Abstract
Objective
Aging is associated with reduced neural integrity, yet there are remarkable individual differences in brain health among older adults (OA). One factor that may attenuate age-related neural decline is cardiorespiratory fitness (CRF). The primary aim of this study was to link CRF to neural white matter microstructure using diffusion tensor imaging in OA.
Methods
Young adults (YA; n = 32) and OA (n = 27) completed a graded maximal exercise test to evaluate CRF and diffusion tensor magnetic resonance imaging to examine neural white matter integrity.
Results
As expected, pervasive age-related declines in white matter integrity were observed when OA were compared to YA. Further, peak VO2 was positively associated with fractional anisotropy (FA), an indicator of white matter integrity, in multiple brain regions in OA, but not YA. In multiple posterior regions such as the splenium, sagittal stratum, posterior corona radiata, and superior parietal white matter, FA values were similar in YA and OA classified as higher fit, with both groups having greater FA than lower fit OA. However, age-related differences in FA values remained in other regions, including the body and genu of the corpus callosum, precuneus, and superior frontal gyrus.
Interpretation
CRF is positively associated with neural white matter microstructure in aging. The relationship between peak VO2 and FA appears to be tract-specific, as equivalent FA values were observed in higher fit OA and YA in some white matter tracts, but not others. Further, the association between peak VO2 and FA appears to be age-dependent.
Introduction
Age-related decline in cerebral macrostructure, such as reductions in gray and white matter volume, is well-documented.1–4 More recently, diffusion tensor imaging (DTI) has been used to assess in vivo cerebral white matter microstructure and to evaluate specific white matter fiber bundles that underpin information transmission between gray matter regions.5,6 Age-related reductions in white matter microstructure have been reported in healthy older adults (OA),7,8 and decreased white matter microstructure in OA has been linked to poorer performance on tasks tapping processing speed, executive functions, and episodic memory.9,10
The pervasive evidence for neural decline in OA has led to substantial interest in individual difference factors that are associated with age-related reductions in cerebral integrity. One such factor is cardiorespiratory fitness (CRF), an indicator of the ability of one’s circulatory and respiratory systems to supply oxygen to skeletal muscle during sustained moderate to vigorous physical activity. CRF is a modifiable attribute and can be enhanced with aerobic physical activities such as walking, running, swimming, or dancing. CRF has been linked to neural structure and function in the aging brain,11 and as such, may modulate cognitive performance12,13 as well as progression of neurodegenerative disease.14–16 Yet, evidence for a relationship between CRF and white matter microstructure in the aging brain is currently limited. A study in rodents has demonstrated that aerobic exercise attenuates age-dependent alterations in a protein marker of myelin (myelin basic protein) as well as in astrocytes, which promote myelination via oligiodendrogial cells.17 In humans, CRF has been positively associated with enhanced white matter integrity in fiber tracts including the cingulum bundle,18,19 corpus callosum,20 and regions of interest encompassing the frontal and temporal lobes.21 Some limitations of previous work include the indirect assessment of CRF19,22 and the use of imaging approaches that preclude consideration of multiple white matter tracts.18,21 Other studies of CRF or physical activity have focused exclusively on OA.18,20,21,23–25 In the current study, we aim to extend the extant literature by implementing a direct assessment of CRF, whole-brain DTI analyses to examine white matter tracts throughout the brain, and inclusion of a group of young adults (YA) to specifically identify age-related decline in white matter microstructure in our sample of OA.
The primary goals of the current study were (1) to examine the relationship between CRF and white matter microstructure in OA using a direct measure of peak VO2 obtained during a treadmill-based maximal exercise protocol (the gold standard for assessment of CRF); (2) to assess whether CRF is differentially associated with white matter microstructure in YA and OA; and (3) to directly compare white matter microstructure in YA, higher fit OA and lower fit OA.
