Abstract
The official WHO/IUIS database (www.allergen.org) currently lists 77 mould allergens from a variety of protein families. To date, only eight recombinant single allergens from three mould species are available for molecular allergy diagnosis of mould sensitization. These include rAlt a 1, the major allergen in Alternaria alternata-sensitized individuals, and enolase rAlt a 6 with it potential cross-reactivity to mould, food and natural latex allergens. rAsp f 1, 2, 3, 4 and 6 from Aspergillus fumigatus are available for diagnostic purposes; specific IgE to rAsp f 2, 4 and 6 is often positive in allergic bronchopulmonary aspergillosis (ABPA). The dehydrogenase rCla h 8 is considered a major allergen of Cladosporium herbarum with possible cross-reactivity to other dehydrogenase allergens. The narrow range of commercially available individual mould allergens should be expanded to include marker allergens typical for mould (e.g., serine proteases). In addition, standardization of total extracts needs to be improved in the future to guarantee valid mould products with defined allergen content for diagnostic and therapeutic purposes.
Key words: mould species, alternaria, aspergillus, diagnostic, recombinant single allergens
Mould allergen sources
The health risks posed by exposure to mould include:
Infectious diseases
Irritative and toxic effects
Sensitization and allergy development
Of the more than 100,000 known species of fungi, approximately 350 are listed on www.allergome.org as having sensitizing potential. At present, 107 fungal allergens from 43 fungal species fulfil the criteria of World Health Organization (WHO) and International Union of Immunological Societies (IUIS) for allergen classification (www.allergen.org). From a phylogenetic perspective, moulds belong to the Ascomycota (sac fungi). Basidiomycota (Club fungi) are also capable of inducing IgE-mediated diseases (Fig. 1). A total of 84 single allergens fulfilling WHO/IUIS criteria from ten fungal species have been characterized in the Ascomycota phylum to date. A total of 23 allergens from five fungal species have been characterized (Fig. 1) in the Basidiomycota; of these, ten belong to the most prominent species, Malassezia sympodialis.
Fig. 1.
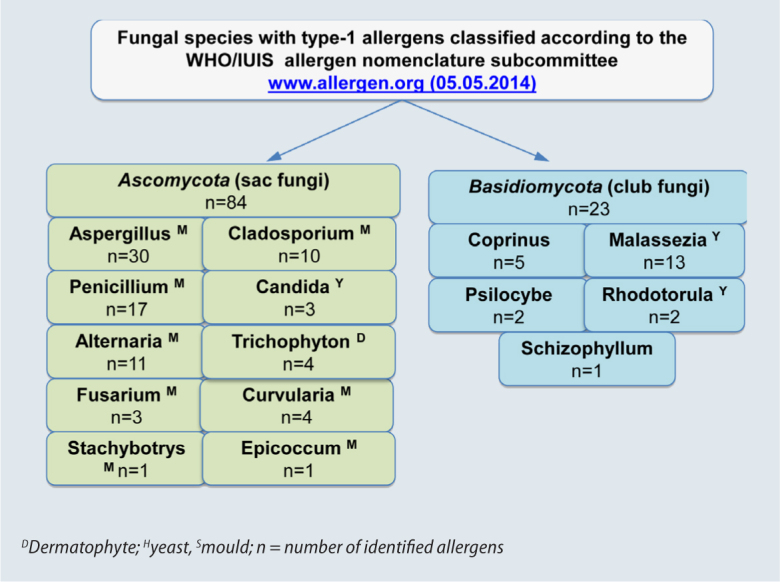
Fungal species with immediate-type allergens classified according to the WHO/IUIS allergen nomenclature subcommittee
In medical mycology, Ascomycota and Basidiomycota, irrespective of their taxonomic classification, are subdivided into dermatophytes, yeasts and moulds:
Dermatophytes include the clinically relevant genera Microsporum, Trichophyton and Epidermatophyton.
Among the yeasts, the genera Candida and Malassezia are relevant in allergology.
The term “mould” subsumes all fungal genera of the phylum Ascomycota, excluding the genera Trichophyton und Candida (Fig. 1).
