Abstract
Drug hypersensitivity reactions are unpredictable adverse drug reactions. They manifest either within 1–6 h following drug intake (immediate reactions) with mild to life-threatening symptoms of anaphylaxis, or several hours to days later (delayed reactions), primarily as exanthematous eruptions. It is not always possible to detect involvement of the immune system (allergy). Waiving diagnostic tests can result in severe reactions on renewed exposure on the one hand, and to unjustified treatment restrictions on the other. With this guideline, experts from various specialist societies and institutions have formulated recommendations and an algorithm for the diagnosis of allergies. The key principles of diagnosing allergic/hypersensitivity drug reactions are presented. Where possible, the objective is to perform allergy diagnostics within 4 weeks–6 months following the reaction. A clinical classification of symptoms based on the morphology and time course of the reaction is required in order to plan a diagnostic work-up. In the case of typical symptoms of a drug hypersensitivity reaction and unequivocal findings from validated skin and/or laboratory tests, a reaction can be attributed to a trigger with sufficient confidence. However, skin and laboratory tests are often negative or insufficiently reliable. In such cases, controlled provocation testing is required to clarify drug reactions. This method is reliable and safe when attention is paid to indications and contraindications and performed under appropriate medical supervision. The results of the overall assessment are discussed with the patient and documented in an „allergy passport“ in order to ensure targeted avoidance in the future and allow the use of alternative drugs where possible.
Key words: drug hypersensitivity, diagnosis, skin test, in vitro test, provocation test
Introduction
Whilst type-A adverse drug reactions (ADR) are caused by known pharmacological or toxic reactions, hypersensitivity drug reactions are caused by individual predisposing factors in the patient and are essentially unpredictable (type-B reactions) [1]. Drug allergy is distinct from a non-immunological drug hypersensitivity reaction (Tab.1).
|
Adverse drug reaction (ADR)
A noxious and unintended reaction that occurs alongside the intended principal effect of a drug, for which a causal relationship between the use of the drug and the adverse reaction is suspected |
|
Adverse drug reaction (ADR)
ADRs can be both type A (pharmacological/toxic) and type B (hypersensitivity) |
|
Type A („augmented“: pharmacological/toxic drug reaction)
Disease manifestations due to predictable, dose-dependent pharmacological/toxic effects typical for a drug at the recommended dose (e.g., sedative effect of older antihistamines, hair loss caused by cytostatics) or at higher doses (intoxication) |
|
Type B („bizarre“: hypersensitivity reactions)
Individual, unpredictable clinical reaction to a drug, i.e., disease manifestations occur in specifically predisposed patients; a distinction is made between two forms: |
|
Drug allergy:
Hypersensitivity is based on an immunological reaction (types I–IV according to Coombs and Gell) |
|
Non-immunological drug hypersensitivity:
An immunological (allergic) reaction mechanism cannot be detected. This form of reaction was formerly further subdivided into: _ Drug intolerance: typical symptoms of the pharmacological effect (toxicity) develop already at low doses, which are usually tolerated _ Drug idiosyncrasy: Symptoms differ from the pharmacological effect of the substance. Reactions involving symptoms that correspond to allergic disease were also referred to as pseudo-allergies |
All cases of suspected hypersensitivity reactions associated with the use of drugs in any age group should undergo diagnostic investigation with the aim of identifying the trigger and possibly the underlying pathomechanism, assessing the patient’s risk for further reactions, and advising the patient accordingly on this risk [2]. Dispensing with a diagnostic work-up can result in severe reactions on renewed exposure on the one hand, and to unjustified restrictions in terms of treatment options on the other.
The DGAKI and the DDG assigned the working group Arzneimittelallergie („drug allergy“) with the task of updating the current version of the guideline [1]. Following constitutive meetings on 11 October 2012 and 5 September 2013, K. Brockow, B. Przybilla, and H. Merk compiled a draft version of the new guideline by updating and revising the existing guideline [2]. Together with experts from other specialist societies and institutions largely involved in the treatment of patients with drug hypersensitivity reactions, recommendations were developed based on literature searches, an assessment of participants’ experiences, and theoretical considerations. At a consensus conference held on 15 April 2014, each recommendation was discussed and agreed on in a structured consensus-finding process under neutral moderation in order to solve open decision-making problems and to provide a final assessment of the recommendations.
This guideline is addressed to all physicians, as well as other professionals working in the medical field, involved in the diagnosis of and counseling on drug hypersensitivity reactions in patients of all age groups. The general principles of clarifying drug hypersensitivity reactions are presented. It is beyond the scope of this guideline to provide details on diagnostic methods or specific procedures in the case of hypersensitivity reactions to individual drugs or drug groups, or on rare diseases induced by drug hypersensitivity.