Materials and Methods
Participants
Thirty-four YA (age 18–31 years) and 33 OA (age 55–82 years) participated in the study. Six OA were excluded from data analysis (four due to incidental findings on the magnetic resonance imaging (MRI), one requested to terminate MRI session prior to DTI scan, and one failed to meet criteria for valid peak VO2) as well as two YA (one was a statistical outlier based on high peak VO2 value, the other requested to terminate MRI session prior to DTI). The final sample included in the analyses consisted of 32 YA (23 Caucasian, seven Asian, two African-American) and 27 OA (25 Caucasian, two African-American). Four OA reported a diagnosis of hypertension, of which one also reported diabetes. Participant characteristics of the sample included in the analyses are reported in Table1.
Table 1.
Characteristics (mean and SD) of young and older adults, as well as sub-groups of older adults based on median split of peak VO2
| YA | OA | LFOA | HFOA | |
|---|---|---|---|---|
| Number of participants | 32 (17 F) | 27 (15 F) | 12 (6 F) | 12 (6 F) |
| Age (years)1 | 21.1 (3.1) | 63.4 (6.4) | 64.3 (6.9) | 64.3 (5.7) |
| Education (years)1,2 | 14.4 (1.8) | 16.4 (2.6) | 15.4 (2.7) | 17.1 (2.4) |
| CES-D | 6.2 (4.1) | 5.7 (4.2) | 6.2 (3.9) | 5.1 (4.0) |
| MOCA3,4 | 28.5 (1.5) | 27.7 (1.8) | 26.7 (2.2) | 28.4 (1.0) |
| BMI1,5,6 | 23.0 (2.9) | 26.0 (4.6) | 29.1 (5.1) | 23.4 (2.4) |
| Peak VO2 (mL/kg per min)3,6,9 | 39.0 (7.1) | 30.3 (8.4) | 23.7 (6.6) | 37.0 (5.3) |
| Peak VO2 ACSM percentile4,5,6 | 41.9 (25.7) | 46.9 (29.3) | 18.3 (12.7) | 74.6 (11.6) |
| ACSM VO2 percentile min-max | 10–90 | 10–90 | 10–40 | 60–90 |
ASCM, American College of Sports Medicine; F, number of females; BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; HFOA, higher fit older adults; LFOA, lower fit older adults; MOCA, Montreal cognitive assessment; OA, older adults; YA, young adults.
OA > YA.
HFOA > YA.
HFOA > LFOA.
YA > LFOA.
LFOA > HFOA.
LFOA > YA.
YA > OA.
To ensure recruitment of participants with a wide range of CRF levels, participants were recruited from general participant pools (Boston University for YA and the Boston University Memory Disorders Research Center at VA Boston, Boston University Alzheimer’s Disease Center, the Massachusetts Alzheimer’s Disease Research Center, and the Alzheimer’s Association TrialMatch for OA) as well as through local libraries, YMCAs, and track (running) meets. Participants included in the study did not have any notable medical or neurological illness or history of head trauma. Participants completed a telephone-based comprehensive health screen consisting of ∼150 questions to rule out major medical (e.g., myocardial infarction, vascular disease), neurological (e.g., Alzheimer’s disease, Parkinson’s disease, multiple sclerosis), psychiatric (e.g., bipolar disorder, schizophrenia), or substance abuse issues that might affect cognition. Additional exclusion criteria included education less than grade 12, and contraindications to cardiopulmonary exercise testing (CPX) or MRI. Participants were screened for depression using a cut-off score of 16 on the Center for Epidemiologic Studies Depression Scale (CES-D) 20-item version. Mental status was assessed using the Montreal Cognitive Assessment (MOCA; http://www.mocatest.org/), and participants with scores ≤23 were excluded.
Cognitive data are reported elsewhere.26 All participants gave written informed consent and received financial compensation. The VA Boston Healthcare System institutional review board approved all experimental procedures.
