Summary
Anaphylaxis is a potentially fatal, immediate hypersensitivity reaction. Mast cells and basophils, by elaborating vasoactive mediators and cytokines, are the main primary effector cells of anaphylaxis. Mast cells have been identified in human heart between myocardial fibers, perivascularly, in the adventitia, and in the arterial intima. Mast cells isolated from human heart tissue (HHMC) of patients undergoing cardiac transplantation express high affinity immunglobulin E (IgE) receptors (FcεRI), C3a, C5a, and kit receptors (KIT). Anti-IgE, anti-FcεRI, and immunoglobulin superallergens induce in vitro secretion of preformed mediators (histamine, tryptase, chymase, and renin) and the de novo synthesis of cysteinyl leukotriene C4 (LTC4) and prostaglandin D2 (PGD2) from HHMC. Complement is activated and anaphylatoxin forms during anaphylaxis. C5a and C3a cause the in vitro release of histamine and tryptase from HHMC. Therapeutic (general anesthetics, protamine, etc.) and diagnostic agents (radio contrast media, etc.), which can cause anaphylactoid reactions, activate HHMC in vitro. Low concentrations of histamine and cysteinyl leukotrienes given to subjects undergoing diagnostic catheterisation caused significant systemic and coronary hemodynamic effects. These data indicate that human heart mast cells and their mediators play a role in severe anaphylactic reactions.
Keywords: Anaphylaxis, heart, histamine, leukotrienes, mast cells, tryptase
Cite this as Marone G, Genovese A, Varricchi G, Granata F. Human heart as a shock organ in anaphylaxis. Allergo J Int 2014; 23: 60–6
Introduction
Anaphylaxis is the most dramatic and potentially fatal manifestation of immediate hypersensitivity, accounting for more than 500 deaths annually [1, 2]. Anaphylaxis is characterized by laryngeal edema, bronchoconstriction, hypotension, and vascular leakage. It is well known that the heart is directly and/or indirectly involved in severe human anaphylaxis [3, 4, 5, 6, 7, 8, 9]. Cardiac anaphylaxis in humans is characterized by coronary spasm, decreased left ventricular contractility, and/or arrhythmias [10, 11, 12, 13]. Myocardial lesions might be the anatomical basis for the irreversible cardiac failure occasionally associated with systemic anaphylaxis [14].
Coronary spasm or myocardial infarction can occur after insect stings [15, 16] and as a consequence of idiopathic anaphylaxis [17]. Moreover, patients with anaphylaxis may present profound myocardial depression presumably due to the negative inotropic effects of mast cell-derived mediators [18].
In this review we draw together evidence that cardiac mast cells and their mediators play a role in systemic and cardiac human anaphylaxis.
Mast cells are present in human heart
Several years ago Vincenzo Patella, in collaboration with Bärbel Lamparter-Schummert and Monika Adt, established in our laboratory an elegant technique to isolate and purify mast cells from heart tissue of patients undergoing heart transplantation [19, 20, 21].
Mast cells are present in normal and atherosclerotic human intima of coronary arteries [12, 13, 22, 23, 24]. We have identified and characterized mast cells around coronary blood vessels and between myocardial fibers by electron microscopy in all sections of human hearts [19, 20, 25]. Tab. 1 summarizes the three different localizations of mast cells in human cardiac tissue we have found examining sections of more than 50 hearts [19, 20]. A small percentage (about 5 %) of cardiac mast cells appears partially degranulated [23, 19].
| Interstitial mast cells | Close proximity to myocytes Increased density in ischemic and idiopathic cardiomyopathy |
| Perivascular and adventitial mast cells | Always found in the adventitia Increased density in coronary spasm and thrombosis Few mast cells in the arterial media |
| Intimal mast cells | Present in the human arterial intima, at the site of atherogenesis Preferentially located in the shoulder region of atheromas Increased density of activated mast cells in human coronary atheroma |
Fig. 1 shows the electron micrograph of a human cardiac mast cell in close contact with the capillary vessel wall. The presence of mast cells close to coronary vessels suggests that circulating antigens, superallergens, autoantibodies (anti-IgE, anti-FcεRI, etc.), complement (C3a and C5a), drugs (general anesthetics, protamine, etc.), and diagnostic agents (radio contrast media, etc.) can easily reach perivascular HHMC. Activated mast cells can in turn release vasoactive substances (histamine, cysteinyl leukotrienes, PGD2, platelet-activating factor; PAF, etc.) that can affect blood vessels [26].
