Summary
The present guideline (S2k) on allergen-specific immunotherapy (AIT) was established by the German, Austrian and Swiss professional associations for allergy in consensus with the scientific specialist societies and professional associations in the fields of otolaryngology, dermatology and venereology, pediatric and adolescent medicine, pneumology as well as a German patient organization (German Allergy and Asthma Association; Deutscher Allergie- und Asthmabund, DAAB) according to the criteria of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF).
AIT is a therapy with disease-modifying effects. By administering allergen extracts, specific blocking antibodies, toler-ance-inducing cells and mediators are activated. These prevent further exacerbation of the allergen-triggered immune response, block the specific immune response and attenuate the inflammatory response in tissue.
Products for SCIT or SLIT cannot be compared at present due to their heterogeneous composition, nor can allergen concentrations given by different manufacturers be compared meaningfully due to the varying methods used to measure their active ingredients. Non-modified allergens are used for SCIT in the form of aqueous or physically adsorbed (depot) extracts, as well as chemically modified allergens (allergoids) as depot extracts. Allergen extracts for SLIT are used in the form of aqueous solutions or tablets.
The clinical efficacy of AIT is measured using various scores as primary and secondary study endpoints. The EMA stipulates combined symptom and medication scores as primary endpoint. A harmonization of clinical endpoints, e. g., by using the combined symptom and medication scores (CSMS) recommended by the EAACI, is desirable in the future in order to permit the comparison of results from different studies. The current CONSORT recommendations from the ARIA/GA2LEN group specify standards for the evaluation, presentation and publication of study results.
According to the Therapy allergen ordinance (TAV), preparations containing common allergen sources (pollen from grasses, birch, alder, hazel, house dust mites, as well as bee and wasp venom) need a marketing authorization in Germany. During the marketing authorization process, these preparations are examined regarding quality, safety and efficacy. In the opinion of the authors, authorized allergen preparations with documented efficacy and safety, or preparations tradeable under the TAV for which efficacy and safety have already been documented in clinical trials meeting WAO or EMA standards, should be preferentially used. Individual formulations (NPP) enable the prescription of rare allergen sources (e.g., pollen from ash, mugwort or ambrosia, mold Alternaria, animal allergens) for specific immunotherapy. Mixing these allergens with TAV allergens is not permitted.
Allergic rhinitis and its associated co-morbidities (e. g., bronchial asthma) generate substantial direct and indirect costs. Treatment options, in particular AIT, are therefore evaluated using cost-benefit and cost-effectiveness analyses. From a long-term perspective, AIT is considered to be significantly more cost effective in allergic rhinitis and allergic asthma than pharmacotherapy, but is heavily dependent on patient compliance.
Meta-analyses provide unequivocal evidence of the efficacy of SCIT and SLIT for certain allergen sources and age groups. Data from controlled studies differ in terms of scope, quality and dosing regimens and require product-specific evaluation. Therefore, evaluating individual preparations according to clearly defined criteria is recommended. A broad transfer of the efficacy of certain preparations to all preparations administered in the same way is not endorsed. The website of the German Society for Allergology and Clinical Immunology (www.dgaki.de/leitlinien/s2k-leitlinie-sit; DGAKI: Deutsche Gesellschaft für Allergologie und klinische Immunologie) provides tables with specific information on available products for AIT in Germany, Switzerland and Austria. The tables contain the number of clinical studies per product in adults and children, the year of market authorization, underlying scoring systems, number of randomized and analyzed subjects and the method of evaluation (ITT, FAS, PP), separately given for grass pollen, birch pollen and house dust mite allergens, and the status of approval for the conduct of clinical studies with these products.
Strong evidence of the efficacy of SCIT in pollen allergy-induced allergic rhinoconjunctivitis in adulthood is well-documented in numerous trials and, in childhood and adolescence, in a few trials. Efficacy in house dust mite allergy is documented by a number of controlled trials in adults and few controlled trials in children. Only a few controlled trials, independent of age, are available for mold allergy (in particular Alternaria). With regard to animal dander allergies (primarily to cat allergens), only small studies, some with methodological deficiencies are available. Only a moderate and inconsistent therapeutic effect in atopic dermatitis has been observed in the quite heterogeneous studies conducted to date. SCIT has been well investigated for individual preparations in controlled bronchial asthma as defined by the Global Initiative for Asthma (GINA) 2007 and intermittent and mild persistent asthma (GINA 2005) and it is recommended as a treatment option, in addition to allergen avoidance and pharmacotherapy, provided there is a clear causal link between respiratory symptoms and the relevant allergen.
The efficacy of SLIT in grass pollen-induced allergic rhinoconjunctivitis is extensively documented in adults and children, whilst its efficacy in tree pollen allergy has only been shown in adults. New controlled trials (some with high patient numbers) on house dust mite allergy provide evidence of efficacy of SLIT in adults.
Compared with allergic rhinoconjunctivitis, there are only few studies on the efficacy of SLIT in allergic asthma. In this context, newer studies show an efficacy for SLIT on asthma symptoms in the subgroup of grass pollen allergic children, adolescents and adults with asthma and efficacy in primary house dust mite allergy-induced asthma in adolescents aged from 14 years and in adults.
Aspects of secondary prevention, in particular the reduction of new sensitizations and reduced asthma risk, are important rationales for choosing to initiate treatment early in childhood and adolescence. In this context, those products for which the appropriate effects have been demonstrated should be considered.
SCIT or SLIT with pollen or mite allergens can be performed in patients with allergic rhinoconjunctivitis using allergen extracts that have been proven to be effective in at least one double-blind placebo-controlled (DBPC) study. At present, clinical trials are underway for the indication in asthma due to house dust mite allergy, some of the results of which have already been published, whilst others are still awaited (see the DGAKI table “Approved/potentially completed studies” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit (according to www.clinicaltrialsregister.eu)). When establishing the indication for AIT, factors that favour clinical efficacy should be taken into consideration. Differences between SCIT and SLIT are to be considered primarily in terms of contraindications. In individual cases, AIT may be justifiably indicated despite the presence of contraindications.
SCIT injections and the initiation of SLIT are performed by a physician experienced in this type of treatment and who is able to administer emergency treatment in the case of an allergic reaction. Patients must be fully informed about the procedure and risks of possible adverse events, and the details of this process must be documented (see “Treatment information sheet”; available as a handout via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit). Treatment should be performed according to the manufacturer‘s product information leaflet. In cases where AIT is to be performed or continued by a different physician to the one who established the indication, close cooperation is required in order to ensure that treatment is implemented consistently and at low risk. In general, it is recommended that SCIT and SLIT should only be performed using preparations for which adequate proof of efficacy is available from clinical trials.
Treatment adherence among AIT patients is lower than assumed by physicians, irrespective of the form of administration. Clearly, adherence is of vital importance for treatment success. Improving AIT adherence is one of the most important future goals, in order to ensure efficacy of the therapy.
Severe, potentially life-threatening systemic reactions during SCIT are possible, but – providing all safety measures are adhered to – these events are very rare. Most adverse events are mild to moderate and can be treated well.
Dose-dependent adverse local reactions occur frequently in the mouth and throat in SLIT. Systemic reactions have been described in SLIT, but are seen far less often than with SCIT. In terms of anaphylaxis and other severe systemic reactions, SLIT has a better safety profile than SCIT.
The risk and effects of adverse systemic reactions in the setting of AIT can be effectively reduced by training of personnel, adhering to safety standards and prompt use of emergency measures, including early administration of i. m. epinephrine. Details on the acute management of anaphylactic reactions can be found in the current S2 guideline on anaphylaxis issued by the AWMF (S2-AWMF-LL Registry Number 061-025).
AIT is undergoing some innovative developments in many areas (e. g., allergen characterization, new administration routes, adjuvants, faster and safer dose escalation protocols), some of which are already being investigated in clinical trials.
Cite this as Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, Friedrichs F, Fuchs T, Hamelmann E, Hartwig-Bade D, Hering T, Huttegger I, Jung K, Klimek L, Kopp MV, Merk H, Rabe U, Saloga J, Schmid-Grendelmeier P, Schuster A, Schwerk N, Sitter H, Umpfenbach U, Wedi B, Wöhrl S, Worm M, Kleine-Tebbe J. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases – S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int 2014;23:282–319
Key words: allergen-specific immunotherapy, AIT, Hyposensitization, guideline, allergen, allergen extract, allergic disease, allergic rhinitis, allergic asthma
1. Objectives and development of the guideline
The present guideline was developed on behalf of and financed by the German Society for Allergology and Clinical Immu-nology (Deutsche Gesellschaft für Allergologie und klinische Immunologie, DGAKI) and replaces the S2 guideline published in 2009 [1]. It has been conceived as a S2k guideline according to the methodological requirements set out by the German Working Group of Scientific Medical Societies (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaf-ten, AWMF). A detailed guideline report in line with AWMF policy (DELBI criteria 1–7) can be found on the AWMF website (www.awmf.org/leitlinien/detail/ll/061-004.html)
In summary, it was decided by the board of the DGAKI in 2012 that the corresponding author should take over the task of coordinating the revision of the guideline. In addition to members of the DGAKI (Oliver Pfaar, Jörg Kleine-Tebbe, Eckard Hamelmann, Bettina Wedi, Claus Bachert and Margitta Worm), representatives of the following bodies were involved in drawing up the guideline: the Medical Association of German Allergologists (Ärzteverband Deutscher Allergologen, AeDA: Thomas Fuchs, Hans Merk, Uta Rabe), the Society for Pediatric Allergy and Environmental Medicine (Gesellschaft für Pädiatrische Allergologie und Umweltmedizin, GPA: Albrecht Bufe, Matthias Volkmar Kopp, Antje Schuster), the Austrian Society for Allergy and Immunology (Österreichische Gesellschaft für Allergologie und Immunologie, ÖGAI: Christof Ebner, Isidor Huttegger, Stefan Wöhrl), the Swiss Society for Allergy and Immunology (Schweizerische Gesellschaft für Allergologie und Immunologie, SGAI: Peter Eng, Peter Schmid-Grendelmeier), the German Society of Dermatology (Deutsche Gesellschaft für Dermatologie, DDG: Joachim Saloga), the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie, DGHNO-KHC: Ludger Klimek), the German Society of Pediatrics and Adolescent Medicine (Deutsche Gesellschaft für Kinder-und Ju-gendmedizin, DGKJ: Ulrich Umpfenbach), the Society for Pediatric Pneumology (Gesellschaft für Pädiatrische Pneumolo-gie, GPP: Nikolaus Schwerk), the German Respiratory Society (Deutsche Gesellschaft für Pneumologie, DGP; Roland Buhl), the German Association of ENT Surgeons (Berufsverband der HNO-Ärzte, BV-HNO: Doris Hartwig-Bade), the Profes-sional Federation of Paediatricians and Youth Doctors (Berufsverband der Kinder- und Jugendärzte, BVKJ: Frank Frie-drichs), Federal Association of Pulmonologists (Bundesverband der Pneumologen, BdP: Thomas Hering) and the German Dermatologists Association (Berufsverband der Deutschen Dermatologen, BVDD: Kirsten Jung). The Paul-Ehrlich Institute (PEI: Susanne Kaul) and the German Allergy and Asthma Association/Patient Organization (Deutscher Allergie- und Asthmabund, DAAB: Anja Schwalfenberg) were involved in the consensus process in an advisory capacity.
The guideline was updated at a consensus conference in Wiesbaden, Gemany, in April 2013, as well as by written consent and using a web-based guidelines portal especially set-up and authorized by the AWMF (www.leitlinienentwicklung.de). The final consensus process took place on July 18th 2014. The guideline was then presented to all responsible board members to be authorized and recommended for adoption. This final authorization process was formally completed by October 1st 2014.
The guideline is addressed to all physicians with a board certification or subspeciality in allergy as well as all physicians that treat and/or monitor allergic patients in the context of AIT, and can be used for all patient groups with allergic rhinoconjunctivitis with/without allergic asthma and allergic sensitization to inhaled allergens.
The validity of the guideline shall be reviewed by the authors 5 years following its publication. The guideline coordinator shall be responsible for this task. Further details can be found in the separate guideline report.
The guideline will be published and distributed by the allergy societies in their official associated journals; it will also be published (in German language) in the AWMF guideline register, recommended for adoption by other involved societies and made available for reprint to interested journals with allergy-related content.
2. Immunological mechanisms of action
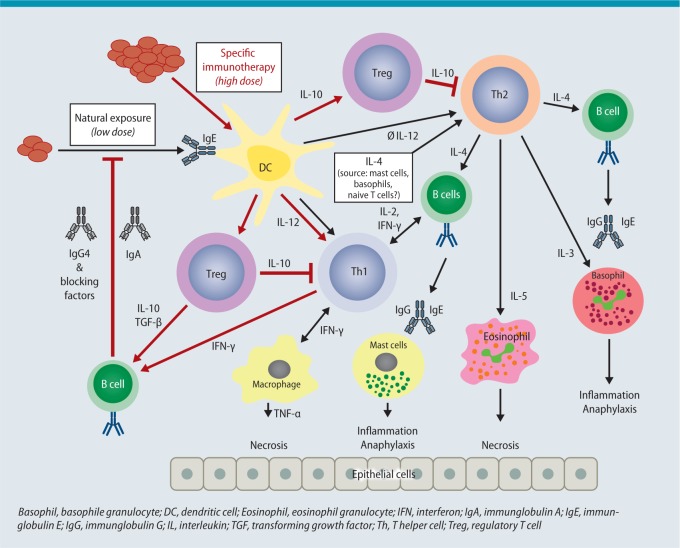
With AIT, allergen extracts in the form of molecule mixtures are presented to the immune system either subcutaneously (SCIT) or sublingually (SLIT). The patient is already sensitized to the allergens and reacts upon renewed exposure to allergens with inflammation of the skin and mucosa. The allergen extracts first diffuse into local tissue, where they are taken up by antigen-presenting cells (APC) [2]. The speed of this process depends on the dose and composition of the extracts, particularly when depot preparations are used [3]. Following administration, the allergens are found in local lymph nodes. They arrive there either unbound via free diffusion or are taken up by dendritic (DC) or B cells [4]. At the same time, immuncomplexes made up of allergens and IgE antibodies may form in the tissue, by which allergens can be intercepted, mast cells activated or allergens transported to lymph nodes. At present, the literature [4, 5, 6, 7, 8, 9] favors the following immunological mechanisms to explain the effect of AIT (Fig. 1):
Fig. 1.
Complex model of the immunological effects during AIT
© Authors of the guideline
Activation of new and boosting of existing antibodies that block the allergen-antibody-mediated immune response. In par-ticular, these include IgG antibodies that are able to prevent binding of IgE-allergen complexes to B cells and DC. An in-crease in these antibodies correlates to a certain extent with the success of treatment, an effect that cannot be seen when measuring the total fraction of IgG and IgG4 serum-antibodies.
Activation of regulatory T cells (Tregs) that inhibit the T cell-mediated activation of B cells and the specific T-cell response to the allergen. Tregs migrate from their site of formation in the lymph nodes back to the area of inflammation and release IL-10 and TGF-ß, thereby reducing local inflammation. These effects can only be measured after 6 months of treatment and have not as yet been confirmed in all studies.
Induction of mediators and cytokines that attenuate local allergic inflammation. Allergens primarily activate local APC (e.g., DC). These release IL-10 and TGF-ß in particular. Both cytokines can have a local anti-inflammatory effect and are able to inhibit T-cell proliferation. In addition, the release of IL-10 serves to reinforce the above-mentioned production of blocking IgG antibodies. Cytokines released locally also attenuate local mast cell activity and the activation of other effector cells that contribute to allergic inflammation.
Conclusion: AIT is a therapy with disease-modifying effects. By administering allergen extracts, specific blocking antibodies, tolerance-inducing cells and mediators are activated. These prevent further exacerbation of the allergen-triggered immune response, block the specific immune response and attenuate the inflammatory response in tissue.
3. Allergen extracts: assessment and marketing authorization
3.1. Production and composition of allergen extracts
Due to manufacturer-specific processing, the allergen extracts produced differ in terms of composition and allergen activity and are therefore not comparable even if the same allergen sources are used. Standardized allergen extracts should preferentially be used for AIT, as otherwise extracts vary significantly in their biological activity [10]. The total activity of the extracts is determined using in-vitro methods [11]. Determining individual allergens (e. g., major allergens) using standardized, validated methods is endorsed in international guidelines [12]. Two recombinant major allergens, rBet v 1 from birch pollen (Betula verrucosa, http://crs.edqm.eu/db/4DCGI/View=Y0001565) and rPhl p 5a from timothy grass pollen (Phleum pratense, http://crs.edqm.eu/db/4DCGI/View=Y0001566), were adopted as reference preparations by the European Pharmacopoeia Commission in 2012. These reference preparations are intended for the determination of the Bet v 1 and Phl p 5a content in corresponding allergen preparations (native and recombinant) [13].
The use of these references is voluntary until suitable ELISA systems are available and requested by the European Pharmacopoeia. Thus, it is not possible at present to compare the allergen concentrations in various preparations, as manufacturers use different antibodies and measuring systems to determine major allergens.
Non-modified (native) extracts with unaltered allergen conformation and chemically modified extracts (allergoids) can be used for SCIT. The concept is that allergoids possess less reactive B-cell epitopes and thus reduced IgE binding, while their T-cell epitopes and their immunogenic effect remain unaltered [14]. In addition to aqueous extracts, which are commonly used as the initial treatment in insect venom allergy, depot extracts are primarily used in Europe for SCIT. Here, allergens or allergoids are physically adsorbed to a carrier, such as aluminum hydroxide, tyrosine or calcium phosphate (Fig. 2).
Fig. 2.
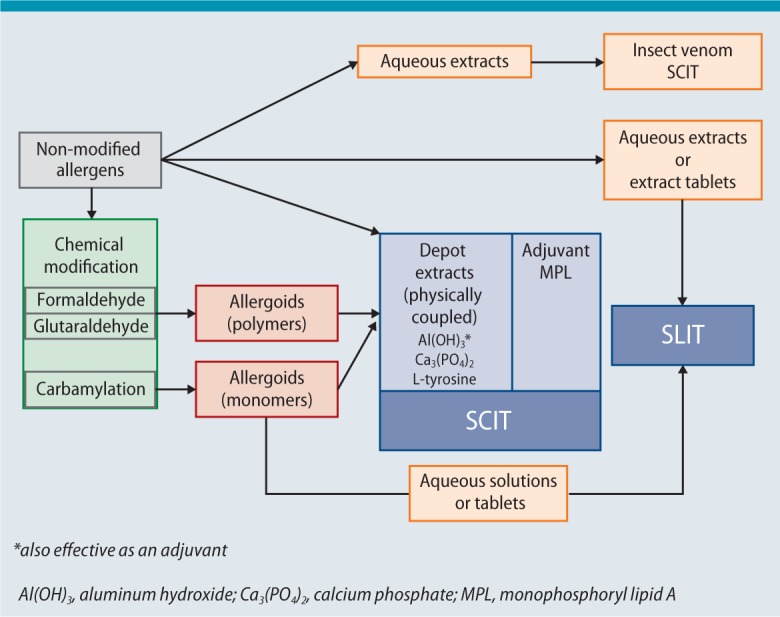
Allergen extracts available for AIT (see Sect. 3.1 for more details)
© Authors of the guideline
Preparations for SLIT are available with allergens in unmodified conformation or as chemically modified extracts in the form of aqueous solutions or tablets (Fig. 2). Some preparations need to be refrigerated, others can be stored at room temperature.
Conclusion: Products for SCIT or SLIT cannot be compared at present due to their heterogeneous composition, nor can allergen concentrations given by different manufacturers be compared meaningfully due to the varying methods used to measure their active ingredients. Non-modified allergens are used for SCIT in the form of aqueous or physically adsorbed (depot) extracts, as well as chemically modified allergens (allergoids) as depot extracts. Allergen extracts for SLIT are used in the form of aqueous solutions or tablets.