Cardiopulmonary exercise testing
An exercise physiologist and a cardiologist of the VA Boston Healthcare system supervised all sessions. Graded maximal exercise testing in association with air-gas-exchange was conducted using a 2-min Bruce protocol27 on a motor driven Woodway Barimill treadmill. A lightweight disposable pneumotach device was positioned in the participant’s mouth during exercise for gas exchange assessments (MedGraphics Ultima II). Peak oxygen consumption (VO2) and respiratory exchange ratio were measured, as well as maximum heart rate, blood pressure, and ECG waveforms. Self-reported ratings of perceived exertion were collected at 1 min intervals using the 20-point Borg Scale. Peak VO2 was considered valid if at least two of the following criteria were met: (1) respiratory exchange ratio ≥1.0, (2) Maximum heart rate equivalent to 85% of the age-predicted maximum (i.e., 220 – age), (3) Ratings of perceived exertion ≥17, which corresponds to an exertion level of “very hard” (a rating of 20 represents “maximal exertion”).
Image acquisition and processing
All participants underwent structural MRI on a 3 T Siemens Tim Trio scanner with a 12-channel head coil located at the Jamaica Plain campus of the VA Boston Healthcare System. A whole-brain T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequence, empirically optimized for a high contrast to noise ratio, was acquired in the sagittal plane: TR = 2530 msec, TE = 3.32 msec, TI = 1100 msec, flip angle = 7°, slices = 176, slice thickness = 1 mm, FOV = 256, matrix = 2562, voxel size = 1 mm3. A whole-brain high-resolution diffusion-weighted sequence was acquired in the axial plane with the following parameters: directions = 60 with an additional 10 T2-weighted “low b” images (b value = 0 sec/mm2) for anatomical reference, TR = 10 sec, TE = 103 msec, slices = 64, slice thickness = 2 mm, FOV = 256, matrix = 1282, voxel size = 2 mm3, b value = 700 sec/mm2.
Diffusion data were processed using The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) software library (FSL version 4.1; www.fmrib.ox.ac.uk/fsl)28 and the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu). The dt_recon command, as implemented in FreeSurfer, was used for eddy current and motion correction. A 12-parameter affine transformation process with FMRIB’s Linear Image Registration Tool (FLIRT) was used for within-subject image registration (e.g., the low b volume was collected using identical parameters as the diffusion-weighted volumes and thus registered with the diffusion maps, within-subject registration of the MP-RAGE and diffusion images). Diffusion tensor reconstruction (including the six components, three eigenvectors and three eigenvalues) were calculated for each voxel using a least-squares fit to the diffusion signal.29 To remove non-brain voxels from the analysis, the low b volume was skull stripped using FSL’s brain extraction tool (BET), and the resulting brain mask applied to the fractional anisotropy (FA) maps. FA, the most common metric reported in diffusion studies, measures the primary direction of diffusion of water molecules, and is considered to reflect myelination, fiber density, and axonal diameter. FA values range between 0 and 1, with higher values assumed to reflect greater white matter integrity. Voxelwise statistical analysis of the FA data was carried out using Tract-Based Spatial Statistics (TBSS30) as implemented in FSL. All subjects’ FA data were aligned into MNI152 space using the nonlinear registration tool FNIRT, which uses a b-spline representation of the registration warp field. Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the centers of all tracts common to the group. Maps were threshold to exclude voxels with FA values <0.2 that may reflect multiple tissue types or crossing fibers. Each subject’s aligned FA data was then projected onto this skeleton, manually inspected to confirm accurate registration, and the resulting data fed into voxelwise cross-subject statistics.
Statistical approach
Whole-brain DTI analyses were implemented using FSL’s Randomise, a tool designed for permutation testing with nonparametric values. Whole-brain analyses were completed using the threshold-free cluster enhancement option (which allows for the identification of significant clusters of voxels without choosing an arbitrary threshold), 5000 permutations for each contrast (e.g., OA < YA), variance smoothing (5 mm), and threshold at P < 0.05, corrected for multiple comparisons (family-wise error rate). Age-related white matter decline was examined by comparing FA values in OA and YA (OA < YA), controlling for gender, education (years), and depression (CES-D) score. Differential effects of CRF on FA within YA and OA were examined by modeling a continuous covariate interaction term (peak VO2 by age group), controlling for gender, education, and depression score. Statistical analyses of behavioral and region of interest (ROI) data were performed using SPSS, version 19 (IBM Corp., Armonk, NY), with the alpha level for results set at P < 0.05.