Mould exposure
Although the air we breathe contains thousands of mould spores, sensitization rates for both indoor (e. g., Aspergillus, Penicillium) and outdoor fungal species (e. g., Cladosporium, Alternaria) is below 5 %; however, this rate is subject to regional variation [1, 2, 3, 4]. Moulds present no real hazard to the majority of the population, except in the case of high exposure, e. g., resulting from massive distribution. The situation is different for risk groups such as allergics or asthmatics. Alternaria alternata (= Alternaria tenuis), which is classified as an airborne outdoor mould in the northern hemisphere, appears to be particularly relevant in the development and severity of asthma [5]. In addition to allergic rhinoconjunctivitis and allergic asthma, moulds can also induce hypersensitivity pneumonitis (dominated by the antigen-IgG complexes) and ABPA. The latter is often caused by the mould Aspergillus fumigatus, which is therefore considered in differential diagnosis and patient history, as well as in in-vitro and in-vivo diagnostic methods, as a potential allergen source.
Characterized mould allergens
To date, 77 mould allergens (excluding dermatophytes and yeasts) have been described and officially recognized (www.allergen.org). The associated protein families are clearly distinct both biometrically and structurally from the allergen families found in pollen, food or pet dander. The most prominent mould allergens are:
Proteases (n = 18, of which 16 are serine proteases)
Ribosomal proteins (n = 9)
Enolases (n = 5),
Dehydrogenases (n = 4)
Thioredoxin (n = 3)
Heat shock proteins (HSP) 70/90 (n = 3)
Peroxisomal proteins (n = 2)
Isomerases (n = 2)
Manganese Superoxide dismutases (MnSOD) (n = 2)
Flavodoxin (n = 2)
Other mould allergens are found among the mitogillin, cyclophilins, fibrinogen-binding proteins and proteins of unknown biochemical function (Tab. 1).
Tabelle 1.
Selected fungal allergen families and commercially available fungal test allergens for IgE diagnosis
| Allergen families | Fungal species | Allergen |
|---|---|---|
| Proteases | Aspergillus flavus | Asp fl 13 |
| Aspergillus fumigatus | Asp f 5b, Asp f 10c, Asp f 13, Asp f 18 | |
| Aspergillus niger | Asp n 18 | |
| Aspergillus oryzae | Asp o 13 | |
| Aspergillus versicolor | Asp v 13 | |
| Cladosporium cladosporidae | Cla c 9 | |
| Cladosporium herbarum | Cla h 9 | |
| Curvularia lunata | Curl 1, Curl 4 | |
| Epicoccum purpurascens | Epi p 1 | |
| Penicillium brevicompactum | Pen b 13 | |
| Penicillium chrysogenum | Pen ch 13 | |
| Penicillium citrinum | Pen c 13, Pen c 18, Pen o 18 | |
| Ribosomal proteins | Alternaria alternata | Alt a 5, Alt a 12 |
| Aspergillus fumigatus | Asp f 8, Asp f 23 | |
| Cladosporium herbarum | Cla h 5, Cla h 12 | |
| Fusarium culmorum | Fus c 1 | |
| Penicillium brevicompactum | Pen b 26 | |
| Penicillium crustosum | Pen cr 26 | |
| Enolases | Alternaria alternata | Alt a 6 |
| Aspergillus fumigatus | Asp f 22 | |
| Cladosporium herbarum | Cla h 6 | |
| Curvularia lunata | Cur l 12 | |
| Penicillium citrinum | Pen c 22 | |
| Dehydrogenases | Alternaria alternata | Alt a 8, Alt a 10 |
| Cladosporium herbarum | Cla h 8, Cla h 10 | |
| Thioredoxins | Aspergillus fumigatus | Asp f 28, Asp f 29 |
| Fusarium culmorum | Fus c 2 | |
| Heat shock proteins (HSP 70/90) | Alternaria alternata | Alt a 3 |
| Aspergillus fumigatus | Asp f 12 | |
| Penicillium citrinum | Pen c 19 | |
| Peroxisomal proteins | Aspergillus fumigatus | Asp f 3 |
| Penicillium citrinum | Pen c 3 | |
| Isomerases | Alternaria alternata | Alt a 4 |
| Aspergillus fumigatus | Asp f 11 | |
| MnSODs | Aspergillus fumigatus | Asp f 6 |
| Alternaria alternata | Alt a 14 | |
| Flavodoxins | Alternaria alternata | Alt a 7 |
| Cladosporium herbarum | Cla h 7 | |
| Mitogillins | Aspergillus fumigatus | Asp f 1 |
| (Aspergillus restrictus) | (Asp r 1) d | |
| Cyclophilin | Aspergillus fumigatus | Asp f 27 |
| Fibrinogen-binding protein | Aspergillus fumigatus | Asp f 2 |
| Alpha-amylase | Aspergillus oryzae | Asp o 21 e |
| Protein without known function | Aspergillus fumigatus | Asp f 4 |
| Alternaria alternata | Alt a 1 |
a Of 18 proteases, 16 are serine proteases; b metalloprotease; c aspartate protease; d non-WHO/IUIS allergen; e baking enzyme: not a typical test allergen for fungal sensitization Allergens given in bold type are commercially available.
