Summary
Background: Alongside hazel, alder and birch pollen allergies, ash pollen allergy is a relevant cause of hay fever during spring in the European region. For some considerable time, ash pollen allergy was not routinely investigated and its clinical relevance may well have been underestimated, particularly since ash and birch tree pollination times are largely the same. Ash pollen extracts are not yet well standardized and diagnosis is therefore sometimes unreliable. Olive pollen, on the other hand, is strongly cross-reactive with ash pollen and is apparently better standardized. Therefore, the main allergen of olive pollen, Ole e 1, has been postulated as a reliable alternative for the detection of ash pollen sensitization.
Methods: To determine to what extent specific IgE against Ole e 1 in patients with ash pollen allergy is relevant, we included 183 subjects with ash pollen allergy displaying typical symptoms in March/April and positive skin prick test specific IgE against Ole e 1 (t224) and ash pollen (t25) and various birch allergens (Bet v 1, Bet v 2/v 4) in a retrospective study.
Results: A significant correlation was seen between specific IgE against Ole e 1 and ash pollen, but also to a slightly lesser extent between IgE against Ole e 1 and skin prick test with ash pollen, the latter being even higher than IgE and skin prick test both with ash pollen. No relevant correlation was found with birch pollen allergens, demonstrating the very limited cross-reactivity between ash and birch pollen.
Conclusion: It appears appropriate to determine specific IgE against Ole e 1 instead of IgE against ash pollen to detect persons with ash pollen allergy. Our findings may also support the idea of using possibly better standardized or more widely available olive pollen extracts instead of ash pollen extract for allergen-specific immunotherapy.
Key words: betulaceae, ash, pollen, Ole e 1, olive pollen
Introduction
Pollinosis is the most common allergic disease in the European region and its prevalence is rising, currently reaching between 12% and 20% [1]. Alongside grass and herb pollen, tree pollen is also responsible for pollen allergies, primarily birch-like plants, with birch trees predominant among these, followed by alders and hazel, known triggers of spring pollinosis. However, various studies over recent years suggest that the relevance of ash pollen ought not to be underestimated. Studies carried out in France and Italy indicate that 18 %–34 % of allergies can be attributed to the ash tree [1, 2].
The common European ash (Fraxinus excelsior) is a tall, wind-pollinated tree. Geographical distribution of the ash is restricted to mild central European zones, generally north of the Alps, and southern Scandinavia. Therefore, most studies on ash pollen are carried out in Spain, France (Alsace), Switzerland and Austria. For a long time, ash pollen was not routinely investigated, due on the one hand to generally overlapping pollination periods, whilst on the other due to its cross-reactivity with trees in the Betulaceae family [3, 4]. The significance of ash pollen allergy was only recognized more recently. The reason for this lies in the only partial cross-reactivity with the Betulaceae family and evidence that — most notably in Switzerland — the higher level of airborne ash pollen has led to increasing sensitization and a greater number of monovalent ash pollen allergy sufferers [6].
From a phylogenetic perspective, ashes belong to the olive family (Oleaceae), which is widespread in the Mediterranean region, where it is of considerable importance. The common privet (Ligustrum vulgare), lilacs (Syringa spp.), forsythias (Forsythia spp.) and jasmine (Jasminus spp.) also belong to the Oleaceae family. A number of studies have demonstrated high cross-reactivity within this family [2][7, 8]. However, there is only partial cross-reactivity between the Betulaceae and the Oleaceae plant families [9]. Ole e 1, a glycoprotein of 145-amino acids, is considered the chief marker allergen of Oleaceae, whereas Bet v 1 is considered the major allergen of Betulaceae [10].
Several ash pollen allergens have been identified to date. The major allergen is Fra e 1, also a glycoprotein, which, due to its high sequential similarity with Ole e 1, displays extremely high cross-reactivity [11]. Therefore, Ole e 1 is considered a marker allergen not only for the olive family, but also for ash pollen [7].
Conventional pollen extracts contain mixtures of allergenic components as well as undesired products [12]. Determining these components precisely is complex and time-consuming due to their extremely low concentrations, the significant variability seen and the lack of standardization to date. Nevertheless, it has been possible in recent years to compile actual allergen profiles by means of proteomic investigations and to identify certain protein families, so-called major and minor allergens — thus also for the olive family (Oleaceae), including ashes, and the birch family.
