Abstract
Adverse drug reactions can manifest clinically in a variety of ways. Whilst the majority can be explained by dose-dependent side effects, there is group of unpredictable immunological or non-immunological intolerances that represent a particular diagnostic challenge. Skin tests are frequently negative, whilst challenge tests are time-consuming and often hazardous. Against this backdrop, cellular in vitro tests play a particularly important role in the identification of the causative drug. Whilst basophil tests can be used in the case of immunoglobulin E (IgE)- as well as non-IgE-mediated hypersensitivity reactions, T-cellular test methods assist in the diagnosis of drug eruptions. The reliability of individual tests can be affected by a variety of parameters, such as the pathomechanism underlying the drug reaction, the causative medication, or the point in time of testing. Not only is a sound knowledge of the basic principles of the individual assays an essential prerequisite for correctly indicating and interpreting this test method, but also an awareness of these additional factors.
Keywords: Drug allergy, In vitro tests, Basophils, T-lymphocytes
In vitro testing for adverse drug reactions
Adverse drug reactions (ADR) represent a frequent problem in medical routine. Up to 15 % of all in-patients and 10 % of all out-patients develop ADR [1]. The majority of these reactions are caused by intoxication which occurs as a typical pharmacological effect of a drug and is therefore easy to diagnose. However, approximately 25 % of all ADR present as unpredictable side effects that are either immunologically (as an allergy) or non-immunologically induced [2]. These are challenging to diagnose and the use of in vitro assays, in addition to classic in vivo skin tests (prick, intracutaneous, and patch testing), can be helpful here.
The range of in vitro tests which can be utilized for the diagnosis of ADR includes on the one hand methods to determine drug-specific immunoglobulin-E (IgE) antibodies; however, these test systems are commercially available for only a few drugs and can only be employed for the narrow spectrum of IgE-mediated ADR. On the other hand, there is a variety of different cellular in vitro tests that can be used for the diagnosis of drug-induced immune reactions, such as immediate- and late-type allergies according to Coombs and Gell [3] or drug intolerances. These assays are based on the functional analysis of peripheral blood cells, i. e. basophil granulocytes (also called basophils), and T-lymphocytes. The different cellular test systems will be specified in the following sections, including the field of their application.
Basophil tests
Basophils, alongside mast cells, belong to the main effector cells of the allergic immediate-type reaction. They bind allergen-specific IgE antibodies on the cell surface, which, following cross-linking by the relevant allergen, induce the release of granules that contain preformed mediators such as histamine. In addition, newly synthesized mediators, such as sulfidoleukotrienes, are released. Since basophils, in contrast to tissue-resident mast cells, are an integral part of the peripheral blood, drug-induced stimulation of these cells can be detected by appropriate test systems taking a simple blood sample. These tests include:
Histamine release test (HRT)
Cellular antigen stimulation test (CAST; also CAST-ELISA [ELISA: enzyme-linked immunosorbent assay])
Basophil activation test (BAT), also known as Flow-CAST since antigen-specific basophil stimulation is evaluated using flow cytometry, in contrast to the CAST-ELISA (Fig. 1).
Fig. 1:
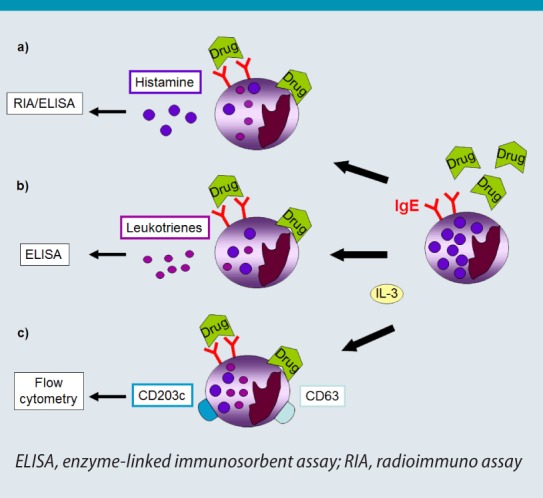
Basophil testing. Basophils are stimulated with interleukin (IL)-3 and incubated with the suspected drug (right). Drug-induced cross-linking of surfacebound IgE antibodies (allergic immediate-type reaction) or direct cell activation can be detected by measuring the release of histamine (a) or leukotrienes (b), as well as the expression of activation markers (c).