Fig. 1.
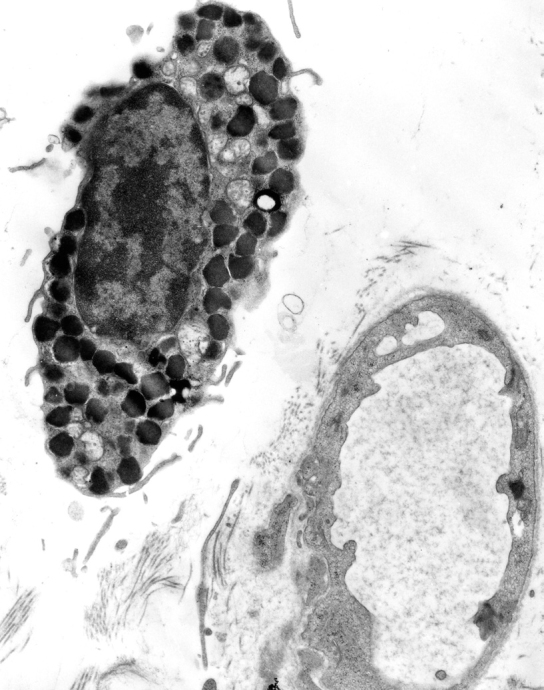
Electron micrograph of a mast cell in human heart tissue. The cytoplasm contains numerous secretory granules. The mast cell is adjacent to a coronary blood vessel. (Stained with uranyl acetate and lead citrate; original magnification 9,000×; reprinted with permission from [13].)
© G. Marone
Cardiac mast cell density and the histamine and tryptase contents of mast cells isolated from failing hearts from patients with idiopathic dilated cardiomyopathy (DCM) and ischemic cardiomyopathy (ICM) undergoing heart transplantation were higher than in control hearts (CH) without cardiovascular disease [27]. Immunologic activation of HHMC induced a significantly greater release of mediators (histamine, tryptase, and LTC4) in patients with failing hearts than in CH. The latter observation suggests that underlying cardiovascular diseases might represent a risk factor for severe anaphylaxis.
Mediators synthesized by HHMC
HHMC contain histamine (≈ 3 pg/cell), tryptase (≈ 24 µg/106 cells) and IgE-mediated activation of HHMC caused tryptase release parallel to histamine secretion [19]. By using the immunogold technique we showed that HHMC contain chymase as well as tryptase [19]. Human chymase generates angiotensin II from angiotensin I, acting as an angiotensin-converting enzyme [28]. Interestingly, supernatants of HHMC challenged in vitro with anti-IgE can convert angiotensin I into angiotensin II, suggesting that chymase released from HHMC play a role in the homeostatic control of blood pressure. Therefore, the activation of HHMC and release of chymase may control blood pressure during anaphylaxis.
We have extended the seminal observation by Roberto Levi´s and Randi B. Silver’s groups [29, 30] by demonstrating that HHMC also contain renin [Marone G., unpublished information]. Thus, there is the possibility that renin released from activated cardiac mast cells can trigger local formation of angiotensin II.
Activation of HHMC with anti-IgE or anti-FcεRI induces the de novo synthesis of PGD2 (≈ 18 ng/106 cells) and LTC4 (≈ 18 ng/106 cells) [19, 20]. This is a relevant observation because both PGD2 and LTC4 have several cardiovascular effects [31, 32], meaning that the release of PGD2 from HHMC can cause coronary vasoconstriction in man.
PAF is an important mediator of anaphylaxis produced by human mast cells [33]. There is preliminary evidence that immunologically activated HHMC synthesize PAF (Triggiani M., unpublished information). Importantly, serum PAF levels are directly correlated and PAF acetylhydrolase levels are inversely correlated with the severity of anaphylaxis [34]. Fig. 2 summarizes the principal receptors present on the surface of HHMC and the mediators released by their activation.
Fig. 2.
