattodea order (cockroaches), which has more than 4,600 species, are also found in homes worldwide and represent a potent allergen source, particularly in the US. Cockroaches are nocturnal and indigenous primarily to the tropics and subtropics. The cockroaches best studied as allergen sources in dwellings include the German cockroach (Blattella germanica), which dominates in the US in terms of numbers, as well as the American cockroach (Periplaneta americana) and the oriental or common cockroach (Blatta orientalis).
As early as in 1995, the working group of Aalberse [54] reported that 30 % of Dutch house dust mite allergy sufferers also exhibited sensitization to silverfish. Inhibition investigations were able to detect cross-reactivity between D. pteronyssinus and silverfish. The silverfish (Lepisma saccharina) (Fig.3) belongs to the Hexapods (class Insecta) and is found as a nocturnal and wingless insect in human dwellings, primarily in kitchens, bathrooms and cellars. They are also commonly accepted to be „humidity indicators“. Only in the case of severe infestation can silverfish contaminate foodstuffs, wallcoverings or books. Exposure to silverfish is not uncommon. Silverfish tropomyosin is named Lep s 1 in the WHO-IUIS allergen database [30] and has also been produced as recombinant allergen (rLep s 1) [55].

Fig. 3: Silverfish (Lepisma saccarina) (image from R. Pospischil; from [3])
© (2) R. Pospischil
The housefly or common housefly (Musca domestica) (Fig.4) belongs to the family of true flies (Muscidae) and is found almost all over the world. Two cases of occupational inhalation allergy to houseflies were documented in conjunction with fly breeding [56]. In both cases, the non-atopic individuals suffered newly manifested perennial rhinoconjunctivitis with symptom onset 30 min following exposure to Musca domestica in the closed breeding areas. High housefly exposure occurs not only during breeding, but also for instance in the context of animal farming, where adjacent residential dwellings can also be affected. Thus, Focke et al. [57] described a female farmer with a specific allergy to Musca domestica or the family of true flies (Muscidae). Protein bands in the molecular ranges of 16, 50 and 70 kDa were displayed in immunoblotting.

Fig. 4: Housefly or common housefly (Musca domestica) (image from R. Pospischil; from [3])
© (2) R. Pospischil
Particularly in autumn, when outdoor temperatures drop and air humidity rises, more spiders are found indoors in our part of the world. Not only the best known order of Araneae (spiders), but also harvestmen, scorpions, pseudo-scorpions and mites (Acari) belong to the class of arachnids. Occupational IgE-mediated allergy to the common house spider (egenaria domestica) has been described by Hasan et al. [58], among others. An arginine kinase from the cellar spider (Holocnemus plucei) was identified as an inhalant allergen in one particular case report [59]. In addition to the 17-kDa arginine kinase, other protein bands in the MW range of 20–70 kDa were displayed in patient serum.
The European pigeon tick Argas reflexus also belongs to the mite order and thus to the Arachnida class. Since Argas reflexus was detected in many homes, e. g. in East Germany, in the early 1990s and the bite of the pigeon tick can cause local inflammatory reactions, as well as anaphylactic systemic reactions, it has been considered a significant indoor allergen. Children, the elderly and also atopic individuals are suspected to have an increased risk of developing IgE-mediated immune reactions to Argas allergens [60]. Arg r 1 has been described as the major allergen and has been cloned and expressed in recombinant form by Hilger et al. [61]. Arg r 1 [30] has an MW of 18–19 kDa. An immunoblot analysis [60] showed that the majority of patients that reacted to a whole-body extract of Argas reacted with a 22-kDa band in the immunoblot.
Although bites from the common bed bug (Cimex lectularius) (Fig.5) are rare in central Europe, bed bugs can nevertheless be found in numerous locations, e. g. old timber-framed houses, hotels, farm buildings or in the vicinity of bird and bat nests [53]. An IgE response to Cimex-lectularius nitrophorin (cNP; a protein with only sparse homology to proteins of other species) was detected in 30 % of individuals who reported having been bitten by bed bugs and showed visible skin reactions in the study by Price et al. [62]. Whilst a specific immune reaction to bed bugs could be assumed on the one hand, many individuals with IgE to Cimex-lectularius extract also showed IgE reactivity to house dust mite and/or cockroach allergens on the other. The partial inhibition of IgE binding to Cimex lectularius by house dust mite or cockroach extracts is evidence of a certain cross-reactivity between bed bug allergens and cockroach or house dust mite allergens.