© W. Pfützner
For these tests, peripheral blood cells are incubated with varying concentrations of the suspected drug together with interleukin (IL)-3. This results in “priming“ of the basophils, leading to a stronger cellular response in the case of a positive reaction, irrespective of whether this response is IgE-mediated or not [4]. In this way, the number of non-responders is minimized, thereby increasing the sensitivity of the assay while only small amounts of blood basophils are needed [5]. Both HRT and CAST detect mediators of basophils stimulated in an allergen-specific manner, i. e. histamine (Fig. 11) or sulfoleukotrienes (leukotrienes C4, D4 and E4; Fig. 1b), whereas the BAT recognizes cell-bound molecules that are expressed in increased numbers on the surface during basophil activation (Fig. 1c and 2) [5, 6]. These molecules include CD63, which is transferred to the cell surface during degranulation [7], and CD203c, which is constitutively expressed at low levels on basophils. Studies have shown that CAST and BAT can be of use not only for the diagnosis of IgE-mediated but also IgE-independent ADR that show the clinical picture of an immediate-type reaction; these include, for example, intolerance reactions to non-steroidal antirheumatic drugs (NSAR) [8, 9].
The BAT assays have been used primarily to diagnose IgE-mediated reactions to β-lactam antibiotics, pyrazolones, and muscle relaxants, as well as intolerance reactions to analgesics. Since sensitivity is generally not higher than 50 %, a negative test does not necessarily circumvent a challenge test (provided this is possible). With a specificity of mostly over 90 %, a positive test has a particularly high predictive value in the presence of a positive medical history. The least helpful test is the HRT which, with the exception of muscle relaxants, has only low sensitivity and specificity in the diagnosis of ADR [10, 11]. Studies into IgE-mediated immediate-type reactions to β-lactam antibiotics and pyrazolones found CAST to have sensitivities of 42 % and 52 %, respectively, and specificities of 80 % and 90 %, respectively [12, 13]. Since increased leukotriene synthesis is considered an essential pathomechanism of intolerance reactions to analgesics [14], it was assumed that CAST is particularly well suited to identify patients affected by non-IgE mediated ADR via an NSAR-induced increase in leukotriene release. However, several studies found sensitivities of (sometimes even remarkably) below 50 % [8, 15, 16]. Attempts to increase leukotriene release in patient samples by adding complement C5a and as a consequence increasing sensitivity yielded inconsistent results [5, 17, 18]. A further problem is presented by the non-specific and already substantially increased basal level of leukotriene release in many samples [19, 20]. Investigations using BAT showed similarly low sensitivities with relatively high specificities. Thus, sensitivities of approximately 50 %, 42–55 %, and 36–64 % were found for IgE-mediated ADR to β-lactam antibiotics, pyrazolones, and muscle relaxants, respectively, at specificities of over 90 % up to 100 % [5, 13, 21]. BAT also yields similar results compared to CAST in the diagnosis of non-IgE mediated intolerance reactions to analgesics, although the results of these two assays do not always correlate [5, 21, 22]. Moreover, it is unclear what diagnostic value should be attributed in cases where tests for drugs suspected on the basis of medical history are negative, but on the other hand positive for other NSAR [23].
In summary, there is evidence that BAT and CAST are able to give additional information in the ADR to certain drugs such as β-lactam antibiotics, analgesics, muscle relaxants, and other distinct medications (Fig. 2), particularly in those cases where skin tests are inconclusive and challenge tests, such as in the case of muscle relaxants, are not feasible. However, diagnostic reliability, as in skin tests, appears to depend on the time interval since the ADR. Therefore, where possible, tests should not be performed later than 6–12 months following ADR, but also not earlier than 2 weeks after the reaction in order to avoid false-positive results due to still strongly pre-activated basophils [21]. Additional studies on the value of CAST and BAT in the diagnosis of ADR, including other groups of drugs that cannot be investigated using challenge tests, e. g., X-ray contrast media, would be desirable.
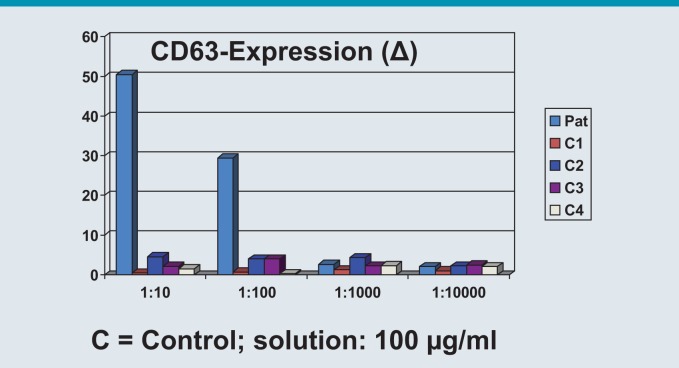
Fig. 2:
Basophil activation test (BAT): a 46-year-old patient with an anaphylactic reaction to carboplatin infusion. The patient reported that urticaria had appeared after starting continuous carboplatin treatment 2 years previously. (Image used with the kind permission of Prof. Dr. Hans Merk, Hautklinik der Medizinischen Fakultät, Universitätsklinikum der RWTH Aachen)
© W. Pfützner
T-lymphocyte tests
T-lymphocytes represent the central players of the adaptive immune system in the initiation of antigen-specific immune reactions [24]. A number of findings suggest that T-lymphocytes take on an important pathophysiological role particularly in the development of skin rashes due to ADR, including acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN). Thus, abundant lesional CD4+ and CD8+ T-lymphocytes can be detected and drug-specific T-cell clones isolated from both affected skin and peripheral blood of these patients [25, 26, 27]. When T-lymphocytes are stimulated by antigen-presenting cells, they differentiate according to the antigen and supporting cytokine milieu into specialized subpopulations that persist as memory T-cells. In the case of recurrent antigen contact, these memory cells are reactivated, causing increased proliferation, expression of certain activation markers, and secretion of cytokines specific for the relevant subpopulation. These effects can be measured quantitatively by employing various test systems and thus used in the identification of allergen-specific sensitization to drugs (Fig. 3) [28].