In the case of ashes, it was revealed that, in addition to Fra e 1 as the major allergen, there is a multitude of other ash pollen allergens (so-called minor allergens, such as profilin, Fra e 2, calmodulin, and Fra e 3). Therefore, the protein families of profilins and calmodulins are responsible for the cross-reactivity to pollen of other families. Moreover, there is evidence to suggest that bioactive lipids could also play a role in the context of the immune response [13].
In addition to typical symptoms (rhinitis, conjunctivitis and possibly also asthma) and skin prick testing, serological detection of Ole e 1 (t224) has been considered adequate up to now due to its high sequence analogy with Fra e 1. However, clinical studies on the correlation between the ImmunoCAP® of Ole e 1 and that of ash pollen are lacking to date. This correlation is not banal, given that the composition of ash pollen has not yet been precisely identified and, in particular, due to the unclear and as yet unknown allergenic potential of these components. The aim of the present retrospective clinical study was to examine this postulate using a patient collective of pollinosis sufferers from 2011/2012 in order to better record data on ash pollen sufferers, not least in terms of immunotherapy.
Patients and methods
The study included 244 pollen allergy sufferers living in the wider Zurich area that were seen in the allergy clinic at the Zurich university hospital in 2011 and, to a lesser extent, 2012. Inclusion criteria comprised typical symptoms (rhinitis, conjunctivitis and/or asthma), a positive skin prick test to ash or birch and ImmunoCAP® detection of Ole e 1 (t224) and/or ash pollen (t25), as well as Bet v 1 (t215) and Bet v 2/v 4 (t221). Where lacking, CAP values for ash pollen were subsequently measured.
Specific immunoglobulin E (IgE) was determined using the commercial ImmunoCAP® method (Thermo Fisher Phadia Diagnostics).
Results were interpreted according to recommendations set out by the manufacturer. The ‘cut-off’ for specific IgE was set at > 0.35 kU/l. In all, 61 subjects were excluded from the study, since insufficient serum was available for the subsequent detection of IgE to ash pollen. Ultimately, the definitive patient collective comprised 183 subjects (n = 183).
Skin prick tests were carried out according to standard international criteria, using a positive (histamine) and a negative control (sodium chloride, NaCl). Commercial skin prick test extracts were used (ash pollen: Allergopharma Esche/Fraxinus excelsior No 116; birch pollen: Stallergenes Alyostal No 615). Evaluation was performed as follows:
Wheal size < 3 mm: negative
Wheal size < 5 mm: + positive
Wheal size to 7 mm: ++ positive
Wheal size > 7 mm: +++ positive
Plus pseudopods: ++++ positive
Additional nasal provocation tests (NPT) using the commercial skin prick test solution for ash pollen were carried out in a subpopulation of nine patients. These were evaluated on the basis of sneezing (maximum of three points), rhinorrhea (maximum of three points) and reduced rhinomanometric flow (maximum of three points). NPTs were deemed positive from a score of four points.
Statistical calculations were performed by a professional statistician and the Spearman’s Rank correlation coefficient was used for statistical analysis.
The investigation of data collected was approved by the ethics commission of the University of Zurich. All patients provided their written consent for the measurement and evaluation of data.
Results
Altogether, 183 subjects aged between 7 and 76 years (mean age, 35.8 years) were included in the study (75 females and 108 males). Tab.1 shows the spectrum of sensitization in the study population. Tab.2 lists the correlation coefficients between the various parameters compared, as well as possible significances. A clear correlation is seen primarily between ImmunoCAP for ash and Ole e 1, as well as ImmunoCAP for Ole e 1 and skin prick test with ash pollen extract.
