Abstract
Objective
The trigeminovascular system plays a central role in migraine, a condition in need of new treatments. The neuropeptide, calcitonin gene-related peptide (CGRP), is proposed as causative in migraine and is the subject of intensive drug discovery efforts. This study explores the expression and functionality of two CGRP receptor candidates in the sensory trigeminal system.
Methods
Receptor expression was determined using Taqman G protein-coupled receptor arrays and immunohistochemistry in trigeminal ganglia (TG) and the spinal trigeminal complex of the brainstem in rat and human. Receptor pharmacology was quantified using sensitive signaling assays in primary rat TG neurons.
Results
mRNA and histological expression analysis in rat and human samples revealed the presence of two CGRP-responsive receptors (AMY1: calcitonin receptor/receptor activity-modifying protein 1 [RAMP1]) and the CGRP receptor (calcitonin receptor-like receptor/RAMP1). In support of this finding, quantification of agonist and antagonist potencies revealed a dual population of functional CGRP-responsive receptors in primary rat TG neurons.
Interpretation
The unexpected presence of a functional non-canonical CGRP receptor (AMY1) at neural sites important for craniofacial pain has important implications for targeting the CGRP axis in migraine.
Introduction
Migraine is a painful and debilitating neurological disorder, which is estimated to affect 11–15% of people worldwide.1 It is characterized by severe headache, nausea, and hyper-sensitivity to light and sound. Migraine also exhibits many forms, including chronic, frequent episodic, acute, with or without aura. Each of these potentially requires distinctive clinical management. Developing new treatments for migraine is an important clinical goal because there are few existing migraine treatments and the majority of sufferers have limited options, tending to utilize non-steroidal anti-inflammatories or the triptans, currently the major class of anti-migraine drug.2
The triptans target the serotonin (5-hydroxytryptamine) subclass of G protein-coupled receptors (GPCRs). As proven targets for ∼30% of medicines, including existing pain medications, GPCRs are a logical choice for further interrogation in the development of new drugs.3 The calcitonin gene-related peptide (CGRP) receptor is a GPCR that has attracted considerable interest for treating migraine. CGRP receptor antagonists were developed that progressed into phase II/III clinical trials for acute migraine and migraine prophylaxis.4 Whilst these molecules were efficacious, the overall response to them was lower than predicted and some suggested that central nervous system (CNS) penetration might be required for improved efficacy.5,6 This suggestion was tested in a recent study which quantified, in vivo, CGRP receptor occupancy by telcagepant in the CNS. This study suggested that at efficacious doses for migraine therapy, telcagepant displayed low receptor occupancy in the CNS. They concluded that telcagepant is unlikely to block CGRP action in the CNS and that CNS penetration is unlikely to be required for efficacy.7 However, the possibility that CNS blockade of CGRP receptor sites may offer enhanced efficacy cannot be ruled out. Recent positive clinical trials with anti-CGRP monoclonal antibodies for chronic or frequent episodic migraine indicate that efficacy can be achieved via actions at the periphery alone.8,9 The CGRP axis clearly has immense potential as a target in various forms of migraine but a greater understanding of its mechanism of action is required to exploit this system to its fullest.
Target engagement for molecules targeting the CGRP axis has utilized dermal vasodilation models and shown that these molecules effectively block vascular CGRP receptors. Although useful models, CGRP-induced dermal vasodilation is not altered in migraine sufferers10 and vasodilation and migraine pain appear to be disconnected.11 Neural models have not routinely been employed because the key neural migraine pain circuitry is much more challenging to study. This has resulted in a relative paucity of information regarding the pharmacology of neural CGRP receptors, their sensitivity to CGRP receptor antagonists and fundamental mechanisms of pain processing involving CGRP.
Although the precise pathophysiology of migraine is unclear, the trigeminal (fifth cranial or V) nerve appears to play a major role in processing signals of migraine pain.12,13 The cell bodies for sensory trigeminal neurons reside in the trigeminal ganglia (TG), with central projections descending into the brainstem via the spinal trigeminal tract (sp5) and terminating within the spinal trigeminal nucleus (Sp5) and at the C1–C2 levels in the spinal cord; we refer to these as the spinal trigeminal complex.14 Neurons derived from the TG therefore provide a useful model for studying molecular mechanisms of pain processing.
We isolated TG neurons and conducted GPCR arrays to identify potential CGRP receptor candidates and also novel GPCR targets for migraine and other forms of head pain. Our ensuing pharmacological and histological characterization revealed that in addition to the CGRP receptor, a second CGRP-responsive receptor was expressed. This finding offers a potential explanation for the modest clinical efficacy of available CGRP receptor antagonists and potential therapeutic opportunities for the future.
Subjects/Materials and Methods
Isolation and culture of TG neurons
Isolation and culture of TG neurons was as previously published.15 All procedures involving the use of animals at the University of Auckland were conducted in accordance with the New Zealand Animal Welfare Act (1999) and approved by the University of Auckland Animal Ethics Committee.
RNA extraction and array methods
TG neurons were prepared from 12 Wistar rat pups as previously published15 and cultured in poly-d-lysine/laminin-coated 3.5 cm dishes. RNA was extracted using Trizol (Sigma-Aldrich, St. Louis, MO) using the manufacturer’s protocol and further purified using an RNeasy kit with DNaseI treatment (Qiagen, Valencia, CA). RNA was converted to cDNA using SuperScript III reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s protocol. As previously described,16 GPCR expression was determined with Taqman GPCR Arrays (Applied Biosystems, Life Technologies Corp., Carlsbad, CA) using 1 ng/μL cDNA according to the recommended protocol, run on an ABI Prism 7900HT system (Applied Biosystems), and analyzed with the Sequence Detection System software and RQ Manager 1.2.1 (Applied Biosystems). For quantification, GPCR expression was expressed relative to 18S rRNA as ΔCt (cycle-threshold values). The ΔCt above which gene expression was considered undetectable represents the difference between 40 cycles (the maximum number of PCR cycles run) and the 18S Ct value. The ΔCt that defined the “undetectable” cutoff value varied among arrays, according to the 18S Ct value, and was between 25.5 and 28.8. Data shown are the average of four independent arrays. Appendix S1 provides the average expression [Δ C(t)] data (with SD) for all GPCRs, control genes, and GPCR-associated genes, in addition to the array-specific identification number, formal gene name (U.S. National Center for Biotechnology Information [NCBI] Reference Sequence [RefSeq], GenBank) and the number of samples in which each transcript was detected.
Gene expression in human TG
The expression of genes in human TG was determined from eight microarray experiments (four males and four females) deposited by Richard Roth (Neurocrine Biosciences, Inc., San Diego, CA). Data were accessed from the NCBI GEO database accession number GSE7307.17,18 Data are expressed as the mean log 2RMA ± SEM.