Fig. 5: Common bed bug (Cimex lectularius) (image from R. Pospischil; from [3])
© (2) R. Pospischil
The book louse (Liposcelis bostrichophila), which belongs to the bark lice family (Psocoptera), is a relevant indoor allergen in Japan [63]. Although the insect is found worldwide, it prefers a habitat with 80 % air humidity and temperatures from 25°C.
In contrast to the book louse (Psocoptera order), both the head louse (Pediculus humanus capitis) and the clothes louse (Pediculus humanus humanus) belong to the body louse family (Pediculidae; genus, Pediculus). Pubic lice (Phthirus pubis) live parasitically only on humans and belong to the genus Phthirus and the family Phthiridae [53]. In 2006, Fernández et al. [64] published the case of a 6-year-old boy with an allergy to Pediculus humanus capitis. The young patient suffered repeated head lice infestations, which caused intensive pruritus, bilateral nasal obstruction, runny nose and difficulty breathing at night. These symptoms resolved after the second application of pyrethrin lotion. Asthma and rhinitis also resolved upon elimination of the lice infestation. According to the family history, the boy had no predisposition to allergic disease. Prick and provocation tests with a protein extract from head lice were positive in this patient. A band in the 20-kDa region was detected with patient serum using IgE immunoblotting with this extract.
Flea allergy dermatitis (FAD) is the most common dermatological disease in cats and dogs [65]. Susceptible animals develop an intensely pruritic papular reaction in response to bites from cat fleas (Ctenocephalides felis) (Fig.6). To date, an 18-kDa protein from cat flea saliva has been identified as a major allergen in FAD (Cte f 1) and has also been produced recombinant form (rCte f 1) [66]. Two further cat flea allergens besides Cte f 1can now also be found in the IUIS allergen database [30], Cte f 2 (27 kDa) and Cte f 3 (25 kDa).

Fig. 6: Cat flea (Ctenocephalides felis) (image from R. Pospischil; from [3]))
© (2) R. Pospischil
Although they do not transfer pathogens, storage pests can also induce secondary infestation with hygiene pests or moulds and, as such, represent a public health risk that should not be underestimated [53].
Mealworm beetles or flour worms (Tenebrio molitor), the corn weevil (Sitophilus granaries), the confused flour beetle (rice flour beetle; Tribolium confusum) or the flour moth (Ephestia kuehniella) and the Indian meal moth (Plodia interpunctella) are ubiquitously occurring insects that feed on diverse stocks such as grain and other plant material, meaning that they are found not only in various work places used for grain processing or storage, but also in homes.
The prevalence of sensitization to these rare indoor allergens depends on geographical and climate conditions, the behaviour and habits of inhabitants, as well as the conditions of the domestic environment. Public health pests, such as lice, bed bugs, fleas and storage pests represent potential allergen sources, whereby they are often documented by case reports and only rarely by systematic investigations. Consequently, only a few allergens have been identified on a protein level and only scant allergen extracts or individual allergens are commercially available for allergy diagnosis. It is important to note that the distribution of species may very well change as a result of climate change and altered living conditions (as in the case of the pigeon tick), as well as worldwide commerce and tourism and the resulting transportation, e.g. of infested containers.
Abbreviations
- AH
Antihistamines
- AIT
Allergen-specific immunotherapy
- AR
Allergic rhinitis
- cNP
Cimex lectularius nitrophorin
- EDC
Electrostatic dust fall collector
- ELISA
Enzyme linked immunosorbent assay
- FAD
Flea allergy dermatitis
- GCS
Glucocorticosteroid
- IgE
Immunoglobulin E
- IUIS
International Union of Immunological Societies
- LRA
Leukotriene receptor antagonists
- MG
Molecular weight
- NPT
Nasal provocation testing
- SIT
Specific immunotherapy
- WHO
World Health Organization
Footnotes
Conflict of interest
The authors state that there are no conflicts of interest.