Fig. 3:
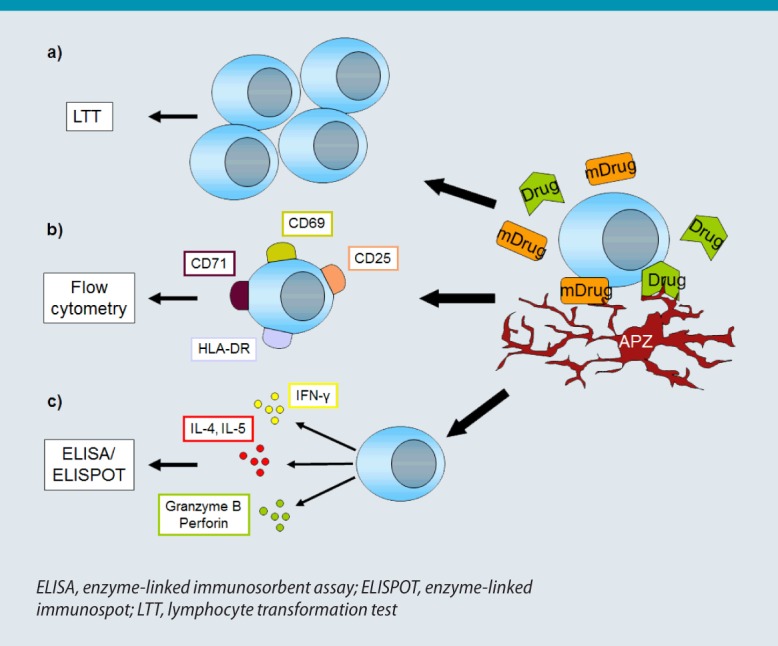
T-Lymphocyte testing. Drugs are taken up and presented to T-lymphocytes directly or following prior metabolization (mDrug). Drug-specific T-cell activation is detected either by increased T-cell proliferation (a), increased expression of activation markers (b), or increased secretion of certain cytokines (c).
Drug-induced T-cell proliferation can be detected using the lymphocyte transformation test (LTT; Fig. 3a). Here, T-lymphocytes or mononuclear cells from peripheral blood are incubated with the suspected drug and the proportion of proliferating T-cells determined by incorporating radioactively labeled thymidine (3H-thymidine) [29]. The ratio of cell proliferation following drug administration to cell proliferation in the absence of drug exposure denotes the drug-specific stimulation index (SI) for the drug in question. This should reach a value of at least SI > 2 (in the case of β-lactams, SI > 3) before drug sensitization can be considered [30]. Since vancomycin, NSAR (especially paracetamol), and X-ray contrast media often induce false-positive T-cell proliferation, an SI > 4 has been recommended for these medications in the detection of a drug-specific reaction [30]. In addition, comparative investigations using control individuals with no drug allergies in whom the SI should be around 1 are advisable. However, such investigations can also produce false-negative results when, for example, a metabolite rather than the drug used is responsible for T-cell activation [31]. Feasible yet technically complex methods to identify the causative drug as the trigger of an ADR are based on the use of sera from non-allergic individuals that have previously received and metabolized the drug, or of cultures of human liver microsomes to which the drug was added [32, 33]. Moreover, the time interval since the original clinical reaction occurred may be too great for sufficient drug-sensitized T-cells still be found in peripheral blood. Ideally, a diagnostic blood sample is taken at between 2 and 6 weeks following the ADR [34]; however, depending on the magnitude of sensitization, positive results may still be detected years later, in some cases up to 20 years later [30]. Finally, there may be a generally reduced capacity to activate T-cells due, for example, to the use of immunosuppressive drugs by the tested patient, whereas glucocorticoids up to a dose of 0.2 mg/kg BW should not interfere with testing. Therefore, it is advisable to test a positive control antigen at the same time, such as tetanus toxoid, the SI of which should be > 5 in individuals vaccinated against tetanus.