3.2. Criteria for evaluating subcutaneous or sublingual administration of specific immunotherapy in clinical studies
The efficacy of AIT is measured using symptom scores [e. g., individual symptoms, total symptom score (TSS)], medication scores, combined symptom and medication scores, health-related quality of life (HRQL) as well as other methods (e. g., visual analog scales, “well” or “severe days”) [15, 16, 17]. It is essential to record allergy exposure over time (e. g., using pollen counts) as well as to collect safety data; moreover, recording laboratory data on IgE, IgG and IgG4 is recommended.
Combined symptom and medication scores are frequently used as primary endpoints in AIT trials and proposed in multiple variations [18, 19, 20]. The lack of validation of primary and secondary efficacy parameters represents a considerable problem in terms of the comparability of study results [15, 16, 17].
The European Medicines Agency (EMA) primarily recommends combined symptom and medication scores for the primary endpoint in AIT trials and accepts (in justified exceptional cases) a positive study result for both scores, as the consumption of rescue medication also affects the symptoms. Therefore, the score should reflect both, severity of symptoms as well as the need for medication [21]. However, the EMA does not provide a precise definition for this parameter [21, 22].
A task force working group of the EAACI recently published specific recommendations on clinical endpoints in AIT trials [17]. Particularly worthy of note is that the EAACI Position Paper provides a definition of a homogeneous, standardized combined symptom and medication score (CSMS) as primary endpoint with the aim of harmonizing this outcome measure in future AIT trials [17].
It is essential that study results are evaluated, represented and published in an appropriate manner. To this end, standards have been established (Consolidated Standards of Reporting Trials [CONSORT]) which, by the use of checklists, are intended to guarantee minimal yet transparent reporting of studies (www.consort-statement.org [23]). This includes the evaluation of clinical data in an intention-to-treat (ITT) analysis, which takes all patients included in a study (even those that withdraw early) into account, illustrating the actual effects of AIT under practical conditions [23, 24]. The per-protocol (PP) analysis, on the other hand, is well suited to estimating maximum efficacy under optimal standard conditions. In addition, data on all patients – even those included in the study without fulfilling the specified inclusion criteria or whose treatment deviated from the study protocol – are recorded in the analysis of full-analysis-sets (FAS) in order to depict the safety profile of the treatment.
Conclusion: The clinical efficacy of AIT is measured using various scores as primary and secondary study endpoints. The EMA stipulates combined symptom and medication scores as primary endpoint. A harmonization of clinical endpoints, e. g., by using the CSMS recommended by the EAACI, is desirable in the future in order to permit the comparison of results from different studies. The current CONSORT recommendations from the ARIA/GA2LEN group specify standards for the evaluation, presentation and publication of study results.
3.3. Relevance of marketing authorization for allergen preparations
In Germany, marketing authorization is required for allergen preparations in accordance with the German Medicinal Products Act (Arzneimittelgesetz, AMG). However, there is an exemption excluding individual formulations (named patient products, NPP) of therapy allergens from marketing authorization. Irrespective of this, all preparations are finished medicinal products according to the AMG (Tab. 1).
Table 1.
Important terms in the German Medicinal Products Act (Deutsches Arzneimittelgesetz, AMG) www.gesetze-im-internet.de/bundesrecht/amg_1976/gesamt.pdf, as well as particular features of the Austrian and Swiss drug laws
| Finished medicinal products |
| Section 4 Sub-section 1, AMG § 4 (1): „Finished medicinal products are medicinal products which are manufactured beforehand and placed on the market in packaging intended for distribution to the consumer or other medicinal products intended for distribution to the consumer, in the preparation of which any form of industrial process is used or... are produced commercially“. |
| Marketing authorization |
| Section 21 Sub-section 1, AMG § 21 (1): „Finished medicinal products which are medicinal products as defined in Section 2 sub-section 1 or sub-section 2 number 1, may only be placed on the market within the purview of the present Act, if they have been authorised by the competent higher federal authority...“ |
| Individual formulations (NPP) |
| Section 21 Sub-section 2, AMG § 21 (2): “A marketing authorization (Zulassung) shall not be required for medicinal products which ... No. 1g: ... are therapeutic allergens manufactured to order for individual patients...” |
| Important Terms in the Austrian Drug Law (Österreichisches Arzneimittelgesetz) |
| AMG § 7a(1): “Medicinal products containing antigens or half-antigens intended for the detection of specific antibodies and protective substances for desensitization or hyposensitization, provided they are not always produced in the same composition and under the same designation in a defined form intended for distribution to the consumer or user, are only permitted to be distributed domestically or held ready for domestic distribution if the Federal Office for Safety in Healthcare has approved by notification the manufacturing process including chemical/pharmaceutical documentation, for this medicinal product.“ |
| Situation in Switzerland |
| Under the terms of the Swiss Federal Law on Medicinal Products [Heilmittelgesetz, HMG; Art. 9 (1)] dated December 15th 2000, allergen preparations intended for AIT are considered as medicinal products requiring marketing approval (SR812.21, www.admin.ch/opc/de/classified-compilation/20002716/index.html#a9). Allergen preparations used under the terms of the exemption clause [HMG Art. 9 (2)], for example as individual formulations (patient-specific mixture of allergens, NPP), are exempt from marketing authorization. |
| A new regulation came into force in 2010 to simplify the marketing approval process for allergen preparations (Allergenverordnung, AllergV SR812.216.2, www.admin.ch/opc/de/classified-compilation/20060055/index.html). The simplification of the marketing approval procedure consists of the fact that the market authorization documentation can be based on published literature (from scientifically recognized sources) or on documentation for other allergen preparations (a reference preparation of the same manufacturer). Allergen preparations containing recombinant allergens or genetically modified organisms are excluded from this simplified marketing authorization procedure. |
| In cases where marketing authorization has already been granted in a country with comparable medicinal drug regulations and a comparable marketing authorization process, it is possible, under the terms of Art. 13 of the Swiss HMG to take these results into consideration with regard to marketing authorization in Switzerland. |
Both types of product can be prescribed and are tradeable. Individual formulations have been regulated in Germany since 2008 in addition by the Therapy Allergen Ordinance (Therapieallergene-Verordnung, TAV) [25]. According to the TAV, individual formulations containing at least one extract of an allergen source that frequently triggers allergies (Tab. 2) require a marketing authorization.
Table 2.
A list of therapy allergens requiring marketing authorization in Germany* [25]4
| Species of the Poaceae family excluding Poa mays (grasses excluding maize) |
| Betula sp. (species of the birch genus) |
| Alnus sp. (species of the alder genus) |
| Corylus sp. (species of the hazel genus) |
| Dermatophagoides sp. (species of the house dust mite genus) |
| Bee venom |
| Wasp venom |
*A list of therapy allergens requiring marketing authorization according to the German Therapy Allergen Ordinance [25] and which, once transitional regulations have expired, may not be marketed either as individual preparations or as mixtures without marketing authorization.
At present, there are marketing authorization applications for 96 individual formulations of this kind pending at the Paul-Ehrlich-Institute (PEI) (as of December 2014, PEI communication). These preparations remain equivalent to approved preparations in terms of being prescribable and tradeable until the decision on their application for marketing authorization. All preparations (more than 6,400) containing allergens of this kind and for which no approval was sought needed to be reported to the PEI, remained tradeable until November 2011 at the latest for the treatment of patients already on treatment and were then removed from the market [26]. New regulations related to batch release also came into force with the TAV. Before the TAV became effective, only authorized allergen preparations were subject to governmental batch release; with the TAV, batch release became mandatory for all reported individual preparations. In the case of individual formulations (NPP), testing is performed on the bulk allergen extracts from which the individual formulations are produced (bulk allergen extract batch release), whereas for all other preparations testing is performed primarily on the end product.
All other therapy allergens produced as individual formulations (NPPs that do not contain allergens listed in the TAV appendix; see Tab. 3 for examples) are still exempt from mandatory marketing authorization and are thereby neither subject to official monitoring on quality, efficacy and safety nor governmental batch release. With regard to manufacture, however, according to the AMG, a manufacturing license that fulfills all the criteria of good manufacturing practice (GMP) is required.
Table 3.
Examples of individual formulations (NPP) for specific immunotherapy using allergen sources not subject to the German Therapy Allergen Ordinance* [25]
| Mugwort pollen (Artemisia vulgaris) |
| Ash pollen (Fraxinus excelsior) |
| Alternaria (Alternaria alternata) |
| Animal allergens, e.g., from the cat (Felis domesticus) |
| Storage mites (e.g., Acarus siro) |
*Not mixed with allergen groups subject to the Therapy Allergen Ordinance (Tab. 2), otherwise they would subject to the ordinance.
Authorized preparations (www.pei.de/DE/arzneimittel/allergene/allergene-node.html) can be distinguished from individual formulations (NPP) by their authorization number on the outer packaging and in the summary of product information.
In Germany, the PEI is responsible for the marketing authorization of allergen preparations (Tab. 4) for therapeutic and diagnostic purposes and for batch release. Authorization in Austria is regulated by the Federal Office for Safety in Health Care (Bundesamt für Sicherheit im Gesundheitswesen), which serves the Austrian Agency for Health and Nutrition (Österreichische Agentur für Gesundheit und Ernährung, AGES-PharmMed). Marketing authorization for allergens is supervised in Switzerland by the Swiss Agency for Therapeutic Products Swissmedic. The above-mentioned regulations apply only partially to Austria and Switzerland, especially the TAV applies only for Germany.
Table 4.
Marketing authorization procedures* for medicinal products in the European Union (EU)
| National procedure, when marketing authorization is granted for a medicinal product in the respective member state only |
| Mutual recognition procedure, when a preparation already has marketing authorization in one EU member state and this authorization should be extended to other member states |
| Decentralized procedure, when a medicinal product does not yet have national marketing authorization and seeks parallel marketing authorization in several EU member states |
| Central procedure (simultaneous marketing authorization in all EU member states), necessary in the case of medicinal products cited in the Appendix to EU Regulation 726/2004 (e.g., medicinal products which are manufactured by using biotechnological processes); can also be used for other medicinal products under certain conditions |
*All procedures resulting in marketing authorization in several or all European countries are coordinated by the European Medicines Agency (EMA).
The application for marketing authorization at the competent authority shall include among others information on the production process of the drug, its quality control, the results of all pre-clinical and clinical studies as well as further medical testing. Medicinal products must fulfil the state of the art requirements valid at the time of authorization [27]. Today, these include, e. g., GMP, good clinical practice (GCP), the European pharmacopoeia as well as the relevant EMA guidelines (www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003333.pdf [11], www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003605.pdf [21]).
Preparations are only authorized for those indications and patient groups for which safety and efficacy have been proven in clinical trials.
Since 1993, and with the exception of bee and wasp venom preparations, marketing authorization has only been granted if at least one double-blind placebo-controlled (DBPC) trial complying with the relevant state of the art has been successfully carried out. Placebo control is not required for hymenoptera venom preparations for ethical reasons; in such cases, an established equivalent preparation is generally used for comparative testing. In the case of older authorizations – in accordance with requirements valid at that time – open studies were sometimes also accepted as evidence of efficacy.
Increased requirements set more recently have resulted in a significant improvement in the quality of data obtained in clinical studies and thus also in the evidence of safety and efficacy of preparations authorized on the basis of these studies. Although individual formulations that come under the TAV (Tab. 2) are subject to governmental batch release on bulk allergen extracts for quality assurance purposes, no official inspection of the production process or examination of efficacy and safety is carried out prior to the authorization process.
In the opinion of the authors, authorized allergen preparations with documented efficacy and safety, or preparations tradeable under the TAV for which efficacy and safety have already been documented in clinical trials meeting WAO or EMA standards, should be preferentially used. A current overview intended as a guide for most of the current clinical trials on AIT approved for implementation can be found in the European Clinical Trials Register at: www.clinicaltrialsregister.eu.
Manufacturers have the opportunity to report results on efficacy from relevant studies in the summary of product information under Article 5.1; however, study quality may vary significantly, given the differences in requirements between 1990 and today. In the case of authorized preparations, this information is also examined by the authorities. In the case of current marketing authorizations, manufacturers use this opportunity, which also offers physicians a good chance to inform themselves about the preparation.
Since authorized finished medicinal products are not able to cover the full spectrum of allergen extracts required for AIT, the use of individual formulations (named patient products, NPP) is justified in cases where the extract needs to be individually tailored to the allergy needs of a particular patient [28] (see Tab. 3).
Conclusion: According to the TAV, preparations containing common allergen sources (pollen from grasses, birch, alder, hazel, house dust mites, as well as bee and wasp venom) need a marketing authorization in Germany. During the marketing authorization process, these preparations are examined regarding quality, safety and efficacy. In the opinion of the authors, authorized allergen preparations with documented efficacy and safety, or preparations tradeable under the TAV for which efficacy and safety have already been documented in clinical trials meeting WAO or EMA standards, should be preferentially used.
Individual formulations (NPP) enable the prescription of rare allergen sources (e.g., pollen from ash, mugwort or ambrosia, mold Alternaria, animal allergens) for specific immunotherapy. Mixing these allergens with TAV allergens is not permitted.
3.4. AIT from a socio-economic perspective
Allergic diseases, such as allergic rhinoconjunctivitis, have a significant impact on the individual patients as well as on the national economy as a whole [29, 30, 31].
The healthcare system is burdened not only by the costs caused directly by disease, but also by the indirect costs that are often challenging to measure [32]. One in ten doctors’ certificates for work absence can be attributed to allergy symptoms. The direct disease costs for allergic rhinoconjunctivitis already totalled several hundred million euros in the 1990s [31]. Treatment options consist of symptomatic treatment and allergen avoidance, as well as disease-modifying therapy in the form of AIT. Since AIT is both a somehow curative and a preventive approach, it is able to affect the individual disease course positively (disease modifying effect). Allergic rhinitis patients have a 3.5-fold higher relative risk of developing bronchial asthma within less than 10 years [33]. In this context, AIT is deemed to have a preventive effect in terms of allergic progression (to allergic bronchial asthma) or new sensitizations [24, 34].
The scientific socio-economic evaluation of therapeutic agents is carried out using cost-benefit and cost-effectiveness analyses, which enable healthcare policymakers to compare different methods and products, as well as to identify the advantages and disadvantages of treatment methods from a socio-economic perspective. The results of this kind of analysis are taken into consideration in the evaluation of medicinals and play an important role today in decision-making on the coverage of the treatment costs by state health institutions.
The gain in quality of life per year following intervention with AIT is measured using the standardized quality-adjusted life year (QALY) and employed for incremental cost-effectiveness analysis [35]. Every year of life in perfect health is expressed with a QALY of 1, diminishing according to disease burden to a QALY of 0.0 for death. By dividing the disease course, the difference in costs for various methods or time points in treatment (in this case AIT) by the relevant QALY, one obtains the incremental cost-effectiveness ratio (ICER). Recent examples show that the ICER for AIT, irrespective of the route of administration, falls within the range of treatments accepted in healthcare policy for the treatment of chronic diseases [36, 37]. Another cost-effectiveness analysis carried out in Germany underscores the potential of AIT to save costs [38].
If one takes the cumulative ICER per year as a basis, it becomes clear that the significant investment made in AIT at the beginning of treatment proves to be cost-neutral after 7 years on average [39]. This is consistent with the fact that the principal advantage of AIT lies in its long-term effects. It must be pointed out, however, that these effects depend to a great extent on treatment compliance.
Generally speaking, the prices of individual products valid at the time (according to the official drug price list (LAU-ER-TAXE®) and at dosage according to the manufacturer‘s recommendations) for a treatment period of 3 years should be used to compare the costs of SCIT and SLIT.
Conclusion: Allergic rhinitis and its associated co-morbidities (e. g., bronchial asthma) generate substantial direct and indirect costs. Treatment options, in particular AIT, are therefore evaluated using cost–benefit and cost-effectiveness analyses. From a long-term perspective, AIT is considered to be significantly more cost effective in allergic rhinitis and allergic asthma than pharmacotherapy, but is heavily dependent on patient compliance.
4. Efficacy in clinical studies
4.1. Systematic reviews and meta-analyses for the evaluation of AIT
Systematic reviews and meta-analyses are often referred to as the highest form of statistical evaluation of multiple studies. The reliability of their conclusions depends on the study selection criteria and on quality control measures as the studies evaluated are usually highly heterogenous [40]. Although numerous meta-analyses on AIT have been carried out, recent ones were able to include more studies with large numbers of cases and of higher quality. Reviews of published meta-analyses carried out up to and including 2009 can be found in [41] and [42].
One way to reduce the effect of heterogeneity on study results, while enabling conclusions that are relevant in routine practice, is to select the studies to be included strictly according to predefined criteria. As an example one could only include studies with a minimum of 100 subjects per arm or studies on commercially available products. In their analysis through 2009, Calderon et al. evaluated 33 clinical studies on AIT in grass-pollen allergic patients that fulfilled predefined criteria [43]. 28 recent trials were used for a more up-to-date meta-analysis of studies on SCIT and SLIT in patients with seasonal allergic rhinitis [44]. Another recent systematic review of the efficacy and tolerability of SCIT and SLIT in patients with house dust mite allergy included 44 studies published up to 2013 [45].
In summary, these meta-analyses and reviews demonstrate a well-documented efficacy for AIT. However, due to the heterogeneity of individual studies described in all analyses, the authors stress that it is not possible to make a generic recommendation about the route of application, but rather that evidence of efficacy and tolerability is required for individual AIT preparations.
It is anticipated that, under the TAV (see Sect. 3.3), a large number of studies combined with adequate evidence on various preparations will be available.
Tables providing a preparation-specific list of AIT products on the market in Germany, Switzerland and Austria can be found on the DGAKI website via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit. This list includes all preparations with certain features for some products:
studies are available fulfilling five efficacy criteria that are modified to conform to the recommendations in the WAO consensus paper on the standardization of clinical AIT studies [46]. Since the efficacy of AIT in view of potential side effects and treatment costs should at least be comparable to that of pharmacotherapy, a threshold in efficacy of 20 % above placebo has been selected as acceptable [46]. The currently most effective pharmacotherapy (MP29-02) has an efficacy of 19 % above placebo, thereby justifying this threshold value [47].
marketing authorization has been granted in Germany.
the authorities have granted consent to perform clinical trials and the positive vote of the relevant ethics commission has been submitted to the competent authorities (from www.clinicaltrialsregister.eu).
The table lists studies in adults and children separately, the year of marketing authorization, the clinical endpoints used as a basis, the number of patients randomized and evaluated, the evaluation method used (ITT, FAS, PP) for grass pollen –and birch pollen allergens and house dust mite allergens, as well as the status of consent to conduct clinical trials.
It is important to note that the quality of evidence of efficacy differs according to the year in which approval was granted (in accordance with the PEI criteria applied in the year of approval) and that approval is independent of the five efficacy criteria discussed here (e.g., a fixed percentage improvement above placebo is not endorsed for marketing authorization).
Conclusion: Meta-analyses provide unequivocal evidence of the efficacy of SCIT and SLIT for certain allergen sources and age groups. Data from controlled studies differ in terms of scope, quality and dosing regimens and require product-specific evaluation. Therefore, evaluating individual preparations according to clearly defined criteria is recommended. A broad transfer of the efficacy of certain preparations to all preparations administered in the same way is not endorsed.
4.2. Efficacy of SCIT in inhalant allergies
4.2.1. Efficacy of SCIT in allergic rhinoconjunctivitis
The documentation on the clinical efficacy of SCIT in allergic rhinoconjunctivitis is based on numerous DBPC trials of heterogenous size and quality and which were summarized for seasonal allergens (e.g., grass pollen, birch pollen) in a systematic review and meta-analysis in 2007 [48]. This analysis evaluated 15 studies on SCIT that demonstrated a reduction in the symptom score (Standardized Mean Difference (SMD) -0.73; 95 % Confidence Interval (CI) -0.97 to -0.50; p < 0.00001) and in the medication score (SMD -0.57; 95 % CI -0.82 to -0.33; p < 0.00001; in 13 studies).