Cell culture and transfection
Culture of Cos7 and HEK293S cells was performed as previously described.19 Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 8% heat-inactivated fetal bovine serum and 5% (v/v) penicillin/streptomycin and kept in a 37°C humidified 95% air, 5% CO2 incubator. Cells were seeded into 96-well plates at a density of 15,000 cells per well (determined using a Countess Counter™, Invitrogen) 1 day prior to transfection. Cells were transiently transfected using polyethylenimine as described previously.20 Rat calcitonin receptor (CTR) in pcDNA1 was kindly provided by Professor Patrick Sexton (Monash Institute of Pharmaceutical Sciences). All other vectors were pcDNA3 or 3.1. Rat calcitonin receptor-like receptor (CLR) and rat receptor activity-modifying protein 1 (RAMP1) vectors were used as previously described.20 Hemagglutinin-tagged human CTR and myc-tagged human RAMP1 were used as previously described.21
cAMP assays in rat TG neurons and transfected cells
cAMP assays were performed in TG neurons as previously described.15 Briefly, TG neurons were stimulated by agonist with or without antagonists for 30 min at room temperature. cAMP assays were performed with 1 mmol/L 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich, St. Louis, MO). cAMP content was determined using the LANCE ultra cAMP detection kit (Perkin Elmer Life and Analytical Sciences, Waltham, MA). cAMP was measured in transfected Cos7 cells using the AlphaScreen cAMP assay kit (Perkin Elmer) as described.22 These cAMP assays were also performed with 1 mmol/L IBMX. For both assays, plates were read using an Envision plate reader (Perkin Elmer). All peptides were purchased from American Peptide (Sunnyvale, CA) or Bachem (Bubendorf, Switzerland). Olcegepant was kindly provided by Boehringer-Ingelheim (Ingelheim, Germany). Telcagepant was purchased from Suzhou Rovathin Pharmatech Co. (Jiangsu, China). Forskolin (Tocris, Bristol, UK) was used as a positive control.
Histology in cultured cells
Cultured TG neurons or transfected cells were fixed using 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 30 min (TG neurons) or 10 min (HEK293S cells) at room temperature in cell culture plates. Cells were washed twice with PBS and stored at 4°C. For the detection of CLR and RAMP1, antigen retrieval was performed by incubating cells overnight followed by microwaving cells for 1 min in 0.1 mol/L citric acid (pH 4.5). Antigen retrieval was not required for CTR staining. Irrespective, cells were incubated in PBS containing 0.6% hydrogen peroxide at room temperature for 20 min. Cells were washed twice with PBS and blocked with 10% goat serum or rabbit serum (depending on the species of the secondary antibody) for 1 h. Cells were incubated at 4°C overnight with specific primary antibodies (Table S1) in PBS containing 1% serum. These primary antibodies were selected as they are well characterized and recognize their target proteins with high specificity23–27 (and Fig. S1). Cells were washed with PBS and incubated with secondary antibody (Table S1) at room temperature with gentle shaking for 1 h. Cells were washed in PBS and incubated with streptavidin HRP-polymer (1:1000; Sigma-Aldrich) diluted in PBS containing 5% serum and incubated at room temperature for 1 h. Cells were washed twice in PBS and visualized by incubation for 5 min in SIGMAFAST 3,3′-Diaminobenzidine tablets (Sigma-Aldrich, St. Louis, MO) pre-dissolved in PBS. Cells were washed twice with PBS and then imaged under PBS using a Nikon Ti-E inverted microscope at 200× magnification.
Histology in rat TG
Rat TG histology was performed as described previously.23 Briefly, TG were removed from four male Sprague-Dawley rats weighing 300–350 g (Scanbur, Stockholm, Sweden). The ganglia were placed in 4% PFA and fixed for 2–4 h. After fixation the tissues were rinsed in raising concentrations of sucrose in Sörensen′s phosphate buffer, embedded, sectioned and stored as the human samples (below). The experiments were approved by the University Animal Ethics Committee (M8-09), Lund University, Sweden. Antibody details and detailed immunostaining methodology are described in Table S1.
Human histology
We used post-mortem human tissue from New Zealand and Europe. Details of source, gender and post-mortem delay are specified in Table S2. Tissue samples were processed and stained according to the full methodological details provided in Data S1. In New Zealand, human brainstems were obtained from the Neurological Foundation of New Zealand Human Brain Bank in the Centre for Brain Research at the University of Auckland and ethical approval was obtained from the University of Auckland Human Participants ethics committee. These brains had no history of neurological disease and showed no neurological abnormalities on pathological examination. In Europe, human brainstem and TG samples were obtained at autopsy from adult subjects in accordance with the Faculty of Medicine University of Szeged guidelines for ethics in human tissue experiments and were approved by the local Hungarian Ethics Committee. The procedures for the human samples were conducted according to the principles outlined in the Declaration of Helsinki. None of the subjects had a CNS disorder.
Data analysis
All statistical analysis was performed using GraphPad Prism 5.0 or 6.0 (GraphPad Software, San Diego, CA). Maximal cAMP responses (Emax) were determined and the data expressed as a percentage of the Emax or the response given by the highest agonist concentration. pEC50 values were obtained by fitting a four-parameter logistic equation to the concentration-response curve data. To determine if the Hill slope was significantly different from one for agonist potency curves, F-tests were performed. For the majority of experiments, the Hill slope was not significantly different to unity. The agonist potency curves were therefore re-fitted with a Hill slope constrained to one and pEC50’s obtained. pA2 values were calculated from pEC50 values obtained in the presence or absence of antagonist. Separate experiments comprise individual TG neuron preparations performed in triplicate or individual transient transfections. For statistical analysis, pEC50 and pA2 values from individual experiments were combined and determined using Student’s t-tests or one-way analysis of variance (ANOVA) with post hoc Tukey’s tests. Statistical significance was defined as P < 0.05. All data points represent the mean ± SEM combined from n separate experiments.
Results
GPCR microarray and expression of CGRP receptor components in rat TG neurons
We first determined the expression of CGRP receptors in rat TG neurons. To do this we used a Taqman GPCR array to determine the global expression profile of non-chemosensory GPCRs in four independent samples of TG neurons from neonatal rats.16 In addition to providing CGRP receptor expression, this approach gives greater insight into the expression profile of receptors and G proteins in the TG, and may be a useful resource for those investigating novel trigeminal GPCR drug targets.
Of the 318 GPCRs in this array, detectable expression ranged between 242 and 277 GPCRs across the four samples. GPCR accessory proteins were also detected. The G protein linkage of the detected GPCRs was 97 Gi/o, 37 Gs, 85 Gq/11, 12 G12/13, 10 “other” (e.g. gustducin, transducin)–coupled receptors; a further 60 have unknown coupling. Some receptors reportedly couple to more than one pathway and are listed with multiple G proteins. Table1 reports the 30 highest expressed GPCRs, most of which have been implicated in neural development, pain or sensory neuron function; including GPR56, which is involved in neuron development and polymicrogyria28 and the prostacyclin receptor (IP1), whose activation can induce migraine.29 Thirteen of the 30 most highly expressed GPCRs are classified as orphan receptors, that is, receptors without known physiologic agonists. The full data set is provided in Appendix S1.