The sensitivity of LTT to detect drug sensitization is relatively variable and also depends on the type of allergic reaction investigated, the causative drug, and the point in time of testing. Thus, a number of studies found sensitivities of 50–75 %, whereby positive test results generally were not verified using challenge tests [29, 30, 35, 36]. While positive LTT results for the suspected drugs were obtained in more than 50 % of all analyzed patients who experienced drug-induced skin rashes (maculopapular, AGEP, DRESS) and anaphylaxis, the same was true in less than 10 % of patients investigated for TEN, vasculitis, or non-cutaneous manifestations of ADR [30]. On the other hand, its specificity seems to be high (keeping in mind, that confirmative challenge tests were usually not performed) at almost 100 % for antibiotics (especially β-lactam antibiotics and sulphonamides) and anticonvulsant agents (especially carbamazepine and lamotrigene) [37, 38, 39]. However, a negative LTT for these preparations by no means excludes an ADR. Moreover, the timing of testing following an ADR affects the LTT outcome, with results showing disease-specific differences. Thus, compared with other ADR, detection of the causative drug by LTT in case of DRESS is substantially delayed (weeks to months) following the clinical reaction [40, 41].
It is important to note that the level of SI does not permit any conclusions to be drawn on the degree of severity of the allergic reaction occurring in case of re-exposure to the causative drug [42]. In addition, drug-LTT show marked inter- and intra-individual variability that can be affected by, among others, the time at which blood samples are taken, the ratio of monocytes to lymphocytes in the blood sample, the plasma composition, blood processing, as well as the concentration and formulation of the drug added [30]. Thus, a patients’ SI can vary 30-fold in parallel assays with the same drug [43]. Therefore, performing an LTT in triplicate is recommended, whereby the standard deviation should be below 30 %.
A further option for the detection of drug-induced T-cell reactions is to measure activation markers on the surface of T-lymphocytes incubated with the drug (Fig. 3b). These markers include CD25, CD69, CD71, and HLA-DR, which are expressed on both CD4+ and CD8+ T-cells in drug allergic patients (on CD8+ T-cells particularly in bullous drug eruptions) and can be detected using flow cytometry [43, 44, 45, 46]. One study found a clear correlation between positive LTT results and the expression of CD69 on drug-stimulated T-cells [46]. The advantage of this test method compared with LTT is that it is easier to perform and produces faster test results, i.e., after 2 rather than 6–7 days. However, depending on the surface molecule analyzed, the drug used, or the underlying ADR, the proportion of cells expressing a particular activation marker can vary substantially, making it advisable to measure several markers in order to detect drug-activated T-cells. Nevertheless, too few studies have been carried out to date to allow any conclusions on the reliability of this assay in the diagnosis of ADR.
A third T-lymphocytic approach is based on the determination of drug-specific T-cells by detecting cytokines, or other signal molecules, that are secreted by lymphocytes following stimulation by the relevant medication (Fig. 3c). To this end, peripheral blood mononuclear cells are incubated with the suspected drug under T-cell-propagating culture conditions on plates with nitrocellulose membranes. If T-cell activation occurs, the mediators released are bound to the antibodies (coating the nitrocellulose membranes) directed against them. Antibody-mediated detection according to ELISA then visualizes, by means of an enzyme staining reaction, a drug-specific T-lymphocyte as a spot at every point at which an activated T-cell was located, similar to a negative image in photography (Fig. 4). Hence this method is referred to as an enzyme-linked immunospot (ELISPOT) assay [47].
Fig. 4:
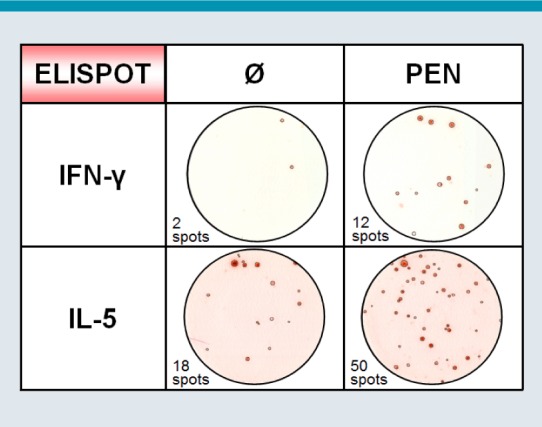
Enzyme-linked immunospot (ELISPOT) assay for the detection of drug-specific interferon (IFN)-γ- or interleukin (IL)-5-secreting T cells following stimulation with penicillin (PEN), compared with unstimulated controls (Ø)
© W. Pfützner
Numerous different mediators have been investigated in studies as potential markers for ADR, including IL-2, -4, -5, -8, -10, -13, -17, interferon (IFN)-γ, tumor necrosis factor-α, perforin, and granzyme B [48, 49, 50, 51, 52, 53, 54]. In this regard, the cytokine IL-5 secreted by CD4+ T-cells represents a parameter frequently positive in a variety of ADR [43, 48], whilst IFN-γ is apparently primarily released by CD4+ T-cells in patients with drug-induced maculopapular eruptions and TEN [49, 55]. Secretion of IL-8 is characteristic for drug-specific T-cells in AGEP [56]. Since cytotoxic immune mechanisms play an important role in all drug-induced skin rashes (albeit in varying degree), both granzyme B and perforin synthesized by CD8+ cytotoxic T-lymphocytes and natural killer cells are potential parameters for diagnosing ADR. On top, their expression profile may also give a hint of the severity of the experienced ADR [52, 57].