Definition and classification
Definitions for the classification of ADR are given in Tab.1 [1]. Diagnostic allergy tests are only useful in allergic or non-allergic hypersensitivity reactions, not, however, in pharmacological or toxic ADR.
Clinical classification on the basis of morphology, chronology, and time course is helpful for further diagnostic planning and permits the differentiation of hypersensitivity reactions based on the time course (Tab.2) [1, 3].
| 1. Time interval to reaction | a) In already sensitized patients | |
| -> immediate reaction | immediate to 60 min | |
| -> delayed (non-immediate) | > 1 h-several weeks | |
| b) In de novo sensitization while on treatment | ||
| -> Typical sensitization latency | 5–10 Days | |
| 2. Clinical manifestations | a) Immediate-type symptoms: e. g., flushing, urticaria, angioedema, bronchospasm, anaphylaxis | |
| b) Delayed-type symptoms: maculopapular drug eruptions, acute generalised exanthematic pustulosis (AGEP), severe cutaneous adverse reactions: Stevens-Johnson-Synrom (SJS), toxische epidermale Nekrolyse (TEN), „drug reaction with eosinophilia and systemic symptoms“ (DRESS) | ||
| c) Specific symptoms: e. g., hepatitis, cytopenias, autoimmune diseases (e.g., lupus erythematosus, Ig-A dermatosis) | ||
| 3. Pathomechanisms | a) Immunological hypersensitivity reaction: immediate-type (type I according to Coombs and Gell, mostly IgE-mediated): typical manifestation, immediate-type symptoms | Reaction time: 0–6 h (in rare cases, up to 12 h) |
| b) Non-immunological hypersensitivity reaction: typical manifestation, immediate-type symptoms | Reaction time: 0–6 h (in rare cases, up to 12 h) | |
| c) Immunological hypersensitivity reaction: delayed-type (type IV according to Coombs and Gell, T cell-mediated): typical manifestation, delayed-type symptoms | Reaction time: 24–72 h (in rare cases, after 6 h) | |
| d) Other immunological hypersensitivity reactions (type II, type III according to Coombs and Gell, IgG-, IgA, or IgM-mediated): cytopenias, serum sickness, allergic vasculitis | Reaction time: from 24 h | |
| In new sensitization under treatment | Typical sensitization latency: 5–10 days in type I–IV, rarely longer: weeks to months, e. g., in SJS/TEN, DRESS, autoimmune diseases (e. g., lupus erythematosus) | |
Diagnostic methods
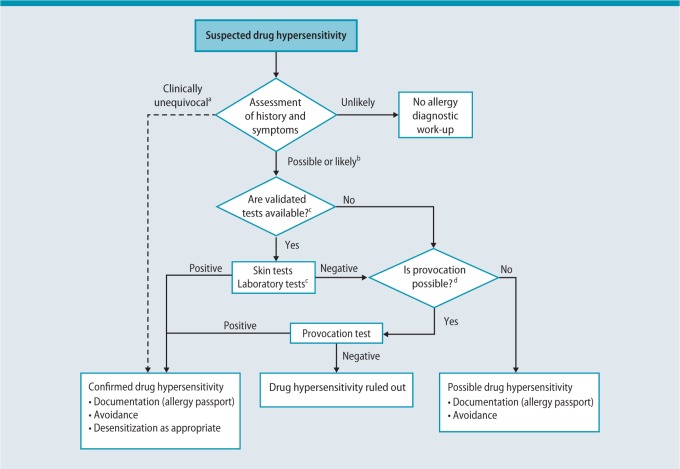
Investigations should be performed by an experienced allergist or at a specialized allergy center. Knowledge of drugs that frequently elicit specific hypersensitivity reactions is essential for diagnostic planning in order to be able to assess the likelihood with which a drug has caused a reaction. Patient history as well as skin, in vitro, and provocation tests are used to identify the trigger. A precise description and classification of the original reaction is of utmost importance. Any diagnostic work-up should take into consideration the particular features of the individual case as well as the diagnostic options available (Fig.1) [4].
Every effort should be made to perform diagnostic allergy testing within 4 weeks–6 months after the resolution of symptoms. There is evidence that successful detection of hypersensitivity several years after the reaction is less frequent [5, 6]. Diagnostic testing for allergy in patients with no previous history of drug hypersensitivity („prophetic testing“) is not beneficial [7].