A current meta-analysis (2013) evaluated 17 clinical trials (up to April 2011) for efficacy of SCIT in patients with seasonal allergic rhinitis [44]. This analysis found a reduction in the symptom scores (SMD -0.65; 95 % CI -0.85 to -0.45; p < 0.00001; all 17 studies), the medication scores (SMD -0.55; 95 % CI -0.75 to -0.34; p < 0.00001; 16 studies), the combined symptom and medication scores (CSMS) (SMD -0.48; 95 % CI -0.67 to -0.29; p < 0.00001; 8 studies) as well as an improvement in the quality-of-life scores (SMD -0.53; 95 % CI -0.66 to -0.39; p < 0.00001; 8 studies).
An evidence-based review of SCIT efficacy based on results from 7 studies on house dust mite-allergic patients was also published in 2013, wherein strong heterogeneity in data on the major allergen doses used, the evaluation parameters selected and the actual study results was seen [45].
A comparison of meta-analyses of DBPC SCIT trials published to date with meta-analyses of pharmacotherapy only in seasonal allergic rhinitis showed that, even in the first year of treatment, SCIT resulted in a reduction in allergic symptoms that was at least equivalent to (purely symptomatic) drug treatment [49].
Despite new, methodologically sound DBPC trials (for example [50]), there is less data to support evidence of the clinical efficacy of SCIT in children.
4.2.2. Efficacy of SCIT in allergic bronchial asthma
In contrast to the use of SCIT in allergic rhinoconjunctivitis, the decision to use SCIT in allergic bronchial asthma is generally made with greater caution [51, 52, 53, 54, 55, 56, 57]. SCIT is not a substitute for adequate anti-asthmatic treatment. On the basis of numerous studies, SCIT can be recommended in intermittent (severity according to the National disease management guideline (NVL) for asthma /Global Initiative for Asthma (GINA) I) and mild persistent bronchial asthma (severity according to NVL/GINA II) [51, 52, 54, 58]. These recommendations are based on data from a meta-analysis in the Cochrane Library [59], which evaluated 88 randomized controlled – yet methodologically heterogeneous – SCIT studies including altogether 3,459 patients with allergic asthma to house dust mite allergens (42), pollen allergens (27), animal dander allergens (10) and other allergens. An analysis of all the articles evaluated showed a significant reduction in both symptom score and medication use. Furthermore, a slight yet significant reduction in non-specific bronchial hyperreactivity was seen. The marked reduction in allergen-specific bronchial hyperreactivity to house dust mite allergens as well as pollen allergens and animal dander allergens in patients treated with SCIT compared with control groups can be considered as evidence of a lower risk of asthma exacerbation on renewed exposure to the relevant allergen. However, the 20 studies that included the measurement of lung function parameters showed only a trend towards improved lung function, without statistical significance [59]. As, there is generally no significant reduction in lung function parameters in patients with intermittent or mild persistent asthma, this clinical endpoint is not suitable for evaluating the efficacy of AIT.
The incidence of systemic side effects was 19.9 % in the actively treated group versus 8.1 % in patients receiving placebo injections. One in nine actively treated patients developed systemic reactions of varying severity to the allergen injections. Unfortunately, this particular Cochrane review did not conduct a separate analysis for children.
The relatively small group of patients with insufficiently controlled asthma represents a high-risk group for systemic side effects, which explains why here particular caution is required when assessing the indication for AIT and its practical implementation [60].
A recent evidence-based analysis of 19 studies (three of which were in children) on the efficacy of SCIT in patients with (house dust mite) allergic asthma found a statistically significant benefit in SCIT compared with placebo in terms of symptom score or symptom-related scores in only 9 studies [45]. Moreover, there was significant heterogeneity in terms of (major allergen) doses used as well as the evaluation parameters and time periods selected [45].
One study conducted solely in children with allergic asthma in which SCIT was employed using an allergoid extract of house dust mite showed improved asthma control as well as significant reduction in the required doses of inhaled corticosteroids compared with the control group not treated with SCIT [61].
4.2.3. Efficacy of SCIT relative to allergen source
4.2.3.1. Grass pollen
Numerous clinical studies in the literature highlight the efficacy of AIT in grass pollen allergic adult patients (amongst others [62, 63, 64, 65, 66]). Not all approved grass pollen extracts available on the market have been tested according to the WAO and EMA efficacy criteria, and specific pediatric studies are lacking for most preparations (see DGAKI table “Trials showing evidence of treatment efficacy: grass pollen” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit). A DBPC trial in 35 children and adolescents with seasonal grass pollen-induced asthma aged 3 to 16 years showed that SCIT using a non-modified (native) allergen extract can significantly reduce asthma symptom–medication scores [67].
4.2.3.2. Tree pollen
A number of efficacy studies on birch pollen allergies have shown a reduction in symptoms and/or medication use (e. g., [68, 69, 70, 71, 72, 73, 74]). The efficacy and safety of most early-flowering (fagales) tree extracts available on the market have not been proven in DBPC trials, and relevant specific pediatric studies are lacking (see the DGAKI table “Trials showing evidence of treatment efficacy: tree pollen” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit).
4.2.3.3. House dust mites
The evaluation of the efficacy of SCIT in house dust mite-induced rhinoconjunctivitis is based on a number of studies (e. g., [75, 76, 77, 78, 79]), but it is true also in this indication that many of the commercially available dust mite extracts have not been investigated for efficacy or safety in DBPC trials, and only scant specific pediatric studies (e. g., [80]) are available (see the DGAKI table “Trials showing evidence of treatment efficacy: house dust mites” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit).
Studies on SCIT using mite extracts in patients with perennial allergic asthma and house dust mite allergy found less symptoms [75, 81, 82, 83], lower medication use [75, 81, 83, 84], a reduction in allergen-specific bronchial hyperreactivity [75, 83] and improved quality of life [75, 83] compared with placebo. These findings were also confirmed in children; SCIT with an allergoid extract of dust mite resulted in improved asthma control with a significant reduction in the dose of inhaled corticosteroids required compared with the non-SCIT control group (see Sect. 4.2.2 [61]).
4.2.3.4. Animal allergens
To date, a small number of studies have provided evidence of efficacy primarily for cat allergen extracts (with only few for dog allergen extracts) [85, 86, 87, 88, 89]. Only isolated reports are available on AIT with allergens from other furry animal species.
4.2.3.5. Other allergen sources
Evidence of clinical efficacy in mold allergy is limited to a small number of studies using Alternaria alternata and Cladosporium herbarum extracts [90, 91, 92]. A 3-year DBPC trial in children with Alternaria alternata allergy showed SCIT to be effective from the second year of treatment onwards [50].
4.2.4. Efficacy of AIT in other indications
Data on the efficacy of AIT with pollen allergens to treat oral allergy syndrome (OAS) are as yet insufficient [93], meaning that further studies are needed before a conclusion is possible. A randomized controlled trial (on 40 tree pollen allergic patients, 20 of whom were treated with SCIT and 20 treated with SLIT) demonstrated an improvement of the OAS in some of the patients [94]. At present, AIT is not indicated in exclusively pollen allergen-associated OAS without airway symptoms.
Recent studies show AIT to have clinical effects in patients with extrinsic atopic dermatitis (AD) as well as corresponding and likely clinically relevant type-I sensitization (e. g., eczema triggered by airborne allergens; reviews in [95, 96]). One randomized double-blind dose-range-finding SCIT trial on 89 adult patients with a chronic form of AD and sensitization to house dust mites revealed a significant improvement of the SCORAD (Scoring Atopic Dermatitis) over a one-year therapy-course [97]. In a more recently published DBPC-Phase-III study (SCIT) on 168 adult patients a significant improvement in the SCORAD was only demonstrated in a subgroup with severe forms of AD [98].
A 2013 meta-analysis on the efficacy of AIT in AD, in which eight randomized and controlled (six SCIT, two SLIT) trials were included, found a positive effect [99]. The authors stress, however, the considerable heterogeneity between the studies conducted, some of which had small patient numbers, thus limiting the validity of this meta-analysis. However, AD is not a contraindication for AIT in patients with allergic airway diseases requiring treatment.
Conclusion: Strong evidence of the efficacy of SCIT in pollen allergy-induced allergic rhinoconjunctivitis in adulthood is well-documented in numerous trials and, in childhood and adolescence, in a few trials.
Efficacy in house dust mite allergy is documented by a number of controlled trials in adults and few controlled trials in children. Only a few controlled trials, independent of age, are available for mold allergy (in particular Alternaria). With regard to animal dander allergies (primarily to cat allergens), only small studies, some with methodological deficiencies are available. Only a moderate and inconsistent therapeutic effect in atopic dermatitis has been observed in the quite heterogeneous studies conducted to date.
SCIT has been well investigated for individual preparations in controlled bronchial asthma (GINA 2007 [56]) and intermittent and mild persistent asthma (GINA 2005 [55]) and it is recommended as a treatment option, in addition to allergen avoidance and pharmacotherapy, provided there is a clear causal link between respiratory symptoms and the relevant allergen.
4.3. Efficacy of SLIT in inhalant allergies
4.3.1. Efficacy of SLIT in allergic rhinoconjunctivitis
Due to new controlled trials in adults [100, 101, 102, 103, 104, 105, 106] and children [107, 108, 109], some with high patient numbers, good data on the efficacy of SLIT is also available. As with SCIT, there are significant differences in the documentation of clinical efficacy depending on the product used. While for certain products no randomized and large controlled trials have been published, extensive data is available for individual preparations and allergens, which have been taken into consideration in a recent Cochrane meta-analysis on SLIT ([110] intended as an update of [111]). The analysis conducted up to August 2009 included for the symptom scores 23 studies in grass pollen allergic patients (SMD -0.35; 95 % CI -0.45 to -0.24; p < 0.00001), 9 studies (including 2 using birch pollen extract) in tree pollen allergic patients (SMD -0.42; 95 % CI -0.77 to -0,06; p = 0.02) and 9 studies in house dust mite allergic patients (SMD -0.97; 95 % CI -1.80 to -0.13; p = 0.02).
A meta-analysis published in 2013 on the efficacy of SLIT in patients with seasonal allergic rhinitis found a reduction in the symptom scores (SMD -0.33; 95 % CI -0.42 to -0.25; p < 0.00001; 42 studies), in the medication scores (SMD -0,27; 95 % CI -0,37 bis -0,17; p < 0.00001; 35 studies), the combined symptom and medication scores (SMD -0,40, 95 % CI -0.55 to -0.25; p < 0.00001; 6 studies) as well as improved quality of life scores (SMD -0.37, 95 %-CI -0.52 to -0.22; p < 0.00001; 7 studies) in SLIT-treated patients compared with placebo [44].
An evidence-based review of SLIT efficacy in patients with (house dust mite-induced) allergic rhinitis found a significant difference in the respective symptom score or symptom-related scores in only two of the eight studies considered, whereby (as with the analysis of SCIT studies in the same publication) the authors described significant heterogeneity in terms of the (major allergen) doses used as well as the evaluation parameters and time periods selected [45].
Although head-to-head comparisons of studies between SLIT and SCIT in adults show both treatment methods to be clinically effective, these studies are methodologically inadequate ([112], reviewed in [41, 44]). Due to scant data and/or methodological deficiencies, it is not possible to draw conclusions either from meta-analyses on the differences between SLIT and SCIT in terms of efficacy [41, 44].
In a recent comparison of DBPC trials in seasonal allergic rhinitis on SLIT grass tablets and pharmacotherapy-only studies published to date, a reduction in allergic symptoms by SLIT at least equivalent to purely symptomatic drug treatment was found [113].
4.3.2. Efficacy of SLIT in allergic bronchial asthma
Compared with allergic rhinoconjunctivitis, there are only a limited number of studies on the efficacy of SLIT in patients with allergic bronchial asthma. A grass tablet study showed efficacy for SLIT in bronchial asthma in a subgroup of children with seasonal allergic asthma [107]. With regard to immunotherapy using dust mite extracts, heterogeneous results were found in clinical trials with methodological limitations [114, 115, 116, 117].
A recent study included 604 house dust mite-allergic patients at least 14 years of age with mild to moderate asthma treated for a 1-year period with SLIT with house dust mite tablets. Compared with placebo, actively treated patients exhibited a significant reduction in the dose of inhaled corticosteroids required to maintain asthma control over the course of the study period [118].
4.3.3. Efficacy of SLIT relative to allergen source
4.3.3.1. Grass pollen
The efficacy of SLIT with grass pollen extracts in allergic rhinoconjunctivitis with or without concomitant asthma has been documented in a number of large studies conducted in Europe [102, 104] and the US [119] (reviewed in [120]) (see the DGAKI table “Trials showing evidence of treatment efficacy: grass pollen” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit). The strongest evidence of clinical efficacy (in terms of the size and methodology of studies) is for the sublingual tablets already approved [43, 102, 104]. In a randomized controlled study in 80 children comparing the clinical efficacy of a co-seasonal versus a perennial (continuous) schedule, SLIT with an aqueous grass pollen extract demonstrated better efficacy of the continuous SLIT during the first year, but the clinical effects of both schedules were comparable in the second and third year of treatment [121].
Studies in grass pollen allergic children at least 5 years of age the course of one season showed comparable efficacy with grass tablet products to the previously conducted adult studies [107, 108, 122]. As a result, both preparations were approved for use in children from the age of 5 years. In addition, a carry-over effect could be shown for both grass tablets in adults: clinical efficacy was confirmed 1 year [123, 124] to 2 years [125] following completion of a 3-year treatment course.
In addition, large DBPC trials showed aqueous grass SLIT preparations to be clinically effective in children as well as in adults [103, 109, 126, 127]. With regard to other grass SLIT preparations, either conflicting study results are available or they have not yet been investigated in DBPC trials.
4.3.3.2. Tree pollen
A handful of efficacy studies also showed a reduction in symptoms and/or medication use in tree pollen allergic patients (e.g.,[105, 112, 128, 129]. An early DBPC trial with a birch pollen extract demonstrated a significant reduction in symptom and medication scores after 1 year of treatment compared with placebo [112]. A recent study in over 570 birch pollen allergic adults found a statistically significant advantage with aqueous tree pollen extract compared with placebo in pre/co-seasonal SLIT over a 2 year period [105] (see the DGAKI table “Trials showing evidence of treatment efficacy: tree pollen” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit).
However, for numerous aqueous tree pollen (birch or birch/alder/hazel mixtures) SLIT preparations, either heterogeneous study results are available or they have not yet been investigated in DBPC trials. Efficacy data for birch tablets in SLIT are not available.
4.3.3.3. House dust mites
Data on the efficacy of SLIT with house dust mite allergens are conflicting. A number of SLIT dust mite products currently available have not as yet been subjected for efficacy in clinical studies.
Most studies have been conducted in patients with mild to moderate asthma (with concomitant dust mite-induced rhinitis). Besides several positive study results (e. g., [130, 131, 117, 114, 132, 133, 134]), negative study results also exist (e.g., [116, 135]) (see the DGAKI table “Trials showing evidence of treatment efficacy: house dust mites” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit). A DBPC trial with a modified dust mite allergen tablet product proved efficacy in mild dust mite-induced rhinitis [134].
A recently published study in 509 adults with house dust mite allergic rhinitis demonstrated a significant improvement in symptom scores following 1-year SLIT with dust mite tablets with a carry-over effect even in the second year of the trial without immunotherapy [106]. Another recently published study on house dust mite tablets in adolescent (aged from 14 years) and adult patients with bronchial asthma also showed clinical efficacy for SLIT (see Sect. 4.3.2. [118]).
4.3.3.4. Efficacy of SLIT with other allergen extracts
While individual studies on other inhalant allergen sources (animal dander, molds, weed pollen) are available, they do not permit a conclusive evaluation of treatment efficacy.
Conclusion: The efficacy of SLIT in grass pollen-induced allergic rhinoconjunctivitis is extensively documented in adults and children, whilst its efficacy in tree pollen allergy has only been shown in adults. New controlled trials (some with high patient numbers) on house dust mite allergy provide evidence of efficacy of SLIT in adults.
Compared with allergic rhinoconjunctivitis, there are only few studies on the efficacy of SLIT in allergic asthma. In this context, newer studies show an efficacy for SLIT on asthma symptoms in the subgroup of grass pollen allergic children, adolescents and adults with asthma and efficacy in primary house dust mite allergy-induced asthma in adolescents aged from 14 years and in adults.
4.4. Prevention of asthma and new sensitizations
For individual products controlled, open studies have shown that, in addition to its primary allergen-specific effect, AIT also has secondary preventive characteristics, thus the potential to have a positive effect on the long-term course of allergic disease. Therefore, young patients with early manifestations of allergic symptoms are an important target group for AIT intervention.
A SCIT preparation containing birch or grass allergens, or a birch–grass mixture in allergic rhinoconjunctivitis was shown to reduce the risk of developing allergic asthma in an open prospective study (“Preventive allergy treatment (PAT) study” [136, 137]). Moreover, this effect was detectable 7 years following discontinuation of SCIT compared with the control group that received symptomatic treatment only [34].
The development of new sensitizations can be reduced in the case of mono- and oligosensitizations [138, 139, 140, 141].
Evidence of these and other secondary preventive effects were described in an open study up to 12 years following discontinuation of SCIT with a modified allergen preparation compared with an untreated control group [139].
A recent SLIT study was able to show a reduction in new sensitizations, whereas this effect was not observed in another (also open) study [142, 143].
The preventive effect of SLIT on lower respiratory tract involvement (asthma onset) has also been demonstrated, but mainly in open studies [142, 144, 145]. A multinational prospective DBPC trial is currently being conducted in over 800 children with grass pollen allergic rhinitis but no evidence of asthma, on whether early intervention using grass pollen tablets can prevent the development of asthma during the 3 years of treatment, as well as during 2 subsequent follow up-years [146]. Preliminary data are expected in 2016.
Conclusion: Aspects of secondary prevention, in particular the reduction of new sensitizations and reduced asthma risk, are important rationales for choosing to initiate treatment early in childhood and adolescence. In this context, those products for which the appropriate effects have been demonstrated should be considered.
5. Indications and contraindications
5.1. SCIT and SLIT
A number of variables influences the success of AIT and should therefore be considered when planning therapy (Tab. 5).
Table 5.
Factors that increase the clinical efficacy of AIT a, b
| Short duration of disease |
| Minor involvement of the lower airways |
| Age (the EMA PDCO recommends that therapy not be commenced before the age of 5 years) |
| Good compliance and adherence |
| A high cumulative AIT dose |
a The more of these points that apply, the higher the probability that administration of AIT will reduce symptoms and medication use, as well as decrease the likelihood of allergic march – the development of bronchial asthma and broadening of the allergen spectrum. b only valid for inhalant allergens
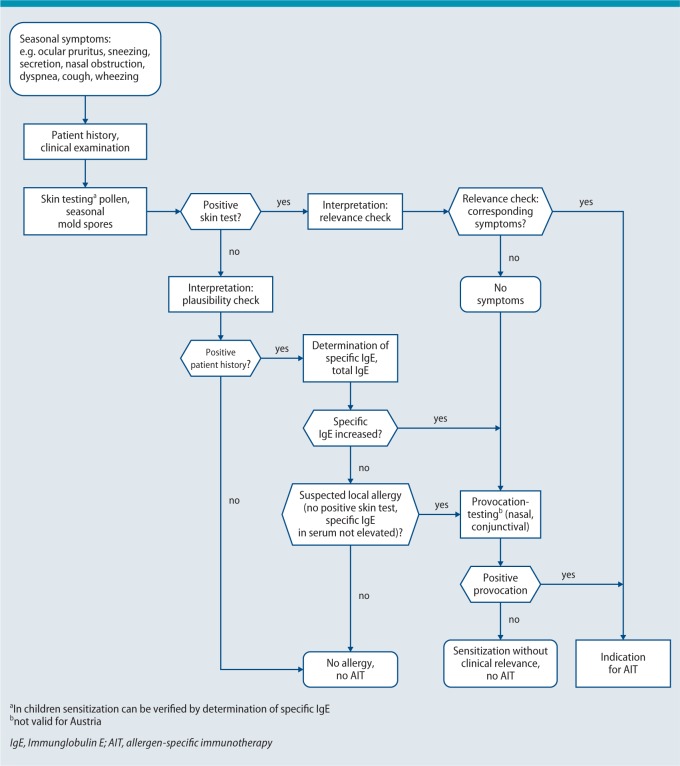
Tab. 6 contains an overview of the indications for specific immunotherapy using inhalant allergens. Fig. 3 outlines the clinical algorithm of the diagnostic work-up for the indication of AIT with seasonal allergens.