Like LTT, the ELISPOT assay also demonstrates high specificities of 85–100 %. Moreover, ar a detection limit of less than 25 secreting cells per 106 of peripheral blood mononuclear cells, it also has high sensitivities [58] of between 70 % and over 90 %, depending on the parameter analyzed [42, 48, 49]. The ELISPOT assay is also believed to yield positive results following considerably longer time intervals (12 to over 20 years later) [49, 50]. However, also in these studies, patient history rather than positive challenge testing was considered as clinical readout.
In summary, a number of cellular tests for the detection of T-cell mediated drug sensitization are available that can help in the diagnosis of ADR presenting as skin rash (maculopapular, AGEP, DRESS, TEN). Compared with patch testing as in vivo test method with its sensitivity of approximately 30–40 % [59, 60], test systems such as LTT or ELISPOT assay appear to have greater sensitivity [42, 48, 49]. It is too early to draw final conclusions on the value of newer methods, such as the ELISPOT assay, compared with the long-established LTT, although comparative studies point to a higher reliability with the ELISPOT assay [49, 58]. However, cellular assays have been insufficiently validated by oral drug challenge to be able to adequately evaluate their specificity — which is high with patch testing (81 % positive oral drug challenge tests following positive prior patch testing compared with only 10 % positive challenge following negative skin testing). Nevertheless, since drug provocation tests are not permissible in the diagnosis of severe ADR, such as AGEP, DRESS, or TEN, it is precisely for these ADR that in vitro T-cell tests represent a promising alternative to other diagnostic measurements [42, 62]. As with basophil testing, T-lymphocyte testing is advisable in the first 6–12 months following the drug reaction, although in some cases positive test results may be seen after even longer time intervals due to the persistence of drug-specific memory T-cells [30, 49].
Conclusion
Cellular in vitro diagnosis comprises basophil and T-lymphocyte tests, which can be used for the diagnosis of immediate-type allergies as well as intolerance reactions and drug-induced skin rashes, respectively. Due to their technical complexity and the sometimes pronounced variations in sensitivity and specificity they are currently not suitable for the routine diagnosis of ADR. However, in the hands of allergy laboratories with the relevant expertise, they constitute important assays in addition to the in vivo test methods (skin testing, challenge testing), particularly in the diagnosis of severe ADR.
Abbreviations
- ADR
Adverse drug reactions
- AGEP
Acute generalized exanthematous pustulosis
- APC
Antigen-presenting cells
- BAT
Basophil activation test
- CAST
Cellular activated antigen stimulation test
- DRESS
Drug rash with eosinophilia and systemic symptoms
- ELISA
Enzyme-linked immunosorbent assay
- ELISPOT
Enzyme-linked immunospot
- HRT
Histamine release test
- IFN
Interferon
- IgE
Immunoglobulin E
- IL
InterleukinE
- LTT
Lymphocyte transformation test
- mDrug
Metabolized drug
- NSAR
Non-steroidal antirheumatic drug
- PEN
Penicillin
- RIA
Radio immunoassay
- SI
Stimulation index
- TEN
Toxic epidermal necrolysis
Footnotes
Conflict of interest
Dr. Christian Möbs states that there are no conflict of interest. Prof. Dr. Wolfgang Pfützner states the following: Cooperation in studies which are financed by Allergopharma and Astellas, consultant and lectures for ALKAbelló and Novartis and research funding from Biomay.