Cite this as
Raulf M, Bergmann KC, Kull S, Sander I, Hilger C, Brüning T, Jappe U, Müsken H, Sperl A, Vrtala S, Zahradnik E, Klimek L. Mites and other indoor allergens — from exposure to sensitization and treatment. Allergo J Int 2015;24:68–80 DOI: 10.1007/s40629-015-0049-1
References
- 1.Heinzerling LM, Burbach GJ, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA2LEN skin test study I: GA2LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 2.Haftenberger M, Laußmann D, Ellert U, Kacklösch M, Langen U, Schlaud M. Prävalenz von Sensibilisierungen gegen Inhalations- und Nahrungsmittelallergene. Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsbl. 2013;56:687–697. doi: 10.1007/s00103-012-1658-1. [DOI] [PubMed] [Google Scholar]
- 3.Raulf M, Sander I, Hilger C, Brüning T, Zahradnik E. Schädlinge und andere „unerwünschte Untermieter“ in Innenräumen — wie relevant sind sie als Allergenquellen? Allergologie. 2015;38:91–102. [Google Scholar]
- 4.Bergmann KC, Müsken H. Milben sind nicht gleich Milben. Allergologie. 2015;38:47–54. [Google Scholar]
- 5.Sander I, Zahradnik E, Brüning T, Raulf M. Quantifizierung der Milbenallergenexposition in Haushalten mit verschiedenen Immunoassays und Luftstaubsammelmethoden. Allergologie. 2015;38:64–69. [Google Scholar]
- 6.Kull S, Vrtala S, Jappe U. Bekannte Einzelallergene der Hausstaubmilben: Struktur, Funktion und Relevanz. Allergologie. 2015;38:55–63. [Google Scholar]
- 7.Klimek L, Sperl A, Raulf M. Diagnostik und Therapie der Milbenallergie. Allergologie. 2015;38:70–82. [Google Scholar]
- 8.Hilger C, Zahradnik E. Hund, Katze und Co — Haustiere als Allergenquellen in Innenräumen. Allergologie. 2015;38:83–90. [Google Scholar]
- 9.Voorhorst R, Spieksma-Boezeman MI, Spieksma FT. Is a mite (Dermatophagoides spp.) the producer of the house dust mite allergen? Allerg Asthma (Leipz) 1964;10:329–334. [PubMed] [Google Scholar]
- 10.Brown HM, Filer JL. Role of mites in allergy to house dust. Br Med J. 1968;3:646–647. doi: 10.1136/bmj.3.5619.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankland AW, McEwen LM, Feinberg JG. Skin reactions to dust and mites. Int Arch Allergy Appl Immunol. 1970;37:351–356. doi: 10.1159/000230797. [DOI] [PubMed] [Google Scholar]
- 12.Smith AP. Hyposensitization with Dermatophagoides pteronyssinus antigen: trial in asthma induced by house dust. Br Med J. 1971;4:204–206. doi: 10.1136/bmj.4.5781.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franz JT, Schulz S, Fuhlendorff J, Masuch G, Bergmann KC, Müsken H. Pheromones — a possible mite avoidance measure? Allergy. 2001;68:178. [Google Scholar]
- 14.de Boer R, Kuller K. Mattresses as a winter refuge for house dust mite populations. Allergy. 1997;52:299–305. doi: 10.1111/j.1398-9995.1997.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 15.Lebrun PH, van Impe G, de Saint Georges-Gridelet D, Wauthy G, Andre HM. The life strategies of mites. In: Schuster R, Murphy PW, editors. The Acari. London: Chapman & Hall; 1991. pp. 3–22. [Google Scholar]
- 16.Gehring U, Brunekreef B, Fahlbusch B, Wichmann HE, Heinrich J, INGA Study Group Are house dust mite allergen levels influenced by cold winter weather? Allergy. 2005;60:1079–1082. doi: 10.1111/j.1398-9995.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- 17.Zock JP, Heinrich J, Jarvis D, Verlato G, Norbäck D, Plana E, et al. Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006;118:682–690. doi: 10.1016/j.jaci.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 18.de Blay F, Heymann PW, Chapman MD, Platts-Mills TA. Airborne dust mite allergens: comparison of group II allergens with group I mite allergen and cat-allergen Fel d I. J Allergy Clin Immunol. 1991;88:919–926. doi: 10.1016/0091-6749(91)90249-N. [DOI] [PubMed] [Google Scholar]