Although α-amylase from the mould Aspergillus oryzae is described as an allergen (Asp o 21), it is not suited to the diagnosis of fungal sensitization, since it is expressed in Aspergillus oryzae and used in a purified form as a baking enzyme (raising agent). It can cause IgE-mediated sensitization in exposed bakers. Thus, detecting IgE to α-amylase (Asp o 21) serves to diagnose sensitization, not however mould sensitization to baking enzyme.
Although Asp r 1 from Aspergillus restrictus is not one of the officially classified allergens, it has been included in the list of mould allergen families (Tab. 1) due to its commercial availability.
Protein families and their function
More than 50 % of the proteases — protein-cleaving enzymes — recorded in the IUIS allergen database are found in moulds. Of these, > 85 % are serine proteases and form a characteristic fungal protein family. The allergens in this family are not yet available for diagnostic purposes. Cross-reactivity has been described for both alkaline and vacuolar serine proteases (group-13 and -18 allergens of Asp f, Asp fl, Pen b, Pen c, Pen ch and Pen o) with positive IgE detection in 20 %–80 % of mould-sensitized individuals [4].
Of the ribosomal proteins that are allergens, 90 % are found in moulds. As cytoplasmic proteins, and together with rRNA, they form the 60S ribosomal subunit. Due to sequence homology, cross-reactive structures are likely [6, 7]. The rate of sensitization to the ribosomal fungal allergen Fus c 1 has been given as 35 % in individuals allergic to Fusarium [4].
To date, the enolase superfamily includes 11 allergens, of which five have been characterized in moulds, one in yeast, two in plants and three in animals. Cross-reactivity has been described for the enolases Alt a 6, Cla h 6 and Hev b 9 [8], as well as for Asp f 22 and Pen c 22 [9]. The rate of sensitization to enolases is between 20 % and 30 % in mould-allergic individuals [4].
Of altogether eight dehydrogenases that oxidize proteins by transferring H+ to reduction equivalents like nicotinamide adenine dinucleotide (NAD) or flavin adenine dinucleotide (FAD), four are found as allergens in moulds. Cross-reactivity between Cla h 8 and Alt a 8 is known [10]. The rate of sensitization to these two allergens is between 40 % and 50 % in mould allergy sufferers [4].
As antioxidants, thioredoxins, small proteins containing around 100 amino acids, can promote the reduction of other proteins and are essential to numerous biochemical processes in animal and plant organisms. Of the eight thioredoxins described as allergens, three come from moulds. Half of all individuals allergic to Fusarium are sensitized to Fus c 2 [4].
Heat shock proteins (HSP) 70/90 or chaperones are involved in the folding and stabilization of secondary protein structures in all organisms. Alt a 3 and Pen c 19 belong to the HSP 70 chaperones with a sensitization rate of 41 % for Pen c 19 and 5 % for recombinant Alt a 3 in affected mould allergics [4].