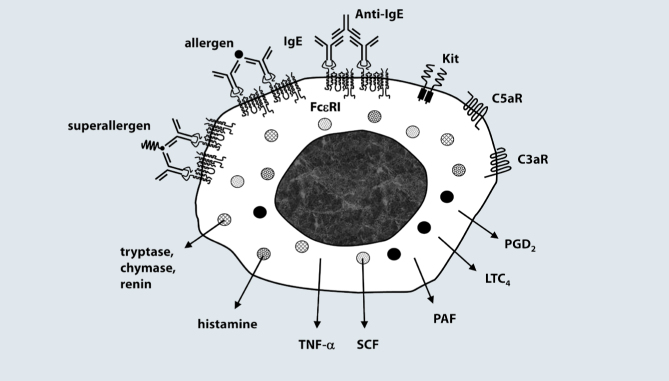
HHMC express the high affinity receptor (FcɛRI) for IgE, the C3a, the C5a, and the Kit receptors. Activation of the IgE receptors on HHMC can be induced by antigens, immunoglobulin superantigens (superallergens), anti-IgE, and anti-FcɛRI autoantibodies. IgE crosslinking and activation of C3aR or C5aR on HHMC induces the release of preformed mediators (histamine, chymase, renin, etc.), of cytokines (TNF-α, SCF, etc.), and the de novo synthesis of lipid mediators (PGD2, LTC4, PAF). Not shown in this figure is a variety of nonimmunological stimuli used for diagnostic (contrast media, etc.) or therapeutic purposes (general anesthetics, protamine, etc.) that can induce the release of mediators from HHMC.
© G. Marone
Cytokines synthesized by HHMC
Cytokines have been implicated in the pathogenesis of a variety of cardiovascular diseases [35]. There is growing evidence of the possible roles of cardiac mast cell-derived cytokines in anaphylaxis. Mast cells in human coronary atheromas contain TNF-α [36]. We have demonstrated the presence of granule-associated stem cell factor (SCF), the principal growth factor for human mast cells [37], in HHMC of patients with dilated cardiomyopathy [27]. Thus, there is the possibility that SCF released by HHMC is an autocrine factor that contributes to mast cell hyperplasia observed in dilated cardiomyopathy [27]. Additional studies are necessary to clarify the roles of other cytokines in anaphylaxis and the contribution of cardiac mast cells to their production.
Stimuli that activate HHMC in vitro
Immunologic activation of HHMC can be induced by IgE-mediated stimuli (antigens, immunoglobulin superantigens, anti-IgE or anti-FcεRI) [19, 20, 38, 39]. Activation of HHMC by anti-IgE and by a monoclonal antibody against an epitope of the α-chain of FcεRI may be clinically important. Histamine-releasing autoantibodies against IgE or against the α-subunit of FcεRI are present in the circulation of some patients with various allergic disorders [40, 41].
Anaphylatoxins (C3a and C5a) are formed when complement is activated during cardiac [42] and systemic anaphylaxis [9]. C5a causes cardiovascular derangement directly or through the release of vasoactive mediators [43, 44]. We demonstrated that C5a and C3a cause rapid histamine release from HHMC [19]. Interestingly, C3a causes cardiac dysfunction in the Langendorff-perfused guinea pig heart system [44]. SCF, which is present in the secretory granules of HHMC [27] and is released after their immunologic activation [45], activates HHMC through the engagement of kit receptor. Eosinophil granule proteins (ECP and MBP) are selective activators of HHMC [20, 46, 47]. The latter observation suggests that eosinophil-mast cell interactions might be clinically relevant in patients with hypereosinophilia.
A variety of non-immunological stimuli can activate HHMC. Some of clinical relevance because they might explain adverse effects observed in vivo when these compounds are used for diagnostic (contrast media, etc.) [3, 7] or therapeutic purposes (general anesthetics, protamine, etc.) [20]. For example, protamine and certain general anesthetics (propofol and atracurium) can cause histamine release from HHMC [20, 48]. Radio contrast media, injected into the coronary arteries for diagnostic purposes, can also activate HHMC in vitro [20]. The proximity of HHMC to coronary blood vessels [12, 13] and the presence of mast cells in human coronary atheromas [23] suggest that intracoronary injection of high doses of contrast media can activate mast cells and induce in vivo release of vasoactive mediators. In fact, in a multicenter study of patients who experienced immediate reactions to the injection of contrast media, the concentrations of plasma histamine and tryptase rose [49].