- 19.Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- 20.Luczynska CM, Arruda LK, Platts-Mills TA, Miller JD, Lopez M, Chapman MD. A two-site monoclonal antibody ELISA for the quantification of the major Dermatophagoides spp. allergens, Der p I and Der f I. J Immunol Methods. 1989;118:227–235. doi: 10.1016/0022-1759(89)90010-0. [DOI] [PubMed] [Google Scholar]
- 21.Chapman MD, Heymann PW, Wilkins SR, Brown MJ, Platts-Mills TA. Monoclonal immunoassays for major dust mite (Dermatophagoides) allergens, Der p I and Der f I, and quantitative analysis of the allergen content of mite and house dust extracts. J Allergy Clin Immunol. 1987;80:184–194. doi: 10.1016/0091-6749(87)90128-X. [DOI] [PubMed] [Google Scholar]
- 22.Antens CJ, Oldenwening M, Wolse A, Gehring U, Smit HA, Aalberse RC, et al. Repeated measurements of mite and pet allergen levels in house dust over a time period of 8 years. Clin Exp Allergy. 2006;36:1525–1531. doi: 10.1111/j.1365-2222.2006.02603.x. [DOI] [PubMed] [Google Scholar]
- 23.Custovic A, Simpson B, Simpson A, Hallam C, Craven M, Woodcock A. Relationship between mite, cat, and dog allergens in reservoir dust and ambient air. Allergy. 1999;54:612–616. doi: 10.1034/j.1398-9995.1999.00062.x. [DOI] [PubMed] [Google Scholar]
- 24.Sander I, Zahradnik E, Kraus G, Mayer S, Neumann HD, Fleischer C, et al. Neues Messverfahren zum Nachweis von Hausmilbenantigenen auch in Luftstaubproben aus Innenräumen von Wohnungen und Arbeitsplätzen. Gefahrstoffe Reinhaltung der Luft. 2013;73:281–284. [Google Scholar]
- 25.Sander I, Zahradnik E, Kraus G, Mayer S, Neumann HD, Fleischer C, et al. Domestic mite antigens in floor and airborne dust at workplaces in comparison to living areas: a new immunoassay to assess personal airborne allergen exposure. PLoS ONE. 2012;7:52981. doi: 10.1371/journal.pone.0052981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krop EJM, Jacobs JH, Sander I, Raulf-Heimsoth M, Heederik Dick JJ. Allergens and β-Glucans in dutch homes and schools: characterizing airborne levels. PloS ONE. 2014;9:88871. doi: 10.1371/journal.pone.0088871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahradnik E, Sander I, Fleischer C, Mayer S, Brüning T, Raulf-Heimsoth M. Entwicklung von Enzymimmunoassays zur Quantifizierung von Vorratsmilbenantigenen in arbeitsplatzbezogenen Staubproben. Gefahrstoffe Reinhaltung der Luft. 2009;69:369–375. [Google Scholar]
- 28.Zahradnik E, Sander I, Kendzia B, Fleischer C, Brüning T, Raulf-Heimsoth M. Passive airborne dust sampling to assess mite antigen exposure in farming environments. J Environ Monit. 2011;13:2638–2644. doi: 10.1039/c1em10430f. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz R, Ellert U, Kalcklösch M, Dahm S, Thamm M. Patterns of sensitization to inhalant and food allergens — findings from the German Health Interview and Examination Survey for Children and Adolescents. Int Arch Allergy Immunol. 2013;162:263–270. doi: 10.1159/000353344. [DOI] [PubMed] [Google Scholar]
- 30.www.allergen.org
- 31.Marsh DG, Goodfriend L, King TP, Lowenstein H, Platts-Mills TA. Allergen nomenclature. Bull World Health Organ. 1986;64:767–774. [PMC free article] [PubMed] [Google Scholar]
- 32.King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TAE, Thomas W. Allergen nomenclature. Int Arch Allergy Immunol. 1994;105:224–233. doi: 10.1159/000236761. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MD, Pomés A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119:414–420. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190:3059–3067. doi: 10.4049/jimmunol.1202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011;41:1468–1477. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weghofer M, Dall’Antonia Y, Grote M, Stöcklinger A, Kneidinger M, Balic N, et al. Characterization of Der p 21, a new important allergen derived from the gut of house dust mites. Allergy. 2008;63:758–767. doi: 10.1111/j.1398-9995.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- 37.Weghofer M, Grote M, Dall’Antonia Y, Fernández-Caldas E, Krauth MT, van Hage M, et al. Characterization of folded recombinant Der p 5, a potential diagnostic marker allergen for house dust mite allergy. Int Arch Allergy Immunol. 2008;147:101–109. doi: 10.1159/000135696. [DOI] [PubMed] [Google Scholar]