To date, five peroxisomal membrane proteins that are allergens have been described only in fungi, two of these in the moulds Asp f 3 und Pen c 3. Asp f 3 is prominent among peroxisomal membrane proteins with a sensitization rate of 32 %–100 % [4].
Isomerases catalyze the conversion of a chemical compound to an isomeric configuration. A sensitization rate of 90 % has been described for Asp f 11 (a peptidyl-prolyl isomerase) in Aspergillus fumigatus-sensitized patients [4].
Two moulds from the MnSOD group have been described: Asp f 6 and Alt a 14. Cross-reactivity was confirmed using IgE inhibition tests. Depending on the patient group (ABPA, cystic fibrosis), sensitization rates to Asp f 6 range from 63 % to 70 % [4].
Fig. 2.
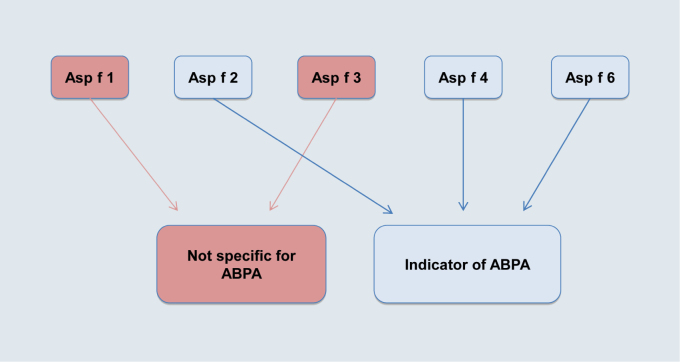
The use of Aspergillus fumigatus single allergens in the diagnosis of allergic bronchopulmonary aspergillosis (ABPA)
Flavodoxin and flavodoxin-like proteins (YCP4 homologs) are gene-regulatory proteins expressed in the late developmental phase of moulds and yeasts. Of the mould allergens, Alt a 7 and Cla h 7 belong in this group, albeit with low sensitization rates of 7 % and 22 %, respectively [4].
Diagnostic methods
In Germany, skin testing solutions and serological allergy tests are available for 30–40 fungal species. The standardization of mould extracts remains a challenge even today. Comparing test solutions from different manufacturers reveals heterogeneous extracts despite identical allergen sources [11], a possible cause of discrepant results in skin tests and specific IgE determination. Thus, depending on the type of mould, concordance between diagnostic methods can be less than 30 % [5].
Despite the numerous mould allergens described to date, only eight single allergens from the three genera Alternaria alternata, Aspergillus fumigatus and Cladosporium herbarum are currently available for molecular diagnostic methods:
rAlt a 1 is available as an allergen on various test platforms (ImmunoCAP, ISAC-Chip, ThermoFisherScientific). Up to 98 % of IgE-mediated Alternaria alternata sensitizations can be detected with this single allergen [4]. Alt a 1 is an acidic glycoprotein of unknown biochemical function and is expressed only after 21–30 days of cultivation. Its unique structure, a “butterfly-like dimer (double protein)”, can only be found in mould proteins [12]. Allergens homologous to Alt a 1 have been identified in other genera of the Pleosporaceae: Biopolaris, Curvularia, Pithomyces, Stemphylium, Ulocladium, Spondylocladium, Crivellia, Embellisia, Nimbya and Sinomyces (www.allergome.org). No allergens homologous to Alt a 1 have been discovered as yet in mould phyla such as Aspergillus, Penicillium or Cladosporium, which belong to other fungal families.
rAlt a 6 (enolase) is only available as a test allergen on the ISAC chip. sIgE to Alt a 6 is detected in 15 %–22 % of Alternaria alternata-sensitized patients [13]. Enolases in other mould species (e. g., Cla h 6), as well as in foods (Gad m 2, Sal s 2, Thu a 2) and natural latex (Hev b 9), represent potential sequence homology-based cross allergens. To date, inhibition studies have demonstrated IgE cross-reactions between rHev b 9, r Alt a 6 and r Cla h 6 [4].