Table 1.
Thirty highest expressed GPCRs (top) and the expression of calcitonin-family receptor components (bottom) from Taqman GPCR arrays in rat TG neurons
| Receptor name | Gene | Primary transduction | GPCR class | Average ΔC(t) | SD |
|---|---|---|---|---|---|
| GPR56 | Gpr56 | Gq/11, G12/13 | Adhesion (O) | 12.9 | 0.5 |
| GABAB1 | Gabbr1 | Gi/0 [Requires Gabbr2] | C | 13.1 | 0.4 |
| IP1 | Ptgir | Gs | A | 13.3 | 0.6 |
| D2 | Drd2 | Gi/0 | A | 13.4 | 1.5 |
| CD97 | Cd97 | G12/13 | Adhesion (O) | 13.6 | 0.7 |
| LPHN1 | Lphn1 | Gq/11 | Adhesion (O) | 13.6 | 0.6 |
| A1 | Adora1 | Gi/0 | A | 13.8 | 0.8 |
| LPHN3 | Lphn3 | Unknown | Adhesion (O) | 14.1 | 0.8 |
| LPA1 | Lpar1 | Gi/0, Gq/11, G12/13 | A | 14.4 | 1.0 |
| S1P3 | S1pr3 | Gi/0, Gq/11, G12/13 | A | 14.4 | 0.8 |
| LGR4 | Lgr4 | Unknown | A (O) | 14.5 | 0.8 |
| GPR161 | Gpr161 | Unknown | A (O) | 14.6 | 0.8 |
| PAR3 | F2rl2 | Unknown | A | 14.7 | 0.6 |
| mGlu7 | Grm7 | Gi/0 | C | 14.7 | 0.7 |
| CELSR3 | Celsr3 | Unknown | Adhesion (O) | 14.7 | 0.8 |
| FZD3 | Fzd3 | WNT | F | 14.8 | 0.6 |
| PKR2 | Prokr2 | Gq/11 | A | 14.8 | 0.8 |
| MRGPRX3 | Mrgprx3 | Gq/11 | A (O) | 14.8 | 1.0 |
| FZD8 | Fzd8 | Unknown | F | 15.0 | 0.9 |
| GPR68 | Gpr68 | Gi/0, Gq/11 | A (O) | 15.0 | 0.7 |
| GPR158 | Gpr158 | Unknown | C (O) | 15.0 | 0.8 |
| PKR1 | Prokr1 | Gq/11 | A | 15.0 | 0.8 |
| GABAB2 | Gabbr2 | Gi/0 [Requires GABBR1] | C | 15.1 | 0.5 |
| LPA5 | Lpar5 | Gq/11, G12/13 | A | 15.2 | 1.0 |
| B2 | Bdkrb2 | Gs, Gi/G0, Gq/11 | A | 15.3 | 0.7 |
| CELSR2 | Celsr2 | Unknown | Adhesion (O) | 15.3 | 0.4 |
| MRGPRE | Mrgpre | Unknown | A (O) | 15.3 | 0.5 |
| Y1 | Npy1r | Gi/0 | A | 15.4 | 0.8 |
| GPR149 | Gpr149 | Unknown | A (O) | 15.4 | 0.5 |
| ETB | Ednrb | Gs, Gi/0, Gq/11 | A | 15.4 | 0.6 |
| Calcitonin-family receptor components | |||||
| CTR | Calcr | Gs | B | 21.2 | 0.8 |
| CLR | Calrl | Gs | B | 19.5 | 0.6 |
| RAMP1 | Ramp1 | NA | Accessory | 18.2 | 1.0 |
| RAMP2 | Ramp2 | NA | Accessory | 15.9 | 0.7 |
| RAMP3 | Ramp3 | NA | Accessory | 15.7 | 0.9 |
| RCP | Crcp | NA | Accessory | 17 | 0.7 |
| Selected housekeeping genes | |||||
| ACTB | Actb | NA | Housekeeping | 7.2 | 0.6 |
| GAPDH | Gapdh | NA | Housekeeping | 8.0 | 0.8 |
GPCR, G protein-coupled receptor; TG, trigeminal ganglia; NA, not applicable; O, orphan; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein.
Although not amongst the mostly highly expressed GPCRs, 11 of 12 serotonin receptors, including 5-HT1B, 5-HT1D, and 5-HT1F were expressed. Other GPCRs that are potential targets for anti-migraine drugs, currently in phase II (or later) clinical trials were also present.30 Notably, this includes Calcrl, the gene for the CGRP receptor component, the class B GPCR CLR and its essential accessory protein (RAMP1) (Table1). The CGRP receptor has been heralded as one of the most promising migraine targets, although no agents are as-yet approved that target this receptor.4,31
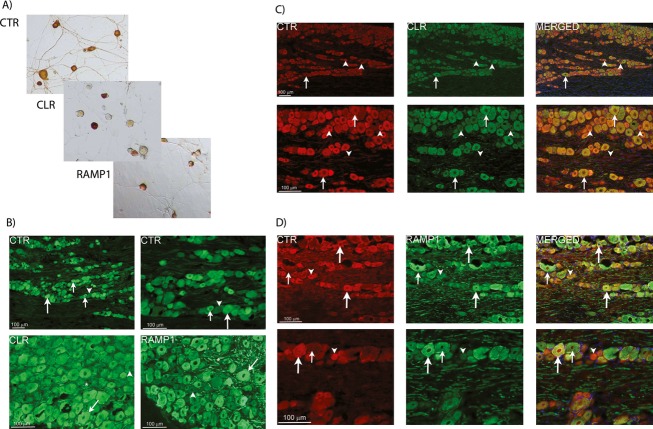
Other class B GPCRs were also expressed, including Calcr – the CTR (Table1). This receptor can act as a CGRP receptor in the presence of RAMP1 and has been proposed to be a candidate for the “elusive” CGRP2 receptor; the CTR/RAMP1 complex is known as the AMY1 receptor.32 Using specific antibodies (Table S1, Fig. S1), we confirmed the expression of CLR, CTR, and RAMP1 in isolated neonatal rat TG neurons (Fig.1A) and in the adult rat TG (Fig.1B) (Table S1). In adult rat TG, we observed co-localization of CTR and RAMP1 but not of CLR and CTR (Fig.1C and D). In light of these findings, we pursued the hypothesis that CTR and RAMP1 form functional CGRP-responsive complexes in the trigeminovascular system and that the AMY1 receptor may be a potential mediator of actions related to CGRP.