Table 6.
Indications for AIT with allergens a
| Verification of an IgE-mediated sensitization (preferablyb from skin testing andc/ord in vitro diagnostics) with a clear relationship to clinical symptoms (if indicated, challenge testing) |
| Availability of standardized or high-quality allergen extracts |
| Proof of efficacy of the planned AIT for the respective indication and age group |
| Allergen avoidance not possible or inadequate |
| Patient age ≥ 5 years |
a All points should be fulfilled. b In Switzerland, verification of sensitization preferably by skin testing.
c “And” refers to rare allergens or uncertain results. d “Or” refers to situations in which skin testing is not possible and to diagnostic work-up in children below 5 years.
Fig. 3.
Diagnostic work-up for AIT with seasonal allergens (clinical algorithm)
© Authors of the guideline
Tab. 7 outlines the possible advantages of a molecular allergy based diagnostic work-up to establish the indication for AIT. In some situations (polysensitized patients), the use of in vitro component-based IgE diagnostics can increase the likelihood of AIT being successful as early on as at the time of making the indication. Patients without sensitization to major allergens may receive less therapeutic benefit from AIT [147]; although detailed prospective studies on this topic are not currently available. Sensitizations solely to pollen-panallergens do not constitute indications for AIT.
Table 7.
Allergen components helpful in establishing the indication for AIT (major allergens a versus panallergens b )
| Major allergensa |
| Bet v 1 ➾ Birch, Betula pendula (formerly Be tula ve rrucosa) |
| Phl p 1/5 ➾ Grasses, Ph leum p ratense (timothy grass) |
| Der p 1/2 ➾ House dust mites, Der matophagoides p teronyssinus |
| Alt a 1 ➾ Alternaria, Alt ernaria a lternata |
| Ole e 1 ➾ Ash – no actual components instead due to high cross-reactivity: olive tree: Ole a e uropaea |
| Art v 1 ➾ Mugwort, Art emisia v ulgaris |
| Amb a 1 ➾ Ragweed, Ambrosia artemisifolia (common ragweed) |
| Components that explain positive skin tests but are not valid in the indications for AIT (panallergensb) |
| Profilins: e.g.: Amb a 8 (ragweed), Ara h 5 (peanut), Bet v 2 (birch), Cor a 2 (hazelnut), Hev b 8 (latex), Phl p 12 (grass), Tri a 12 (wheat) |
| Polcalcins: e.g.: Aln g 4 (alder), Amb a 9 (ragweed), Art v 5 (mugwort), Bet v 4 (birch), Phl p 7 (grass) |
a The name of an allergen component is derived from the first three letters of the genus and the first letter of the species names, e.g., timothy grass Phleum pratense ⇨ Phl p 1. The numbering often follows the chronological order of first description; thus, identical numbers unfortunately do not automaticallysignify cross-reactivity. Cross-reactivity is so high in some allergen-families that it is not necessary to determine the individual components separately: Beech-like (PR10 proteins): Bet v 1 (birch) ⇦⇨ Aln a 1 (alder) ⇦⇨ Cor a 1 (hazelnut); Grasses (grass group 1 allergen): Phl p 1 (timothy) ⇦⇨ Cyn d 1 (Bermuda grass) ⇦⇨ Lol p 1 (ryegrass) ⇦⇨ Tri a 1 (wheat); House dust and flour mites: cysteine proteases, Der p 1 ⇦⇨ f1, NPC2 family: Der p 2 ⇦⇨ f 2. The up-to-date, international WHO/IUIS list of all allergen components is available at: www.allergen.org.
b Definition: a major allergen is an allergen component to which more than 50% of sensitized allergy sufferers exhibit specific IgE (e.g., in grass allergy: major components, Phl p 1, 2, 5, 6; minor component: Phl p 11). Panallergens are found in many species and are generally clinically insignificant, but nevertheless explain irrelevant positive extract-based skin and/or blood tests, e. g., profilins from 48 plant species are currently described, and new ones are being added daily; for an up-to-date list see: www.meduniwien.ac.at/allergens/allfam.
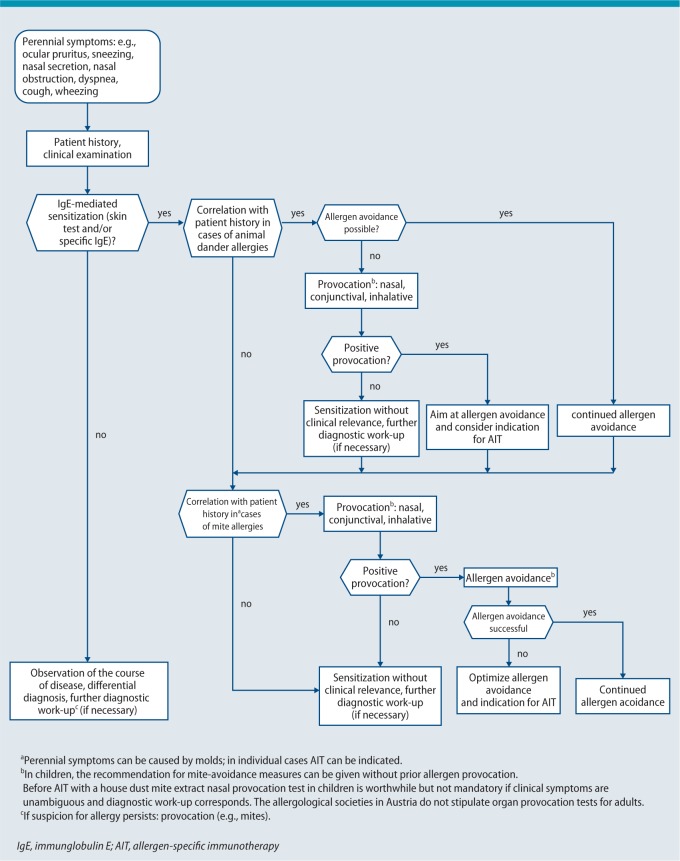
In case of a confirmed house dust mite allergy, AIT is an option if measures for mite avoidance (mite allergen-proof mattress encasings, washable blankets and further measures to reduce house dust mite allergens) are insufficient (Fig. 4) and no improvement in symptoms is observed after 3 months of mite avoidance. A meta-analysis published in 2008 questioned the efficacy of mite control measures [148]. In only 17 of 54 included studies evaluated a significant reduction in the number of house dust mites could be documented. Overall, the intervention measures applied in the investigated studies were very heterogeneous and no subgroup analysis was carried out for children. Due to the methodological deficiencies of this meta-analysis, the conclusion drawn by the authors is questionable. Therefore, in patients with a clinically relevant house dust mite allergy, the aforementioned intervention measures are primarily indicated [149, 150]. The German S3 guideline on allergy prevention also underscores the value of mite control measures for secondary and tertiary allergy prevention [150].
Fig. 4.
Diagnostic work-up to establish the indication for AIT with perennial allergens
© Authors of the guideline
Allergen avoidance is the treatment of choice for animal dander allergies. If allergen avoidance cannot be ensured, SCIT with animal allergen extracts can be considered in individual cases (in particular in the case of a cat allergy; Fig. 4).
In the case of mold allergy, total allergen avoidance is only possible in exceptional cases. SCIT using mold allergens can be considered in the case of seasonal mold allergy with a corresponding indication and a well-characterized therapeutic allergen preparation (Alternaria, Cladosporium) [50, 90, 91].
The efficacy of AIT depends on the optimal therapeutic dose of each clinically relevant allergen. Current knowledge on the clinical efficacy and immunological effects of AIT is based primarily on studies in which monotherapy with a single allergen extract was administered. Therefore, no different (non-homologous) allergen groups should be mixed in an allergen preparation used for therapy, if the use of the particular combination is not supported by data from clinical trials. A current SCIT DBPC study with a chemically modified mixture of tree pollen and grass pollen allergens found significant (albeit moderate) clinical efficacy throughout the entire tree and grass pollen season in the second year of treatment [151].
In general, seasonal and perennial allergens are not combined in one extract. One reason for this is to avoid an unnecessary reduction in the perennial allergen fraction during the pollen season. Similarly, due to enzymatic degradation reactions [152], mite and animal dander allergens, mite and mold allergens and extracts containing pollen and mold allergens should never be combined in one preparation.
Before one opts for SCIT, several contraindications need to be considered (Tab. 8). For safety reasons, partially controlled or uncontrolled bronchial asthma (Tab. 9) (classification according to NVL-Asthma [153] or the GINA guidelines, 2007 [56]) represents a contraindication to AIT in adults. In the German NVL, “partially controlled asthma” is defined more restrictively for the pediatric age group than for adults; therefore AIT may be performed in children in case of partially controlled asthma (NVL definition [153]) – provided they rarely experience asthma symptoms.
Table 8.
Contraindications a, d to AIT with allergens
| Subcutaneous administration (SCIT) | Sublingual administration (SLIT) |
|---|---|
| Partially controlled or uncontrolled bronchial asthma (classification according to the GINA guidelines, 2007 or NVL, see Tab. 9) | Partially controlled or uncontrolled bronchial asthma (classification according to the GINA guidelines, 2007 or NVL, see Tab. 9) |
| Diseases in which administration of epinephrine is contraindicated (except in the case of insect venom allergies) | no contraindication |
| Treatment with β-blockers (local or systemic application)b | preparation-specific differences, see product information leaflet |
| severe autoimmune diseasesc, immune defects, immunodeficiencies, immunosuppression | severe autoimmune diseasesc, immune defects, immunodeficiencies, immunosuppression |
| malignant neoplastic diseases with current disease relevance | malignant neoplastic diseases with current disease relevance |
| serious systemic reactions to AIT in the past | serious systemic reactions to AIT in the past |
| acute, severe inflammatory disorder of the oral cavity | |
| insufficient compliance | insufficient compliance |
a In justified individual cases and on the basis of a risk-benefit analysis, AIT may also be possible even with existing contraindications.
b In Germany, treatment with ACE inhibitors is also currently a contraindication to SCIT with insect venom.
c Diseases not among those severe autoimmune diseases that represent a contraindication to AIT include: Hashimoto thyroiditis, rheumatoid arthritis, ulcerative colitis and Crohn‘s disease, type-1 diabetes mellitus; see also Sect. 5.2
d When evaluating contraindications, the product information leaflet corresponding to the particular product must be consulted.
GINA, global initiative for Asthma ; NVL, Nationale Versorgungsleitlinie, National disease management guideline; AIT, specific immunotherapy
Table 9.
Level of asthma control (translated by the authors, © of the German version ÄZQ, BÄK, KBV and AWMF 2013, NVL [153], modified according to GINA 2007 [56], www.ginasthma.org)
| Criterion | controlled asthma (all criteria fulfilled) | partially controlled asthma (one or two criteria fulfilled within 1 week) | uncontrolled asthma |
|---|---|---|---|
| Daytime symptoms | ≤ 2x per week no |
> 2x per week yes |
three or more criteria of „partially controlled asthma“ fulfilled within 1 week |
| Restrictions in activities of daily living | no | yes | |
| Nighttime symptoms/awakening | No | yes | |
| Use of reliever medication/emergency treatment | ≤ 2x per week no |
> 2x per week yes |
|
| Lung function (PEF or FEV1) | normal | < 80 % of the predicted value (FEV1) or personal best value (PEF) | |
| Exacerbation1 | no | one or more per year | one per week |
Information relates to any one week within the preceding 4 weeks.
green = applies only to adults, red = applies only to children and adolescents, blue = general recommendations
1 Any exacerbation in 1 week signifies by definition “uncontrolled asthma“. Definition of exacerbation: an episode involving increased dyspnea, coughing, wheezing and/or chest tightness, associated with a deterioration in PEF or FEV. 1
FEV 1, forced expiratory volume in 1 second; NVL, nationale Versorgungsleitlinie, National disease management guideline; PEF, peak expiratory flow
In addition to guideline recommendations, practicioners should also be aware of the product information leaflet issued by the product manufacturer. This information has been approved by the PEI and is binding with respect to product-specific contraindications.
Although pregnancy is considered to be a contraindication to initiating AIT, continuation of SCIT in the case of life-threatening allergies to insect venom (bee/wasp venom) is advisable as well in the case of allergies to inhalant allergens, AIT is permissible, if the treatment is well tolerated by the patient (and in case it is in accordance with the product information leaflet) [154, 155]. Only in isolated cases (e. g., life-threatening insect venom allergy), can SCIT be initiated during pregnancy.
Medication with β-blockers (also in topical preparations, such as eye drops) is listed as a contraindication to SCIT in the specialist information. An increased risk of adverse airway reactions (bronchial constriction) and the risk that potentially required emergency treatment with epinephrine might be less effective [156] are discussed. The decision as to whether it is, under the circumstances, necessary to continue therapy with β-blockers has to be made on an individual basis, together with the prescribing physician. Although specific data are lacking, it is logical to assume that treatment with immunosuppressants or (immunomodulatory) biologicals may reduce the efficacy of AIT [156].
Indications and contraindications also need to be considered with the sublingual application of AIT (see Tab. 6 and Tab. 8). Systemic adverse events are observed less frequently with SLIT than with SCIT. Patients with chronic disease of the oral mucosa are not suitable for SLIT. Furthermore, similar contraindications to those for SCIT (Tab. 8) also apply, although the product manufacturer‘s information leaflet must be consulted.
5.2. AIT despite contraindications
In selected cases, immunotherapy can also be initiated despite the existence of relative contraindications. A typical example of burnt-out autoimmune disease that can be well compensated by drug-based treatment is Hashimoto‘s thyroiditis. If controlled by drug-based treatment, this disease need not contraindicate AIT. In the case of other autoimmune diseases, such as multiple sclerosis, myasthenia gravis, lupus erythematosus, rheumatoid arthritis and Crohn‘s disease, for example, initiating AIT may be judged possible on an individual basis considering activity and course of the disease.
An exception among the contraindications listed in Tab. 8 under immunodeficiency is represented by acquired immunodeficiency in stable, well-controlled HIV (human immunodeficiency virus) infection under highly active antiretroviral therapy (HAART), with negative HIV replication and normal CD4 counts. A case report and one small series of three patients have been described in the literature to date [157, 158]. Although SCIT during HAART is safe and does not negatively influence the disease course, there are currently no data to support the clinical efficacy of SCIT in HIV-positive patients. In the case of a clear indication, SCIT may be initiated in individual HIV-positive patients with stable disease who are undergoing HAART.
Because advanced age no longer represents a contraindication to AIT and the incidence of cancer increases with increasing age, there is a growing population of allergic rhinitis/asthma patients with a past history of neoplastic disease. Even relatively recent, but currently stable, malignant disease need not necessarily represent a contraindication. In a case study of four patients with melanoma and an insect venom allergy and one patient with breast cancer and seasonal allergic rhinitis, it was possible to complete AIT. Moreover, in most of these patients, no reactivation of malignant disease was observed even after more than 5 years of cancer follow-up [159].
A Swiss case series of 25 patients (with a clear and strictly defined indication for SCIT due to a history of severe anaphylactic reactions to insect venom) with cardiac disease who were taking β-blockers observed no increase in the instance of severe adverse events during SCIT [160].
Evidence for the triggering of autoimmune diseases by AIT is based on case studies (15 articles reporting 22 cases, of which 12 cases were vasculitis) [161].
In contrast, a registry-based observational study conducted in Denmark showed that SCIT was associated with reduced mortality [hazard ratio (HR) 0.71; 95 % CI 0.62–0.81), as well as with a lower incidence of myocardial infarction (HR 0.70; 95 % CI 0.52–0.93) and autoimmune diseases (HR 0.86; 95 % CI 0.74–0.99) over a 10-year observation period (1997 to 2006) [162].
Although the risk of SCIT causing an autoimmune disease is probably very low, this risk should be considered, particularly in light of the fact that the therapy lasts several years. Where relevant suspicions are raised, AIT should be interrupted until any possible relation has been ruled out.
Conclusion: SCIT or SLIT with pollen or mite allergens can be performed in patients with allergic rhinoconjunctivitis using allergen extracts that have been proven to be effective in at least one double-blind placebo-controlled (DBPC) study. At present, clinical trials are underway for the indication in asthma due to house dust mite allergy, some of the results of which have already been published, whilst others are still awaited (see the DGAKI table “Approved/potentially completed studies” via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit (according to www.clinicaltrialsregister.eu)).
When establishing the indication for AIT, factors that favour clinical efficacy should be taken into consideration (Tab. 5). Differences between SCIT and SLIT are to be considered primarily in terms of contraindications. In individual cases, AIT may be justifiably indicated despite the presence of contraindications.
6. Performing specific immunotherapy
AIT is carried out by physicians with a (sub)speciality in allergy or adequate relevant treatment experience. Furthermore, the treating physician must be capable of dealing with adverse events (including anaphylactic shock and severe asthma attacks) [163, 164]. Since January 1st 1996, product information leaflets for desensitization solutions used in Germany are required to include the following warning: “Desensitization injections may be prescribed and administered only by physicians with specialized allergological training or physicians experienced in allergy.” (PEI, communication dated April 5th 1995). In Austria, solutions for AIT may be prescribed and administered by physicians specialized or experienced in allergy. The continuation of AIT can then be delegated to a general practitioner. In Switzerland, AIT can also be performed by primary care physicians, provided an allergological work-up with a specialist has been undertaken before commencing treatment.
Before initiating AIT, patients must be informed about the following: the practical procedure, type and duration of treatment; expected effects; as well as possible risks of and alternatives to treatment [165]. The process of providing patients with this information must be documented (Patients‘ Rights Act of the German Civil Code [Patientenrechtegesetz des BGB]; §630f BGB: Documentation of Treatment [Dokumentation der Behandlung]; available at www.patienten-rechte-gesetz.de/bgb-sgbv/dokumentation.html).
Printed information (see “Treatment information sheet”, available as a handout at www.dgaki.de/Leitlinien/s2k-Leitlinie-sit; Fig. 5 and Fig 6) on how AIT is carried out and how to deal with possible adverse events should be made available to patients. Adequate documentation of patient counseling is obligatory and written informed consent from the patient (or the patient‘s parent or legal guardian, as appropriate) is advisable.
Fig. 5.

Treatment information sheet for subcutaneous specific immunotherapy (SCIT), available at www.dgaki.de/Leitlinien/s2k-Leitlinie-sit
Fig. 6.

Treatment information sheet for sublingual specific immunotherapy (SLIT), available at www.dgaki.de/Leitlinien/s2k-Leitlinie-sit
In cases where AIT is to be administered or continued by a different physician other than the one who initially established the indication, close cooperation is required in order to ensure that treatment is implemented consistently and at low risk. If necessary, the patient should be referred back to the physician who originally made the indication.
If the treatment shows no signs of success after 1 to a maximum of 2 years, it should be critically reassessed – if possible by the physician who made the indication. Where indicated a change of preparation or a change from preseasonal to perennial treatment can be considered. Discontinuation of treatment is also an option. In general, it is recommended that SCIT and SLIT should only be performed using preparations for which adequate clinical trial data are available (see Sect. 4.).
6.1. SCIT with inhalant allergens
Prior to injection, the patient is interviewed regarding current allergic, or other relevant symptoms: pyrexia or other signs of infection; the tolerability of the last injection; any current or recent illnesses; or new or altered medications or vaccinations. The time interval since the last injection should be checked [165]. Confusion can be avoided by, for example, reading the names of the allergen preparation and of the patient out loud in the patient‘s presence.