HHMC in systemic and cardiac anaphylaxis
Roberto Levi’s group has provided extensive evidence that the heart is involved in experimental anaphylaxis [29, 30, 43, 50] through the release of chemical mediators from cardiac mast cells. There is increasing evidence of cardiac involvement in human anaphylaxis too [8, 9, 12, 13]. Initially this has been attributed to mediators originating from the lung and reaching the heart. However, it is likely that the local release of vasoactive mediators by mast cells around coronary arteries and in human coronary atheromas, particularly in patients with ischemic heart disease [23], can contribute to cardiovascular derangements during anaphylaxis. In addition, C5a formation has been documented during anaphylaxis in man [9]. HHMC possess FcεRI, C3a receptor (C3aR), and C5a receptor (C5aR) bound to their membrane surface. Therefore, it is likely that IgE-, C5a-, and C3a-mediated activation of these cells could play a role in systemic and cardiac anaphylaxis in humans [9, 12, 13].
In addition, several agents injected intravenously (general anesthetics, protamine, radio contrast media, etc.) can cause non-IgE-mediated anaphylactic reactions in vitro [20, 48] and in vivo [49]. Therefore, the release of mediators caused by these agents from cardiac mast cells may well be involved in anaphylactic reactions related to these compounds.
Cardiovascular effects of histamine in man
Several years ago, in collaboration with our colleagues of the Division of Cardiology at the University of Naples Federico II, we have investigated the effects of histamine on peripheral and coronary hemodynamics in man. Low doses of histamine (0.4 µg/kg/min) infused in patients with normal left ventricular (LV) function undergoing diagnostic catheterisation induced significant drops in systolic, diastolic, and mean aortic pressure, systemic vascular resistance, LV end-diastolic pressure, and stroke index [51]. Hemodynamic changes started 1–2 min after the infusion started and reverted to normal within 5 min after the infusion. In one subject there was a transient progression from first to third degree atrioventricular block, with prompt recovery of atrioventricular conduction at the end of the infusion. Thus, exogenous histamine in man causes significant transient hemodynamic changes, mainly systemic hypotension, tachycardia, and increased LV performance. These changes can be partly attributed to the related increase in sympathoadrenergic activity, although it cannot be excluded that histamine has some direct cardiac effect.
In a subsequent study, the effects of selective activation of histamine H1 receptors on coronary hemodynamics were examined in two groups: patients with atypical chest pain and normal coronary arteries and patients with vasospastic angina [52, 53]. H1 receptor stimulation was achieved by infusing histamine intravenously for 5 min after pretreatment with cimetidine to antagonize the H2 receptors. Heart rate was kept constant by coronary sinus pacing. In the first group mean aortic pressure and coronary vascular resistance (CVR) dropped, while coronary blood flow (CBF) and myocardial oxygen consumption remained unchanged during histamine infusion. No patient in this group developed angina during histamine infusion. By contrast, a percentage of the second group developed angina during histamine infusion (≈40 %), with a decrease in CBF and an increase in CVR. These findings suggest that stimulation of the H1 receptor in subjects with normal coronary arteries reduces CVR, probably because of vasodilation of small coronary resistance vessels. However, in some patients with vasospastic angina, H1 receptor activation can cause vasoconstriction of large-capacitance coronary arteries.
The results of these studies support the hypothesis that the endogenous release of histamine during anaphylactic reactions may precipitate coronary spasm in a subset of patients with vasospastic angina. Interestingly, it has been reported that premedication with an H2 receptor antagonist increases the risk of heart block in patients who develop anaphylaxis [54].
Studies have now started to clarify the role of H3 receptors in the cardiovascular system. Roberto Levi and collaborators have identified H3 receptors as inhibitory heteroreceptors in cardiac adrenergic nerve endings [55, 56] suggesting a mechanism by which endogenous histamine can activate norepinephrine release in normal and ischemic conditions [57]. The presence of H3 receptors in human heart [55] suggests that these receptors might be directly and/or indirectly involved in anaphylactic reactions.
Cardiovascular effects of cysteinyl leukotrienes in man
In collaboration with cardiologists we have evaluated the effects of cysteinyl leukotriene D4 (LTD4) on systemic and coronary hemodynamic in patients with normal coronary arteries [17]. LTD4 induced an early (20 s) transient fall in mean arterial pressure paralleled by rises in heart rate and plasma levels of catecholamines which returned to baseline after 10 min. CVR rose at 10 and 15 min. Thus, small doses of cysteinyl leukotrienes may induce both an early transient fall in mean arterial pressure, with secondary sympatho-adrenergic activation, and a later increase in small coronary arteriolar resistance. Although high levels of cysteinyl leukotriene receptor CysLT2 mRNA were detected in the human heart and coronary artery, while cysteinyl leukotriene receptor CysLT1 mRNA was barely detectable [58], it is not clear which receptor was involved in these cardiovascular effects.