Figure 1.
Immunostaining of CGRP-responsive receptor components in rat TG. (A) Expression of CLR, CTR, and RAMP1 (CGRP and AMY1 receptor components) in isolated TG neurons by immunostaining. Images are representative of those obtained in at least three independent preparations. (B) Expression of CGRP receptor components in rat TG. CTR is expressed in small to medium sized cells (small arrows) and some larger neurons (large arrows). CLR and RAMP1 are expressed mostly in larger neurons (arrows) and thick nerve fibers (asterisks). Arrowheads indicate negative neurons. (C) Double-staining of CTR and CLR in rat TG. No clear co-expression is found between CTR (red) and CLR (green) in the TG neurons. CTR-positive neurons and fibers (arrowheads) lacking CLR expression are found. Arrows point at neurons only expressing CLR. DAPI, (blue) staining nuclei, is used in the merged picture. (D) Double-staining of CTR and RAMP1 in rat TG. Large arrows indicate CTR and RAMP1-positive neurons and their co-localization in the merged pictures. Large neurons only expressing RAMP1 are found (small arrows). Neurons only expressing CTR are also found (arrowheads). DAPI, (blue) staining nuclei, is used in the merged pictures. For (B–D), data are representative images from four rats. CGRP, calcitonin gene-related peptide; TG, trigeminal ganglia; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP1, receptor activity-modifying protein 1; DAPI, 4’6-diamidino-phenylindole.
Functional characterization of CGRP-responsive receptors in primary cultured rat TG neurons
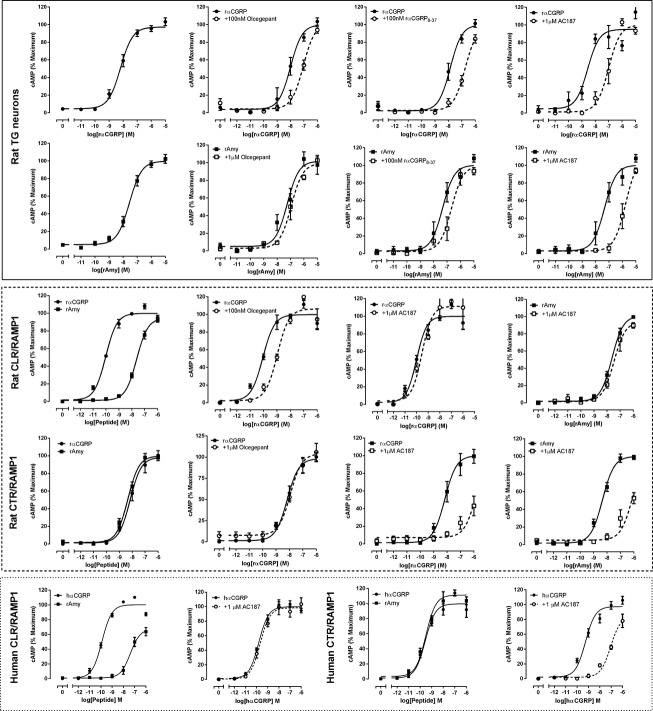
We recently established methodologies to measure GPCR signaling in primary TG neurons in 384 well plates, thereby facilitating far more comprehensive pharmacological analysis in these neurons than has been possible with earlier methodologies.15,33 Using these techniques, we characterized putative CGRP-responsive receptors in rat TG neuron-enriched cultures by measuring cAMP, a signaling pathway to which both CLR and CTR robustly couple.34,35 We selected agonists and antagonists that can distinguish between different populations of CGRP-responsive receptors.
In TG neurons, rat (r) αCGRP (a potent agonist of both CLR/RAMP1 and CTR/RAMP120) and rat amylin (rAmy, a selective agonist of CTR/RAMP complexes20,21) were both effective agonists (Table2, Fig.2). Two other agonists of CGRP-responsive receptors, rat adrenomedullin (AM) and rat calcitonin, increased cAMP accumulation with lower potency in the TG neurons (Table2). The key pharmacological discriminator is rAmy. In transfected cells that express human or rat CLR/RAMP1, CGRP has significantly higher potency than rAmy (∼250-fold, Fig.2 and Table2). In contrast, in cells transfected with CTR/RAMP1, CGRP and rAmy are equipotent (Fig.2, Table2). Therefore, the pharmacological profile of the TG neurons (equipotent CGRP and rAmy) is consistent with CTR/RAMP1 being the principal receptor that mediates CGRP-stimulated cAMP production in these cells. However, this does not exclude the possibility that CLR/RAMP1 is also responsible for CGRP responses, especially given the immunohistochemistry data showing the presence of this receptor (Fig.1). Therefore, to further evaluate the relative contributions of CLR/RAMP1 and CTR/RAMP1 to the observed agonist responses, we tested a series of antagonists against both receptor complexes in TG neurons and transfected cells.
Table 2.
Summary of agonist potencies (pEC50) for cAMP accumulation in response to agonists in neuron-enriched rat TG cultures and Cos7 cells transfected with rat or human CGRP receptor components
| Rat TG neurons1 | Rat CLR/RAMP1 | Rat CTR/RAMP1 | Human CLR/RAMP1 | Human CTR/RAMP1 | |
|---|---|---|---|---|---|
| αCGRP | 8.07 ± 0.09 (12) | 10.04 ± 0.05 (8) | 8.44 ± 0.23 (4) | 9.92 ± 0.06 (4) | 9.63 ± 0.12 (4) |
| rAmy | 7.69 ± 0.06 (6) | 7.66 ± 0.07 (6) *** | 8.37 ± 0.13 (4) | 7.40 ± 0.14 (4) *** | 9.46 ± 0.05 (4) |
| rAM | 6.68 ± 0.25 (3) ***,++ | 9.40 ± 0.17 (9) **,+++ | 7.20 ± 0.15 (4) **,++ | – | – |
| rCT | 7.25 ± 0.24 (4) ** | – | – | – | – |
Data represent mean ± SEM of n individual experiments. Comparisons were performed by one-way ANOVA followed by post-hoc multiple comparisons tests. TG, trigeminal ganglia; CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein; rAmy, rat amylin; rAM, rat adrenomedullin; rCT, rat calcitonin; –, not performed.
The amount of cAMP produced in TG neurons was as follows (Emax fmol/well [n]): rαCGRP 144.4 ± 24.7 (12); rAmy 108.3 ± 11.3 (6); rAM 123.5 ± 47.5 (3); rCT 179.8 ± 51.8 (4). There were no significant differences between these values.
P < 0.01
P < 0.001 versus αCGRP.
P < 0.01
P < 0.001 versus rAmy.
Figure 2.