For AIT injection – which represents a medical task and should thus be performed by the physician – a 1 ml syringe with fine graduation down to 0.01 ml with an injection needle (size 14–18, short bevel, sufficient length) is used. First, the area of skin where the injection is to be administered is disinfected. The injection is made strictly subcutaneously: following prior or, depending on the injection volume, repeated aspiration, injections are made into a lifted skin fold a hand‘s width above the olecranon on the extensor side of the upper arms. Details of the injection site and dose are documented. The patient must remain under medical observation for at least 30 min after injection [165]. During this period, the patient must report any symptoms that may indicate an allergic reaction to the medical staff. Once the waiting time has elapsed, the injection site should be examined. If a strong local reaction develops, the diameter should be documented, since a dose adjustment according to the product information leaflet of the administered SCIT preparation may be required (see Sect 8.1).
After a 3-year transitional period, the national policy for the prevention of injury from sharp/pointed instruments in hospitals and the health sector came into force in Germany on May 11th 2013, within the context of the Biological Agents Act (Biostoffverordnung) BGR 250/TRBA 250 (rules of the employers‘ liability insurance association (BG)/technical rules for biological materials; overview in [166]). This policy regulates the use of, e. g., hypodermic needles such as those used for subcutaneous allergen-specific immunotherapy (SCIT) according to state regulations covering occupational safety and accident prevention. Since the introduction of these regulations, the use of injection systems less likely to cause injury (including SCIT syringes with retraction systems, needle shields etc.) is obligatory for employees active in the field of allergy. As an employer, the allergist is not only specifically obliged to exercise due care personally, but also has a special obligation to ensure that his employees also exercise due care. It can be assumed that official checks on the implementation of the Biological Agents Act in allergy practices will be heightened in the future.
Shortly before and for the remainder of the day of injection, factors augmenting allergic reactions (e. g., physical exertion, whirlpool or sauna, alcohol) should be avoided. The time interval between a SCIT injection and a planned vaccination should be at least 1 week [165]. Vaccinations should, therefore, be carried out during the SCIT maintenance phase and administered between two SCIT injections performed at a 4-week interval. Emergency vaccinations (e. g., tetanus due to injury) can of course be administered at any time. Thereafter, SCIT is continued either according to the product information leaflet, or 2 weeks after the vaccination using the previously administered dose (overview in [167]).
Treatment is usually carried out on an outpatient basis. In the case of rush desensitization protocols (see below) or high-risk patients (pronounced systemic reactions, relative contraindications), it may be appropriate to initiate SCIT in an inpatient setting.
Allergen extracts for SCIT are mainly applied as depot solutions. During the dose escalation period (frequently doubling of the previous dose; see product information leaflet), treatment intervals are between 3 and 7 days for aqueous solutions and between 1 and 2 weeks for depot solutions. If cluster or rush titration protocols are applied, several injections are administered on each day of treatment (reviewed in [168, 169]). Once the maximum tolerated dose has been reached, the injection intervals can be increased to 4 to 8 weeks, according to the corresponding product information leaflet. In the case of seasonal aeroallergens, treatment escalation to the maximum dose is initiated outside of the allergen season and continued for at least 3 additional years [165]. The general implementation of purely intra-seasonal dose escalation has recently been investigated. This study employed a single preparation for which good tolerability to this type of approach could be demonstrated [170]. Scientific data on the efficacy of such a strategy are currently not available in published form; therefore, no general recommendations for intra-seasonal initiation of SCIT in pollen allergic patients can be made at present. Co-seasonally performed SCIT (continuation during the allergy season) without dose reduction is possible where this is in line with the preparation‘s product information leaflet and there are no allergic symptoms at the time of injection. Precise clinical documentation is necessary.
Due to potential differences in biological activity and where stated in the product information leaflet, a reduction in the intended dose may be necessary at the beginning of a new batch during treatment continuation. However, preparations which no longer require this are now available from various manufacturers.
If the injection interval is exceeded, the dose is reduced according to the product information leaflet. The greater the discrepancy between the intended and actual intervals, the more the dose needs to be reduced [165]. In the case of airway allergies, the duration of SCIT should be at least 3 years. Although no controlled studies on parallel immunotherapy with two different allergen extracts administered during the same sitting exist, safety considerations mandate a time interval of at least 30 min between injections/sublingual administrations for safety reasons. After the final injection, the usual observation period of 30 min must be adhered to.
In patients with allergic bronchial asthma it is recommended that a peak flow protocol be run during treatment and that lung function tests are performed at regular intervals.
6.2. SLIT with inhalant allergens
SLIT is performed on an outpatient basis according to the manufacturer‘s product information leaflet. Recommendations for practical use can be found in [120, 165].
Depending on the preparation and the manufacturer, the initial dose should be administered (and followed-up) under the supervision of a physician experienced in allergy [120, intended as an update of 171]. With some SLIT preparations, and in accordance with their product information leaflets, SLIT can be initiated during the pollen season (intra-seasonal start).
In cases of viral infections of the respiratory tract, it may be possible to continue administration based on a physician‘s recommendation or it may be necessary to interrupt treatment (see product information leaflet). According to the product information leaflet of the particular preparation, administrations can then once again be increased to the maximal dose. No SLIT allergen extracts should be administered in cases of acute inflammation or injury to the oral or pharyngeal mucosa, significant surgical interventions (tooth extraction) in the oral cavity, acute gastroenteritis or uncontrolled asthma (consult corresponding product information leaflet).
Co-seasonally performed SLIT (continuation during the allergy season) without dose reduction is possible where this is in line with the preparation‘s product information leaflet and there are no, or only minor, allergic symptoms at the time of administration. Precise clinical documentation is necessary. Following an unintended break in administrations of several days, the dose should be reduced according to the product information leaflet. The greater the lapsed interval, the more the dose needs to be reduced.
Based on experience with SCIT, the duration of SLIT should also be at least 3 years. If the treatment is continued in another practice, close cooperation with the physician who made the initial indication should be maintained, particularly in the event of questions relating to efficacy and safety.
Conclusion: SCIT injections and the initiation of SLIT are performed by a physician experienced in this type of treatment and who is able to administer emergency treatment in the case of an allergic reaction. Patients must be fully informed about the procedure and risks of possible adverse events, and the details of this process must be documented (see “Treatment information sheet”, Fig.5 and Fig. 6; available as a handout via www.dgaki.de/Leitlinien/s2k-Leitlinie-sit). Treatment should be performed according to the manufacturer‘s product information leaflet. In cases where AIT is to be performed or continued by a different physician to the one who established the indication, close cooperation is required in order to ensure that treatment is implemented consistently and at low risk.
In general, it is recommended that SCIT and SLIT should only be performed using preparations for which adequate proof of efficacy is available from clinical trials.
6.3. Compliance and adherence
The term compliance describes a patient‘s passive observance of the physician‘s instructions, whereby the patient is primarily responsible for the success or failure of therapy [172, 173]. The modern concept of adherence, however, describes the extent of agreement between physician and patient on jointly made treatment decisions and therapeutic goals, as well as the extent to which patients take medications as prescribed by their physician on the basis of these decisions [173, 174].
Since the success of AIT depends on the duration of appropriately performed treatment, it is particularly important that AIT is carried out in accordance with prescriber’s recommendations. Analogous to other types of treatment, the likelihood of treatment success and adherence to therapy is improved by thoroughly informing the patient about the way in which AIT works [165, 175, 176].
As SCIT is administered by a physician, it would initially seem that adherence is easier to monitor for SCIT than for SLIT. However, exactly how much better this renders adherence to SCIT than to SLIT is currently controversial. There is a general lack of suitable independent studies addressing the important issue of compliance/adherence under real-life conditions.
In a review by Senna, data from clinical studies on SCIT and SLIT were pooled together [177]. This review reports adherence rates of approximately 70 % for SCIT and 75 % for SLIT. However, these results have only limited significance, since data from studies from the US and Europe – with differing treatment regimens, indications and patient groups – were pooled together.
In a randomized controlled trial, 271 patients (age 15 to 65 years) with allergic rhinitis with/without accompanying asthma were to receive SLIT over a course of three years [145]. The authors found in 72 % of the patients treated over the whole course of three years an adherence-rate of more than 80 % and in 18 % of the patients an adherence-rate between 60 % and 80 %.
No differences in compliance rates between SCIT and SLIT were reported in a comprehensive review of Incorvaia et al. [30], independently of the form of administration compliance rates in recent trials varied between 75 % and 90 %. However, these data are from clinical trials and are not likely to predict treatment adherence in a real-world setting [120].
Low treatment adherence oviously jeopardizes therapeutic success. This conclusion was confirmed by an analysis of real German statutory healthcare insurance SCIT prescription data conducted by Claes et al. [178]. This study demonstrated persistence rates (consecutive average prescription rates) that dropped off over the years: in only 24 % of patients treated with established SCIT products was SCIT continued into the third year. Similarly negative results from Germany and Italy for SLIT over 3 years have also been published as posters or letters (13,2 % to 22,7 %) [179, 180, 181]. Another analysis of real German statutory healthcare insurance prescription data investigated persistence rates among 1,409 patients treated with market-leading SCIT and SLIT products [182]. This analysis found unsatisfactory persistence rates in the third year of therapy in 34 % to 51 % of patients. An evaluation of German statutory healthcare insurance prescription data from 562 children and adolescents aged between 4 and 18 years demonstrated a persistence rate of 44.1 % in the third year [183].
Following therapy, a study by Sondermann et al. questioned SCIT and SLIT patients on what they perceived as disadvantageous aspects of the treatment in order to explain the unsatisfactory adherence to therapy [173]. The heavy time demands of the treatment were considered a problem by 69.5 % of patients, while 62.5 % reported adverse effects of the therapy as a problem. Furthermore, 60.7 % of patients experienced no relief of symptoms and 53.7 % had not received adequate information about the therapy.
According to these data, reasons for early discontinuation of therapy may be based on inadequate patient information, on the way in which treatment is carried out and on practice management (see Tab. 10). Patient compliance rates should be increased by better organization in the medical office and an attempt to better educate the patient. In order to ensure adequate therapeutic adherence and compliance, recall systems are necessary for both SLIT and SCIT. Improvements in AIT adherence represent one of the most important goals for the future, in order to ensure success of the therapy. Additional motivation and support measures for physicians (e. g., the “Bavarian Selective Contract”) are desirable.
Table 10.
Reasons for non-compliance in AIT (modified from [173])
| Patient information |
| – Patient inadequately informed/motivated |
| – no understanding of primary and secondary preventative effects of AIT (allergic march, new sensitization) |
| Therapeutic procedure |
| – Adverse events |
| – no reduction in symptoms or self-medication use |
| – incorrect patient selection |
| Clinic/Practice Management |
| – heavy time demands on patients (particularly for SCIT) |
| – treatment insufficiently integrated into daily life |
| – no recall system or patient counselling, possibly due to lack of financial resources |
Conclusion: Treatment adherence among AIT patients is lower than assumed by physicians, irrespective of the form of administration. Clearly, adherence is of vital importance for treatment success. Improving AIT adherence is one of the most important future goals, in order to ensure efficacy of the therapy.
7. Subcutaneous immunotherapy with insect venom allergens
A systemic allergic reaction with symptoms of an immediate-type allergy (anaphylaxis) occurs in about 3.5 % of the population following a hymenoptera sting (e. g., bee, wasp) [184].
Reactions can occur with various degrees of severity, which should be considered when making the indication for AIT. The success rate of guideline-oriented AIT performed with the standard maintenance dose lies between 75 % and 85 % for bee-venom allergic patients and between 90 % and 95 % in wasp-venom allergic patients. In non-responders an increased maintenance dose results in a therapeutic success in almost all the cases [184].
For information on indications, contraindications, performing treatment, possibilities for monitoring treatment as well as treatment duration, the reader is referred to the current guideline on “Diagnosis and treatment of bee and wasp venom allergy” (S2-AWMF-LL registry number 061-020 [184]).
8. Safety, risk factors and adverse events
8.1. SCIT
When administered correctly to properly selected patients, in a medical office/hospital with experience in this type of treatment, allergen-specific immunotherapy with SCIT preparations is safe and well tolerated [165, 185, 186].
Local reactions including redness, swelling or itching at the injection site occur very frequently, but can be treated using local measures (e. g., cooling or topical glucocorticoids) or systemic antihistamines.
When increased local reactions (redness and/or swelling >10 cm in diameter) occur at the injection site, the specific information contained in the manufacturer‘s product information leaflet for the corresponding SCIT preparation should be consulted for the dosage of the subsequent injection. However, in a retrospective evaluation of their own patient data, an American group was able to show that increased local reactions do not predict an increased individual risk of a systemic reaction [187].
In the case of Al(OH)3-containing SCIT products, rarely and particularly with incorrect intradermal administration, but also as a result of Al(OH)3-contact allergy, protein contact dermatitis, or a vasculitic inflammatory reaction, granulomas may result from a foreign body reaction [188, 189, 190]. In such cases, is it recommended that treatment continues with an allergen extract that does not contain Al(OH)3. The possible systemic risks from adjuvant aluminum have been the topic of critical discussion for some time. In response to the increasing number of requests, the PEI published a safety evaluation of aluminum in therapeutic allergens on its website in 2014 (www.pei.de/DE/arzneimittelsicherheit-vigilanz/archiv-sicherheitsinformationen/2014/ablage2014/2014-01-21-sicherheitsbewertung-von-aluminium-in-therapieallergenen.html). The statement addresses not only local tolerability, but also sensitization potential, toxicity and German pharmacovigilance data. According to this publication, the overall sensitization potential of aluminum is to be considered low; only isolated cases of sensitization in SCIT patients have been reported [189, 190, 191]. Toxic effects depend on the quantity of aluminum absorbed [191]. The contribution of SCIT to the lifelong accumulation of aluminum in the human body is low compared with other sources. The specific evaluation of all reports of treatment-related AEs between 1986 and 2013 also raised no alarms regarding the safety of these preparations.
The PEI concludes that the currently available scientific data do not suggest that children or adults are put at risk by undergoing SCIT with aluminum adjuvanted allergens and that, on the basis of current knowledge, there is no reason to reconsider the use of licensed therapy allergens containing aluminum adjuvants.
Systemic allergic reactions to SCIT can take the form of mild to severe reactions of the skin, gastrointestinal tract, airways or cardiovascular system. In a retrospective analysis of a large patient population (2,206 patients) and a large total number of injections (192,505 injections) over a 10-year observation period, a total of 115 systemic reactions (5.2 % of patients or 0.06 % of all injections) were observed, almost all of which occurred within the 30 min post-injection observation phase (no fatalities) ([192], reviewed in [186]). A more recent analysis conducted between 2008 and 2011 by the American Academy of Allergy, Asthma and Immunology (AAAAI), based on an average of 6.3 million injection visits per year, found systemic reactions in approximately 0.1 % of all injection visits. Once again, no fatal reactions were observed [193, 194]. Fatal reactions with clear causal relation to SCIT were estimated in a survey of American allergists for the period of 1990 to 2001 to have a frequency of 1 in 2.5 million injections [195].
According to PEI data (1991 to 2000), the incidence of severe reactions is calculated to be 0.002 % to 0.0076 % (in terms of injections) for non-modified (“native”) allergen extracts and 0.0005 % to 0.01 % for chemically modified allergen extracts (allergoids) [196]. If risk factors are considered, severe reactions are sometimes predictable and can usually be avoided with appropriate care and prophylactic measures [165, 186, 197].
Tab. 11 provides an overview of possible risk factors that may be related to the occurrence of systemic reactions during AIT.
Table 11.
Risk factors for systemic reactions during AIT (modified from [165, 186, 197, 202])
| Current allergy symptoms and potential allergen exposure |
| Current infections |
| Mast cell disease |
| Hyperthyroidism |
| Unstable or insufficiently treated asthma |
| A high degree of sensitization |
| Inadequate dose escalation during initiation |
| Pharmaceutical use (β-blockers) |
| Inadequate circulatory stress, excessive alcohol consumption, high-intensity physical exercise, sauna (shortly before and for the rest of the day of injection, augmenting factors should be avoided) |
| Poor technique of injection |
| Allergen extract overdose |
| Manufacturer’s recommendation for dose reduction upon changing to a new production batch was overlooked |
In 2010, the WAO has published a new standardized five-grade classification of systemic adverse events in SCIT (Tab. 12 [198]).
Table 12.
Grading system of systemic adverse events for subcutaneous immunotherapy (SCIT) according to the World Allergy Organization (WAO) 2010 [198] and for sublingual immunotherapy (SLIT) according to WAO 2013 [205]
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
|
Symptom(s)/sign(s) of one organ system present
Cutaneous Generalized pruritus, urticaria, flushing, or sensation of heat or warmtha or Angioedema (not laryngeal, tongue or uvular) or Upper respiratory Rhinitis (e. g., sneezing, rhinorrhea, nasal pruritus and/or nasal congestion) or Throat-clearing (itchy throat) or or Cough perceived to originate in the upper airway, not the lung, larynx, or trachea or Conjunctival Erythema, pruritus or tearing or other Nausea, metallic taste, or headache |
Symptom(s)/sign(s) of more than one organ system present
or Lower respiratory Asthma: cough, wheezing, shortness of breath (e. g., less than 40 % PEF or FEV1 drop, responding to an inhaled bronchodilator) or Gastrointestinal Abdominal cramps, vomiting, or diarrhea or other Uterine cramps |
Lower respiratory
Asthma (e. g., 40 % PEF or FEV1 drop not responding to an inhaled bronchodilator) or Upper respiratory Laryngeal, uvula, or tongue edema with or without stridor |
Lower or upper respiratory
Respiratory failure with or without loss of consciousness or Cardiovascular Hypotension with or without loss of consciousness |
Death |
Patients may also have a feeling of impending doom, especially in grades 2, 3, or 4.
Note: Children with anaphylaxis seldom convey a sense of impending doom and their behavior changes may be a sign of anaphylaxis; eg, becoming very quiet or irritable and cranky. Scoring (grade 1-4) includes a suffix (a–d or z) that denotes if and when epinephrine is or is not administered in relationship to onset of symptom(s)/sign(s) of the SR:a, ≤ 5 minutes; b, >5 minutes-to ≤ 10 minutes; c: > 10 to 20 minutes; d: > 20 minutes; z, epinephrine not administered.
(further details in [198])
The final grade of the reaction will not be determined until the event is over, regardless of the medication administered. The final report should include the first symptom(s)/sign(s) and the time of onset after the subcutaneous allergen immunotherapy injectionb and a suffix reflecting if and when epinephrine was or was not administered, e. g., Grade 2a; rhinitis: 10 minutes
Final Report: Grade a–d, or z_________, First symptom(s)/sign(s)_____________ Time of onset of first symptom_______ Comments (on reaction and treatment):
a This constellation of symptoms may rapidly progress to a more severe reaction.
b Symptoms occurring within the first minutes after the injection may be a sign of severe anaphylaxis. Mild symptoms may progress rapidly to severe anaphylaxis and death.
In the case of recurrent severe reactions or insufficient compliance (e.g., the patient does not stay in the medical office long enough, intervals between injections are too long, inappropriate physical exertion or avoidable contact with allergens around the time of injection), the decision on whether to continue or discontinue therapy should be made by an allergist. When making this decision, the allergist should weigh the risks associated with continuing treatment against the urgency of the indication and possible treatment alternatives (see also Sect. 6.).
In order to aid decision-making, the patient should be referred back to the physician who established the original indication for SCIT where required. The risk factors described above should be investigated and avoided in relation to SCIT. In cases where treatment is continued, it is recommended that the dose be reduced in accordance with the individual preparation‘s product information leaflet.
Where adverse effects occur, it is possible to premedicate with an antihistamine in order to reduce the frequency and severity of possible systemic reactions; however, premedication does not eliminate the possibility of systemic reactions [156, 168, 169, 199, 200].
The management of severe adverse events is described in detail in Sect. 9. (“Emergency treatment”).
Conclusion: Severe, potentially life-threatening systemic reactions during SCIT are possible, but – providing all safety measures are adhered to – these events are very rare. Most adverse events are mild to moderate and can be treated well.
8.2. SLIT
When administered correctly to patients selected based on the given indications, allergen-specific immunotherapy with SLIT preparations is safe and well tolerated [110, 120].