- 38.Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119:247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 39.Lopata AL, Lehrer SB. New insights into seafood allergy. Curr Opin Allergy Clin Immunol. 2009;9:270–277. doi: 10.1097/ACI.0b013e32832b3e6f. [DOI] [PubMed] [Google Scholar]
- 40.Van R R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996;51:108–113. doi: 10.1111/j.1398-9995.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee S, Resch Y, Chen KW, Swoboda I, Focke-Tejkl M, Blatt K, et al. Der p 11 is a major allergen for house dust mite allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2015;135:102–109. doi: 10.1038/jid.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klimek L, Sperl A, Raulf M. Diagnostik und Therapie der Milbenallergie. Allergologie. 2015;38:70–82. [Google Scholar]
- 43.Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med. 2008;102:1371–1378. doi: 10.1016/j.rmed.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Ledford DK. Omalizumab: overview of pharmacology and efficacy in asthma. Expert Opin Biol Ther. 2009;9:933–943. doi: 10.1517/14712590903036060. [DOI] [PubMed] [Google Scholar]
- 45.Morgenstern JP, Griffith IJ, Brauer AW, Rogers BL, Bond JF, Chapman MD, et al. Amino acid sequence of Fel dI, the major allergen of the domestic cat: protein sequence analysis and cDNA cloning. Proc Natl Acad Sci USA. 1991;88:9690–9694. doi: 10.1073/pnas.88.21.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spitzauer S, Pandjaitan B, Söregi G, Mühl S, Ebner C, Kraft D, et al. IgE cross-reactivities against albumins in patients allergic to animals. J Allergy Clin Immunol. 1995;96:951–959. doi: 10.1016/S0091-6749(95)70233-4. [DOI] [PubMed] [Google Scholar]
- 47.de Lucca SD, O’Meara TJ, Tovey ER. Exposure to mite and cat allergens on a range of clothing items at home and the transfer of cat allergen in the workplace. J Allergy Clin Immunol. 2000;106:874–879. doi: 10.1067/mai.2000.110804. [DOI] [PubMed] [Google Scholar]
- 48.Karlsson A-S, Renstrom A. Human hair is a potential source of cat allergen contamination of ambient air. Allergy. 2005;60:961–964. doi: 10.1111/j.1398-9995.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 49.Krop EJM, Doekes G, Stone MJ, Aalberse RC, van der Zee JS. Spreading of occupational allergens: laboratory animal allergens on hair-covering caps and in mattress dust of laboratory animal workers. Occup Environ Med. 2006;64:267–272. doi: 10.1136/oem.2006.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zahradnik E, Sander I, Bruckmaier L, Flagge A, Fleischer C, Schierl R, et al. Development of a sandwich ELISA to measure exposure to occupational cow hair allergens. Int Arch Allergy Immunol. 2011;155:225–233. doi: 10.1159/000319839. [DOI] [PubMed] [Google Scholar]
- 51.Raulf M, Buters J, Chapman M, Cecchi L, de Blay F, Doekes G, et al. Monitoring of occupational and environmental aeroallergens — EAACI Position Paper. Allergy. 2014;69:1280–1299. doi: 10.1111/all.12456. [DOI] [PubMed] [Google Scholar]
- 52.Hilger C, Kuehn A, Raulf M, Jakob J. Allergien auf Schaben, Zecken, Vorratsmilben und andere Gliederfüßer: Wie weit ist die molekulare Allergiediagnostik? Allergo J. 2014;6:18–24. doi: 10.1007/s15007-014-0649-y. [DOI] [Google Scholar]