Automated ROI segmentation
To localize significant voxels and extract FA data for ROI analyses and plotting purposes, we used a neuroanatomy atlas31 that merged the parcellations from the John Hopkins White Matter Parcellation Atlas and the FreeSurfer atlas. These atlases provide complementary data, with the John-Hopkins White Matter Parcellation Atlas providing detailed labeling of deeper white matter structures, regions, and tracts, and the FreeSurfer atlas providing parcellations of white matter that extends to the cortical surface. The data plotted in the figures represent voxels that were significant in the voxel-wise analysis within a particular ROI, rather than FA from the entire ROI.
Results
Cardiorespiratory fitness
There was no difference in the American College of Sports Medicine (ACSM) percentile scores in YA and OA t (57) <1, P = ns, indicating that both groups were equivalently fit relative to their age and gender-based norms. ACSM percentile scores for both YA and OA ranged from the 10th–90th percentile (Table1), and mean percentile scores for YA and OA were not significantly different from the 50th percentile. Thus, both the YA and OA were representative samples in terms of CRF levels based on peak VO2.
Effect of age on white matter microstructure
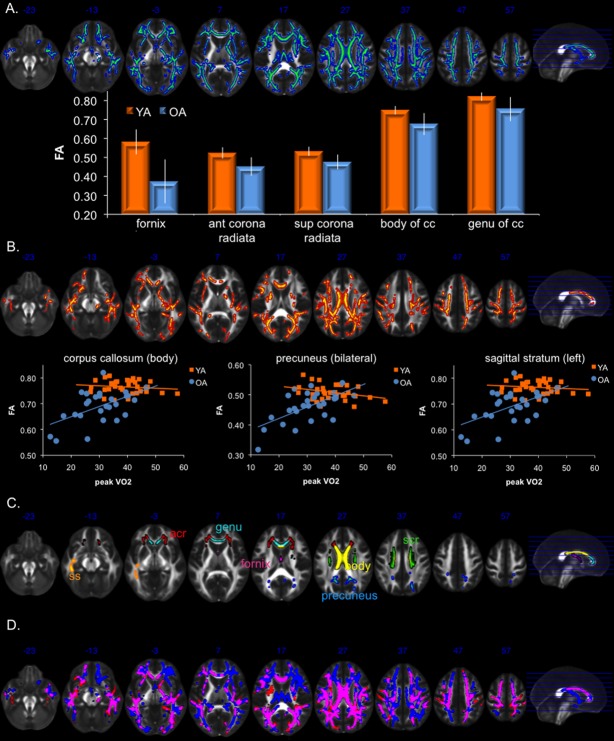
Controlling for gender, education, and depression score, widespread reductions in white matter microstructure were evident with aging (Fig.1A), as OA showed lower FA relative to YA in the genu and body of the corpus callosum, corona radiata, and fornix, among other regions. Table S1 provides a comprehensive list of white matter regions showing age-related decline, as well as the percent of the region showing an age-effect (calculated by dividing the number of voxels showing an age effect by the total number of voxels of the ROI within the TBSS skeleton). No regions showed greater FA values in OA relative to YA.
Figure 1.