|
Number
Patients |
Ash
(t25) |
Ole e 1
(t224) |
Bet v 1
(t215) |
Bet v 2/v 4
(t221) |
Prick
Ash |
Prick
Birch |
|
|---|---|---|---|---|---|---|---|
| Number (n) | 183 | 183 | 181 | 163 | 145 | 183 | 183 |
| Positive values (> 0,35 kU/l or positive prick test) | 129 | 113 | 121 | 32 | 127 | 125 |
Prick, skin prick test
| CAP Ole e 1 | CAP Bet v 1 | CAP Bet v 2 | Prick birch | Prick ash | ||
|---|---|---|---|---|---|---|
| CAP ash | Spearmans p p-Wert n |
0,896* 0,000 181 |
0,374* 0,000 163 |
0,478* 0,000 145 |
0,386* 0,000 183 |
0,604* 0,000 183 |
| CAP Ole e 1 | Spearmans p p-Wert n |
1,000 181 |
0,346* 0,000 161 |
0,324* 0,000 143 |
0,299* 0,000 181 |
0,664* 0,000 181 |
| CAP Bet v 1 | Spearmans p p-Wert n |
0,346* 0,000 161 |
1,000 163 |
-0,013 0,877 145 |
0,566* 0,000 163 |
0,355* 0,000 163 |
| CAP Bet v 2 | Spearmans p p-Wert n |
0,324* 0,000 143 |
-0,013 0,877 145 |
1,000 145 |
0,046 0,579 145 |
0,138 0,099 0,099 |
| Prick birch | Spearmans p p-Wert n |
0,299* 0,000 181 |
0,566* 0,000 163 |
0,046 0,579 145 |
1,000 183 |
0,358* 0,000 183 |
aCorrelation is signifi cant at 0.001
CAP, ImmunoCAP® (Thermo Fisher Phadia Diagnostics); IgE, Immunglobulin E; Prick, skin prick test
Fig.1 shows the comparison of ImmunoCAP values for ash (t25) and Ole e 1 (t224). The relationship is significant and shows a clear correlation (correlation coefficient of 0.896).
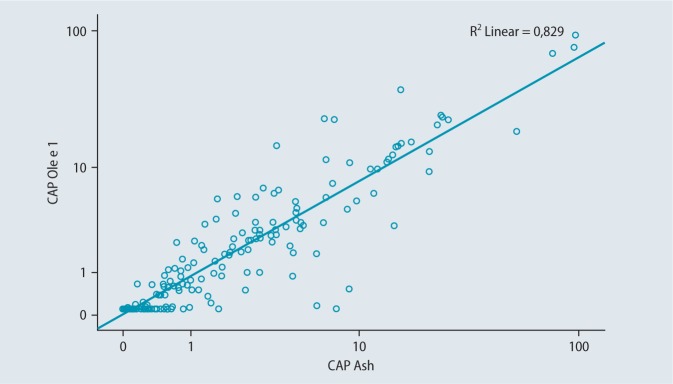
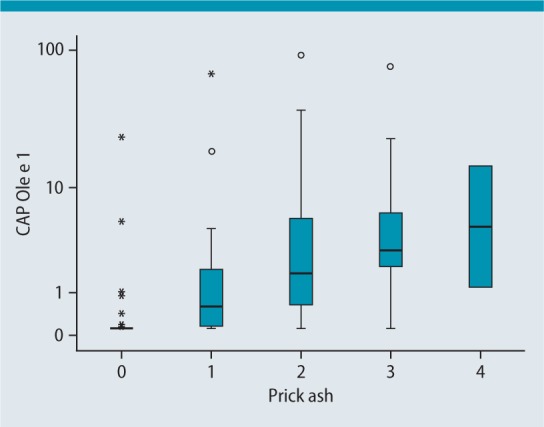
Fig.2–4 show the relationships between skin prick tests and ImmunoCAP values. Fig.2 shows the relationship between the ash skin prick test and ImmunoCAP values for Ole e 1 (t224), which is once again significant here with a correlation coefficient of 0.664.
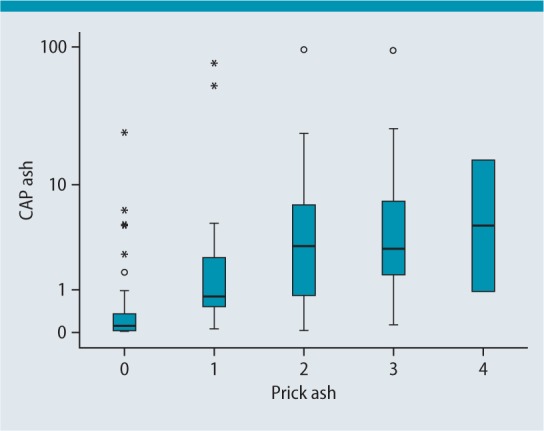
The relationship between the ash skin prick test and ash ImmunoCAP (t25) can be seen in Fig.3: although the relationship is much less marked here with a correlation coefficient of 0.604 than between ash skin prick test and ImmunoCAP Ole e 1, it is still significant. Nevertheless, it is valid to say that the skin prick test for ash pollen tends to correlate better with specific IgE for Ole e 1 than with specific IgE to ash.
