Abstract
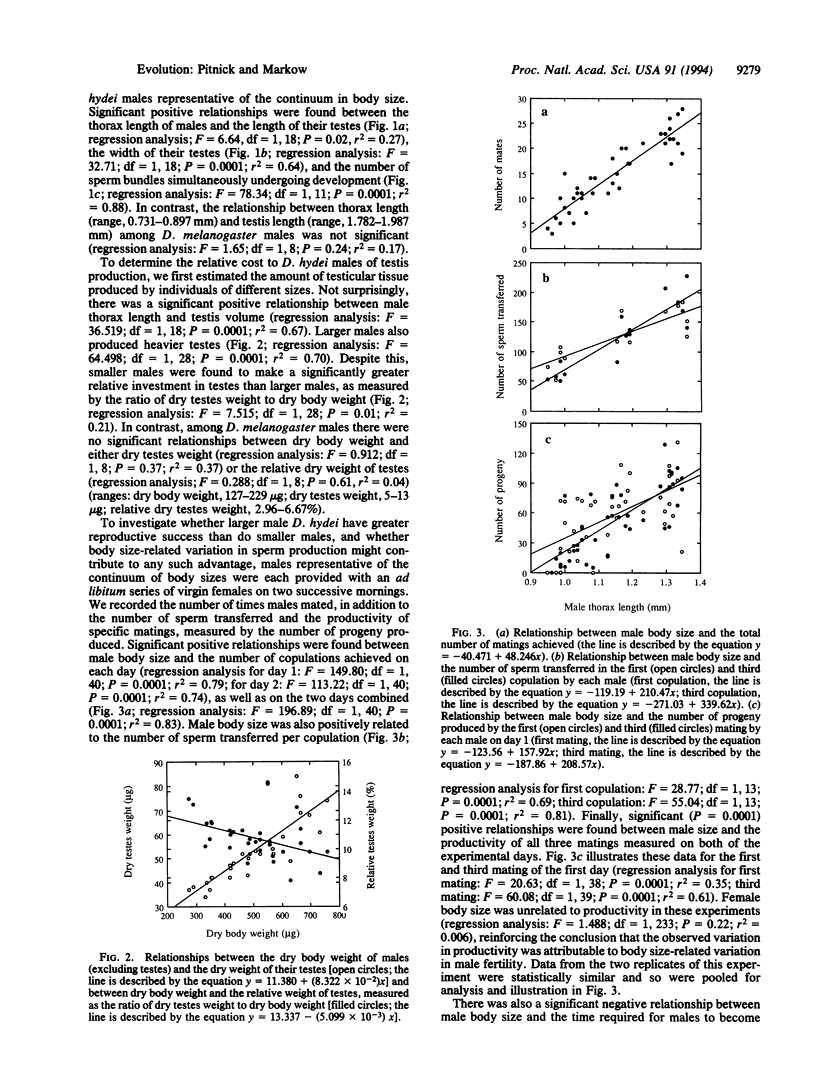
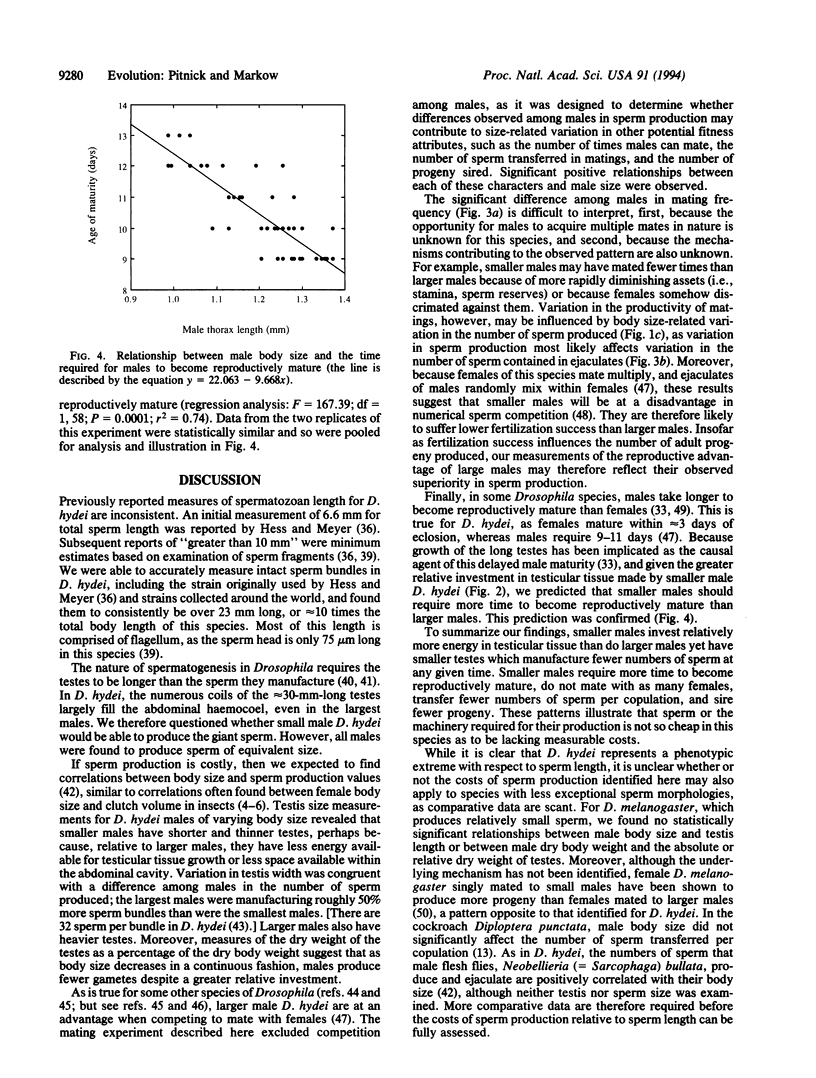
Males of the fruit fly Drosophila hydei were found to produce 23.47 +/- 0.46-mm-long spermatozoa, the longest ever described. No relationship was found between male body size and sperm length. We predicted that if these giant gametes are costly for males to produce, then correlations should exist between male body size, rates of sperm production, and fitness attributes associated with the production of sperm. Smaller males were found to make a greater relative investment in testicular tissue growth, even though they have shorter and thinner testes. Smaller males were also found to (i) be maturing fewer sperm bundles within the testes at any point in time than larger males, (ii) require a longer period of time post-eclosion to become reproductively mature, (iii) mate with fewer females, (iv) transfer fewer sperm per copulation, and (v) produce fewer progeny. The significance of these findings for body size-related fitness and the question of sperm size evolution are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almquist J. O., Branas R. J., Barber K. A. Postpuberal changes in semen production of Charolais bulls ejaculated at high frequency and the relation between testicular measurements and sperm output. J Anim Sci. 1976 Mar;42(3):670–676. doi: 10.2527/jas1976.423670x. [DOI] [PubMed] [Google Scholar]
- Bongso T. A., Hassan M. D., Nordin W. Relationship of scrotal circumference and testicular volume to age and body weight in the swamp buffalo (Bubalus bubalis). Theriogenology. 1984 Aug;22(2):127–134. doi: 10.1016/0093-691x(84)90425-4. [DOI] [PubMed] [Google Scholar]
- Bongso T. A., Jainudeen M. R., Zahrah A. S. Relationship of scrotal circumference to age, body weight and onset of spermatogenesis in goats. Theriogenology. 1982 Nov;18(5):513–524. doi: 10.1016/0093-691x(82)90184-4. [DOI] [PubMed] [Google Scholar]
- Briskie J. V., Montgomerie R. Sperm size and sperm competition in birds. Proc Biol Sci. 1992 Feb 22;247(1319):89–95. doi: 10.1098/rspb.1992.0013. [DOI] [PubMed] [Google Scholar]
- Elmore R. G., Bierschwal C. J., Youngquist R. S. Scrotal circumference measurements in 764 beef bulls. Theriogenology. 1976 Nov;6(5):485–494. doi: 10.1016/0093-691x(76)90115-1. [DOI] [PubMed] [Google Scholar]