(A) White matter regions with reduced FA in older adults relative to younger adults (blue), as well as bar graphs of mean FA (error bars represent SD) of selected regions showing some of the strongest age effects. Blue numbers near the anterior portion of the brain represent MNI coordinates of the image in the z-direction. (B) White matter regions showing a significant peak VO2 by age group interaction (red), driven by a positive relationship between FA and peak VO2 in older adults and no relationship in younger adults. Scatter plot and best-fit line for YA (orange) and OA (blue) in selected regions showing the strongest interaction. (C) ROIs used to extract data plotted in (A and B). (D) Overlap (pink) of age-effect (blue) and peak VO2 by age group interaction (red). Pink regions show both the main effect of age-related decline and the peak VO2 by age group interaction. Statistical maps were dilated using tbss_fil to facilitate visualization. acr, anterior corona radiata; ant, anterior; cc, corpus callosum; FA, fractional anisotropy; ROI, region of interest; L, left; OA, older adults; scr, superior corona radiata; sup, superior; ss, sagittal stratum; YA, young adults.
Differential effect of CRF in YA and OA
A significant peak VO2 by age group interaction was observed in multiple white matter regions, in which peak VO2 was more strongly associated with FA in OA relative to YA, again controlling for gender, education, and depression score (Fig.1B; Table2). This pattern was observed in regions associated with age- and AD-related white matter decline, including the genu and body of the corpus callosum, fornix, precuneus, as well as other regions (see Table S2 for a comprehensive list; see Fig.1C for the ROIs used to extract FA values plotted in Fig.1A and B).
Table 2.
White matter regions showing peak VO2 by age group interaction (listed by descending t-value within each lobe), as well as results of ANCOVA comparing YA, HFOA, and LFOA
| White matter region | Hem | Volume (mm3) | % ROI | Mean t-value | SD | ANCOVA F-value | ANCOVA follow-up |
|---|---|---|---|---|---|---|---|
| Frontal | |||||||
| Pars orbitalis | L | 30 | 47.6 | 1.84 | 0.57 | 2.77 | YA > LFOA |
| Superior frontal | R | 922 | 30.7 | 1.81 | 0.60 | 12.17 | YA > HFOA > LFOA |
| Body of cc | M | 1964 | 62.6 | 1.86 | 0.63 | 10.82 | YA > HFOA > LFOA |
| Ant limb of ic | L | 90 | 11.0 | 1.81 | 0.53 | 6.28 | YA and HFOA > LFOA |
| External capsule | L | 642 | 44.9 | 1.80 | 0.64 | 3.67 | YA and HFOA > LFOA |
| Pars opercularis | L | 325 | 35.5 | 1.76 | 0.61 | 11.65 | YA > HFOA > LFOA |
| Genu of cc | M | 776 | 44.1 | 1.68 | 0.53 | 9.44 | YA > HFOA > LFOA |
| Parietal | |||||||
| Pos corona radiata | L, R | 409, 524 | 54.5, 63.4 | 1.74, 1.88 | 0.60, 0.68 | 5.52, 6.73 | YA and HFOA > LFOA, YA and HFOA > LFOA |
| Postcentral | R | 445 | 34.2 | 1.79 | 0.70 | 2.47 | YA and HFOA > LFOA |
| Pos thalamic radiation | R | 540 | 47.2 | 1.75 | 0.64 | 6.73 | YA > HFOA and LFOA |
| Paracentral | R | 312 | 40.4 | 1.72 | 0.49 | 4.83 | YA and HFOA > LFOA |
| Superior parietal | L | 857 | 26.3 | 1.71 | 0.61 | 6.88 | YA and HFOA > LFOA |
| Precuneus | L | 293 | 22.8 | 1.70 | 0.51 | 9.01 | YA > HFOA > LFOA |
| Temporal | |||||||
| Temporal pole | R | 39 | 32.2 | 2.48 | 0.96 | 1.52 (ns) | |
| Fornix (stria) | L, R | 104, 150 | 25.9, 47.9 | 1.81, 1.94 | 0.64, 0.63 | 4.14, 4.70 | YA > LFOA, YA > HFOA and LFOA |