Patient history and findings clinical manifestation
The diagnostic success rate is higher in patients presenting during the acute phase of a reaction, since it is possible at that point to establish differential diagnoses, classify clinical manifestations, and reliably interpret the course of symptoms in relation to drug use. Creating a timeline diagram is recommended in cases where multiple drugs are taken. Typical time intervals between first drug intake and symptom onset are shown in Tab.3. Although information provided by the patient, as well as all available medical records relating to the reaction (e. g., discharge letter, medical chart, anesthetic chart) need to be consulted, these are sometimes unreliable. A standardized questionnaire to collect relevant information is available [8, 9]. A test plan is formulated on the basis of all available information.
| Hypersensitivity reaction | Time interval |
|---|---|
| Urticaria, asthma, anaphylaxis | typically within 1 h, in rare cases up to 12 h after exposure |
| Maculopapular drug eruption | 4–14 Days after start of usea |
| AGEP | 1–12 Days after start of useb |
| SJS/TEN | 4–28 Days after start of usec |
| DRESS | 2–8 Weeks after start of use |
AGEP, acute generalized exanthematous pustulosis; SJS, Stevens-Johnson syndrome; TEN: toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms.
a Time interval in repeat reactions typically shorter compared with the first reaction. In maculopapular drug eruptions, reaction typically seen after 1–4 days, typical time interval for repeat reactions has not been investigated in AGEP, SJS, TEN, and DRESS; b mostly 1–2 days with antibiotics, often 7–12 days with other medications; c sometimes longer with allopurinol
To this end, the following information should be documented:
A. Clinical manifestation:
Documentation of clinical manifestations and/or organ systems involved, e. g., skin, respiratory tract, cardiovascular reactions, gastrointestinal reactions, liver, kidneys
Precise description of clinical and morphological findings (particularly in the case of skin manifestations/mucosal reactions), in addition to photodocumentation
General symptoms: fever, fatigue
Course of the reaction (reaction onset in temporal relation to drug use, duration of the reaction, morphological change of the reaction)
Laboratory findings (e. g., changes in blood count, such as eosinophilia, neutrophilia, thrombocytopenia; liver and kidney function; serum tryptase level)
Where appropriate, histological findings (especially in skin manifestations)
B. Additional factors associated with the reaction:
Acute diseases at the time of the reaction (e. g., intercurrent infections)
Patient whereabouts and activities
Cofactors for allergic reactions: stress, exertion, food intake, alcohol consumption, ultraviolet (UV) exposure
C. Documentation of drugs used in temporal relation to the reaction:
Indication for drug use
Trade name (ideally with batch no.; is a sample available?)
Mode of application
Ingredients (active substance, excipients)
Duration of use
Dosage
Tolerance in the case of earlier or repeated use
D. General patient history and clinical findings:
Basic data (sex, age, profession)
Known hypersensitivity reactions (allergy passport)
Similar reactions in the absence of drug use (e. g., natural latex allergy)
Atopic disease, food allergy/intolerance
Predisposing diseases [e. g., bronchial asthma, nasal polyps, chronic urticaria, mastocytosis, infections such as human immunodeficiency virus (HIV), Epstein-Barr virus (EBV)]
Other relevant previous or current diseases (e. g., somatoform disorders or mental illness)
Noxious agents: nicotine, alcohol, illicit drugs
Current medication
E. Chronology of the ADR:
Timing in relation to drug use
First onset
Course and resolution
Therapeutic measures and response in terms of the clinical course
F. Diagnosis and pathophysiological classification of the clinical reaction taking into account (see Tab.1):
Morphology and symptoms
Time course
Note: In the case of multiple reactions information is required for each individual reaction.
Skin tests
Skin tests are carried out in the context of hypersensitivity reactions involving symptoms consistent with allergy in order to determine a sensitization [10, 11]. As yet, there is no uniform standard for skin testing with drugs. The European Network on Drug Allergy (ENDA) methods, which are currently being investigated in studies, are recommended [11]. In cases where provocation tests are not possible, e. g., as with muscle relaxants, skin testing plays a particularly important role in the diagnosis of allergy [12, 13].
However, diagnostically useful skin test reactions occur only in some patients with hypersensitivity reactions. Test substances in high concentrations can cause reactions even in healthy individuals. It is essential that non-irritant test concentrations are used. However, for many drugs optimal test concentrations are not known. Recommendations on a number of drugs have been developed recently; examples are given in Tab.4 [14]. Where appropriate, natural rubber latex allergy should be excluded.