Pharmacological characterization of CGRP responses in rat TG neurons and transfected cells. Cyclic AMP accumulation of rat TG neurons (solid box) or Cos7 cells transfected with rat (dashed box) or human (dotted box) CLR/RAMP1 or CTR/RAMP1. Elevation in cAMP accumulation in response to rat (r) or human (h) αCGRP and rAmy in the absence or presence of rαCGRP8–37, olcegepant or AC187. Data are expressed as a percentage of the maximum cAMP response and are mean ± SEM of 3–12 combined experiments, performed in duplicate or triplicate. CGRP, calcitonin gene-related peptide; TG, trigeminal ganglia; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein.
We used olcegepant and telcagepant, which are small molecule CLR/RAMP1 antagonists that have been evaluated in clinical trials for the treatment of migraine.36 These antagonists are more selective for the CLR/RAMP1 CGRP receptor (Table3). We also tested the classic peptidic CGRP antagonist CGRP8–37, which can antagonize both receptors,20 and AC187, which is a higher affinity antagonist of CTR/RAMP1 (Table3, Fig.2).19–21 For most antagonists, we determined their relative affinities using both rαCGRP and rAmy as agonists in separate experiments; the results are shown in Table3. Olcegepant and AC187 showed the greatest ability to discriminate between the receptors. In TG neurons, AC187 and olcegepant were equally effective as antagonists when rαCGRP was the agonist (Table3, Fig.2). This profile changed with rAmy as the agonist, for which AC187 was significantly more effective than olcegepant. These results were akin to those we obtained in cells transfected with rat receptors (Fig.2, Tables2, 3) and support the conclusion that both CLR/RAMP1 and CTR/RAMP1 are functional in TG neurons. To assess the translatability of these findings, we examined the pharmacology of human CLR/RAMP1 and CTR/RAMP1 in transfected cells and found that the antagonism of hαCGRP by AC187 at human CLR/RAMP1 and CTR/RAMP1 mirrored that in rat receptors (Fig.2). Olcegepant had ∼260-fold higher affinity at human CLR/RAMP1 than human CTR/RAMP1. On the other hand, Telcagepant had less selectivity and had only 40-fold higher affinity at human CLR/RAMP1 compared to human CTR/RAMP1 (Table3).
Table 3.
Summary of ability of antagonists to block αCGRP or rAmy-stimulated cAMP accumulation in neuron-enriched rat TG cultures and Cos 7 cells transfected with rat or human CGRP receptor components
| Rat TG neurons | Rat CLR/RAMP1 | Rat CTR/RAMP1 | Human CLR/RAMP1 | Human CTR/RAMP1 | |
|---|---|---|---|---|---|
| rαCGRP | hαCGRP | ||||
| AC187 | 7.54 ± 0.36 (3)*** | <6 (4) | 8.21 ± 0.57 (3)** | <6 (4) | 8.22 ± 0.16 (4) *,++ |
| Olcegepant | 7.79 ± 0.21 (6) *** | 7.95 ± 0.12 (4) *** | <6 (4) | 9.65 ± 0.26 (5) | 7.23 ± 0.14 (4) |
| Telcagepant | 5.72 ± 0.14 (4) | 5.34 ± 0.14 (4) | 4.17 ± 0.65 (3) | 9.00 ± 0.17 (10) | 7.36 ± 0.12 (3) |
| rαCGRP8–37 | 8.12 ± 0.07 (5) *** | 8.12 ± 0.27 (3)1 | 7.20 ± 0.16 (3)1 | 7.79 ± 0.14 (6)***,+++ | 7.72 ± 0.18 (6) |
| rAmy | |||||
| AC187 | 8.07 ± 0.21 (4)+++ | <6 (4) | 8.06 ± 0.29 (4)*** | – | – |
| Olcegepant | 6.26 ± 0.06 (3) | 7.46 ± 0.16 (4) ** | <6 (3) | – | – |
| Telcagepant | – | 5.84 ± 0.30 (4) | 4.27 ± 0.31 (3) | – | – |
| rαCGRP8–37 | 7.64 ± 0.18 (4)++ | – | 7.07 ± 0.15 (4)1 | – | – |
Data are pA2 values, representing antagonist potency. Where pA2 values could not be determined they are defined as less than the highest concentration of antagonist used. Data represent mean ± SEM of n individual experiments. Comparisons were performed by Student’s t-test or one-way ANOVA followed by Tukey’s tests. TG, trigeminal ganglia; CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein; –, not performed.
CGRP8–37 data in transfected cells as previously reported.20 Previously reported data were not included in statistical analysis.
Comparisons shown are against telcagepant (**P < 0.01, ***P < 0.001) or olcegepant (++P < 0.01, +++P < 0.001).
Expression of CTR and RAMP1 in the human sensory trigeminal system
Our data from rat TG prompted us to question whether CTR/RAMP1 complexes might also be present in relevant loci in adult humans, especially given the clinical importance of CGRP in migraine.31 CLR and RAMP1 have previously been identified in post-mortem human tissue from TG and the spinal trigeminal complex of the brainstem; functionally significant locations for pain processing in migraine.23,37
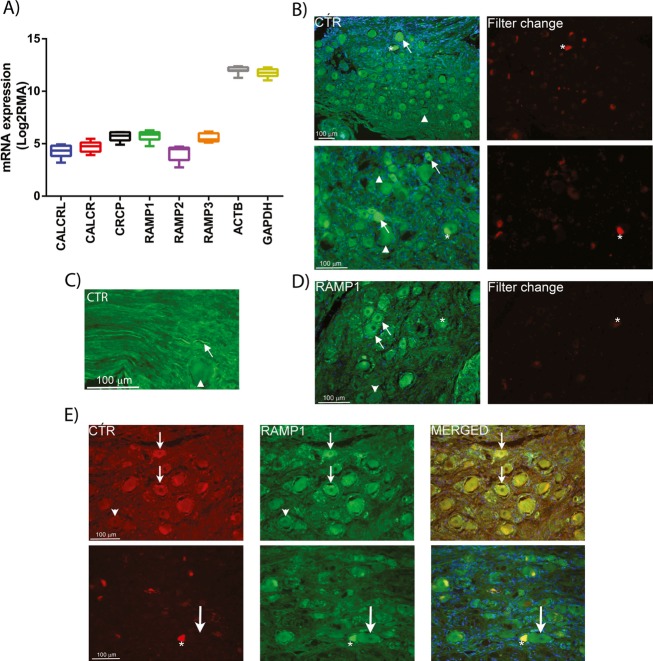
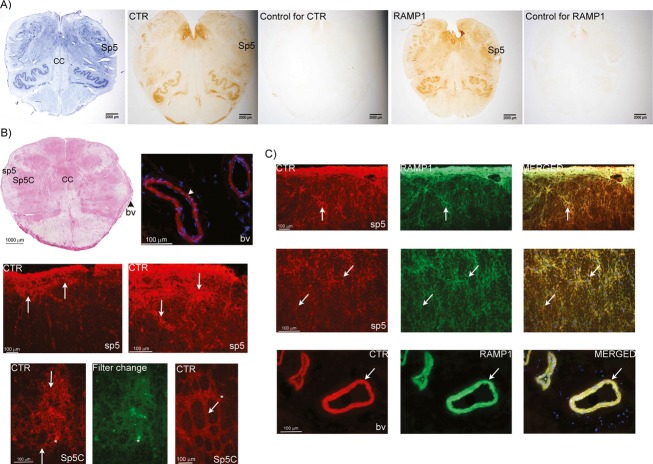
Available GEOset (Gene Expression Omnibus repository data, National Centre for Biotechnology Information) data suggested that mRNA for CTR is expressed in adult human TG at similar levels to that for CLR (Fig.3A). We observed CTR and RAMP1 staining in human TG (Fig.3B–D). The RAMP1 staining was similar to that observed in previous studies23 and co-localized with CTR in discrete neurons (Fig.3E). In the brainstem, we found CTR and RAMP1 expression in multiple regions, including the spinal trigeminal complex (Fig.4). Specifically, CTR was expressed in the fibers of sp5 and around fiber bundles in Sp5 (Fig.4B). Interestingly, blood vessels were also stained for CTR and RAMP1. RAMP1-positive fibers were mostly observed in sp5 (Fig.4C). We detected co-expression of CTR and RAMP1 in fibers of sp5 and in the vessels. The detection of RAMP1 in Sp5 depended on the samples used. Those used for DAB staining showed some evidence for RAMP1 in Sp5 (Fig.4A), but immunofluorescence techniques did not detect RAMP1 in the nucleus, as has been reported previously.37 It is possible that this relates to the sampling point within the medulla. Thus, for DAB staining we tended to use sections from higher up in the medulla, as compared to immunofluorescence. There could be discrete differences in RAMP1 expression, and thus co-localization with CTR between these regions and different sub-nuclei of Sp5. Details of antibodies can be found in Table S1.