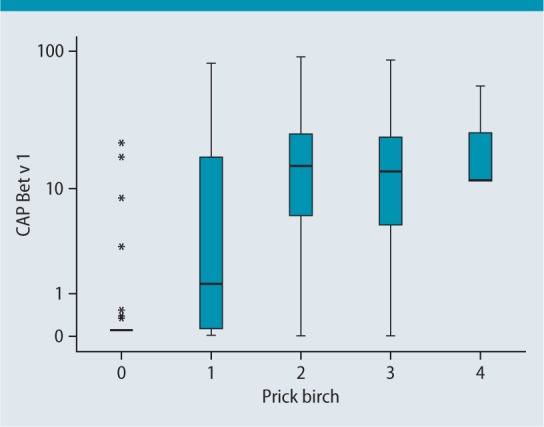
However, the correlation coefficient (0.604) is still greater than that between skin prick birch and ImmunoCAP Bet v 1 (t215), which is 0.566 (Fig.4).
All nine patients in whom an NPT was carried out showed sensitization to ash pollen in the skin prick test, but no sensitization to birch pollen. ImmunoCAP for Ole e 1 (t224) was > 0.7 kU/l (i.e., at least class 2) in six patients, whilst ImmunoCAP for Ole e 1 was > 0.35 kU/l in the other three patients. NPT with ash pollen was positive in five of six patients positive for IgE to Ole e 1; however, none of the three patients with an ImmunoCAP to Ole e 1 of < 0.35 kU/l tested positive on NPT with ash pollen.
The data in Tab.2 permit analysis of the relationship between ImmunoCAP values for ash (t25) and Bet v 1 and Bet v 2. In this context, and as expected given the only partial cross-reactivity between Oleaceae and Betulaceae, no significant relationship could be seen between ImmunoCAP values for ash and Bet v 1 (correlation coefficient of 0.374) or ash and Bet v 2 (correlation coefficient of 0.478).
Thus, the data suggest that the relationship between the ImmunoCAP for Ole e 1 and both the ImmunoCAP for ash and the ash skin prick test is relevant.
Discussion
Due to their complex structure, natural pollen extracts in general and ash pollen in particular have been only partially standardized to date. They differ not only in terms of their total protein volume, but also their major allergen content [14, 15, 16]. However, standardization is desirable and indeed necessary for the purposes of diagnosis and specific immunotherapy. In Europe at least, there is a regulation whereby commercial batches need to be comparable based on the use of in-house reference preparation (IHRP); however, this regulation only applies within and not between companies. Hrabina et al. [4] established an IHRP of this kind for ash pollen, comprising a particular molecular characterization (including Fra e 1, Fra e 2, Fra e 3, a 15-kDa doublet and high-molecular weight proteins). In this way, it was possible to significantly reduce variability at least compared with non-standardized batches.
Several studies to date have created allergen profiles of ash pollen allergy sufferers [2, 5]. Using immunoblotting of ash pollen in 2010, Poncet [1] detected 200 spots, of which at least 100 proteins have allergenic characteristics. It has also been demonstrated that, due to a polymorphism, several isoforms — which can also be glycosylated to varying degrees — are formed from Fra e 1 [17]. Moreover, it was also possible to detect the other known panallergens, such as profilins (Fra e 2) and calcium-binding proteins (Fra e 3), that are mainly responsible for cross-reactivity to other trees and grasses.
The results presented here reveal that Ole e 1, and thus also the Fra e 1 allergen largely identical in sequence, are of the utmost relevance as major allergens. The minor allergens found in ash pollen (t25), such as profilin (Fra e 2), calcium-binding protein (Fra e 3) and HMW proteins, appear to play a subordinate role, at least in terms of detecting sensitization. This is also true to a certain extent of the comparison between ImmunoCAP Ole e 1 and the ash pollen skin prick test, although here too a significant correlation was seen in our patient collective. NPT with ash pollen was only positive in those patients who tested positive for IgE to Ole e 1. This also supports the significant clinical relevance of the Ole e 1 allergen in ash pollen allergy sufferers.