| Drug or drug class | Prick test | Intradermal test h | Patch test |
|---|---|---|---|
| β-Lactam antibiotics | |||
| Penicilloyl poly-L-lysine | 5x10-5 mM | 5x10-5 mM | NA |
| Minor determinant mixture | 2x10-2 mM | 2x10-2 mM | NA |
| Benzylpenicillin | 10,000 UI/ml | 10,000 UI/ml | 5 % |
| Amoxicillin | 20 mg/ml | 20 mg/ml | 5 % |
| Ampicillin | 20 mg/ml | 20 mg/ml | 5 % |
| Cephalosporins | 2 mg/ml | 2 mg/ml | 5 % |
| Anticoagulants | |||
| Heparinsa | undilutedh | 1/10 diluted | undilutedh |
| Heparinoidsb | undilutedh | 1/10 diluted | undilutedh |
| Platinum salts | |||
| Carboplatin | 10 mg/ml | 1 mg/ml | NA |
| Oxaliplatin | 1 mg/ml | 0.1 mg/ml | NA |
| Cisplatin | 1 mg/ml | 0.1 mg/ml | NA |
| NSAIDs | |||
| Pyrazolonesc | Suspensioni | 0,1–1 mg/ml | 10 % |
| Coxibsd | Suspensioni | NA | 10 % |
| Other NSAIDe | Suspensioni | 0.1–1 mg/ml | 10 % |
| Biologicals | |||
| Adalimumab | 50 mg/ml | 50 mg/ml | undilutedh |
| Etanercept | 25 mg/ml | 5 mg/ml | NA |
| Infliximab | 10 mg/ml | 10 mg/ml | NA |
| Omalizumab | 1.25 μg/ml | 1.25 μg/ml | NA |
| Others | |||
| Local anesthetics | Undilutedh | 1/10 diluted | undilutedh |
| Iodinated contrast media | Undilutedh | 1/10 diluted | undilutedh |
| Gadolinium chelates | Undilutedh | 1/10 diluted | NA |
| Patent blue | Undiluted | 1/10 diluted | NA |
| Methylene blue | Undiluted | 1/100 diluted | NA |
| Fluorescein | Undilutedh | 1/10 diluted | undilutedh |
| Proton pump inhibitorsf | Undilutedh | 40 mg/ml | 10 % |
| Anticonvulsantsg | NA | NA | 10 % |
| Chlorhexidine digluconate | 5 mg/ml | 0.002 mg/ml | 1 % |
NA, not applicable or no recommended concentration; NSAID, non-steroidal anti-inflammatory drugs
a Heparins: unfractionated heparin, nadroparin, dalteparin, enoxaparin; testing contraindicated in heparin-induced thrombocytopenia; b heparinoids: danaparoid, fondaparinux; c pyrazolones: metamizole, propyphenazone, aminopyrine, phenazone, phenylbutazone; d coxibs: celecoxib, etoricoxib, valdecoxib; e other NSAIDs: e. g., aspirin, ibuprofen, naproxen, indomethacin, diclofenac, fenoprofen, meloxicam, mefenamic acid, nimesulide; f no intravenous solution available for intradermal testing with lansoprazole and rabeprazole, only for prick testing; g test initially with 1 % in the case of severe reactions; h use of the commercially available solution for intravenous infusion or subcutaneous injection; i tablet is ground to a powder and a suspension prepared using physiological saline solution
In extremely rare cases, skin testing with the trigger of a hypersensitivity reaction can cause systemic, occasionally life-threatening reactions. The physician performing the skin test, as well as nursing staff must be prepared to deal with potential emergency situations [11, 15, 16, 17].
De novo sensitization as a result of skin testing is possible, whereby this risk depends on the substance tested, its concentration, and the test method used. Therefore, intradermal tests and patch tests should only be performed with the drug suspected of triggering a reaction or relevant alternatives.
The indication for skin testing with non-standardized substances must be established according to particularly strict criteria. Tests with incremental increases in the test substance concentration (threshold tests, e. g., 1 : 1000, 1 : 100, 1 : 10) can reduce the risk of severe allergic reactions during cutaneous testing.
Test material
Medicinal preparations used, active substances, and excipients
Positive and negative controls depending on the test method
Appropriate test concentrations to avoid irritative/toxic or pharmacological reactions (e. g., to morphine derivatives, gyrase inhibitors) or false-negative test failure (where appropriate, threshold tests)
Substance should be prepared in a manner suited to skin testing
Test procedure
Sufficient interval since drug reaction and allergy medication.
In the case of tests at risk for eliciting a systemic reaction, adequate medical supervision of the patient over a sufficient time period; where appropriate, threshold testing with diluted solutions.
Sequence of test procedures: prick test prior to intradermal test; patch test (where appropriate, open prior to closed prior to tape-stripping patch test); in the case of suspected photo-induced reactions, additional tests in combination with UV light (e. g., photo-patch test).
Painful intradermal tests should be used sparingly in children, especially infants and young children.
Timing of tests (simultaneous or consecutive testing of different substances or substance concentrations) is selected according to the suspected pathomechanism, the severity of the reaction, and the risks associated with the chosen skin test method.Prick and intradermal test reactions are typically read after 15–20 min, patch tests after 24–48 h and 72 h. In the case of drug eruptions, late readings after 24 and 48 h (or 48 and 72 h) should be performed for prick and intradermal tests (e. g., when investigating amoxicillin eruptions). In the case of anaphylactic symptoms and high-risk of systemic test reactions, it is possible to perform an open patch test with an early reading after 20–30 min (e. g., when investigating anaphylaxis following topical application of bacitracin). Additional readings can be helpful, e. g., patch test readings after 7 days in the case of glucocorticoid allergy.