Figure 3.
Expression of AMY1 receptor components in human TG. (A) GEOset data showing CTR family mRNA expression. (B and C) CTR expression is found in neurons and nerve fibers (arrows). Not all neurons express CTR (arrowheads). Asterisks point at auto-fluorescent lipofuscin in some neurons, which is visible in both filter channels. (D) Expression of RAMP1 in human TG neurons (arrows). Some neurons are negative for RAMP1 (arrowheads). Asterisks point at auto-fluorescent lipofuscin in some neurons, which is visible in both filter channels. (E) Double-staining of CTR and RAMP1 showing co-expression in human TG neurons (small arrows). Arrowheads indicate negative neurons for CTR and RAMP1. Lower panel shows RAMP1-positive neurons that lack CTR expression (large arrows). Auto-fluorescent lipofuscin is found in some neurons (asterisks). Nuclei staining (DAPI, blue) is used in the merged pictures. Data represent mean (min to max) of eight combined postmortem human samples (A) and representative images from three postmortem human samples (B–E). TG, trigeminal ganglia; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein; DAPI, 4’6-diamidino-phenylindole.
Figure 4.
Identification of AMY1 receptor components in human brainstem. (A) Whole brainstem images. From left to right; Nissl, CTR and RAMP1 staining. The spinal trigeminal nucleus (Sp5) is labeled. (B) Hematoxylin-Eosin staining of a whole brainstem section; spinal trigeminal tract (sp5) and spinal trigeminal nucleus, caudal part (Sp5C), central canal (CC). Fluorescence image shows CTR expression in vessels (arrowheads) (bv). Beneath these images CTR expression in sp5 and Sp5C is shown; CTR expression is found in fibers and around fiber bundles (arrows). In Sp5C, CTR is expressed around fiber bundles (arrows). Asterisks identify examples of auto-fluorescent lipofuscin, visible in both filter channels. (C) CTR and RAMP1 double-staining in the sp5 region. Co-expression is observed in the fibers (arrows). Co-expression of CTR and RAMP1 is also observed in adjacent vessels (arrows). DAPI, (blue) staining nuclei, is used in the merged picture. CTR, calcitonin receptor; RAMP, receptor activity-modifying protein; DAPI, 4’6-diamidino-phenylindole.
Discussion
New migraine treatments are vital to improve the quality of life for sufferers worldwide.31 The 37 amino acid neuropeptide CGRP has been a subject of intense scrutiny as a potential mediator of migraine pain. Several antagonists of the CGRP receptor (CLR/RAMP1) have been developed and entered clinical trials.4 Although showing great promise for the treatment of acute migraine, questions regarding the efficacy of the “gepants” (as these agents are termed) have been raised.6,38 It has been proposed that factors, other than CGRP, may be involved in migraine.5 Our data question whether the path chosen so far – the CGRP receptor (CLR/RAMP1) – is the only one and suggest that a closer examination of CGRP receptor biology is required to exploit the CGRP system to its fullest potential.
Two CGRP receptors have been proposed: the CGRP1 receptor (CLR/RAMP1) which is now classified as the “CGRP receptor” and the CGRP2 receptor which is not officially recognized by IUPHAR.32,35 These two CGRP-responsive receptors were originally described primarily on the basis of the relative sensitivity of the CGRP antagonist CGRP8–37, which shows greater antagonism of the CGRP1 receptor.35 This receptor is present in the vasculature, the TG and the spinal trigeminal complex of the brainstem and has hence been the subject of drug-discovery efforts for migraine.23,37,39–41
The relatively CGRP8–37-insensitive CGRP2 receptor has not been studied as a potential mediator of CGRPs actions in migraine, or elsewhere, perhaps because the molecular components of this receptor have been harder to identify. It has been speculated that CTR/RAMP1 is a particularly strong candidate for this receptor.32 This complex is currently classified as an amylin receptor – AMY1 – based on its high affinity for amylin.35 However this receptor also has similarly high affinity for CGRP as the CGRP receptor and is antagonized by CGRP8–37.20,21 Our GPCR array, protein expression and functional analysis of receptors in TG neurons suggest that the AMY1 receptor could be a relevant target for CGRP, and perhaps should be considered as a dual CGRP/amylin receptor.
Our agonist potency data in rat TG neurons showed that CGRP and amylin are equipotent in this preparation, and results with the amylin receptor antagonist AC187 imply that CGRP can act via the AMY1 receptor in rat TG neurons. This idea was supported by detection of CTR and RAMP1 mRNA and protein in the rat TG, and co-localization of CTR with RAMP1, presumably generating AMY1 receptor complexes, in both rat and human TG. On the other hand, expression of CLR protein and antagonism by olcegepant (∼200-fold more selective for the CGRP receptor) implicate the CGRP receptor in the response to CGRP. Therefore, populations of both receptors may be important for CGRP actions in the TG, perhaps in individual neurons, as supported by the lack of co-localization of CLR and CTR in the rat TG. Moreover, we found expression for both CTR and RAMP1 in the spinal trigeminal complex, potentially also implicating the AMY1 receptor in the central processing of CGRP signaling.