Thus Poncet’s results are confirmed. In the present study, of 114 pollinosis sufferers — sensitized to ash, olive or privet — 86 % demonstrated an IgE response to Fra e 1. Although this result was presumed and expected, it had not as yet been investigated and demonstrated in a collective of this size.
Based on the results of this study, the significance of Ole e 1 and ash (t25) becomes apparent on the one hand, while on the other there are also scant differences in results, which could possibly be attributed to the relevance of panallergens or a lack of pollen standardization.
This will be illustrated in more detail using individual patients (Tab.3).
|
Ash
(t25) |
Ole e 1
(t224) |
Bet v 1
(t215) |
Bet v 2/v 4
(t221) |
Prick
Ash |
Prick
Birch |
|
|---|---|---|---|---|---|---|
| Patient no. 47 | 95,5 kU/l | 76,2 kU/l | 10,3 kU/l | 0,26 kU/l | +++ | +++ |
| Patient no. 95 | 2,52 kU/l | 0,99 kU/l | < 0,10 kU/l | 0,14 kU/l | 0 | 0 |
| Patient no. 151 | 6,13 kU/l | 0,16 kU/l | < 100 kU/l | 0,21 kU/l | 0 | +++ |
Prick, skin prick test
Patient no. 47 had the following results in ImmunoCAP for ash (t25): 95.5 kU/l, Ole e 1 (t224): 76.2 kU/l, Bet v 1: 10.3 kU/l, Bet v 2/v 4: 0.26 kU/l; both ash and birch were +++ in the skin prick test. In addition, NPT with ash and birch pollen were both positive in this 37-year-old patient. This example highlights the high correlation between ash and Ole e 1.
In contrast, patient no. 95 had apparently differing results. ImmunoCAP yielded the following values: ash: 2.52 kU/l, Ole e 1: 0.99 kU/l, Bet v 1: < 0.10 kU/l, Bet v 2/v 4: 0.14 kU/l. Skin prick tests for both ash and birch were negative. To what can this be attributed? Patient no. 95 had previously completed 3 years of specific immunotherapy with 100 % ash. Clearly, immunomodulation was responsible for the negative skin prick test with ash. The negative skin prick test with birch can be explained by the respective ImmunoCAP results.
There is also an interesting case (patient no. 151) that nicely illustrates residual cross-reactivity between birch and ash: ash: 6.13 kU/l, Ole e 1: 0.16 kU/l, Bet v 1: >100 kU/l, Bet v 2/v 4: 0.21 kU/l; the skin prick test was positive for birch (+++) and negative for ash. Due to the high ImmunoCAP value for Bet v 1, there was residual cross-reactivity to ash, hence the false-positive CAP result for ash pollen.
Since Ole e 1 plays such a dominant role at least in the diagnosis of ash pollen allergy, olive pollen extracts with a proven high Ole e 1 content can also be used as an alternative for the treatment of ash pollen allergies. Although based on small collectives, recent studies also suggest that Ole e 1 as an allergen could on its own indeed be sufficient to treat olive pollen allergy [11].
Thus one can conclude that, on the basis of the available results, the determination of specific IgE to Ole e 1 is as effective at detecting patients with clinically relevant ash pollen allergy as the determination of IgE to ash pollen (t25).
Abbreviations
- Bet v
Betula verrucosa
- CAP
Capacity, ImmunoCAP®
- Fra e
Fraxinus excelsior
- HMW
High molcular weight
- IgE
Immunglobulin E
- IHRP
In-house reference preparation
- Ole e
Olea europaea
- NaCl
Sodiumchloride
- NPT
Nasal provocation test
- SIT
Specific immunotherapy
Footnotes
German version www.springermedizin.de/allergo-journal
Conflict of interest
The authors state that there are no conflict of interest.