Note: Skin test reactions can occur at other points in time, sometimes also after more than 1 week. Patients need to be informed that, in such cases, they should seek immediate medical advice from the treating physician.
Interpreting test results
Readings should be made according to the recommended criteria for the test procedure used, and unusual morphological features documented.
In the case of reactions to medicinal preparations, further testing of the individual ingredients, where available, should be performed.
In the case of skin reactions, a nonspecific reaction should be excluded where possible.
Only when non-irritant test concentrations are used is it sometimes possible to definitively diagnose an individual allergy (e. g., β-lactam antibiotic or heparin allergy) on the basis of „positive“ skin tests combined with patient history. In all other cases, additional investigations (in vitro tests, provocation tests) are required.
In vitro investigations
Particularly in the case of negative skin tests or severe life-threatening reactions, laboratory investigations can be helpful, most notably when provocation testing is not possible or where the skin test itself poses a risk, as in anaphylactic reactions to β-lactam antibiotics, for instance [18, 19].
In vitro diagnosis with drugs
Tests to measure specific immunoglobulin E (IgE) antibodies to various drugs are available (Tab.5). Although cellular tests are sometimes helpful, they are only available at a limited number of centers and can cause problems in early childhood due to the large volumes of blood required [20, 21].
| Adrenocorticotropic hormone (ACTH)d |
| Amoxicilloyla |
| Ampicilloyla |
| Cefaclorb |
| Chlorhexidineb |
| Chymopapainb |
| Gelatin (bovine) a |
| Galactose-α-1,3-galactose (α-Gal) c, e |
| Insulin (bovine)b |
| Insulin (human)a |
| Insulin (porcine)b |
| Morphineb |
| Penicilloyl Ga |
| Penicilloyl Va |
| Pholcodineb |
| Protamineb |
| Suxamethonium (succinylcholine)b |
| Tetanus toxoidd |
*It is important to ensure that test methods have been validated when determining sIgE to drugs. CE certification requires at least five, the US Food and Drug Administration (FDA) at least 30 positive patient sera, as well as studies on stability and reproducibility. Where these criteria have not been fulfilled, test reagents are offered for research purposes where appropriate. Particular attention should be paid here to the quality of the available literature. Determination of sIgE against substances for which no IgE-mediated allergic reactions have been described as yet should not be performed in routine diagnostics.
a CE-certified and FDA-registered b CE-certified; c CE certification in preparation; d for research purposes only; e α-Gal, this is an IgE-reactive sugar epitope held responsible for anaphylactic reactions to cetuximab and infusion solutions containing gelatin
Validated tests to detect specific IgE (sIgE) antibodies in serum are available for only a small number of drugs (Tab.5; most notably β-lactam antibiotics); other than that, there are no standardized and evaluated in vitro procedures.
Other immunological laboratory methods [e. g., basophile histamine release test, basophile activation test, cysteinyl-leukotriene release test (cellular antigen stimulation test, CAST), lymphocyte transformation test, lymphocyte activation test, Enzyme-Linked ImmunoSpot assay (ELISpot test)] may be helpful in selected cases; however, they should not be used as a rule in routine practice, not least since standardization is not guaranteed.
It is not possible to conclusively detect or exclude drug hypersensitivity solely on the basis of in vitro tests. In vitro test results can only be interpreted in conjunction with patient history/clinical findings and possibly in vivo tests.
Additional in vitro investigations
In the presence of relevant clinical symptoms, measurement of drug-metabolizing enzymes to detect metabolic disorders associated with hypersensitivity to certain drugs [e. g., dihydropyrimidine dehydrogenase (methotrexate), thiopurine S-methyltransferase (azathioprine)]
Where appropriate, pharmacogenetic investigations, e. g., human leukocyte antigen (HLA) status, in the case of abacavir use in Caucasians (B*5701) or carbamazepine use in Asians (B*1502)
Where appropriate, mast cell mediator detection (in particular tryptase) to confirm anaphylaxis preferably 1–2 h after the start of a reaction and for comparison with baseline tryptase value (after 2–3 days)
Provocation tests
Provocation tests are indicated when the drug triggering hypersensitivity cannot be identified with sufficient reliability on the basis of history, skin testing, and in vitro investigations and when the benefit of information obtained from prvovocation testing outweighs the risks [22]. This is often the case. Particularly in the case of suspected reactions to substances in drug groups that are essential or that cannot be permanently avoided (e. g., analgesics, antibiotics, local anesthetics), provocation tests also serve to identify tolerated drugs (alternative preparations in the case of possible drug cross-reactivity). Drug provocation testing is indicated for the purposes of [22]:
Excluding hypersensitivity when the history is unclear
Confirming the diagnosis when the history is suggestive but includes negative, unconvincing, or unavailable results from other diagnostic tests
Excluding cross-reactivity of related drugs
The patient should be informed about the goal of diagnostic testing, the risks involved, the alternatives, as well as the test procedure, including the use of placebo. Informed consent should be given in writing.