Adverse events during SLIT are dose-dependent and, depending on the preparation, manifest in 40 % to 75 % of the cases as temporary local mucosal reactions (pruritus or dysesthesia in the oral cavity, swelling of the oral mucusa, throat irritation) [120, 201, 202, 203]. These reactions usually occur during the initiation of SLIT, are mostly mild and generally subside 1 to 3 weeks after the start of treatment [120]. However, particularly in the early treatment phase, these reactions can lead to self-discontinuation of therapy. It is thus particularly important that the patient is thoroughly informed at the start of treat-ment (see also “treatment information sheet for SLIT”, Fig. 6; available as a handout at www.dgaki.de/Leitlinien/s2k-Leitlinie-sit). Gastrointestinal symptoms during SLIT are described as occurring at a frequency of 14 % [110].
Premedication with antihistamines may also be suitable for SLIT in order to reduce the extent of local reations. A lack of compliance, newly arising contraindications, persisting unaccaptable local adverse events, severe reactions after administration and a lack of clinical response after two years of SLIT are indications for early discontinuation of therapy [165].
In regards to the safety profile of SLIT, it is important to note that most adverse events occur at home, where there is no possibility of immediate medical intervention in the (very rare) case of a systemic reaction. It is therefore important to in-form patients – and, when applicable, their parents – thoroughly on how to react if adverse events occur or if administration of the SLIT preparation is forgotten, as well as about situations in which SLIT should be temporarily interrupted. Examples of the latter include elective maxillofacial surgery, as well as the existence of oropharyngeal infections and lesions (ulcers, gingivitis, periodontitis), gastroenteritis and asthma exacerbations [120].
Although the risk of severe systemic adverse reactions is lower with SLIT compared with SCIT [120, 196], 11 incidences—in some cases with severe anaphylaxis—are described in the literature following sublingual administration of allergens in droplet or tablet form (reviewed in [202]). However, treatment in these cases was not administered according to the standards that apply today (non-standardized extracts, rush protocols, excessive allergen dose, patients in whom SCIT had previously been interrupted due to severe reactions) [202]. An important risk factor for severe systemic adverse events during SLIT – as is also the case for SCIT – is insufficiently controlled asthma [202].
The recommendation of one expert team that patients with severe adverse events during SCIT should switch to SLIT [176] cannot be supported: a history of severe systemic reations after subcutaneous application of allergens also constitutes a risk factor for possible severe systemic reactions during SLIT [204].
The WAO recommends the adoption of the grading system for systemic reactions on SCIT also for SLIT and, moreover, proposes a new, standardized classification of local reactions during SLIT (Tab. 13) [120, 205]. The aim of both classification systems is to provide a worldwide standardized reporting system that should enable the frequency and severity of adverse events of AIT (SLIT and SCIT) to be more precisely defined.
Table 13.
Grading system for local adverse events in sublingual immunotherapy (SLIT) according to the WAO (modified from [205])
| Symptom/sign | Grade 1: mild | Grade 2: moderate | Grade 3: severe | unknown severity |
|---|---|---|---|---|
| Pruritus/swelling of mouth, tongue, or lip; throat irritation*, nausea, abdominal pain, vomiting, diarrhea, heartburn, or uvular edema | not troublesome and no symptomatic treatment required and no discontinuation of SLIT because of local side effects |
troublesome oder requires symptomatic treatment and no discontinuation of SLIT because of local side effects |
Grade 2 and SLIT discontinued because of local side effects |
Treatment is discontinued, but there is no subjective, objective, or both description of severity from the patient/physician. |
Each local adverse events can be early (< 30 minutes) or delayed, *for example, itchy palate, burning or swelling of the throat (added by guideline authors)
Conclusion: Dose-dependent adverse local reactions occur frequently in the mouth and throat in SLIT. Systemic reactions have been described in SLIT, but are seen far less often than with SCIT. In terms of anaphylaxis and other severe systemic reactions, SLIT has a better safety profile than SCIT.
8.3. Reporting adverse events from AIT in Germany, Austria and Switzerland
In Germany, in accordance with §63c (2) of the German AMG (www.gesetze-im-internet.de/amg_1976/__63c.html), the marketing authorization holder of a particular drug is legally obliged to report within 15 days every suspected serious adverse event (SAE) of which he gains knowledge to the competent higher federal authority and dditionally, in the case of a SAE occurring in third countries, to the European EudraVigilance database. Moreover, it is planned that suspected non-serious adverse events are to be reported to the European database by the marketing authorization holder within 90 days.
Reporting suspected AEs occurring in daily practice is of great importance for collecting as much data as possible pertaining to the safety of a drug, as well as for allowing continued monitoring of its risk–benefit ratio. Physicians, pharmacists and other healthcare professionals, as well as patients, parents, legal guardians and other relatives should report every suspected AE via the national reporting system (in Germany, for allergen preparations located at the PEI; in accordance with AMG §11a Sect. 1). Patients in Germany can report an AE via https://verbraucher-uaw.pei.de/fmi/iwp/cgi?-db=Verbraucher-UAW&-loadframes.
In Austria, the Institute of Pharmacovigilance of the Austrian Medicines and Medical Devices Agency (BASG/AGES Medizinmarktaufsicht) is responsible for operational tasks. According to the Austrian Medicinal Products Act and Pharmacovigilance Regulations, 2006 (AMG §75j; www.basg.gv.at/ueber-uns/gesetzliche-grundlagen/arzneimittel), members of the following professions are legally obliged to report AEs to the Institute of Pharmacovigilance of the Austrian Medicines and Medical Devices Agency: physicians, dentists, veterinarians, midwives, pharmacists and druggists, as well as tradespersons who are authorized for the manufacture or wholesale of drugs in accordance with the Austrian Commercial Code of 1994 and the marketing authorization holders of proprietary medicinal products. As in Germany, marketing authorization holders are legally obliged to report all information on suspected serious AEs arising within the European Economic Area and third countries electronically to the European EudraVigilance database within 15 days after gaining knowlegde. Information on all suspected non-serious AEs occurring within the European Economic Area must be reported electronically by the marketing authorization holder to the EudraVigilance Database (AMG §75j Sect. 3] within 90 days after gaining knowledge. Patients in Austria also have the option to report AEs electronically via www.basg.gv.at/pharmakovigilanz/elektronische-meldung/registrierung/patientangehoeriger.
Since the introduction of the new Medicinal Products Act (HMG) 2002, medical professionals in Switzerland are legally obliged to report particular AEs that are fatal or life-threatening, cause severe or permanent damage and those which are either not mentioned in or are inadequately addressed by drug information (drug compendium) [206]. Reports are made to regional pharmacovigilance centers by means of a special form. These centers take over the data entry and electronically forward the information (anonymously regarding patient and primary reporter) to Swissmedic. Swissmedic administers the central Swiss AE database and forwards information on severe and new AEs to the relevant pharmaceutical companies. Further to this, Swissmedic conveys all reports to the World Health Organization (WHO). Marketing authorization holders, manufacturers and distributors are also legally obliged to report AEs and quality complaints. AEs covered by this legal obligation are serious or previously unknown AEs, accumulation of known or previously unknown AEs, quality complaints and unusual constraints on distribution. Manufacturers are required to send quarterly safety update reports on each of their approved drugs to the national competent authority.
9. Emergency treatment
Systemic reactions following AIT generally occur within the first 30 min following administration. Therefore, in the case of SCIT, it is essential that patients remain under medical observation for at least 30 min following injection and that they report immediately any symptoms that may arouse suspicion of an allergic reaction [165].
Systemic reactions require immediate treatment due to the risk of rapid exacerbation [207, 208].
The personnel involved need to be familiar with the obligatory medications and with the equipment used in an allergic emergency (see Tab. 14) [207]. Initial measures include: suitable positioning of the patient, i.m. epinephrine (150 µg for patients weighing 15 to 30 kg, 300 µg for patients weighing > 30 kg), infusion therapy via a large-lumen intravenous access as well as O2 administration. Early use of i.m. epinephrine in the acute management of anaphylactic reactions helps guarantee rapid efficacy of medication (stabilization of the cardiovascular system) [208]. For practical reasons, having an epinephrine auto-injector available is recommended in order to ensure prompt therapeutic intervention. Regular training in immediate procedures for allergic systemic reactions is to be recommended [207].
Table 14.
Emergency equipment for the treatment of anaphylactic reactions (S2-AWMF-LL registry number 061–025, 2014 [207])
| Stethoscope, blood pressure monitor |
| Tourniquet, syringes, indwelling venous catheters, infusion set |
| Oxygen with face mask/nasal cannula |
| Guedel-tube, bag valve mask, suction unit, intubation set |
| Adrenaline for injection |
| H1-antihistamines for intravenous injection |
| infusion solutions (0.9 % NaCl solutions, balances electrolytes/colloids ) |
| Glucocorticoids for intravenous injection |
| Bronchiodilator (rapidly acting β2 adrenoreceptor agonist for inhalation or intravenous injection) |
| Automated external defibrillator (optional) |
| Pulse oximeter (optional) |
NaCl, Sodium chloride
Early signs of a severe reaction include: a burning sensation and pruritus of the palms and soles; perianal or perigenital pruritus; an urge to defecate and urinate; sneezing attacks and generalized pruritus. In addition, further respiratory and/or cardiovascular symptoms may occur rapidly.
Although treatment recommendations for the emergency management of anaphylaxis are based on only limited data from clinical studies, they are nonetheless consistent on an international, European [209] as well as national level [207] regarding the use of Adrenalin i.m., which is also valid in the acute management of emergencies in the AIT setting.
The described recommendations also apply in the case of anaphylactic reactions occurring in regard to SLIT.
Conclusion: The risk and effects of adverse systemic reactions in the setting of AIT can be effectively reduced by training of personnel, adhering to safety standards and prompt use of emergency measures, including early administration of i.m. epinephrine. Details on the acute management of anaphylactic reactions can be found in the current S2 guideline on anaphylaxis issued by the AWMF (S2-AWMF-LL Registry Number 061-025 [207]).
10. Future perspectives for AIT
By using novel or optimized adjuvants, it is possible to achieve stronger stimulation of the immune system at otherwise unchanged doses or higher doses can be implemented without increased risk [210, 211]. With the aid of recombinant allergens, immunotherapeutics can be produced in precisely defined concentrations and quality in a highly standardized manner [212].
By modifying such allergens, novel preparations that also offer potential in terms to optimize effects/side effects profiles can be produced. The first products of this kind are currently being investigated in phase-II trials [213].
Furthermore, with recombinant allergens, attention can also be turned to new indications for AIT, e. g., food allergies.
By using allergens at other administration sites [epidermal immunotherapy (EPIT) or intralymphatic immunotherapy (ILIT)], it is possible to achieve similarly good immune responses compared with conventional AIT (with patch applications or only a few (3 to 6) injections) [214].
These approaches are also undergoing clinical investigation in phase-II and also in early phase-III trials with altogether promising results to date [215, 216, 217].
Combination therapy, using in particular the humanized anti-IgE antibody omalizumab, makes AIT possible in patients with moderate to severe bronchial asthma or hymenoptera venom allergy to whom AIT was previously inaccessible due to allergic side effects [218, 219].
Conclusion: AIT is undergoing some innovative developments in many areas (e. g., allergen characterization, new administration routes, adjuvants, faster and safer dose escalation protocols), some of which are already being investigated in clinical trials.
11. Requirements on future AIT trials
Despite the fact that AIT has been used for the disease-modifying treatment of type-I allergic diseases (such as allergic rhinitis and allergic asthma) for over a century [220], a number of important questions remain to be answered with large multicenter trials (see Tab. 15, modified according to [193] and [221]).
Table 15.
Requirements on future AIT trials (modified according to the ”PRACTALL“ consensus report (EAACI and AAAAI [193] as well as the current ARIA report [221])
| Development and implementation of clinical studies | – Standardization and validation of clinical endpoints (e.g., CSMS) in AIT studies to ensure future comparability of clinical documentation on differing preparations, including independently for children, adolescents and adults – Clear definition of the period during which data on clinical symptoms are recorded (standardized classification of the severity of measured pollen exposure) – Further validation and standardization of the use of allergen exposure chambers, in order that these can be used in AIT not only in phase-II AIT studies – More detailed investigation of underlying immunological mechanisms of AIT |
| Specific questions | –AIT in polysensitized patients – (Secondary) preventive effects, such as preventing allergic march and new sensitizations with which AIT preparations – Long-term effects of AIT (adults and children) – Recording and analyzing the safety of AIT in patients that receive AIT in the case of particular co-factors or (relative) contraindications – More data from non-interventional observational trials in order to better assess the efficacy of AIT under practical conditions – Direct comparison of different preparations [unmodified (native) versus chemically modified], treatment regimens and modes of administration in a direct head-to-head comparison |
| Patient selection for clinical studies | – Development of methods/biomarkers to select patients ideally suited for AIT on the basis of responder phenotypes – Phenotyping according to indication (allergic rhinitis, bronchial asthma and atopic dermatitis) |
| Measurement of allergen content in various extracts | – Standardization, validation and general acceptance of measuring methods to determine the (major) allergen content |
| Biomarkers | – Identification and validation of biomarkers as predictive factors for the success of AIT |
| Efficacy and safety of AIT at different ages | – More studies complying with up-to-date quality standards on clinical efficacy, immunological effects and safety stratified according to age (children, adults, >65 years) |
| New approaches in AIT | – Investigation and confirmation of the efficacy and safety of AIT by using new adjuvants, synthetically produced peptides, recombinants or modified therapy allergens as well as by means of new modes of allergen administration, such as intralymphatic or epicutaneous immunotherapy |
| Safety and tolerability of AIT | – Clear international definition of contraindications in AIT – Central register to record systemic reactions to AIT in everyday practice |
| Adherence | – Development of further programs to improve patient compliance |
| Socio-economics | – Long-term (>3 years) cost-effectiveness of AIT |
Final
10th October 2014 (ed: Pfaar), following a consensus process and approval by the guideline authors on 18-07-2014 and by board members of the scientific medical societies involved and professional associations by 01-10-2014
Co-ordinator
Prof. Dr. Oliver Pfaar
Abbreviations
- AAAAI
American Academy of Allergy, Asthma & Immunology
- ACE
Angiotensin converting enzyme
- AD
Atopic dermatitis
- ADR
Adverse drug reactions
- AGES
Austrian Agency for Health and Nutrition, Österreichische Agentur für Gesundheit und Ernährungssicherheit GmbH
- AIT
Allergen-specific immunotherapy
- Al(OH)3
Aluminum hydroxide
- AMG
Arzneimittelgesetz, Medicinal Products Act
- APC
Antigen-presenting cells
- BASG
Austrian Medicines and Medical Devices Agency, Bundesamt für Sicherheit im Gesundheitswesen (Österreich)
- Baso
Basophile granulocyte
- BGB
Bundesgesetzbuch, german civil code
- BGR
professional association regulations, Berufsgenossenschaftsregeln
- Ca3(PO4)2
Calcium phosphate
- CI
Confidence interval
- CONSORT
Consolidated standards of reporting trials
- CSMS
Combined symptom and medication score
- DAAB
German Allergy and Asthma Association, Deutscher Allergie- und Asthmabund
- DBPC
Double-blind placebo-controlled
- DC
Dendritic cells
- DELBI
German instrument for methodological guideline appraisal, Deutsches Leitlinien-Bewertungsinstrument
- EAACI
European Academy of Allergy and Clinical Immunology
- EMA
European Medicines Agency
- Eos
Eosinophile granulocyte
- EPIT
Epidermal immunotherapy
- FAS
Full analysis set
- FEV1
Forced expiratory volume in 1 second
- GCP
Good clinical practice
- GINA
Global Initiative for Asthma
- GKV
Statutory health insurance, Gesetzliche Krankenversicherung
- GMP
Good manufacturing practice
- HAART
Highly active antiretroviral therapy
- HIV
Human immunodeficiency virus
- HMG
Medicinal Products Act (Switzerland), Heilmittelgesetz (Schweiz)
- HR
Hazard ratio
- HRQL
Health-related quality of life
- ICER
Incremental cost-effectiveness ratio
- IFN
Interferon
- IgA
Immunglobulin A
- IgE
Immunglobulin E
- IgG
Immunglobulin G
- IL
Interleukin
- ILIT
Intralymphatic immunotherapy
- ITT
Intention to treat
- MPL
Monophosphoryl lipid A
- NaCl
Sodium chloride
- NPP
Named patient product
- NVL
National disease management guideline, Nationale Versorgungsleitlinie
- O2
Oxygen
- OAS
Oral allergy syndrome
- PAT
Preventive allergy treatment
- PDCO
Paediatric Committee
- PEF
Peak expiratory flow
- PP
Per protocol
- QALY
Quality-adjusted life year
- SCIT
Subcutaneous immunotherapy
- SCORAD
Scoring Atopic Dermatitis
- SLIT
Sublingual immunotherapy
- SMD
Standardised mean difference
- TAV
Therapy allergen ordinance, Therapieallergene-Verordnung
- TGF
Transforming growth factor
- Th
T helper cell
- TRBA
Technical regulations for biological agents
- Treg
Regulatory T cell
- TSS
Total symptom score
- VAS
Visual analogue scale
- WAO
World Allergy Organization
- WHO
World Health Organization
Footnotes
Disclosure/ Conflict of Interest
The information on potential conflicts of interest have been reviewed by a steering-group which did not find any conflicts of interest which may affect the authors’ independency in the process of the development of this guideline. The authors’ disclosures are available in a table (together with the working-report on this guideline) on the AWMF-page of the S2k- Guideline on “allergen-specific immunotherapy in IgE-mediated allergic diseases” via www.awmf.org/leitlinien/detail/ll/061-004.html.
Acknowledgement
The authors and contributing medical and professional societies gratefully acknowledge the translation of this guideline by Christine Schäfer, BA (Hons) PGDip (in Technical and Specialised Translation), Heidelberg, Germany. For careful review of the translated guideline and final editorial assistance we gratefully acknowledge native speakers Dr. Steve Love, PhD, Laguna Niguel, CA, USA, and Dr. Sabine Müller, MD, Allergy Research Group, Department of Dermatology, University Medical Center, Freiburg, Germany.