| Sagittal stratum | L, R | 311, 382 | 64.9, 64.1 | 1.94, 1.76 | 0.81, 0.59 | 3.66, 3.65 | YA and HFOA > LFOA, YA and HFOA > LFOA |
| Superior temporal | L | 1111 | 40.8 | 1.81 | 0.67 | 4.17 | YA and HFOA > LFOA |
| Banks sts | L | 185 | 33.6 | 1.77 | 0.51 | 9.95 | YA and HFOA > LFOA |
| Occipital | |||||||
| Cuneus | L, R | 152, 122 | 37.7, 53.3 | 1.75, 1.71 | 0.55, 0.56 | 2.49, 2.18 (ns) | YA > LFOA |
| Pericalcarine | R | 409 | 49.7 | 1.74 | 0.60 | 6.14 | YA and HFOA > LFOA |
| Fusiform | L, R | 317, 684 | 19.4, 43.7 | 1.70, 1.71 | 0.60, 0.50 | 3.49, 3.10 | YA and HFOA > LFOA, YA and HFOA > LFOA |
| Splenium of cc | M | 784 | 34.1 | 1.71 | 0.53 | 3.83 | YA and HFOA > LFOA |
| Lateral occipital | L | 312 | 22.9 | 1.69 | 0.59 | 7.00 | YA > HFOA > LFOA |
Percent of ROI represents the percentage of the TBSS skeleton ROI showing the peak VO2 by age group interaction. Mean and SD are based on t-values of each voxel within the ROI. Two values within a single cell represent the left and right region, respectively. ANCOVA, analysis of covariance; YA, young adults; HFOA, higher fit older adults; LFOA, lower fit older adults; ROI, region of interest; L, left; R, right; cc, corpus callosum; M, mid-line; ant, anterior; ic, internal capsule; pos, posterior; sts, superior temporal sulcus; TBSS, Tract-Based Spatial Statistics.
For comparison purposes, the FA maps showing a main effect of age and a significant interaction were overlaid on the same template, illustrating regions that overlap (Fig.1D). No brain region showed a pattern in which CRF was more strongly associated with FA in YA relative to OA. Furthermore, a whole-brain TBSS analysis examining the relationship between FA and peak VO2 in YA only did not reveal any significant FA-peak VO2 associations in the YA.
To further illuminate the relationship between CRF and white matter microstructure in OA, FA values from the five ROIs showing the strongest interaction effect were extracted and entered into separate hierarchical regression models. This approach allows for the simultaneous examination of multiple predictors of FA (peak VO2, age, gender, education, and depression score) in OA in familiar statistical terms, with the caveat that these values likely represent an over-estimation because the voxels were selected based on the results of the TBSS voxel-wise analysis. In Step 1 of each model, peak VO2 was entered to examine whether CRF was a significant predictor of FA in OA. In Step 2, age, gender, education, and depression score were entered as predictors of FA. This approach allows one to determine whether CRF by itself can account for the majority of variance associated with FA values in OA. Indeed, this pattern was observed in all five ROIs, as Step 1 (peak VO2) was significant in the model for each ROI (Table3), whereas Step 2 (age, gender, education, and depression score) did not significantly improve model fit for any of the ROIs. When the order of variable entry into the model was reversed (age, gender, education, and depression entered in Step 1 and peak VO2 entered in Step 2), peak VO2 remained a significant predictor of FA in four of the five ROIs, accounting for an additional 20–26% of the variance over and above age, gender, education, and depression score (Table S3). The results were not significantly altered when diagnosis of hypertension or diabetes were also included in the models.
Table 3.