Cite this as Möbs C, Pfützner W. Cellular in vitro diagnosis of adverse drug reactions. Allergo J Int 2014;23:164–71 DOI 10.1007/s40629-014-0020-6
References
- 1.Merk HF. Allergische Arzneimittelreaktionen der Haut: Epidemiologie, Klinik und Pathogenese. Allergo J. 2006;5:476–91. [Google Scholar]
- 2.Pfützner W. Arzneimittelreaktionen. In: Plewig G, Landthaler M, Burgdorf WC, Hertl M, Ruzicka T, eds. Braun Falco’s Dermatologie, Venerologie und Allergologie. 6. Aufl. Berlin - Heidelberg: Springer; 2012. p. 559-81
- 3.Coombs RRA, Gell PHG. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In: Gell PGH, Coombs RRA, Lachmann PJ, eds. Clinical aspects of immunology. 3rd edn. Oxford: Blackwell Scientific Publications; 1975. p. 761-81
- 4.Kurimoto Y, De Weck AL, Dahinden CA. The effect of interleukin 3 upon IgE-dependent and IgE-independent basophil degranulation and leukotriene generation. Eur J Immunol. 1991;21:361–8. doi: 10.1002/eji.1830210217. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann SM, Ventocilla S, Moll-Slodowy S, Sauer I, Merk HF. Basophilenaktivierungstests in der Diagnostik von Arzneimittelreaktionen. Hautarzt. 2005;56:38–43. doi: 10.1007/s00105-004-0871-8. [DOI] [PubMed] [Google Scholar]
- 6.Mayorga C, Sanz ML, Gamboa PM, Garcia BE, Caballero MT, Garcia JM, et al. In vitro diagnosis of immediate allergic reactions to drugs: an update. J Investig Allergol Clin Immunol. 2010;20::103–9. [PubMed] [Google Scholar]
- 7.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 8.De Weck AL, Sanz ML, Gamboa PM, Aberer W, Blanca M, Correia S, et al. Nonsteroidal anti-inflammatory drug hypersensitivity syndrome. A multicenter study. I. Clinical findings and in vitro diagnosis. J Investig Allergol Clin Immunol. 2009;19:355–69. [PubMed] [Google Scholar]
- 9.Gamboa P, Sanz ML, Caballero MR, Urrutia I, Antepara I, Esparza R, et al. The flow-cytometric determination of basophil activation induced by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is useful for in vitro diagnosis of the NSAID hypersensitivity syndrome. Clin Exp Allergy. 2004;34:1448–57. doi: 10.1111/j.1365-2222.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 10.Demoly P, Lebel B, Messaad D, Sahla H, Rongier M, Daures JP, et al. Predictive capacity of histamine release for the diagnosis of drug allergy. Allergy. 1999;54:500–6. doi: 10.1034/j.1398-9995.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 11.Mata E, Gueant JL, Moneret-Vautrin DA, Bermejo N, Gerard P, Nicolas JP, et al. Clinical evaluation of in vitro leukocyte histamine release in allergy to muscle relaxant drugs. Allergy. 1992;47:471–6. doi: 10.1111/j.1398-9995.1992.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 12.Gamboa PM, Sanz ML, Caballero MR, Antepara I, Urrutia I, Jauregui I, et al. Use of CD63 expression as a marker of in vitro basophil activation and leukotriene determination in metamizol allergic patients. Allergy. 2003;58:312–7. doi: 10.1034/j.1398-9995.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 13.De Weck AL, Sanz ML, Gamboa PM, Aberer W, Sturm G, Bilo MB, et al. Diagnosis of immediate-type beta-lactam allergy in vitro by flow-cytometric basophil activation test and sulfidoleukotriene production: a multicenter study. J Investig Allergol Clin Immunol. 2009;19:91–109. [PubMed] [Google Scholar]
- 14.Nasser SM, Lee TH. Leukotrienes in aspirin-sensitive asthma. In: Szczeklik A, Gryglewski RJ, Vane J, eds. Eicosanoids, aspirin and asthma. New York: Marcel Dekker; 1998. p. 317-36
- 15.De Weck AL, Sanz ML. Cellular allergen stimulation test (CAST) 2003, a review. J Investig Allergol Clin Immunol. 2004;14:253–73. [PubMed] [Google Scholar]
- 16.Bavbek S, Dursun AB, Birben E, Kalayci O, Misirligil Z. Cellular allergen stimulation test with acetylsalicylic acid-lysine is not a useful test to discriminate between asthmatic patients with and without acetylsalicylic acid sensitivity. Int Arch Allergy Immunol. 2009;149:58–64. doi: 10.1159/000176307. [DOI] [PubMed] [Google Scholar]
- 17.May A, Weber A, Gall H, Kaufmann R, Zollner TM. Means of increasing sensitivity of an in vitro diagnostic test for aspirin intolerance. Clin Exp Allergy. 1999;29:1402–11. doi: 10.1046/j.1365-2222.1999.00655.x. [DOI] [PubMed] [Google Scholar]
- 18.Czech W, Schopf E, Kapp A. Release of sulfidoleukotrienes in vitro: its relevance in the diagnosis of pseudoallergy to acetylsalicylic acid. Inflamm Res. 1995;44:291–5. doi: 10.1007/BF02032571. [DOI] [PubMed] [Google Scholar]
- 19.Pierzchalska M, Mastalerz L, Sanak M, Zazula M, Szczeklik A. A moderate and unspecific release of cysteinyl leukotrienes by aspirin from peripheral blood leucocytes precludes its value for aspirin sensitivity testing in asthma. Clin Exp Allergy. 2000;30:1785–91. doi: 10.1046/j.1365-2222.2000.00953.x. [DOI] [PubMed] [Google Scholar]