References
- 1.Kleine-Tebbe J, Bufe A, Ebner C [, et al. Specific immunotherapy (hyposensitization) for IgE-mediated allergic diseases. Guideline of the German Society of Allergy and Clinical Immunology (DGAKI), of the Association of German Allergists (ÄDA) and the Society of Pediatric Allergy and Environmental Medicine (GPA), of the Austrian Society of Allergy and Immunology (ÖGAI) and the Swiss Society of Allergy and Immunology. Allergo J. 2009;18:509–37. [Google Scholar]
- 2.Bagnasco M, Altrinetti V, Pesce G, et al. Pharmacokinetics of Der p 2 allergen and derived monomeric allergoid in allergic volunteers. Int Arch Allergy Immunol. 2005;138:197–202. doi: 10.1159/000088719. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Gómez JM, Johansen P, Erdmann I, Senti G, Crameri R, Kündig TM. Intralymphatic injections as a new administration route for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2009;150:59–65. doi: 10.1159/000210381. [DOI] [PubMed] [Google Scholar]
- 4.Allam JP, Würtzen PA, Reinartz M, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–45. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 7.Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174:5024–32. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 8.Reisinger J, Horak F, Pauli G, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–54. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li JT, Lockey RF, Bernstein I, Portnoy JM, Nicklas RA. Allergen immunotherapy: a practice parameter. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 2003;90(1Suppl1):1–40. [PubMed] [Google Scholar]
- 11.European Medicines Agency; Committee for medicinal products for human use (CHMP). Guideline on allergen products: production and quality issues. EMEA/CHMP/BWP/304831/2007. London, 20 November, 2008
- 12.van Ree R, Chapman MD, Ferreira F, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–26. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 13.European Directorate for the Quality of Medicines & HealthCare (EDQM), Council of Europe, eds. Monograph: Allergen Products - Producta Allergenica 07/2014:1063. as implemented of 01.07.2014. Strasbourg: Council of Europe; 2014:p. 3945-47
- 14.Grammer LC, Shaughnessy MA, Patterson R. Modified forms of allergen immunotherapy. J Allergy Clin Immunol. 1985;76:397–401. doi: 10.1016/0091-6749(85)90661-x. [DOI] [PubMed] [Google Scholar]
- 15.Pfaar O, Kleine-Tebbe J, Hörmann K, Klimek L. Allergen-specific immunotherapy: which outcome measures are useful in monitoring clinical trials? Immunol Allergy Clin North Am. 2011;31:289–309. doi: 10.1016/j.iac.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Calderon MA, Eichel A, Makatsori M, Pfaar O. Comparability of subcutaneous and sublingual immunotherapy outcomes in allergic rhinitis clinical trials. Curr Opin Allergy Clin Immunol. 2012;12:249–56. doi: 10.1097/ACI.0b013e32835358b3. [DOI] [PubMed] [Google Scholar]
- 17.Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–67. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 18.Grouin JM, Vicaut E, Jean-Alphonse S, et al. The average adjusted symptom score, a new primary efficacy end-point for specific allergen immunotherapy trials. Clin Exp Allergy. 2011;41:1282–8. doi: 10.1111/j.1365-2222.2011.03700.x. [DOI] [PubMed] [Google Scholar]
- 19.Didier A, Melac M, Montagut A, Lheritier-Barrand M, Tabar A, Worm M. Agreement of efficacy assessments for five-grass pollen sublingual tablet immunotherapy. Allergy. 2009;64:166–71. doi: 10.1111/j.1398-9995.2008.01767.x. [DOI] [PubMed] [Google Scholar]
- 20.Clark J, Schall R. Assessment of combined symptom and medication scores for rhinoconjunctivitis immunotherapy clinical trials. Allergy. 2007;62:1023–8. doi: 10.1111/j.1398-9995.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency; Commitee for medicinal products for human use (CHMP), eds. Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. CHMP/EWP/18504/2006. London, 20. November 2008
- 22.Bousquet J, Schünemann HJ, Bousquet PJ, et al. How to design and evaluate randomized controlled trials in immunotherapy for allergic rhinitis: an ARIA-GA(2)LEN statement. Allergy. 2011;66:765–74. doi: 10.1111/j.1398-9995.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet PJ, Brozek J, Bachert C, et al. The CONSORT statement checklist in allergen-specific immunotherapy: a GA2LEN paper. Allergy. 2009;64:1737–45. doi: 10.1111/j.1398-9995.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- 24.Brehler R, Klimek L, Kopp MV, Christian Virchow J. Specific immunotherapy-indications and mode of action. Dtsch Arztebl Int. 2013;110:148–58. doi: 10.3238/arztebl.2013.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verordnung über die Ausdehnung der Vorschriften über die Zulassung der Arzneimittel auf Therapieallergene, die für einzelne Personen auf Grund einer Rezeptur hergestellt werden, sowie über Verfahrensregelungen der staatlichen Chargenprüfung (Therapieallergene-Verordnung). Bundesgesetzbl 2008;51:2177-8
- 26.Englert S, May S, Kaul S, Vieths S [ The therapy allergens ordinance (“Therapieallergene-Verordnung”) - Background and effects. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2012;55:351–57. doi: 10.1007/s00103-011-1434-7. [DOI] [PubMed] [Google Scholar]
- 27.Kaul S, Jappe U, Vieths S, May S [ Surveillance of allergen extracts for specific immunotherapy: legal regulations and procedures. Allergo J. 2008;17:385–93. [Google Scholar]
- 28.May S, Haustein D [ Named patient products in specific immunotherapy. Necessities and sources of error. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2001;44:719–23. [Google Scholar]
- 29.Incorvaia C, Agostinis F, Amoroso S, et al. Pharmacoeconomics of subcutaneous allergen immunotherapy. Eur Ann Allergy Clin Immunol. 2007;39:17–20. [PubMed] [Google Scholar]
- 30.Incorvaia C, Mauro M, Ridolo E, et al. Patient’s compliance with allergen immunotherapy. Patient Prefer Adherence. 2008;2:247–51. doi: 10.2147/ppa.s3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bocking C, Renz H, Pfefferle PI. Prevalence and socio-economic relevance of allergies in Germany. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2012;55:303–7. doi: 10.1007/s00103-011-1427-6. [DOI] [PubMed] [Google Scholar]
- 32.Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr Med Res Opin. 2006;22:1203–10. doi: 10.1185/030079906X112552. [DOI] [PubMed] [Google Scholar]
- 33.Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372:1049–57. doi: 10.1016/S0140-6736(08)61446-4. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 35.Hagen A, Gorenoi V, Schonermark MP. Specific immunotherapy (SIT) in the treatment of allergic rhinitis. GMS Health Technol Assess. 2010;6:Doc01. doi: 10.3205/hta000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronaldson S, Taylor M, Bech PG, Shenton R, Bufe A. Economic evaluation of SQ-standardized grass allergy immunotherapy tablet (Grazax(®)) in children. Clinicoecon Outcomes Res. 2014;6:187–96. doi: 10.2147/CEOR.S44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brüggenjürgen B, Reinhold T, Brehler R, et al. Cost-effectiveness of specific subcutaneous immunotherapy in patients with allergic rhinitis and allergic asthma. Ann Allergy Asthma Immunol. 2008;101:316–24. doi: 10.1016/S1081-1206(10)60498-X. [DOI] [PubMed] [Google Scholar]
- 38.Westerhout KY, Verheggen BG, Schreder CH, Augustin M. Cost effectiveness analysis of immunotherapy in patients with grass pollen allergic rhinoconjunctivitis in Germany. J Med Econ. 2012;15:906–17. doi: 10.3111/13696998.2012.688904. [DOI] [PubMed] [Google Scholar]
- 39.Schädlich PK, Brecht JG. Economic evaluation of specific immunotherapy versus symptomatic treatment of allergic rhinitis in Germany. Pharmacoeconomics. 2000;17:37–52. doi: 10.2165/00019053-200017010-00003. [DOI] [PubMed] [Google Scholar]
- 40.Bachert C. Comparison of solutions for sublingual immunotherapy. Int Arch Allergy Immunol. 2007;142:89–90. doi: 10.1159/000096033. [DOI] [PubMed] [Google Scholar]
- 41.Calderon MA, Casale TB, Togias A, Bousquet J, Durham SR, Demoly P. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. J Allergy Clin Immunol. 2011;127:30–8. doi: 10.1016/j.jaci.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Compalati E, Penagos M, Tarantini F, Passalacqua G, Canonica GW. Specific immunotherapy for respiratory allergy: state of the art according to current meta-analyses. Ann Allergy Asthma Immunol. 2009;102:22–8. doi: 10.1016/S1081-1206(10)60103-2. [DOI] [PubMed] [Google Scholar]
- 43.Calderon M, Mösges R, Hellmich M, Demoly P. Towards evidence-based medicine in specific grass pollen immunotherapy. Allergy. 2010;65:420–34. doi: 10.1111/j.1398-9995.2009.02292.x. [DOI] [PubMed] [Google Scholar]
- 44.Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–6. doi: 10.1016/j.jaci.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132:1322–36. doi: 10.1016/j.jaci.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Canonica GW, Baena-Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317–24. doi: 10.1111/j.1398-9995.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 47.Carr W, Bernstein J, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012;129:1282–9. doi: 10.1016/j.jaci.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 48.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1:CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matricardi PM, Kuna P, Panetta V, Wahn U, Narkus A. Subcutaneous immunotherapy and pharmacotherapy in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791–9. doi: 10.1016/j.jaci.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 50.Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. 2011;127:502–8. doi: 10.1016/j.jaci.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 51.Bergmann KC [ Specific immunotherapy in allergic asthma. Pneumologie. 2003;57:84–90. doi: 10.1055/s-2003-37151. [DOI] [PubMed] [Google Scholar]
- 52.Bousquet J, Lockey RF, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. WHO Position paper. Allergy. 1998;53(44Suppl):1–42. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 53.Buhl R, Berdel D, Criée C [, et al. Guidelines for diagnosis and treatment of asthma patients. Pneumologie. 2006;60:139–83. doi: 10.1055/s-2005-919153. [DOI] [PubMed] [Google Scholar]
- 54.Gillissen A, Bergmann KC, Kleine-Tebbe J [, et al. Specific immunotherapy in allergic asthma. Dtsch Med Wochenschr. 2003;128:204–9. doi: 10.1055/s-2003-36983. [DOI] [PubMed] [Google Scholar]
- 55.Global Initiative for Asthma (GINA), ed. Global strategy for asthma management and prevention (updated 2005). www.ginasthma.org
- 56.Global Initiative for Asthma (GINA), ed. Global strategy for asthma management and prevention (updated 2007). www.ginasthma.org
- 57.Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177–200. doi: 10.1111/j.1365-2222.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 58.Bousquet J. Specific immunotherapy in asthma. Allergy. 1999;54(Suppl56):37–8. doi: 10.1111/j.1398-9995.1999.tb04438.x. [DOI] [PubMed] [Google Scholar]
- 59.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;8:CD001186. doi: 10.1002/14651858.CD001186.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117:169–75. doi: 10.1016/j.jaci.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126:942–9. doi: 10.1016/j.jaci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Frew AJ, Powell RJ, Corrigan CJ, Durham SR. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–25. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005;60:801–7. doi: 10.1111/j.1398-9995.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 64.Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo-controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67:272–9. doi: 10.1111/j.1398-9995.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- 65.Dubuske L, Frew A, Horak F, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239–47. doi: 10.2500/aap.2011.32.3453. [DOI] [PubMed] [Google Scholar]
- 66.Brewczynski P, Kroon A [ Efficacy and safety of immunotherapy with modified grasspollen allergens. Results of a placebo-controlled study. Allergologie. 1999;22:411–20. [Google Scholar]
- 67.Roberts G, Hurley C, Turcanu V, Lack G. Grass pollen immunotherapy as an effective therapy for childhood seasonal allergic asthma. J Allergy Clin Immunol. 2006;117:263–8. doi: 10.1016/j.jaci.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 68.Arvidsson MB, Löwhagen O, Rak S. Effect of 2-year placebo-controlled immunotherapy on airway symptoms and medication in patients with birch pollen allergy. J Allergy Clin Immunol. 2002;109:777–83. doi: 10.1067/mai.2002.123868. [DOI] [PubMed] [Google Scholar]
- 69.Balda BR, Wolf H, Baumgarten C, et al. Tree-pollen allergy is efficiently treated by short-term immunotherapy (STI) with seven preseasonal injections of molecular standardized allergens. Allergy. 1998;53:740–8. doi: 10.1111/j.1398-9995.1998.tb03969.x. [DOI] [PubMed] [Google Scholar]
- 70.BØdtger U, Poulsen LK, Jacobi HH, Malling HJ. The safety and efficacy of subcutaneous birch pollen immunotherapy - a one-year, randomised, double-blind, placebo-controlled study. Allergy. 2002;57:297–305. doi: 10.1034/j.1398-9995.2002.1o3532.x. [DOI] [PubMed] [Google Scholar]
- 71.van Neerven RJ, Arvidsson M, Ipsen H, Sparholt SH, Rak S, Würtzen PA. A double-blind, placebo-controlled birch allergy vaccination study: inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation. Clin Exp Allergy. 2004;34:420–8. doi: 10.1111/j.1365-2222.2004.01899.x. [DOI] [PubMed] [Google Scholar]
- 72.Hoiby AS, Strand V, Robinson DS, Sager A, Rak S. Efficacy, safety, and immunological effects of a 2-year immunotherapy with Depigoid birch pollen extract: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy. 2010;40:1062–70. doi: 10.1111/j.1365-2222.2010.03521.x. [DOI] [PubMed] [Google Scholar]
- 73.Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614–21. doi: 10.1111/j.1398-9995.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- 74.Drachenberg KJ, Heinzkill M, Urban E [ Short-term immunotherapy with tree pollen allergoids and the adjuvant monophosphoryl lipid-A - results from a multicentre, placebo-controlled, randomised, double-blind study. Allergologie. 2002;25:466–74. [Google Scholar]
- 75.Ameal A, Vega-Chicote JM, Fernández S, et al. Double-blind and placebo-controlled study to assess efficacy and safety of a modified allergen extract of Dermatophagoides pteronyssinus in allergic asthma. Allergy. 2005;60:1178–83. doi: 10.1111/j.1398-9995.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 76.Pichler CE, Marquardsen A, Sparholt S, et al. Specific immunotherapy with Dermatophagoides pteronyssinus and D. farinae results in decreased bronchial hyperreactivity. Allergy. 1997;52:274–83. doi: 10.1111/j.1398-9995.1997.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 77.Riechelmann H, Schmutzhard J, van der Werf JF, Distler A, Kleinjans HA. Efficacy and safety of a glutaraldehyde-modified house dust mite extract in allergic rhinitis. Am J Rhinol Allergy. 2010;24:e104–9. doi: 10.2500/ajra.2010.24.3508. [DOI] [PubMed] [Google Scholar]
- 78.Varney VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003;33:1076–82. doi: 10.1046/j.1365-2222.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 79.Blainey AD, Phillips MJ, Ollier S, Davies RJ. Hyposensitization with a tyrosine adsorbed extract of Dermatophagoides pteronyssinus in adults with perennial rhinitis. A controlled clinical trial. Allergy. 1984;39:521–8. doi: 10.1111/j.1398-9995.1984.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 80.Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol. 2012;157:288–98. doi: 10.1159/000327566. [DOI] [PubMed] [Google Scholar]
- 81.Olsen OT, Larsen KR, Jacobsan L, Svendsen UG. A 1-year, placebo-controlled, double-blind house-dust-mite immunotherapy study in asthmatic adults. Allergy. 1997;52:853–9. doi: 10.1111/j.1398-9995.1997.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Lin X, Hao C, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. 2006;61:191–7. doi: 10.1111/j.1398-9995.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Robaina JC, Sanchez I, de la Torre F, Fernandez-Caldas E, Casanovas M. Successful management of mite-allergic asthma with modified extracts of Dermatophagoides pteronyssinus and Dermatophagoides farinae in a double-blind, placebo-controlled study. J Allergy Clin Immunol. 2006;118:1026–32. doi: 10.1016/j.jaci.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 84.Blumberga G, Groes L, Haugaard L, Dahl R. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy. 2006;61:843–8. doi: 10.1111/j.1398-9995.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- 85.Bucur J, Dreborg S, Einarsson R. Immunotherapy with dog and cat allergen preparations in dog-sensitive and cat-sensitive asthmatics. Ann Allergy. 1989;62:355–61. [PubMed] [Google Scholar]
- 86.Hedlin G, Graff-Lonnevig V, Heilborn H, et al. Immunotherapy with cat- and dog-dander extracts. V. Effects of 3 years of treatment. J Allergy Clin Immunol. 1991;87:955–64. doi: 10.1016/0091-6749(91)90417-m. [DOI] [PubMed] [Google Scholar]
- 87.Hedlin G, Heilborn H, Lilja G, et al. Long-term follow-up of patients treated with a three-year course of cat or dog immunotherapy. J Allergy Clin Immunol. 1995;96:879–85. doi: 10.1016/s0091-6749(95)70223-7. [DOI] [PubMed] [Google Scholar]
- 88.Nanda A, O’Connor M, Anand M, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114:1339–44. doi: 10.1016/j.jaci.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 89.Sundin B, Lilja G, Graff-Lonnevig V, et al. Immunotherapy with partially purified and standardized animal dander extracts. I. Clinical results from a double-blind study on patients with animal dander asthma. J Allergy Clin Immunol. 1986;77:478–87. doi: 10.1016/0091-6749(86)90183-1. [DOI] [PubMed] [Google Scholar]
- 90.Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double-blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation. I. Clinical results. Allergy. 1986;41:131–40. doi: 10.1111/j.1398-9995.1986.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 91.Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85:460–72. doi: 10.1016/0091-6749(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 92.Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy. V. Clinical efficacy and side effects of immunotherapy with Cladosporium herbarum. Allergy. 1986;41:507–19. doi: 10.1111/j.1398-9995.1986.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 93.Mari A, Ballmer-Weber BK, Vieths S. The oral allergy syndrome: improved diagnostic and treatment methods. Curr Opin Allergy Clin Immunol. 2005;5:267–73. doi: 10.1097/01.all.0000168793.27948.b0. [DOI] [PubMed] [Google Scholar]
- 94.Mauro M, Russello M, Incorvaia C, et al. Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156:416–22. doi: 10.1159/000323909. [DOI] [PubMed] [Google Scholar]
- 95.Bussmann C, Böckenhoff A, Henke H, Werfel T, Novak N. Does allergen-specific immunotherapy represent a therapeutic option for patients with atopic dermatitis? J Allergy Clin Immunol. 2006;118:1292–8. doi: 10.1016/j.jaci.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 96.Novak N. Allergen specific immunotherapy for atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007;7:542–46. doi: 10.1097/ACI.0b013e3282f1d66c. [DOI] [PubMed] [Google Scholar]
- 97.Werfel T, Breuer K, Rueff F, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006;61:202–5. doi: 10.1111/j.1398-9995.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 98.Novak N, Bieber T, Hoffmann M, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. 2012;130:925–31. doi: 10.1016/j.jaci.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110–7. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 100.Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardized grass allergen tablets in asthmatics with rhinoconjunctivitis. Allergy. 2006;61:185–90. doi: 10.1111/j.1398-9995.2005.00949.x. [DOI] [PubMed] [Google Scholar]
- 101.Dahl R, Kapp A, Colombo G, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118:434–40. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 102.Didier A, Malling HJ, Worm M, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–45. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 103.Worm M. Efficacy and tolerability of high dose sublingual immunotherapy in patients with rhinoconjunctivitis. Allerg Immunol (Paris) 2006;38:355–60. [PubMed] [Google Scholar]
- 104.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–9. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 105.Worm M, Rak S, de Blay F, et al. Sustained efficacy and safety of a 300IR daily dose of a sublingual solution of birch pollen allergen extract in adults with allergic rhinoconjunctivitis: results of a double-blind, placebo-controlled study. Clin Transl Allergy. 2014;4:7. doi: 10.1186/2045-7022-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bergmann KC, Demoly P, Worm M, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–14. doi: 10.1016/j.jaci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 107.Bufe A, Eberle P, Franke-Beckmann E, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–73. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 108.Wahn U, Tabar A, Kuna P, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–6. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 109.Wahn U, Klimek L, Ploszczuk A, et al. High-dose sublingual immunotherapy with single-dose aqueous grass pollen extract in children is effective and safe: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 2012;130:886–93. doi: 10.1016/j.jaci.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 110.Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;12:CD002893. doi: 10.1002/14651858.CD002893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;2:CD002893. doi: 10.1002/14651858.CD002893. [DOI] [PubMed] [Google Scholar]