Conclusion
Within human cardiac tissue, mast cells are located between myocytes and are in close contact with blood vessels [12, 13, 19, 20, 22, 45]. They are also found in the coronary adventitia and in the shoulder regions of coronary atheroma [22, 23, 24].
Circulating antigens, superantigens, autoantibodies (anti-IgE, anti-FcεRI, etc.), eosinophil granule proteins (major basic protein, MBP, and eosinophil cationic protein ECP), therapeutic (e. g. protamine, general anesthetics) or diagnostic substances (e. g. radio-contrast media) can induce the release of mediators from HHMC. These cells express FcεRI, C5aR, and C3aR, which could explain the involvement of cardiac mast cells in systemic and cardiac anaphylaxis.
The increases in cardiac mast cell density and release of vasoactive mediators in patients with cardiomyopathy might also have clinical implications given the marked cardiovascular effects of histamine, cysteinyl leukotrienes, and PGD2 [31, 32, 51, 52, 53].
Pioneering studies have started to shed light on the cardiovascular effects in humans caused by such mast cell-derived mediators as histamine [51, 52, 53] and leukotrienes [12]. Interestingly, these studies provided the important information that the hemodynamic effects of mediators depend on both the underlying cardiovascular conditions and pharmacologic treatment (e. g., H2 blockade). Using specific antagonists of CysLT1 and CysLT2 [58] it will be possible to assess the each receptor’s contribution to the cardiovascular effects of these vasoactive mediators. Immunologically activated human mast cells synthesize PAF [33], which can play a role in human [34] and experimental anaphylaxis [59].
In conclusion, there is extensive experimental and clinical evidence that the human heart can be viewed as both a site and a target in anaphylaxis. Pharmacological targeting of cardiac mast cells might offer novel therapeutic opportunities to prevent and/or modulate the dramatic and potentially fatal manifestations of anaphylaxis.
Abbreviations
- C3aR
C3a receptor
- C5aR
C5a receptor
- CH
Control heart
- CBF
Coronary blood flow
- CVR
Coronary vascular resistance
- CysLT2
Cysteinyl leukotriene receptor 2
- CysLT1
Cysteinyl leukotriene receptor 1
- DCM
Dilated cardiomyopathy
- ECP
Eosinophil cationic protein
- FcɛRI
High affinity immunglobulin E receptors
- HHMC
Mast cells isolated from human heart tissue
- ICM
Ischemic cardiomyopathy
- IgE
Immunglobulin E
- LTC4
Cysteinyl leukotriene C4
- LTD4
Cysteinyl leukotriene D4
- LV
Left ventricular
- MBP
Major basic protein
- PAF
Platelet-activating factor
- PGD2
Prostaglandin D2
- SCF
Stem cell factor
Footnotes
Conflicts of interest
The authors states that there are no conflicts of interest.
Acknowledgments
This paper is dedicated to Professor Johannes Ring in recognition of his pioneering studies on anaphylaxis. This work was supported in part by grants from the Ministero dell’Istruzione, Università e Ricerca (MIUR; Marone G. and Genovese A.), the Istituto Superiore di Sanità AIDS Project „Role of basophils in HIV-1 infection“ (Marone G.), Regione Campania „CISI-Lab Project“ and CRÈME Project (Marone G.). Marone G. is the recipient of the Paul Ehrlich Award 2011.