rAsp f 1, 2, 3, 4, 6 are available as test allergens on ImmunoCAP and rAsp f 1, 3, 6 are also on the ISAC chip. However, a typical major allergen, comparable to Alt a 1, is lacking in Aspergillus fumigatus, as in all other mould species. Testing with certain recombinant Aspergillus fumigatus single allergens (rAsp f) can provide serological evidence of ABPA [14]. rAsp f 2-, rAsp f 4- and rAsp f 6-specific IgE was detected significantly more frequently in the serum of patients with clinically relevant ABPA compared with asthmatic or healthy controls. The single allergens rAsp f 1 and rAsp f 3 were recognized by ABPA patients as well as by asthmatic patients and sensitized individuals without symptoms. The combination of rAsp f 2+rAsp f 4+rAsp f 6 appears to make discrimination between ABPA and allergic asthma possible, whilst sensitization to rAsp f 1 and/or rAsp f 3 does not provide unequivocal evidence of allergic asthma. Serologically positive results for rAsp f allergens can also be found in other diseases, such as mucoviscidosis (cystic fibrosis).
rCla h 8 (dehydrogenase) is only available as a test allergen on the ISAC chip. The sensitization rate for Cla h 8 is 57 % in Cladosporium herbarum-sensitized patients [4]. Cross-reactivity to a dehydrogenase in Alternaria alternata (Alt a 8) has been demonstrated [4]. Potential cross-reactions with other dehydrogenases classified as allergens from ladybugs (Har a 2) and wheat (Tri a 34) have not been demonstrated as yet.
Although two other individual components from the moulds Aspergillus restrictus and Aspergillus oryzae are commercially available, they are used very rarely in the molecular diagnosis of mould sensitization for good reason.
nAsp r 1 is available as a test allergen in the 3gAllergy IMMULITE system (Siemens Healthcare Diagnostics). Like Asp f 1, this protein belongs to the mitogen family and shares sequential homologies with other ribonucleases, such as Bet v 1. However, no data on the incidence of sensitization or on cross-reactivity have been published for nAsp r 1 as yet.
nAsp o 21 is available as a fungal α-amylase (k87) in the 3gAllergy IMMULITE system and in the ImmunoCAP system. Asp o 21 is not a primary mould allergen, but rather the α-amylase expressed in Aspergillus oryzae is used as a baking enzyme in bakeries. Thus, nAsp o 21 belongs to the spectrum of occupational allergens, particularly those found in the baking industry, and should be included as a potential allergen when performing allergy tests in bakers with allergic respiratory symptoms.
Perspectives and conclusion
The current repertoire of commercially available single mould allergens is restricted to three species, Alternaria alternata, Aspergillus fumigatus and Cladosporium herbarum. Only total extracts are available for the diagnosis of all other forms of mould sensitization.
Improving fungal IgE diagnosis through greater availability and use of mould-typical marker allergens with stronger IgE binding is desirable. Members of the protease allergen families typical of mould come into question here, such as Asp f 13 and Cla h 9. Moreover, single allergens of the ribosomal protein family, such as Alt a 5/Cla h 5 and Alt a 12/Cla h 12, would be conceivable as mould-specific marker allergens.
At the same time, better standardization of total mould extracts is required in order that mould extracts with a defined allergen content are available in the future for skin testing, serological testing methods and, where required, in allergen-specific immunotherapy.
Abbreviations
- ABPA
Allergic bronchopulmonary aspergillosis
- FAD
Flavin adenine dinucleotide
- HSP
Heat shock proteins
- IgE
Immunglobulin E
- IUIS
International Union of Immunological Societies
- MnSOD
Manganese superoxide dismutases
- NAD
Nicotinamide adenine dinucleotide
- WHO
World Health Organization
Footnotes
Conflict of interest
The authors declare that no conflicts of interests exist.