Cite this as Imhof K, Probst E, Seifert B, Regenass S, Schmid-Grendelmeier P. Ash pollen allergy — reliable detection of sensitization on the basis of IgE to Ole e 1. Allergo J Int 2014; 23: 78–83 DOI 10.1007/s40629-014-0010-8
References
- 1.Poncet P, Senechal H, Clement G, Purohit A, Sutra JP, Desvaux FX, et al. Evaluation of ash pollen sensitization pattern using proteomic approach with individual sera from allergic patients. Allergy. 2010;65:571–580. doi: 10.1111/j.1398-9995.2009.02231.x. [DOI] [PubMed] [Google Scholar]
- 2.Hemmer W, Focke M, Wantke F, Götz M, Jarisch R, Jäger S. Ash (Fraxinus excelsior)-pollen allergy in central Europe: specific role of pollen panallergens and the major allergen of ash pollen, Fra e 1. Allergy. 2000;55:923–930. doi: 10.1034/j.1398-9995.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 3.Barderas R, Purohit A, Papanikolaou I, Rodriguez R, Pauli G, Villalba M. Cloning, expression, and clinical significance of the major allergen from ash pollen, Fra e 1. J Allergy Clin Immunol. 2005;115:351–357. doi: 10.1016/j.jaci.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Hrabina M, Purohit A, Oster JP, Papanikolaou I, Jain K, Pascal P, et al. Standardization of an ash (Fraxinus excelsior) pollen allergen extract. Int Arch Allergy Immunol. 2007;142:11–18. doi: 10.1159/000095994. [DOI] [PubMed] [Google Scholar]
- 5.Niederberger V, Purohit A, Oster JP, Spitzauer S, Valenta R, Pauli G. The allergen profile of ash (Fraxinus excelsior) pollen: cross-reactivity with allergens from various plant species. Clin Exp Allergy. 2002;32:933–941. doi: 10.1046/j.1365-2222.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Grendelmeier P, Peeters AG, Wahl R, Wüthrich P. Zur Bedeutung der Eschenpollenallergie. Allergologie. 1994;17:535–542. [Google Scholar]
- 7.Palomares O, Swoboda I, Villalba M, Balic N, Spitzauer S, Rodriguez R, et al. The major allergen of olive pollen Ole e 1 is a diagnostic marker for sensitization to Oleaceae. Int Arch Allergy Immunol. 2006;141:110–118. doi: 10.1159/000094713. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Guerin B, Hewitt B, Lim S, Michel FB. Allergy in the Mediterranean area. III: Cross reactivity among Oleaceae pollens. Clin Allergy. 1985;15:439–448. doi: 10.1111/j.1365-2222.1985.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 9.Wahl R, Schmid-Grendelmeier P, Cromwell O, Wüthrich B. In vitro investigation of cross-reactivity between birch and ash pollen allergen extracts. J Allergy Clin Immunol. 1996;98:99–106. doi: 10.1016/S0091-6749(96)70231-2. [DOI] [PubMed] [Google Scholar]
- 10.Asero R. Analysis of hypersensitivity to oleaceae pollen in an olive-free and ash-free area by commercial pollen extracts and recombinant allergens. Eur Ann Allergy Clin Immunol. 2011;43:77–80. [PubMed] [Google Scholar]
- 11.Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–184. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez R, Villalba M, Batanero E, Palomares O, Quiralte J, Salamanca G, et al. Olive pollen recombinant allergens: value in diagnosis and immunotherapy. J Invest Allergol Clin Immunol. 2007;17(1):4–10. [PubMed] [Google Scholar]
- 13.D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 14.Alche JD, Castro AJ, Jimenez-Lopez JC, Morales S, Zafra A, Hammam-Khalifa AM, et al. Differential characteristics of olive pollen from different cultivars: biological and clinical implications. J Invest Allergol Clin Immunol. 2007;17(1):17–23. [PubMed] [Google Scholar]
- 15.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–1408. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 16.Focke M, Marth K, Valenta R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Invest. 2009;39:429–436. doi: 10.1111/j.1365-2362.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 17.Barderas R, Purohit A, Rodriguez R, Pauli G, Villalba M. Isolation of the main allergen Fra e 1 from ash (Fraxinus excelsior) pollen: comparison of the natural and recombinant forms. Ann Allergy Asthma Immunol. 2006;96:557–563. doi: 10.1016/S1081-1206(10)63550-8. [DOI] [PubMed] [Google Scholar]