References
- 1.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–32. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 2.Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–9. doi: 10.1016/S0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 3.Brockow J, Ring J. Anaphylaxis to radiographic contrast media. Curr Opin Allergy Clin Immunol. 2011;11:326–331. doi: 10.1097/ACI.0b013e32834877c3. [DOI] [PubMed] [Google Scholar]
- 4.Criep LH, Woehler TR. The heart in human anaphylaxis. Ann Allergy. 1971;29:399–409. [PubMed] [Google Scholar]
- 5.Hanashiro PK, Weil MH. Anaphylactic shock in man. Report of two cases with detailed hemodynamic and metabolic studies. Arch Intern Med. 1967;119:129–40. doi: 10.1001/archinte.1967.00290200053001. [DOI] [PubMed] [Google Scholar]
- 6.Matucci A, Vultaggio A, Fassio F, Rossi O, Maggi E. Heart as the early main target of severe anaphylactic reactions: two case reports. Intern Emerg Med. 2011;6:467–9. doi: 10.1007/s11739-010-0482-6. [DOI] [PubMed] [Google Scholar]
- 7.Ring J. Chem Immunol Allergy. Basel: Karger; 2010. Anaphylaxis. [Google Scholar]
- 8.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–84. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 9.Smith PL, Kagey-Sobotka A, Bleecker ER, Traystman R, Kaplan AP, Gralnick H, et al. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980;66:1072–80. doi: 10.1172/JCI109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernreiter M. Electrocardiogram of patient in anaphylactic shock. JAMA. 1959;170:1628–30. doi: 10.1001/jama.1959.03010140008003. [DOI] [PubMed] [Google Scholar]
- 11.Booth BH, Patterson R. Electrocardiographic changes during human anaphylaxis. JAMA. 1970;211:627–31. doi: 10.1001/jama.1970.03170040031006. [DOI] [PubMed] [Google Scholar]
- 12.Marone G, de Crescenzo G, Patella V, Genovese A. Human cardiac mast cells and their role in severe allergic reactions. In: Marone G, Austen KF, Holgate ST, Kay AB, Lichtenstein LM, editors. Asthma and Allergic Diseases. Physiology, Immunopharmacology and Treatment. London: Academic Press; 1998. p. 237. [Google Scholar]
- 13.Marone G, de Crescenzo G, Patella V, Granata F, Verga L, Arbustini E, et al. Human heart mast cells: immunological characterization in situ and in vitro. In: Marone G, Lichtenstein LM, Galli SJ, et al., editors. Mast Cells and Basophils. London: Academic Press; 2000. p. 454. [Google Scholar]
- 14.Delage C, Irey NS. Anaphylactic deaths: a clinicopathologic study of 43 cases. J Forensic Med. 1972;17:525–40. [PubMed] [Google Scholar]
- 15.Bristow MR, Ginsburg R, Kantrowitz NE, Baim DS, Rosenbaum JT. Coronary spasm associated with urticaria: report of a case mimicking anaphylaxis. Clin Cardiol. 1982;5:238–40. doi: 10.1002/clc.4960050307. [DOI] [PubMed] [Google Scholar]
- 16.Levine HD. Acute myocardial infarction following wasp sting. Am Heart J. 1976;91:365–74. doi: 10.1016/S0002-8703(76)80222-0. [DOI] [PubMed] [Google Scholar]
- 17.Wong S, Greenberger PA, Patterson R. Nearly fatal idiopathic anaphylactic reaction resulting in cardiovascular collapse and myocardial infarction. Chest. 1990;98:501–03. doi: 10.1378/chest.98.2.501. [DOI] [PubMed] [Google Scholar]
- 18.Raper RF, Fisher MM. Profound reversible myocardial depression after anaphylaxis. Lancet. 1988;1:386–88. doi: 10.1016/S0140-6736(88)91184-1. [DOI] [PubMed] [Google Scholar]
- 19.Patella V, Marinò I, Lamparter B, Arbustini E, Adt M, Marone G. Human heart mast cells. Isolation, purification, ultrastructure and immunologic characterization. J Immunol. 1995;154:2855–65. [PubMed] [Google Scholar]
- 20.Patella V, de Crescenzo G, Ciccarelli A, Marinò I, Adt M, Marone G. Human heart mast cells: a definitive case of mast cell heterogeneity. Int Arch Allergy Immunol. 1995;106:386–93. doi: 10.1159/000236871. [DOI] [PubMed] [Google Scholar]
- 21.Patella V, de Crescenzo G, Marino I, Genovese A, Adt M, Gleich GJ, et al. Eosinophil granule proteins are selective activators of human heart mast cells. Int Arch Allergy Immunol. 1997;113:200–2. doi: 10.1159/000237546. [DOI] [PubMed] [Google Scholar]