CGRP has been implicated in nociception and in many painful human conditions, including arthritis,42 tumor-associated pain43 and chronic lower back pain.44 CGRP induces pain, and can be blocked by capsaicin-induced desensitization or administration of CGRP8–37.45 The endogenous role of CGRP was elegantly confirmed in mice deficient in αCGRP (attenuated pain and inflammation responses).46 However, the receptor involved in these responses to CGRP remains unclear and is difficult to study, due to the dimeric nature of these receptors. Hence, no pain-related studies have been conducted in CGRP or AMY1 receptor-deficient animal models. Since CGRP8–37 is frequently used at concentrations that can block both CGRP and AMY1 receptors, most studies using this peptide cannot effectively distinguish between the actions of these receptors.32 Even studies with olcegepant and telcagepant can be difficult to interpret because both antagonists are often used at concentrations that can antagonize the AMY1 receptor as well as the CGRP receptor. This is clearly evident from our functional data showing that telcagepant is only 40-fold selective for the human CGRP receptor over the human AMY1 receptor. There is only one other report of the affinity of telcagepant at the AMY1 receptor; in this binding study, telcagepant had a Ki of 190 nmol/L.36 Therefore there is currently insufficient data on the receptor selectivity of this compound. Efficacious doses of olcegepant and telcagepant used in clinical trials apparently achieve blood concentrations of 200 nmol/L and 4–6 μmol/L, respectively.6 These concentrations are sufficient to antagonize the CGRP receptor and potentially also peripheral AMY1 receptors.36 In interpreting these studies, other factors including target accessibility, the antagonist-free fraction and alternative receptors should be considered. The results of clinical trials using small molecules or antibodies with greater specificity for the CGRP receptor may help elucidate this complexity.
The relative role of the AMY1 receptor in CGRP and/or amylin biology is not yet clear because ours is the first study to co-localize CTR and RAMP1 protein in any tissue. Identifying precisely where this receptor is expressed and how expression may be altered in disease will be important for future studies. We identified the AMY1 receptor in the TG and brainstem of normal subjects but it is possible that migraine sufferers, who display an aberrant response to CGRP,31 may have altered patterns of expression that contribute to the disease. In support of this idea, RAMP1 overexpression enhanced responses to both CGRP and amylin.33,47 Our results raise the possibility that CGRP and amylin may have cooperative activities in migraine, although this will depend on whether either peptide is able to access its receptor(s). Amylin has been detected in a subset of CGRP-positive neurons in the TG, the brainstem and in nerve fibers innervating the pial vasculature.48,49 There has been no attempt to localize amylin-binding sites in the TG. Efforts to identify amylin-binding sites in the brainstem are inconclusive regarding the spinal trigeminal complex.50,51 On the other hand, 125I-salmon calcitonin, which labels both calcitonin and amylin-binding sites,52 shows binding in this region of rat and monkey brain.50,53
Despite the importance of CGRP in migraine and other painful conditions, the precise CGRP-responsive receptor(s) involved in each aspect of these conditions has not been well characterized. We report the presence of the AMY1 receptor, a CGRP-responsive receptor, at sites important for craniofacial pain. If the AMY1 receptor (CTR and RAMP1) is a genuine (patho)physiological target for CGRP it has important and far-reaching implications for targeting the CGRP system in migraine and other forms of pain. A greater understanding of the role of each receptor to CGRP biology is urgently required. Furthermore, our results obtained using a GPCR array identify many additional GPCRs within the TG as potential pharmacological targets for migraine than have been previously appreciated.
Acknowledgments
This work was supported by a grant from the Auckland Medical Research Foundation (to C. S. W. and D. L. H.), by the University of Auckland Biopharma Thematic Research Initiative (to D. L. H.), by the National Institutes of Health (to A. F. R.; NS075599) and by the Swedish Research Council (to L. E.; grant no. 5958). Anti-CLR and anti-RAMP1 antibodies were kindly provided by Christopher Salvatore (Merck and Co., USA). Olcegepant was kindly provided by Henri Doods (Boehringer Ingelheim Pharma GmbH & Co., Germany). The authors thank Liam Williams (Center for Genomics, Proteomics and Metabolomics, The University of Auckland, New Zealand) and Peter Wookey for their technical advice. Amorita Petzer, Ignacio Flores and Anthony Philips for their assistance with animal husbandry and ethics applications. Professor David Poyner is thanked for his helpful discussions.
Conflict of Interest
Dr. Jamaluddin reports grants from Health Research Council New Zealand, from null during the conduct of the study.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Supplementary methods.
Figure S1. 9b4 antibody controls. The specificity of the anti-human CTR antibody (9B4; N-terminal epitope) was confirmed in transfected HEK 293S cells. Cells transfected with human CTR, human CLR/RAMP1 or pcDNA3.1 (vector) underwent antigen retrieval (citric acid and microwaving). The receptors were probed with 9B4 antibody (1:100) and visualized using DAB (brown). Similar results were obtained with HA-tagged human CLR and CTR. Images are representative of at least three independent transfections.
Table S1. Details of antibodies used in this study.
Table S2. List of human cases used in this research.
Appendix S1. Expression [ΔC(t)] data for all GPCRs, control genes and GPCR-associated genes.