- 20.Lebel B, Messaad D, Kvedariene V, Rongier M, Bousquet J, Demoly P. Cysteinyl-leukotriene release test (CAST) in the diagnosis of immediate drug reactions. Allergy. 2001;56:688–92. doi: 10.1034/j.1398-9995.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 21.Sanz ML, Gamboa PM, Mayorga C. Basophil activation tests in the evaluation of immediate drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2009;9:298–304. doi: 10.1097/ACI.0b013e32832d5311. [DOI] [PubMed] [Google Scholar]
- 22.De Weck AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Arch Allergy Immunol. 2008;146:177–89. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 23.Kleine-Tebbe J, Erdmann S, Knol EF, MacGlashan DW, Jr., Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141:79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- 24.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125:S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Rozieres A, Vocanson M, Said BB, Nosbaum A, Nicolas JF. Role of T cells in nonimmediate allergic drug reactions. Curr Opin Allergy Clin Immunol. 2009;9:305–10. doi: 10.1097/ACI.0b013e32832d565c. [DOI] [PubMed] [Google Scholar]
- 26.Yawalkar N, Hari Y, Frutig K, Egli F, Wendland T, Braathen LR, et al. T cells isolated from positive epicutaneous test reactions to amoxicillin and ceftriaxone are drug specific and cytotoxic. J Invest Dermatol. 2000;115:647–52. doi: 10.1046/j.1523-1747.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- 27.Hertl M, Geisel J, Boecker C, Merk HF. Selective generation of CD8+ T-cell clones from the peripheral blood of patients with cutaneous reactions to beta-lactam antibiotics. Br J Dermatol. 1993;128:619–26. doi: 10.1111/j.1365-2133.1993.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 28.Beeler A, Pichler WJ. In vitro tests of T cell-mediated drug hypersensitivity. Expert Rev Clin Immunol. 2006;2:887–900. doi: 10.1586/1744666X.2.6.887. [DOI] [PubMed] [Google Scholar]
- 29.Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. 1997;27:175–81. doi: 10.1111/j.1365-2222.1997.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 30.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–20. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 31.Maria VA, Pinto L, Victorino RM. Lymphocyte reactivity to ex-vivo drug antigens in drug-induced hepatitis. J Hepatol. 1994;21:151–8. doi: 10.1016/S0168-8278(05)80388-5. [DOI] [PubMed] [Google Scholar]
- 32.Sachs B, Erdmann S, Al-Masaoudi T, Merk HF. In vitro drug allergy detection system incorporating human liver microsomes in chlorazepate-induced skin rash: drug-specific proliferation associated with interleukin-5 secretion. Br J Dermatol. 2001;144:316–20. doi: 10.1046/j.1365-2133.2001.04021.x. [DOI] [PubMed] [Google Scholar]
- 33.Weber P, Scheurlen M, Irkin I, Viebahn R, Scharek W, Becker W, et al. Lebertransplantation bei halothaninduzierter Lebernekrose. Zentralbl Chir. 1986;119:305–08. [PubMed] [Google Scholar]
- 34.Anonymous. Diagnostische Relevanz des Lymphozytentransformationstestes in der Umweltmedizin. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 2002;45:745-49
- 35.Luque I, Leyva L, Jose Torres M, Rosal M, Mayorga C, Segura JM, et al. In vitro T-cell responses to beta-lactam drugs in immediate and nonimmediate allergic reactions. Allergy. 2001;56:611–8. doi: 10.1034/j.1398-9995.2001.000115.x. [DOI] [PubMed] [Google Scholar]
- 36.Perez T, Dayer E, Girard JP. Hypersensitivity reactions to drugs: correlation between clinical probability score and laboratory diagnostic procedures. J Investig Allergol Clin Immunol. 1995;5:276–82. [PubMed] [Google Scholar]
- 37.Hertl M, Schneider R, Merk HF. In vitro-lymphocyte responses to beta-lactam antibiotics in penicillin allergy: cross-reactivity between penicillins and cephalosporins. Dermatosen. 1992;40:102–07. [Google Scholar]
- 38.Koponen M, Pichler WJ, de Weck AL. T cell reactivity to penicillin: phenotypic analysis of in vitro activated cell subsets. J Allergy Clin Immunol. 1986;78:645–52. doi: 10.1016/0091-6749(86)90083-7. [DOI] [PubMed] [Google Scholar]
- 39.Zakrzewska JM, Ivanyi L. In vitro lymphocyte proliferation by carbamazepine, carbamazepine-10, 11-epoxide, and oxcarbazepine in the diagnosis of drug-induced hypersensitivity. J Allergy Clin Immunol. 1988;82:110–5. doi: 10.1016/0091-6749(88)90059-0. [DOI] [PubMed] [Google Scholar]
- 40.Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439–44. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 41.Ständer S, Metze D, Luger T, Schwarz T. Drug reaction with eosinophilia and systemic symptoms (DRESS) Hautarzt. 2013;64:611–24. doi: 10.1007/s00105-013-2615-0. [DOI] [PubMed] [Google Scholar]