- 22.Forman MB, Oates JA, Robertson D, Robertson RM, Roberts LJ, Virmani R. Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med. 1985;313:1138–41. doi: 10.1056/NEJM198510313131807. [DOI] [PubMed] [Google Scholar]
- 23.Kaartinen M, Penttilä A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90:1669–78. doi: 10.1161/01.CIR.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 24.Kamat BR, Galli SJ, Barger AC, Lainey LL, Silverman KJ. Neovascularization and coronary atherosclerotic plaque: cinematographic localization and quantitative histologic analysis. Hum Pathol. 1987;18:1036–42. doi: 10.1016/S0046-8177(87)80220-4. [DOI] [PubMed] [Google Scholar]
- 25.Marone G, Triggiani M, Cirillo R, Vigorito C, Genovese A, Spampinato N, et al. Chemical mediators and the human heart. Prog Biochem Pharmacol. 1985;20:38–54. [PubMed] [Google Scholar]
- 26.Marone G, de Crescenzo G, Florio G, Granata F, Dente V, Genovese A. Immunological modulation of human cardiac mast cells. Neurochem Res. 1999;24:1195–202. doi: 10.1023/A:1020776807187. [DOI] [PubMed] [Google Scholar]
- 27.Patella V, Marinò I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971–78. doi: 10.1161/01.CIR.97.10.971. [DOI] [PubMed] [Google Scholar]
- 28.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–57. [PubMed] [Google Scholar]
- 29.Mackins CJ, Kano S, Sevedi N, Schafer U, Reid AC, Machida T, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–70. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, et al. Mast cells: a unique source of renin. Proc Natl Acad Sci USA. 2004;101:13607–12. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hattori Y, Levi R. Effect of PGD2 on cardiac contractility: a negative inotropism secondary to coronary vasoconstriction conceals a primary positive inotropic action. J Pharmacol Exp Ther. 1986;237:719–24. [PubMed] [Google Scholar]
- 32.Vigorito C, Giordano A, Cirillo R, Genovese A, Rengo F, Marone G. Metabolic and hemodynamic effects of peptide leukotriene C4 and D4 in man. Int J Clin Lab Res. 1997;27:178–84. doi: 10.1007/BF02912454. [DOI] [PubMed] [Google Scholar]
- 33.Triggiani M, Hubbard WC, Chilton FH. Synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine by an enriched preparation of the human lung mast cell. J Immunol. 1990;144:4773–80. [PubMed] [Google Scholar]
- 34.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-Activating Factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 35.Sasayama S, Matsumori A, Kihara Y. New insights into the pathophysiological role for cytokines in heart failure. Cardiovasc Res. 1999;42:557–64. doi: 10.1016/S0008-6363(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaartinen M, Penttilä A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-α. Circulation. 1996;94:2787–92. doi: 10.1161/01.CIR.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 37.Columbo M, Horowitz EM, Botana LM, MacGlashan DW, Jr, Bochner BS, Gillis S, et al. The human recombinant c-kit receptor ligand, rhSCF, induces mediator release from human cutaneous mast cells and enhances IgE-dependent mediator release from both skin mast cells and peripheral blood basophils. J Immunol. 1992;149:599–608. [PubMed] [Google Scholar]
- 38.Genovese A, Bouvet JP, Florio G, Lamparter-Schummert B, Björck L, Marone G. Bacterial immunoglobulin superantigen proteins A and L activate human heart mast cells by interacting with immunoglobulin E. Infect Immun. 2000;68:5517–24. doi: 10.1128/IAI.68.10.5517-5524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genovese A, Borgia G, Bouvet JP, Detoraki A, de Paulis A, Piazza M, et al. Protein Fv produced during viral hepatitis is an endogenous immunoglobulin superantigen activating human heart mast cells. Int Arch Allergy Immunol. 2003;132:336–45. doi: 10.1159/000074901. [DOI] [PubMed] [Google Scholar]
- 40.Hide M, Francis DM, Grattan CEH, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 41.Marone G, Casolaro V, Paganelli R, Quinti I. IgG anti-IgE from atopic dermatitis induces mediator release from basophils and mast cells. J Invest Dermatol. 1989;93:246–52. doi: 10.1111/1523-1747.ep12277582. [DOI] [PubMed] [Google Scholar]
- 42.del Balzo U, Polley MJ, Levi R. Activation of the third complement component (C3) and C3a generation in cardiac anaphylaxis: histamine release and associated inotropic and chronotropic effects. J Pharmacol Exp Ther. 1988;246:911–16. [PubMed] [Google Scholar]