Results of hierarchical regression models to elucidate the relationship between peak VO2 and FA in OA
| R-value | ΔR2 | ΔF | Model F-value | |
|---|---|---|---|---|
| Right temporal pole | ||||
| Step 1 (peak VO2) | 0.59 | 0.35 | 13.26, P < 0.001 | 13.26, P < 0.001 |
| Step 2 (age, gender, educ, CES-D) | 0.71 | 0.15 | 1.58, P = ns | 4.17, P < 0.01 |
| Right fornix (stria terminalis) | ||||
| Step 1 (peak VO2) | 0.47 | 0.22 | 6.95, P < 0.05 | 6.95, P < 0.05 |
| Step 2 (age, gender, educ, CES-D) | 0.68 | 0.24 | 2.31, P = ns | 3.53, P < 0.05 |
| Left sagittal stratum | ||||
| Step 1 (peak VO2) | 0.63 | 0.39 | 16.20, P < 0.001 | 16.20, P < 0.001 |
| Step 2 (age, gender, educ, CES-D) | 0.76 | 0.18 | 2.22, P = ns | 5.65, P < 0.005 |
| Right posterior corona radiata | ||||
| Step 1 (peak VO2) | 0.61 | 0.37 | 14.51, P < 0.001 | 14.51, P < 0.001 |
| Step 2 (age, gender, educ, CES-D) | 0.74 | 0.18 | 2.02, P = ns | 4.99, P < 0.01 |
| Body of the cc | ||||
| Step 1 (peak VO2) | 0.54 | 0.29 | 10.16, P < 0.005 | 10.16, P < 0.005 |
| Step 2 (age, gender, educ, CES-D) | 0.72 | 0.23 | 2.43, P = ns | 4.45, P < 0.01 |
FA, fractional anisotropy; OA, older adults; cc, corpus callosum; CES-D, Center for Epidemiologic Studies Depression Scale.
Comparison of FA in YA, higher fit OA, and lower fit OA
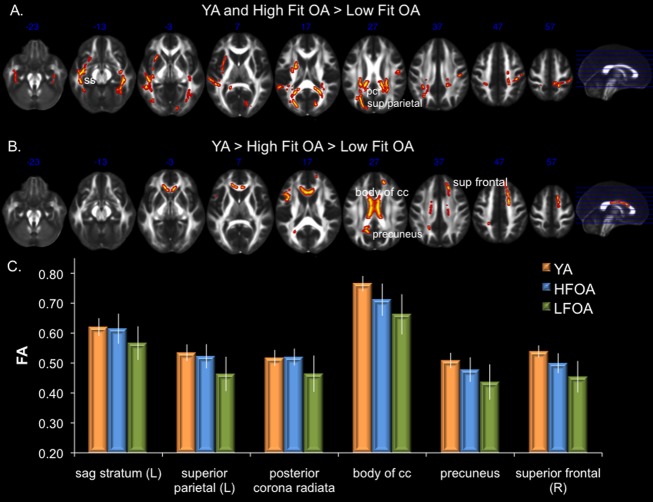
Although the continuous interaction approach demonstrated a positive association between peak VO2 and FA in OA, it does not allow us to directly compare white matter integrity in OA with peak VO2 values similar to those of YA. In order to do so, OA were classified as lower fit or higher fit and both groups were compared to YA. Classification of OA was based on their peak VO2 percentile scores (each subject’s percentile score is derived from their peak VO2 (mL/kg per min) relative to age and gender-matched norms from the ACSM), with OA who scored in the 10th–40th percentile classified as lower fit and OA in the 60th–90th percentile classified as higher fit. OA who scored in the 50th percentile (n = 3) were excluded from this analysis given that classification as lower or higher fit would have been arbitrary. Table1 shows the characteristics of the lower and higher fit OA, and the last row of Table1 shows the mean percentile score as well as the min and max percentile scores for lower and higher fit OA. FA values of YA, higher fit OA, and lower fit OA were directly compared using analyses of covariance (ANCOVAs) (gender, education, and depression score were entered as covariates) followed up with Tukey’s HSD test. ROIs were based on regions showing a significant peak VO2 by age group interaction. In a number of regions, including the sagittal stratum, posterior corona radiata, right superior parietal white matter, and splenium of the corpus callosum, there were no differences in FA values in YA and higher fit OA, and both groups had higher FA values than lower fit OA (Table2; Fig.2A and C). This pattern is consistent with the notion that directional diffusion was equivalent in these regions between higher fit OA and YA. In other regions, such as the body and genu of the corpus callosum, precuneus, and right superior frontal white matter, FA values were greater in higher fit OA relative to lower fit OA, but both groups had lower FA values than YA. Interestingly, regions that differed in FA between high and lower fit OA were predominantly posterior white matter regions (Table2; Fig.2B and C).