- 42.Porebski G, Gschwend-Zawodniak A, Pichler WJ. In vitro diagnosis of T cell-mediated drug allergy. Clin Exp Allergy. 2011;41:461–70. doi: 10.1111/j.1365-2222.2011.03701.x. [DOI] [PubMed] [Google Scholar]
- 43.Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J Immunol. 1995;155:462–72. [PubMed] [Google Scholar]
- 44.Yawalkar N, Egli F, Hari Y, Nievergelt H, Braathen LR, Pichler WJ. Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy. 2000;30:847–55. doi: 10.1046/j.1365-2222.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- 45.Hari Y, Frutig-Schnyder K, Hurni M, Yawalkar N, Zanni MP, Schnyder B, et al. T cell involvement in cutaneous drug eruptions. Clin Exp Allergy. 2001;31:1398–408. doi: 10.1046/j.1365-2222.2001.01164.x. [DOI] [PubMed] [Google Scholar]
- 46.Beeler A, Zaccaria L, Kawabata T, Gerber BO, Pichler WJ. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy. 2008;63:181–8. doi: 10.1111/j.1398-9995.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 47.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibodysecreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 48.Sachs B, Erdmann S, Baron JM, al Neis M M ^, Merk HF. Determination of interleukin-5 secretion from drugspecific activated ex vivo peripheral blood mononuclear cells as a test system for the in vitro detection of drug sensitization. Clin Exp Allergy. 2002;32:736–44. doi: 10.1046/j.1365-2222.2002.01382.x. [DOI] [PubMed] [Google Scholar]
- 49.Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera+Saad N, Berard F, et al. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–42. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 50.Tanvarasethee B, Buranapraditkun S, Klaewsongkram J. The potential of using enzyme-linked immunospot to diagnose cephalosporin-induced maculopapular exanthems. Acta Derm Venereol. 2013;93:66–9. doi: 10.2340/00015555-1386. [DOI] [PubMed] [Google Scholar]
- 51.Lochmatter P, Beeler A, Kawabata TT, Gerber BO, Pichler WJ. Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFNgamma in patients with delayed-type drug hypersensitivity. Allergy. 2009;64:1269–78. doi: 10.1111/j.1398-9995.2009.01985.x. [DOI] [PubMed] [Google Scholar]
- 52.Zawodniak A, Lochmatter P, Yerly D, Kawabata T, Lerch M, Yawalkar N, et al. In vitro detection of cytotoxic T and NK cells in peripheral blood of patients with various drug-induced skin diseases. Allergy. 2010;65:376–84. doi: 10.1111/j.1398-9995.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 53.Naisbitt DJ, Farrell J, Wong G, Depta JP, Dodd CC, Hopkins JE, et al. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J Allergy Clin Immunol. 2003;111:1393–403. doi: 10.1067/mai.2003.1507. [DOI] [PubMed] [Google Scholar]
- 54.Polak ME, Belgi G, McGuire C, Pickard C, Healy E, Friedmann PS, et al. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol. 2013;168:539–49. doi: 10.1111/bjd.12109. [DOI] [PubMed] [Google Scholar]
- 55.Fu M, Gao Y, Pan Y, Li W, Liao W, Wang G, et al. Recovered patients with Stevens-Johnson syndrome and toxic epidermal necrolysis maintain long-lived IFN-gamma and sFasL memory response. PLoS One. 2012;7:e45516. doi: 10.1371/journal.pone.0045516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002;2:325–31. doi: 10.1097/00130832-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Posadas SJ, Padial A, Torres MJ, Mayorga C, Leyva L, Sanchez E, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol. 2002;109:155–61. doi: 10.1067/mai.2002.120563. [DOI] [PubMed] [Google Scholar]
- 58.Ebo DG, Leysen J, Mayorga C, Rozieres A, Knol EF, Terreehorst I. The in vitro diagnosis of drug allergy: status and perspectives. Allergy. 2011;66:1275–86. doi: 10.1111/j.1398-9995.2011.02661.x. [DOI] [PubMed] [Google Scholar]
- 59.Barbaud A, Reichert-Penetrat S, Trechot P, Jacquin-Petit MA, Ehlinger A, Noirez V, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49–58. doi: 10.1046/j.1365-2133.1998.02313.x. [DOI] [PubMed] [Google Scholar]
- 60.Osawa J, Naito S, Aihara M, Kitamura K, Ikezawa Z, Nakajima H. Evaluation of skin test reactions in patients with nonimmediate type drug eruptions. J Dermatol. 1990;17:235–9. doi: 10.1111/j.1346-8138.1990.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 61.Lammintausta K, Kortekangas-Savolainen O. The usefulness of skin tests to prove drug hypersensitivity. Br J Dermatol. 2005;152:968–74. doi: 10.1111/j.1365-2133.2005.06429.x. [DOI] [PubMed] [Google Scholar]
- 62.Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127:S67–73. doi: 10.1016/j.jaci.2010.11.047. [DOI] [PubMed] [Google Scholar]