Cite this as Kespohl S, Raulf M. Mould allergens: Where do we stand with molecular allergy diagnostics? Part 13 of the series Molecular Allergology. Allergo J Int 2014; 23: 120–5
References
- 1.Haftenberger M., Laußmann D., Ellert U., Kalcklösch M., Langen U., Schlaud M., et al. Prävalenz von Sensibilisierungen gegen Inhalations- und Nahrungsmittelallergene — Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsblatt. 2013;56:687–97. doi: 10.1007/s00103-012-1658-1. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz R., Ellert U., Kalcklösch M., Dahm S., Thamm M. Patterns of sensitization to inhalant and food allergens - findings from the German Health Interview and Examination Survey for Children and Adolescents. Int Arch Allergy Immunol. 2013;162:263–70. doi: 10.1159/000353344. [DOI] [PubMed] [Google Scholar]
- 3.Heinzerling L.M., Burbach G.J., Edenharter G., Bachert C., Bindslev-Jensen C., Bonini S., et al. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 4.Simon-Nobbe B., Denk U., Pöll V., Rid R., Breitenbach M. T. he spectrum of fungal allergy. Int Arch Allergy Immunol. 2008;145:58–86. doi: 10.1159/000107578. [DOI] [PubMed] [Google Scholar]
- 5.O’Driscoll B.R., Powell G., Chew F., Niven R.M., Miles J.F., Vyas A., et al. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clin Exp Allergy. 2009;39:1677–83. doi: 10.1111/j.1365-2222.2009.03339.x. [DOI] [PubMed] [Google Scholar]
- 6.Mayer C., Appenzeller U., Seelbach H., Achatz G., Oberkofler H., Breitenbach M., et al. Humoral and cell-mediated autoimmune reactions to human acidic ribosomal P2 protein in individuals sensitized to Aspergillus fumigatus P2 protein. J Exp Med. 1999;189:1507–12. doi: 10.1084/jem.189.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achatz G., Oberkofler H., Lechenauer E., Simon B., Unger A., Kandler D., et al. Molecular cloning of major and minor allergens of Alternaria alternata and Cladosporium herbarum. Mol Immunol. 1995;32:213–27. doi: 10.1016/0161-5890(94)00108-D. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S., Breiteneder H., Simon-Nobbe B., Susani M., Krebitz M., Niggemann B., et al. Eur J Biochem. 2000. Hev b 9, an enolase and a new cross-reactive allergen from Hevea latex and molds. Purification, characterization, cloning and expression; pp. 7006–14. [DOI] [PubMed] [Google Scholar]
- 9.Lai H.Y., Tam M.F., Tang R.B., Chou H., Chang C.Y., Tsai J.J., et al. cDNA cloning and immunological characterization of a newly identified enolase allergen from Penicillium citrinum and Aspergillus fumigatus. Int Arch Allergy Immunol. 2002;121:181–90. doi: 10.1159/000053862. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P.B., Denk U., Breitenbach M., Richter K., Schmid-Grendelmeier P., Nobbe S., et al. Alternaria alternata NADPdependent mannitol dehydrogenase is an important fungal allergen. Clin Exp Allergy. 2006;36:1513–24. doi: 10.1111/j.1365-2222.2006.02582.x. [DOI] [PubMed] [Google Scholar]
- 11.Kespohl S., Maryska S., Zahradnik E., Sander I., Brüning T., Raulf-Heimsoth M. Biochemical and immunological analysis of mould skin prick test solution: current status of standardization. Clin Exp Allergy. 2013;43:1286–96. doi: 10.1111/cea.12186. [DOI] [PubMed] [Google Scholar]
- 12.Chruszcz M., Chapman M.D., Osinski T., Solberg R., Demas M., Porebski P.J., et al. Alternaria alternata allergen Alt a 1: a unique β barrel protein dimer found exclusively in fungi. J Allergy Clin Immunol. 2012;130:241–7. doi: 10.1016/j.jaci.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger A., Stöger P., Simon-Nobbe B., Susani M., Crameri R., Ebner C., et al. Clinical testing of recombinant allergens of the mold Alternaria alternata. Int Arch Allergy Immunol. 1999;118:220–1. doi: 10.1159/000024076. [DOI] [PubMed] [Google Scholar]
- 14.Kurup V.P., Banerjee B., Hemmann S., Greenberger P.A., Blaser K., Crameri R. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy. 2000;30:988–93. doi: 10.1046/j.1365-2222.2000.00837.x. [DOI] [PubMed] [Google Scholar]