- Gomendio M., Roldan E. R. Sperm competition influences sperm size in mammals. Proc Biol Sci. 1991 Mar 22;243(1308):181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- Hahn J., Foote R. H., Seidel G. E., Jr Testicular growth and related sperm output in dairy bulls. J Anim Sci. 1969 Jul;29(1):41–47. doi: 10.2527/jas1969.29141x. [DOI] [PubMed] [Google Scholar]
- Hennig W., Kremer H. Spermatogenesis of Drosophila hydei. Int Rev Cytol. 1990;123:129–175. doi: 10.1016/s0074-7696(08)60673-7. [DOI] [PubMed] [Google Scholar]
- Hess O., Meyer G. F. Genetic activities of the Y chromosome in Drosophila during spermatogenesis. Adv Genet. 1968;14:171–223. doi: 10.1016/s0065-2660(08)60427-7. [DOI] [PubMed] [Google Scholar]
- Karr T. L. Intracellular sperm/egg interactions in Drosophila: a three-dimensional structural analysis of a paternal product in the developing egg. Mech Dev. 1991 Jun;34(2-3):101–111. doi: 10.1016/0925-4773(91)90047-a. [DOI] [PubMed] [Google Scholar]
- Markow T. A., Ricker J. P. Male size, developmental stability, and mating success in natural populations of three Drosophila species. Heredity (Edinb) 1992 Aug;69(Pt 2):122–127. doi: 10.1038/hdy.1992.104. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen K., de Neef K. J., van der Werff ten Bosch J. J., Slob A. K. Testosterone, testis size, seasonality, and behavior in group-living stumptail macaques (Macaca arctoides). Horm Behav. 1987 Jun;21(2):153–169. doi: 10.1016/0018-506x(87)90041-9. [DOI] [PubMed] [Google Scholar]
- Roldan E. R., Gomendio M., Vitullo A. D. The evolution of eutherian spermatozoa and underlying selective forces: female selection and sperm competition. Biol Rev Camb Philos Soc. 1992 Nov;67(4):551–593. doi: 10.1111/j.1469-185x.1992.tb01193.x. [DOI] [PubMed] [Google Scholar]
- Taylor V. A., Luke B. M., Lomas M. B. The giant sperm of a minute beetle. Tissue Cell. 1982;14(1):113–123. doi: 10.1016/0040-8166(82)90011-8. [DOI] [PubMed] [Google Scholar]
- Thompson D. L., Jr, Pickett B. W., Squires E. L., Amann R. P. Testicular measurements and reproductive characteristics in stallions. J Reprod Fertil Suppl. 1979;(27):13–17. [PubMed] [Google Scholar]