Medical supervision during the follow-up period, with the possibility of providing prompt intensive medical care if required, should be maintained for as long as severe reactions (e. g., anaphylaxis) can be expected. For this reason, provocation tests likely to cause systemic reactions should be performed in an in-patient setting equipped to provide immediate emergency care (experienced medical and nursing staff, appropriate drugs and technical equipment). Determining the procedure of drug provocation testing should always remain a case-by-case medical decision that takes numerous individual factors into consideration (e. g., type of drug, estimated likelihood of a reaction, expected severity of the reaction, patient expectations/anxiety).
The basic principle of provocation testing is to administer substances in the form in which they caused hypersensitivity reactions in the past. Oral administration can be attempted with some substances, even though a different mode of administration was originally used (e. g., i.m., i. v., rectal). In some forms of reaction a local test at the reaction site in the sense of a „localized provocation test“ is possible (e. g., in fixed drug eruptions with patch testing in loco). As a basic rule, in-patient provocation tests should be performed in a placebo-controlled manner, since a large number of reactions are also seen with placebo tests.
Test material
Drugs, active ingredients, excipients
Test materials should be prepared in a form suited to single-/double-blind and fractionated administration
Note: When investigating reactions to some drugs (e. g., non-steroidal anti-inflammatory drugs [NSAIDs]), it is advisable to also test alternative substances or preparations.
Test procedure
Sufficient interval since drug reaction and allergy medication.
In-patient medical supervision in the case of provocation tests with the potential to trigger systemic reactions.
Appropriate medical supervision for the entire duration of the provocation test, as well as a safety interval determined by the reaction type, following administration of the final test dose.
Consideration of the pharmacological effects of drugs (e. g., narcotics, antidiabetic agents, neuroleptic agents, heparins) and their respective maximum doses, as well as possible altered pharmacokinetics in the patient (e.g., impaired liver or kidney function).
In the case of systemic administration, drugs should be administered in incremental doses (e.g., [1%] – 10 % - 50 % - 100 % or [1%] - 3 % - 10 % - 30 % - 100 % of the usual single dose, possibly up to the daily dose or the dose given in the patient history) at an interval determined according to the suspected reaction mechanism (30 min–2 days), possibly with the additional administration at therapeutic daily doses for several days (e. g., drug eruptionsexanthems). In the case of eruptions eczematous reactions to external agents, a non-fractionated application test is possible.
Co-exposure to the co-stimulus when a reaction to a combination of triggers is suspected (e. g., exercise-induced anaphylaxis).
Single-blind (double-blind) tests with appropriate placebo controls.
The results of non-blind provocation tests are of diagnostic use only in the case of negative results or unequivocal clinical symptoms.
Placebo controls must be performed in precisely the same manner as verum controls in multiple dosages (for examples, see Tab.5).
Management of hazardous test reactions.
Note: It is essential that drugs and medical equipment required for emergency treatment is available and that personnel are experienced in the management of acute emergencies.
Sufficient interval between test reaction and subsequent tests (e. g., refractory phase).
Patients must be informed on how to react if a reaction occurs after the period of medical supervision.
Assessment
Provocation test results should be assessed preferably on the basis of objective parameters; nevertheless, subjective symptoms should also be recorded.
Symptoms, as well as the evolution of a reaction over time, should be documented and, where possible, quantitative parameters measured (e. g., blood pressure, respiratory parameters, serum tryptase levels).
In the case of drug reactions, further tests should be performed with active ingredients and excipients, where available
If a preparation of the suspected active substance as produced by a center’s own pharmacy dispensary fails to induce a reaction, provocation should then be performed with the drug preparation used in the patient history.
In the case of a reaction to placebo, perform reverse placebo provocation tests where appropriate (Tab.6)
| Procedure: Testing of | Patient information | Result | Further procedure |
|---|---|---|---|
| 1. Verum | Verum | Reaction | 2. |
| 2. Placebo | “Verum” | Reaction | 3. |
| 3. Verum | „Control„ | No reaction | 4. |
| Reaction | Hypersensitivity to verum confirmed | ||
| 4. Verum | Verum (once informed about 1.–3.) | No reaction | - |
Note: A negative provocation test does not reliably exclude hypersensitivity. In particular, effects exerted by the disease originally treated (e. g., viral infections), drug interactions, or a reduction in sensitivity over time can be responsible for false-negative results. The negative predictive value of negative provocation testing to various drugs is > 95 % in most cases, and the severity of the rare reactions seen under renewed re-exposure despite tolerance in provocation tests was mostly mild [24, 25].