- 112.Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59:45–53. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 113.Devillier P, Dreyfus JF, Demoly P, Calderon MA. A meta-analysis of sublingual allergen immunotherapy and pharmacotherapy in pollen-induced seasonal allergic rhinoconjunctivitis. BMC medicine. 2014;12:71. doi: 10.1186/1741-7015-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bousquet J, Scheinmann P, Guinnepain MT, et al. Sublingual-swallow immunotherapy (SLIT) in patients with asthma due to house-dust mites: a double-blind, placebo-controlled study. Allergy. 1999;54:249–60. doi: 10.1034/j.1398-9995.1999.00916.x. [DOI] [PubMed] [Google Scholar]
- 115.Pajno GB, Morabito L, Barberio G, Parmiani S. Clinical and immunologic effects of long-term sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, placebo-controlled study. Allergy. 2000;55:842–9. doi: 10.1034/j.1398-9995.2000.00495.x. [DOI] [PubMed] [Google Scholar]
- 116.Hirsch T, Sahn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol. 1997;8:21–7. doi: 10.1111/j.1399-3038.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 117.Bahceciler NN, Isik U, Barlan IB, Basaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double-blind, placebo-controlled study. Pediatr Pulmonol. 2001;32:49–55. doi: 10.1002/ppul.1088. [DOI] [PubMed] [Google Scholar]
- 118.Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568–75. doi: 10.1016/j.jaci.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 119.Nelson HS, Nolte H, Creticos P, Maloney J, Wu J, Bernstein DI. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80. doi: 10.1016/j.jaci.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 120.Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. The World Allergy Organization journal. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pajno GB, Caminiti L, Crisafulli G, et al. Direct comparison between continuous and coseasonal regimen for sublingual immunotherapy in children with grass allergy: a randomized controlled study. Pediatr Allergy Immunol. 2011;22:803–7. doi: 10.1111/j.1399-3038.2011.01196.x. [DOI] [PubMed] [Google Scholar]
- 122.Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71. doi: 10.1016/j.jaci.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 123.Didier A, Malling HJ, Worm M, et al. Post-treatment efficacy of discontinuous treatment with 300IR 5-grass pollen sublingual tablet in adults with grass pollen-induced allergic rhinoconjunctivitis. Clin Exp Allergy. 2013;43:568–77. doi: 10.1111/cea.12100. [DOI] [PubMed] [Google Scholar]
- 124.Durham SR, Emminger W, Kapp A, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol. 2010;125:131–8. doi: 10.1016/j.jaci.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 125.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–25. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 126.Ott H, Sieber J, Brehler R, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:179–86. doi: 10.1111/j.1398-9995.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 127.Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2008;100:256–63. doi: 10.1016/s1081-1206(10)60451-6. [DOI] [PubMed] [Google Scholar]
- 128.Drachenberg K, Pfeiffer P, Urban E [ Sublingual immunotherapy - results from a multi-centre, randomised, double-blind, placebocontrolled study with a standardised birch and grass/rye pollen extract. Allergologie. 2001;24:525–34. [Google Scholar]
- 129.Horak F, Stübner P, Berger UE, Marks B, Toth J, Jäger S. Immunotherapy with sublingual birch pollen extract. A short-term double-blind placebo study. J Investig Allergol Clin Immunol. 1998;8:165–71. [PubMed] [Google Scholar]
- 130.Tonnel AB, Scherpereel A, Douay B, et al. Allergic rhinitis due to house dust mites: evaluation of the efficacy of specific sublingual immunotherapy. Allergy. 2004;59:491–7. doi: 10.1111/j.1398-9995.2004.00456.x. [DOI] [PubMed] [Google Scholar]
- 131.Passalacqua G, Albano M, Fregonese L, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite-induced rhinocon-junctivitis. Lancet. 1998;351:629–32. doi: 10.1016/S0140-6736(97)07055-4. [DOI] [PubMed] [Google Scholar]
- 132.Lue KH, Lin YH, Sun HL, Lu KH, Hsieh JC, Chou MC. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, randomized, placebo-controlled study. Pediatr Allergy Immunol. 2006;17:408–15. doi: 10.1111/j.1399-3038.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 133.Niu CK, Chen WY, Huang JL, Lue KH, Wang JY. Efficacy of sublingual immunotherapy with high-dose mite extracts in asthma: a multi-center, double-blind, randomized, and placebo-controlled study in Taiwan. Respir Med. 2006;100:1374–83. doi: 10.1016/j.rmed.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 134.Passalacqua G, Pasquali M, Ariano R, et al. Randomized double-blind controlled study with sublingual carbamylated allergoid immunotherapy in mild rhinitis due to mites. Allergy. 2006;61:849–54. doi: 10.1111/j.1398-9995.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 135.Guez S, Vatrinet C, Fadel R, Andre C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000;55:369–75. doi: 10.1034/j.1398-9995.2000.00413.x. [DOI] [PubMed] [Google Scholar]
- 136.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 137.Niggemann B, Jacobsen L, Dreborg S, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61:855–9. doi: 10.1111/j.1398-9995.2006.01068.x. [DOI] [PubMed] [Google Scholar]
- 138.Eng PA, Reinhold M, Gnehm HP. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy. 2002;57:306–12. doi: 10.1034/j.1398-9995.2002.1o3264.x. [DOI] [PubMed] [Google Scholar]
- 139.Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. doi: 10.1111/j.1398-9995.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 140.Purello-D’Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not. A retrospective study. Clin Exp Allergy. 2001;31:1295–302. doi: 10.1046/j.1365-2222.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 141.Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–7. doi: 10.1046/j.1365-2222.2001.01161.x. [DOI] [PubMed] [Google Scholar]
- 142.Di Rienzo V, Marcucci F, Puccinelli P, et al. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003;33:206–10. doi: 10.1046/j.1365-2222.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 143.Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126:969–75. doi: 10.1016/j.jaci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 144.Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851–7. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 145.Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Randomized controlled open study of sublingual immunotherapy for respiratory allergy in real-life: clinical efficacy and more. Allergy. 2004;59:1205–10. doi: 10.1111/j.1398-9995.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 146.Valovirta E, Berstad AK, Blic J d, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ-standardized grass allergy immunotherapy tablet in children with grass pollen-induced allergic rhinoconjunctivitis. Clin Ther. 2011;33:1537–46. doi: 10.1016/j.clinthera.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 147.Schmid-Grendelmeier P [ Recombinant allergens. For routine use or still only science. Hautarzt. 2010;61:946–53. doi: 10.1007/s00105-010-1967-y. [DOI] [PubMed] [Google Scholar]
- 148.Götzsche PC, Johansen HK. House dust mite control measures for asthma: systematic review. Allergy. 2008;63:646–59. doi: 10.1111/j.1398-9995.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- 149.Kopp MV, Niggemann B, Forster J. House dust mite allergy: complete removal of the provoking allergen is a primary therapeutic approach. Allergy. 2009;64:1402–3. doi: 10.1111/j.1398-9995.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- 150.Muche-Borowski C, Kopp M, Reese I [, et al. Evidence-based and consented guideline on allergy prevention - update 2009. Allergo J. 2009;18:332–41. [Google Scholar]
- 151.Pfaar O, Biedermann T, Klimek L, Sager A, Robinson DS. Depigmented-polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy. 2013;68:1306–13. doi: 10.1111/all.12219. [DOI] [PubMed] [Google Scholar]
- 152.Nelson HS. Specific immunotherapy with allergen mixes: what is the evidence? Curr Opin Allergy Clin Immunol. 2009;9:549–53. doi: 10.1097/ACI.0b013e328330ee69. [DOI] [PubMed] [Google Scholar]
- 153.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), eds. Nationale Versorgungs Leitlinie Asthma - Langfassung, 2. Aufl. Version 5, 2009, zuletzt geändert: August 2013. www.leitlinien.de/nvl/asthma/mdb/downloads/nvl/asthma/asthma-2aufl-vers5-lang.pdf
- 154.Zuberbier T, Bachert C, Bousquet PJ, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30. doi: 10.1111/j.1398-9995.2010.02474.x. [DOI] [PubMed] [Google Scholar]
- 155.Demoly P, Piette V, Daures JP. Treatment of allergic rhinitis during pregnancy. Drugs. 2003;63:1813–20. doi: 10.2165/00003495-200363170-00004. [DOI] [PubMed] [Google Scholar]
- 156.Wedi B, Rueff F [ Pharmacoprophylaxis and comedications in allergen-specific immunotherapy. Hautarzt. 2011;62:663–70. doi: 10.1007/s00105-011-2157-2. [DOI] [PubMed] [Google Scholar]
- 157.Fodeman J, Jariwala S, Hudes G, Jerschow E, Rosenstreich D. Subcutaneous allergen immunotherapy in 3 patients with HIV. Ann Allergy Asthma Immunol. 2010;105:320–1. doi: 10.1016/j.anai.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 158.Randhawa IS, Junaid I, Klaustermeyer WB. Allergen immunotherapy in a patient with human immunodeficiency virus: effect on T-cell activation and viral replication. Ann Allergy Asthma Immunol. 2007;98:495–7. doi: 10.1016/S1081-1206(10)60767-3. [DOI] [PubMed] [Google Scholar]
- 159.Wöhrl S, Kinaciyan T, Jalili A, Stingl G, Moritz KB. Malignancy and specific allergen immunotherapy: the results of a case series. Int Arch Allergy Immunol. 2011;156:313–9. doi: 10.1159/000323519. [DOI] [PubMed] [Google Scholar]
- 160.Müller UR, Haeberli G. Use of beta-blockers during immunotherapy for Hymenoptera venom allergy. J Allergy Clin Immunol. 2005;115:606–10. doi: 10.1016/j.jaci.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 161.Linneberg A, Madsen F, Skaaby T. Allergen-specific immunotherapy and risk of autoimmune disease. Curr Opin Allergy Clin Immunol. 2012;12:635–9. doi: 10.1097/ACI.0b013e3283588c8d. [DOI] [PubMed] [Google Scholar]
- 162.Linneberg A, Jacobsen RK, Jespersen L, Abildstrom SZ. Association of subcutaneous allergen-specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol. 2012;129:413–9. doi: 10.1016/j.jaci.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 163.Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ), ed. Stellungnahme der AkdÄ zur allergenspezifischen Immuntherapie. Dtsch Ärztebl 2007;104: A3355-7
- 164.Zylka-Menhorn V. Hyposensibilisierung bald nur noch durch “erfahrene Ärzte”. Dtsch Ärztebl. 1995;92:A–1434. [Google Scholar]
- 165.Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x. [DOI] [PubMed] [Google Scholar]
- 166.Klimek L. Umsetzung der EU-Richtlinie 2010/32/EU fordert verletzungssichere Spritzen: im HNO-Bereich ist hiervon insbesondere auch die subkutane Immun-therapie (SCIT) betroffen. HNO-Mitteilungen. 2013;63:189. [Google Scholar]
- 167.Fischer PJ, Friedrichs F [ Practical parameters of hyposensitization. Monatsschr Kinderheilkd. 2013;161:608–15. [Google Scholar]
- 168.Calabria CW. Accelerated immunotherapy schedules. Curr Allergy Asthma Rep. 2013;13:389–98. doi: 10.1007/s11882-013-0356-x. [DOI] [PubMed] [Google Scholar]
- 169.Calabria CW, Cox L. Accelerated immunotherapy schedules and premedication. Immunol Allergy Clin North Am. 2011;31:251–63. doi: 10.1016/j.iac.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 170.Pfaar O, Wolf H, Klimek L, Schnitker J, Wüstenberg E. Immunologic effect and tolerability of intra-seasonal subcutaneous immunotherapy with an 8-day up-dosing schedule to 10,000 standardized quality-units: a double-blind, randomized, placebo-controlled trial. Clin Ther. 2012;34:2072–81. doi: 10.1016/j.clinthera.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 171.Canonica GW, Bousquet J, Casale T, et al. Sublingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64(Suppl91):1–59. [Google Scholar]
- 172.Haynes R. Einleitung. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance Handbuch. Oldenbourg: München; 1986. pp. 11–8. [Google Scholar]
- 173.Sondermann N, Shah-Hosseini K, Henkel K, Schwalfenberg A, Mösges R [ Factors of success for adherence in hyposensitization. Allergologie. 2011;34:441–6. doi: 10.5414/ALX01430E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 175.Baiardini I, Braido F, Bonini M, Compalati E, Canonica GW. Why do doctors and patients not follow guidelines? Curr Opin Allergy Clin Immunol. 2009;9:228–33. doi: 10.1097/ACI.0b013e32832b4651. [DOI] [PubMed] [Google Scholar]
- 176.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;;63(Suppl86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 177.Senna G, Ridolo E, Calderon M, Lombardi C, Canonica GW, Passalacqua G. Evidence of adherence to allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9:544–8. doi: 10.1097/ACI.0b013e328332b8df. [DOI] [PubMed] [Google Scholar]
- 178.Claes C, Mittendorf T, Graf von der Schulenburg JM. Persistence and frequency of prescriptions of subcutaneous allergen-specific immunotherapy (SCIT) prescribed within the German statutory health insurance. Med Klin (Munich) 2009;104:536–42. doi: 10.1007/s00063-009-1113-8. [DOI] [PubMed] [Google Scholar]
- 179.Aschemann U, Schulte P, Hecker H. Untersuchung zur Compliance eines anwenderfreundlichen Allergoids. Allergo J. 2010;19(S1):63. [Google Scholar]
- 180.Egert-Schmidt A, Martin E, Müller U, Schulte M, Thum-Oltmer S. Patienten-Compliance in der Spezifischen Immuntherapie - Ein Vergleich von SCIT- und SLIT-Präparaten. Allergo J. 2011;20(S1):S40. [Google Scholar]
- 181.Senna G, Lombardi C, Canonica GW, Passalacqua G. How adherent to sublingual immunotherapy prescriptions are patients? The manufacturers’ viewpoint. J Allergy Clin Immunol. 2010;126:668–9. doi: 10.1016/j.jaci.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 182.Sieber J, De Geest S, Shah-Hosseini K, Mösges R. Medication persistence with long-term, specific grass pollen immunotherapy measured by prescription renewal rates. Curr Med Res Opin. 2011;27:855–61. doi: 10.1185/03007995.2011.559538. [DOI] [PubMed] [Google Scholar]
- 183.Eberle P, Schreder H, Shah-Hosseini K, Mösges R [ Medication persistence in children and young people on long-term, grass pollenspecific immunotherapy - measured by prescription renewal rates. Allergologie. 2013;36:9–18. [Google Scholar]
- 184.Przybilla B, Rueff F, Walker A [, et al. Diagnosis and therapy of bee and wasp venom allergy. Allergo J. 2011;20:318–39. [Google Scholar]
- 185.Cox L, Calderon M, Pfaar O. Subcutaneous allergen immunotherapy for allergic disease: examining efficacy, safety and cost-effectiveness of current and novel formulations. Immunotherapy. 2012;4:601–16. doi: 10.2217/imt.12.36. [DOI] [PubMed] [Google Scholar]
- 186.Malling HJ. Minimising the risks of allergen-specific injection immunotherapy. Drug Saf. 2000;23:323–32. doi: 10.2165/00002018-200023040-00005. [DOI] [PubMed] [Google Scholar]
- 187.Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol. 2004;92:225–7. doi: 10.1016/S1081-1206(10)61551-7. [DOI] [PubMed] [Google Scholar]
- 188.Vogelbruch M, Nuss B, Körner M, Kapp A, Kiehl P, Bohm W. Aluminium-induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy. 2000;55:883–7. [PubMed] [Google Scholar]
- 189.Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41–9. doi: 10.1111/j.1600-0536.2008.01474.x. [DOI] [PubMed] [Google Scholar]
- 190.Frost L, Johansen P, Pedersen S, Veien N, Ostergaard PA, Nielsen MH. Persistent subcutaneous nodules in children hyposensitized with aluminium-containing allergen extracts. Allergy. 1985;40:368–72. doi: 10.1111/j.1398-9995.1985.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 191.Lopez S, Pelaez A, Navarro LA, Montesinos E, Morales C, Carda C. Aluminium allergy in patients hyposensitized with aluminium-precipitated antigen extracts. Contact Dermatitis. 1994;31:37–40. doi: 10.1111/j.1600-0536.1994.tb01903.x. [DOI] [PubMed] [Google Scholar]
- 192.Ragusa FV, Passalacqua G, Gambardella R, et al. Nonfatal systemic reactions to subcutaneous immunotherapy: a 10-year experience. J Investig Allergol Clin Immunol. 1997;7:151–4. [PubMed] [Google Scholar]
- 193.Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 194.Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI and ACAAI surveillance study of subcutaneous immunotherapy, year 3: what practices modify the risk of systemic reactions? Ann Allergy Asthma Immunol. 2013;110:274–8. doi: 10.1016/j.anai.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 195.Bernstein DI, Wanner M, Borish L, Liss GM. Immunotherapy Committee AAoAA, and Immunology: Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113:1129–36. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 196.Lüderitz-Püchel U, Keller-Stanislawski B, Haustein D [ Risk reevaluation of diagnostic and therapeutic allergen extracts. An analysis of adverse drug reactions from 1991 to 2000. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2001;44:709–18. [Google Scholar]
- 197.Makatsori M, Calderon MA. Anaphylaxis: still a ghost behind allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2014;14(4):316–22. doi: 10.1097/ACI.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 198.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: The World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125:569–74. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 199.Nielsen L, Johnsen CR, Mosbech H, Poulsen LK, Malling HJ. Antihistamine premedication in specific cluster immunotherapy: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 1996;97:1207–13. doi: 10.1016/s0091-6749(96)70186-0. [DOI] [PubMed] [Google Scholar]
- 200.Reimers A, Hari Y, Müller U. Reduction of side-effects from ultrarush immunotherapy with honeybee venom by pretreatment with fexofenadine: a double-blind, placebo-controlled trial. Allergy. 2000;55:484–8. doi: 10.1034/j.1398-9995.2000.00520.x. [DOI] [PubMed] [Google Scholar]
- 201.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–35. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 202.Calderon MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–11. doi: 10.1111/j.1398-9995.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 203.Passalacqua G, Garelli V, Sclifo F, Canonica GW. Sublingual immunotherapy for allergic rhinitis and conjunctivitis. Immunotherapy. 2013;5:257–64. doi: 10.2217/imt.12.157. [DOI] [PubMed] [Google Scholar]
- 204.de Groot H, Bijl A. Anaphylactic reaction after the first dose of sublingual immunotherapy with grass pollen tablet. Allergy. 2009;64:963–4. doi: 10.1111/j.1398-9995.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 205.Passalacqua G, Baena-Cagnani CE, Bousquet J, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013;132:93–8. doi: 10.1016/j.jaci.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 206.Swissmedic, ed. Neues Heilmittelgesetz: Meldepflicht der Fachleute für unerwünschte Arzneitmittelwirkungen. Schweiz Ärztezeitung 2002;83:819-22
- 207.Ring J, Beyer K, Biedermann T, et al. Guideline for acute therapy and management of anaphylaxis. S2 Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Association of German Allergologists (AeDA), the Society of Pediatric Allergy and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Society for Psychosomatic Medicine (DGPM), the German Working Group of Anaphylaxis Training and Education (AGATE) and the patient organization German Allergy and Asthma Association (DAAB) Allergo J Int. 2014;23:96–112. doi: 10.1007/s40629-014-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Simons FE, Ardusso LR, Bilo MB, et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12:389–99. doi: 10.1097/ACI.0b013e328355b7e4. [DOI] [PubMed] [Google Scholar]
- 209.Dhami S, Panesar SS, Rader T, et al. The acute and long-term management of anaphylaxis: protocol for a systematic review. Clin Transl Allergy. 2013;3:14. doi: 10.1186/2045-7022-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Reeve L, Baldrick P, Hewings S, Skinner M. A battery of genotoxicity studies with an allergy vaccine adjuvanted with monophosphoryl lipid A (MPL(R)) for the treatment of grass pollen allergy. J Appl Toxicol. 2012;32:608–16. doi: 10.1002/jat.1726. [DOI] [PubMed] [Google Scholar]
- 211.Pfaar O, Cazan D, Klimek L, Larenas-Linnemann D, Calderon MA. Adjuvants for immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12:648–57. doi: 10.1097/ACI.0b013e32835a11d6. [DOI] [PubMed] [Google Scholar]
- 212.Jutel M, Solarewicz-Madejek K, Smolinska S. Recombinant allergens: The present and the future. Hum Vaccin Immunother. 2012;8:1534–43. doi: 10.4161/hv.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Patel D, Couroux P, Hickey P, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 214.Johansen P, von Moos S, Mohanan D, Kündig TM, Senti G. New routes for allergen immunotherapy. Hum Vaccin Immunother. 2012;8:1525–33. doi: 10.4161/hv.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Senti G, Prinz Vavricka BM, Erdmann I, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–12. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Senti G, von Moos S, Tay F, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: A double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–35. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 217.Senti G, Crameri R, Kuster D, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129:1290–6. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 218.Kopp MV. Role of immunmodulators in allergen-specific immunotherapy. Allergy. 2011;66:792–7. doi: 10.1111/j.1398-9995.2011.02553.x. [DOI] [PubMed] [Google Scholar]
- 219.Larenas-Linnemann D, Wahn U, Kopp M. Use of omalizumab to improve desensitization safety in allergen immunotherapy. J Allergy Clin Immunol. 2014;133:937. doi: 10.1016/j.jaci.2013.12.1089. [DOI] [PubMed] [Google Scholar]
- 220.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–3. [Google Scholar]
- 221.Bousquet J, Schünemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–62. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]