- 43.del Balzo U, Levi R, Polley MJ. Cardiac dysfunction caused by purified human C3a anaphylatoxin. Proc Natl Acad Sci USA. 1985;82:886–90. doi: 10.1073/pnas.82.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del Balzo U, Polley MJ, Levi R. Cardiac anaphylaxis. Complement activation as an amplification system. Circ Res. 1989;65:847–57. doi: 10.1161/01.RES.65.3.847. [DOI] [PubMed] [Google Scholar]
- 45.de Paulis A, Minopoli G, Arbustini E, de Crescenzo G, Dal Piaz F, Pucci P, et al. Stem cell factor is localized in, released from, and cleaved by human mast cells. J Immunol. 1999;163:2799–808. [PubMed] [Google Scholar]
- 46.Marone G, Patella V, de Crescenzo G, Granata F, Calabrese C. Immunological interactions between human eosinophils and cardiac mast cells. Chem Immunol. 2000;76:118–33. doi: 10.1159/000058784. [DOI] [PubMed] [Google Scholar]
- 47.Patella V, de Crescenzo G, Marinò I, Genovese A, Adt M, Gleich GJ, et al. Eosinophil granule proteins activate human heart mast cells. J Immunol. 1996;157:1219–25. [PubMed] [Google Scholar]
- 48.Stellato C, de Crescenzo G, Patella V, Mastronardi P, Mazzarella B, Marone G. Human basophil/mast cell releasability. XI. Heterogeneity of the effects of contrast media on mediator release. J Allergy Clin Immunol. 1996;97:838–50. doi: 10.1016/S0091-6749(96)80162-X. [DOI] [PubMed] [Google Scholar]
- 49.Laroche D, Aimone-Gastin I, Dubois F, Huet H, Gérard P, Vergnaud MC, et al. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology. 1998;209:183–90. doi: 10.1148/radiology.209.1.9769830. [DOI] [PubMed] [Google Scholar]
- 50.Capurro N, Levi R. The heart as a target organ in systemic allergic reactions: comparison of cardiac anaphylaxis in vivo and in vitro. Circ Res. 1975;36:520–08. doi: 10.1161/01.RES.36.4.520. [DOI] [PubMed] [Google Scholar]
- 51.Vigorito C, Russo P, Picotti GB, Chiariello M, Poto S, Marone G. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. 1983;5:531–37. doi: 10.1097/00005344-198307000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Vigorito C, Poto S, Picotti GB, Triggiani M, Marone G. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation. 1986;73:1175–82. doi: 10.1161/01.CIR.73.6.1175. [DOI] [PubMed] [Google Scholar]
- 53.Vigorito C, Giordano A, De Caprio L, Vitale DF, Maurea N, Silvestri P, et al. Effects of histamine on coronary hemodynamics in man: role of H1 and H2 receptors. J Am Coll Cardiol. 1987;10:1207–13. doi: 10.1016/S0735-1097(87)80120-1. [DOI] [PubMed] [Google Scholar]
- 54.Patterson LJ, Milne B. Latex anaphylaxis causing heart block: role of ranitidine. Can J Anaesth. 1999;46:776–78. doi: 10.1007/BF03013914. [DOI] [PubMed] [Google Scholar]
- 55.Imamura M, Seyedi N, Lander HM, Levi R. Functional identification of histamine H3-receptors in the human heart. Circ Res. 1995;77:206–10. doi: 10.1161/01.RES.77.1.206. [DOI] [PubMed] [Google Scholar]
- 56.Imamura M, Lander HM, Levi R. Activation of histamine H3-receptors inhibits carrier-mediated norepinephrine release during protracted myocardial ischemia. Comparison with adenosine A1 receptors and α2-adrenoceptors. Circ Res. 1996;78:475–81. doi: 10.1161/01.RES.78.3.475. [DOI] [PubMed] [Google Scholar]
- 57.Silver RB, Poonwasi KS, Seyedi N, Wilson SJ, Lovenberg TW, Levi R. Decreased intracellular calcium mediates the histamine H3-receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings. Proc Natl Acad Sci USA. 2002;99:501–06. doi: 10.1073/pnas.012506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamohara M, Takasaki J, Matsumoto M, Matsumoto S-I, Saito T, Soga T, et al. Functional characterization of cysteinyl leukotriene CysLT2 receptor on human coronary artery smooth muscle cells. Biochem Biophys Res Commun. 2001;287:1088–92. doi: 10.1006/bbrc.2001.5695. [DOI] [PubMed] [Google Scholar]
- 59.Choi IW, Kim YS, Kim DK, Choi JH, Seo KH, Im SY, et al. Platelet-activating factor-mediated NF-kappaB dependency of a late anaphylactic reaction. J Exp Med. 2003;198:145–51. doi: 10.1084/jem.20022129. [DOI] [PMC free article] [PubMed] [Google Scholar]