A reduction in the degree of sensitivity over time can be expected with allergic reactions that lie far in the past. In such cases, although a provocation test may be negative, it may have a „booster“ effect. Therefore, in the case of a long interval between a reaction and testing, re-provocation testing after 4–6 weeks may be considered.
Contraindications
The following contraindications exist for tests with suspected culprit drugs, as well as for tests with alternative preparations where cross-reactivity is assumed. In specific cases (e. g., suspected reactions to excipients), provocation testing may be justified, despite contraindications, in order to identify an active substance urgently required for treatment purposes. An individual risk-benefit assessment is essential in all cases.
Pregnancy and breastfeeding
Hypersensitivity reactions that may be beyond medical control (e. g., uncontrolled asthma, agranulocytosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, vasculitis, hepatopathy).
Diseases or the use of drugs that carry an increased risk despite essentially controllable reactions (e. g., drugs contraindicated in anaphylaxis: severe cardiovascular disease, severe asthma, β-blocker use).
Inadequate compliance, lack of understanding of the procedure on the part of the patient.
Overall assessment
The final assessment of findings needs to be made by taking not only results from skin, in vitro, and provocations tests but also, more particularly, the history of the clinical reaction into consideration. Although drug hypersensitivity cannot be reliably ruled out even by applying all available test methods, they do makeenable better risk assessment easier.
The result of the overall assessment is discussed with the patient and documented. Ideally, an allergy passport is issued, representing a medical document. This provides information on the reaction type and substances/preparations not tolerated, along with information on possible cross-reactivities. Possible (tested) alternative substances/preparations, as well as the maximum single and cumulative dose, should be stated (e.g., „paracetamol tolerated up to a single dose of 500 mg [cumulative 800 mg] in oral provocation“). Mention should also be made of the fact that future tolerance of alternative substances/preparations cannot be guaranteed with 100% certainty. Information on possible drug prophylaxis against hypersensitivity reactions (e. g., premedication when using radiocontrast media or for surgical procedures under general anesthesia) as well as on tolerance induction should also be included.
Consensus procedure
Guidelines commission „Adverse drug reactions“
Facilitators
Prof. Dr. K. Brockow, Munich and Prof. Dr. H. F. Merk, Aachen
Abbreviations
- ACTH
Adrenocorticotropic hormone
- AGEP
Acute generalized exanthematous pustulosis
- CAST
Cellular antigen stimulation test
- DRESS
Drug reaction with eosinophilia and systemic symptoms
- EBV
Epstein-Barr virus
- ELISpot
Enzyme-Linked ImmunoSpot
- ENDA
European Network on Drug Allergy
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- Ig
Immunoglobulin
- NA
Not applicable or no concentration recommended
- NSAID
Non-steroidal anti-inflammatory drug
- sIgE
Specific immunoglobulin E
- SJS
Stevens-Johnson syndrome
- TEN
Toxic epidermal necrolysis
- ADR
Adverse drug reactions
- UV
Ultraviolet radiation
Footnotes
Conflict of interests
The information on potential conflicts of interest have been reviewed by a steering-group which did not find any conflicts of interest which may affect the authors’ independency in the process of the development of this guideline. The authors’ disclosures are available in a table (together with the working-report on this guideline) on the AWMF-page of the S2k-Guideline for the diagnosis of drug hypersensitivity reactions via www.awmf.org/leitlinien/detail/ll/061-021.html.