References
- Stovner L, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine–current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Garland SL. Are GPCRs still a source of new targets? J Biomol Screen. 2013;18:947–966. doi: 10.1177/1087057113498418. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S. Monoclonal antibodies for migraine: preventing calcitonin gene-related peptide activity. CNS Drugs. 2014;28:389–399. doi: 10.1007/s40263-014-0156-4. [DOI] [PubMed] [Google Scholar]
- Pascual J. Efficacy of BMS-927711 and other gepants vs triptans: there seem to be other players besides CGRP. Cephalalgia. 2014;34:1028–1029. doi: 10.1177/0333102414526052. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Olesen J. Possible site of action of CGRP antagonists in migraine. Cephalalgia. 2011;31:748–750. doi: 10.1177/0333102411398403. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Joshi AD, Sanabria-Bohorquez S, et al. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther. 2013;347:478–486. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–892. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–1107. doi: 10.1016/S1474-4422(14)70209-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson ML, Edvinsson L. Comparison of CGRP and NO responses in the human peripheral microcirculation of migraine and control subjects. Cephalalgia. 2008;28:563–566. doi: 10.1111/j.1468-2982.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12:454–461. doi: 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Tassorelli C, Nappi G. Peripheral and central activation of trigeminal pain pathways in migraine: data from experimental animal models. Cephalalgia. 2003;23(suppl 1):1–4. doi: 10.1046/j.1468-2982.23.s1.1.x. [DOI] [PubMed] [Google Scholar]
- May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115–127. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia. 2011;31:737–747. doi: 10.1177/0333102411398152. [DOI] [PubMed] [Google Scholar]
- Walker CS, Sundrum T, Hay DL. PACAP receptor pharmacology and agonist bias: analysis in primary neurons and glia from the trigeminal ganglia and transfected cells. Br J Pharmacol. 2014;171:1521–1533. doi: 10.1111/bph.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead AN, Insel PA. Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts. FASEB J. 2012;26:4540–4547. doi: 10.1096/fj.12-213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41(Database issue):D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Abeta1-42 at human amylin receptors. Endocrinology. 2014;155:21–26. doi: 10.1210/en.2013-1658. [DOI] [PubMed] [Google Scholar]
- Bailey RJ, Walker CS, Ferner AH, et al. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol. 2012;166:151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, et al. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- Gingell JJ, Qi T, Bailey RJ, Hay DL. A key role for tryptophan 84 in receptor activity-modifying protein 1 in the amylin 1 receptor. Peptides. 2010;31:1400–1404. doi: 10.1016/j.peptides.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Calamari A, et al. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–696. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Wookey PJ, Turner K, Furness JB. Transient expression of the calcitonin receptor by enteric neurons of the embryonic and early post-natal mouse. Cell Tissue Res. 2012;347:311–317. doi: 10.1007/s00441-011-1303-6. [DOI] [PubMed] [Google Scholar]
- Becskei C, Riediger T, Zund D, et al. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030:221–233. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Qi T, Dong M, Watkins HA, et al. Receptor activity-modifying protein-dependent impairment of calcitonin receptor splice variant Delta(1-47)hCT((a)) function. Br J Pharmacol. 2013;168:644–657. doi: 10.1111/j.1476-5381.2012.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potes CS, Boyle CN, Wookey PJ, et al. Involvement of the extracellular signal-regulated kinase 1/2 signaling pathway in amylin’s eating inhibitory effect. Am J Physiol Regul Integr Comp Physiol. 2012;302:R340–R351. doi: 10.1152/ajpregu.00380.2011. [DOI] [PubMed] [Google Scholar]
- Singer K, Luo R, Jeong SJ, Piao X. GPR56 and the developing cerebral cortex: cells, matrix, and neuronal migration. Mol Neurobiol. 2013;47:186–196. doi: 10.1007/s12035-012-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienecke T, Olesen J, Ashina M. Prostaglandin I2 (epoprostenol) triggers migraine-like attacks in migraineurs. Cephalalgia. 2010;30:179–190. doi: 10.1111/j.1468-2982.2009.01923.x. [DOI] [PubMed] [Google Scholar]
- Anders S. Spotlight on drugs in the pharmaceutical pipeline: agents for migraine. Catamaran RxOutlook. 2013;7:1–3. [Google Scholar]
- Olesen J, Ashina M. Emerging migraine treatments and drug targets. Trends Pharmacol Sci. 2011;32:352–359. doi: 10.1016/j.tips.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Quirion R International Union of P. International Union of Pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev. 2008;60:143–145. doi: 10.1124/pr.108.00372. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Conner AC, Poyner DR, Hay DL. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci. 2010;31:476–483. doi: 10.1016/j.tips.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Moore EL, Salvatore CA. Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol. 2012;166:66–78. doi: 10.1111/j.1476-5381.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011;12:112. doi: 10.1186/1471-2202-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen PC. Is there an inherent limit to the efficacy of calcitonin gene-related peptide receptor antagonists in the acute treatment of migraine? A comment. J Headache Pain. 2009;10:389–391. doi: 10.1007/s10194-009-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki S, Kito S, Kubota Y, et al. Autoradiographic localization of calcitonin gene-related peptide binding sites in human and rat brains. Brain Res. 1986;374:287–298. doi: 10.1016/0006-8993(86)90423-3. [DOI] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Sexton PM, McKenzie JS, Mason RT, et al. Localization of binding sites for calcitonin gene-related peptide in rat brain by in vitro autoradiography. Neuroscience. 1986;19:1235–1245. doi: 10.1016/0306-4522(86)90137-5. [DOI] [PubMed] [Google Scholar]
- Bullock CM, Wookey P, Bennett A, et al. Increased peripheral calcitonin gene-related peptide receptor signalling drives mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014;66:2188–2200. doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Seong J, An JH, et al. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int J Radiat Oncol Biol Phys. 2005;61:1523–1534. doi: 10.1016/j.ijrobp.2004.12.070. [DOI] [PubMed] [Google Scholar]
- Krock E, Rosenzweig DH, Chabot-Dore AJ, et al. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213–1225. doi: 10.1111/jcmm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese N, Diop L, Chevalier E, et al. Involvement of prostaglandins and CGRP-dependent sensory afferents in peritoneal irritation-induced visceral pain. Regul Pept. 1997;70:1–7. doi: 10.1016/s0167-0115(97)02141-1. [DOI] [PubMed] [Google Scholar]
- Salmon AM, Damaj MI, Marubio LM, et al. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci. 2001;4:357–358. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Morgan DA, et al. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes. 2011;60:1063–1071. doi: 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yang J, Chang JK, Dun NJ. Amylin suppresses acetic acid-induced visceral pain and spinal c-fos expression in the mouse. Neuroscience. 2010;165:1429–1438. doi: 10.1016/j.neuroscience.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Goadsby PJ, Uddman R. Amylin: localization, effects on cerebral arteries and on local cerebral blood flow in the cat. ScientificWorldJournal. 2001;1:168–180. doi: 10.1100/tsw.2001.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Chai SY, Christopoulos G, et al. In vitro autoradiographic localization of calcitonin and amylin binding sites in monkey brain. J Chem Neuroanat. 2004;27:217–236. doi: 10.1016/j.jchemneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Paxinos G, Kenney MA, et al. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Olgiati VR, Guidobono F, Netti C, Pecile A. Localization of calcitonin binding sites in rat central nervous system: evidence of its neuroactivity. Brain Res. 1983;265:209–215. doi: 10.1016/0006-8993(83)90334-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary methods.
Figure S1. 9b4 antibody controls. The specificity of the anti-human CTR antibody (9B4; N-terminal epitope) was confirmed in transfected HEK 293S cells. Cells transfected with human CTR, human CLR/RAMP1 or pcDNA3.1 (vector) underwent antigen retrieval (citric acid and microwaving). The receptors were probed with 9B4 antibody (1:100) and visualized using DAB (brown). Similar results were obtained with HA-tagged human CLR and CTR. Images are representative of at least three independent transfections.
Table S1. Details of antibodies used in this study.
Table S2. List of human cases used in this research.
Appendix S1. Expression [ΔC(t)] data for all GPCRs, control genes and GPCR-associated genes.