- 53.Raulf M, Sander I, Gonnissen D, Zahradnik E, Brüning T. Schaben und Co. — Die Rolle von Gesundheitsschädlingen als Allergenquelle. Bundesgesundheitsbl. 2014;57:585–592. doi: 10.1007/s00103-013-1926-8. [DOI] [PubMed] [Google Scholar]
- 54.Witteman AM, van den Oudenrijn S, van Leeuwen J, Akkerdaas J, van der Zee JS, Aalberse RC. IgE antibodies reactive with silverfish, cockroach and chironomid are frequently found in mite-positive allergic patients. Int Arch Allergy Immunol. 1995;108:165–169. doi: 10.1159/000237134. [DOI] [PubMed] [Google Scholar]
- 55.Barletta B, Butteroni C, Puggioni EM, Iacovacci P, Afferni C, Tinghino R, et al. Immunological characterization of a recombinant tropomyosin from a new indoor source, Lepisma saccharina. Clin Exp Allergy. 2005;35:483–489. doi: 10.1111/j.1365-2222.2005.02214.x. [DOI] [PubMed] [Google Scholar]
- 56.Tas E, Jappe U, Beltraminelli H, Bircher A. Berufsbedingte Inhalationsallergie gegen die gemeine Hausfliege (Musca domestica) Hautarzt. 2007;58:156–160. doi: 10.1007/s00105-006-1099-6. [DOI] [PubMed] [Google Scholar]
- 57.Focke M, Hemmer W, Wöhrl S, Götz M, Jarisch R, Kofler H. Specific sensitization to the common housefly (Musca domestica) not related to insect panallergy. Allergy. 2003;58:448–451. doi: 10.1034/j.1398-9995.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 58.Hasan T, Mäkinen-Kiljunen S, Brummer-Korvenkontio H, Pajunen T, Reunala T. Occupational IgE-mediated allergy to a common house spider (Tegenaria domestica) Allergy. 2005;60:1455–1457. doi: 10.1111/j.1398-9995.2005.00924.x. [DOI] [PubMed] [Google Scholar]
- 59.Bobolea I, Barranco P, Pastor-Vargas C, Iraola V, Vivanco F, Quirce S. Arginine kinase from the cellar spider (Holocnemus pluchei): a new asthma-causing allergen. Int Arch Allergy Immunol. 2011;155:180–186. doi: 10.1159/000319822. [DOI] [PubMed] [Google Scholar]
- 60.Kleine-Tebbe J, Heinatz A, Gräser I, Dautel H, Hansen GN, Kespohl S, et al. Bites of the European pigeon tick (Argas reflexus): Risk of IgE-mediated sensitizations and anaphylactic reactions. J Allergy Clin Immunol. 2006;117:190–195. doi: 10.1016/j.jaci.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 61.Hilger C, Bessot JC, Hutt N, Grigioni F, de Blay F, Pauli G, et al. IgE-mediated anaphylaxis caused by bites of the pigeon tick Argas reflexus: cloning and expression of the major allergen Arg r 1. J Allergy Clin Immunol. 2005;115:617–622. doi: 10.1016/j.jaci.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 62.Price JB, Divjan A, Montfort WR, Stansfield KH, Freyer GA, Perzanowski MS. IgE against bed bug (Cimex lectularius) allergens is common among adults bitten by bed bugs. J Allergy Clin Immunol. 2012;129:863–865. doi: 10.1016/j.jaci.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukutomi Y, Kawakami Y, Taniguchi M, Saito A, Fukuda A, Yasueda H, et al. Allergenicity and cross-reactivity of booklice (Liposcelis bostrichophila): a common household insect pest in Japan. Int Arch Allergy Immunol. 2012;157:339–348. doi: 10.1159/000329853. [DOI] [PubMed] [Google Scholar]
- 64.Fernández S, Fernández A, Armentia A, Pineda F. Allergy due to head lice (Pediculus humanus capitis) Allergy. 2006;61:1372. doi: 10.1111/j.1398-9995.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- 65.Bond R, Riddle A, Mottram L, Beugnet F, Stevenson R. Survey of flea infestation in dogs and cats in the United Kingdom during 2005. Vet Rec. 2007;160:503–506. doi: 10.1136/vr.160.15.503. [DOI] [PubMed] [Google Scholar]
- 66.McDermott MJ, Weber E, Hunter S, Stedman KE, Best E, Frank GR, et al. Identification, cloning, and characterization of a major cat flea salivary allergen (Cte f 1) Mol Immunol. 2000;37:361–375. doi: 10.1016/S0161-5890(00)00061-4. [DOI] [PubMed] [Google Scholar]