Cite this as
Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, Fuchs T, Jakob T, Lange L, Pfützner W, Mockenhaupt M, Ott H, Pfaar O, Ring J, Sachs B, Sitter H, Trautmann A, Treudler R, Wedi B, Worm M, Wurpts G, Zuberbier T, Merk HF. Guideline for the diagnosis of drug hypersensitivity reactions. S2K-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Dermatological Society (DDG) in collaboration with the Association of German Allergologists (AeDA), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group, the Swiss Society for Allergy and Immunology (SGAI), the Austrian Society for Allergology and Immunology (ÖGAI), the German Academy of Allergology and Environmental Medicine (DAAU), the German Center for Documentation of Severe Skin Reactions and the German Federal Institute for Drugs and Medical Products (BfArM). Allergo J Int 2015;24:94–105 DOI: 10.1007/s40629-015-0052-6
References
- 1.Demoly P, Adkinson NF, Brockow K, et al. International Consensus (ICON) on Drug Allergy. Allergy. 2014;69:420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 2.Przybilla B, Aberer W, Bircher AJ, et al. Allergologische Diagnostik von Überempfindlichkeitsreaktionen auf Arzneimittel. Allergo J. 2008;17:90–94. [Google Scholar]
- 3.Bircher AJ, Scherer Hofmeier K. Drug hypersensitivity reactions: inconsistency in the use of the classification of immediate and nonimmediate reactions. J Allergy Clin Immunol. 2012;129:263–264. doi: 10.1016/j.jaci.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Gomes E, Pichler W, Demoly P, et al. The drug ambassador project: the diversity of diagnostic procedures for drug allergy around Europe. J World Allergy Org. 2004;17:1–10. [Google Scholar]
- 5.Fernandez TD, Torres MJ, Blanca-Lopez N, et al. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy. 2009;64:242–248. doi: 10.1111/j.1398-9995.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 6.Brockow K, Ring J. Anaphylaxis to radiographic contrast media. Curr Opin Allergy Clin Immunol. 2011;11:326–331. doi: 10.1097/ACI.0b013e32834877c3. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Jo EJ, Kim MY, et al. Clinical value of radiocontrast media skin tests as a prescreening and diagnostic tool in hypersensitivity reactions. Ann Allergy Asthma Immunol. 2013;110:258–262. doi: 10.1016/j.anai.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Rueff F, Bergmann KC, Brockow K, et al. Hauttests zur Diagnostik von allergischen Soforttypreaktionen. Allergo J. 2010;19:402–415. [Google Scholar]
- 9.Brockow K, Romano A, Blanca M, et al. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51. [PubMed] [Google Scholar]
- 10.Mertes PM, Alla F, Trechot P, et al. Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011;128:366–373. doi: 10.1016/j.jaci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Brockow K. Dilemmas of allergy diagnosis in perioperative anaphylaxis. Allergy. 2014;69:1265–1266. doi: 10.1111/all.12485. [DOI] [PubMed] [Google Scholar]
- 12.Brockow K, Garvey LH, Aberer W, et al. Skin test concentrations for systemically administered drugs — an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 13.Riezzo I, Bello S, Neri M, et al. Ceftriaxone intradermal test-related fatal anaphylactic shock: a medico-legal nightmare. Allergy. 2010;65:130–131. doi: 10.1111/j.1398-9995.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 14.Ring J, Beyer K, Biedermann T, et al. Akuttherapie und Management der Anaphylaxie. Allergo J Int. 2014;23:36–52. doi: 10.1007/s15007-014-0542-8. [DOI] [Google Scholar]
- 15.Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026–1045. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 16.Renz H, Becker WM, Bufe A, et al. In-vitro-Allergiediagnostik. Positionspapier der Deutschen Gesellschaft für Allergologie und klinische Immunologie. Allergo J. 2002;11:492–506. [Google Scholar]
- 17.Brockow K. Arzneimittelreaktionen vom Soforttyp. Epidemiologie, Klinik, Auslöser und Management. Hautarzt. 2014;65:409–414. doi: 10.1007/s00105-013-2695-x. [DOI] [PubMed] [Google Scholar]
- 18.Ebo DG, Bridts CH, Mertens CH, et al. Analyzing histamine release by flow cytometry (HistaFlow): a novel instrument to study the degranulation patterns of basophils. J Immunol Methods. 2012;375:30–38. doi: 10.1016/j.jim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Porebski G, Gschwend-Zawodniak A, Pichler WJ. In vitro diagnosis of T cell-mediated drug allergy. Clin Exp Allergy. 2011;41:461–470. doi: 10.1111/j.1365-2222.2011.03701.x. [DOI] [PubMed] [Google Scholar]
- 20.Aberer W, Bircher A, Romano A, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–863. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski ML, Asero R, Bavbek S, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013;68:1219–1232. doi: 10.1111/all.12260. [DOI] [PubMed] [Google Scholar]
- 22.Defrance C, Bousquet PJ, Demoly P. Evaluating the negative predictive value of provocation tests with nonsteroidal anti-inflammatory drugs. Allergy. 2011;66:1410–1414. doi: 10.1111/j.1398-9995.2011.02671.x. [DOI] [PubMed] [Google Scholar]
- 23.Demoly P, Romano A, Botelho C, et al. Determining the negative predictive value of provocation tests with beta-lactams. Allergy. 2010;65:327–332. doi: 10.1111/j.1398-9995.2009.02228.x. [DOI] [PubMed] [Google Scholar]
- 24.Coombs PR, Gell PG. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In: Gell RR, editor. Clinical Aspects of Immunology. Oxford: Oxford University Press; 1968. pp. 575–596. [Google Scholar]