Figure 2.
(A) White matter regions showing equivalent white matter integrity (FA) in YA and higher fit OA, with both groups having higher FA than lower fit OA. (B) Regions showing greater FA in YA, followed by higher fit OA, and then lower fit OA. (C) Examples of mean FA values (error bars represent sd) of YA and OA classified as higher- or lower- cardiorespiratory fitness (CRF) based on a median split of peak VO2 percentiles from the American College of Sports Medicine. cc, corpus callosum; L, left; pcr, posterior corona radiata; R, right; sag, sagittal; sup, superior; FA, fractional anisotropy; YA, young adults; OA, older adults.
Discussion
The current study yielded three main findings. First, CRF was positively associated with white matter microstructure in OA. Second, while peak VO2 was positively associated with white matter microstructure in OA, no relationship was evident in YA. Finally, classification of OA based on peak VO2 revealed that white matter microstructure in multiple brain regions was intact in higher fit OA compared to YA, with higher values observed in both groups relative to lower fit OA. Other regions showed the greatest FA values in YA, followed by higher fit OA and then lower fit OA.
CRF positively linked to white matter microstructure in OA but not YA
Using a direct assessment of peak VO2, we found that CRF was positively associated with white matter microstructure in OA. These results extend and strengthen the extant literature, which has often linked CRF and white matter microstructure using indirect assessments of fitness (physical activity questionnaires) or a ROI approach. Here we implemented a DTI sequence and whole brain tract-based analyses capable of identifying specific white matter tracts and their relationship with peak VO2. Our results indicate that FA values within multiple white matter tracts exhibit positive associations with peak VO2 in OA, and that these associations are evident in some of the same regions that decline with aging (Fig.1D). We further quantified the extent of the relationship between peak VO2 and FA values using hierarchical regressions, and found that peak VO2 accounts for roughly 20–40% of variance in FA in OA, with age, gender, education, and depression scores unable to account for additional variance in the five regions examined. Further, peak VO2 accounted for roughly 20% of the variance in FA in four of the five regions examined when it was entered into the model after age, gender, education, and depression scores, indicating that peak VO2-FA associations are not simply accounted for by correlations with age, gender, education, or depression.
Our results confirm and extend previous studies examining the corpus callosum, a white matter pathway susceptible to age-related decline as well as effects of cardiovascular fitness. First, by including YA, we were able to observe an anterior–posterior gradient in decline in callosal microstructure: both the genu and body of the corpus callosum had significantly lower FA in OA relative to YA, whereas the majority of the splenium was intact (Fig.1A; sagittal section).32,33 Second, CRF was positively associated with integrity of the body and genu of the corpus callosum in OA (Fig.1B; Table2). Our work replicates and extends the findings of a previous DTI study of relatively fit OA (mean ACSM VO2 percentile of nearly 90% for males and 60% for females).20 Like Johnson et al., we found that CRF is positively associated with integrity of the body of the corpus callosum, but in our sample, this positive association also extends to the genu of the corpus callosum (Fig.1B; Table2). Third, we show that despite a positive association with FA, CRF does not eliminate age-related decline in FA in this region, as higher fit OA had lower FA values than YA. Finally, our results demonstrate that this relationship is specific to OA, as a positive association was not observed in our YA sample.
Our finding that CRF was associated with white matter microstructure in OA but not YA is consistent with the age-dependence hypothesis.34 According to the age-dependence hypothesis, CRF impacts cognition and the brain during childhood, exerts minimal influence during young adulthood when indicators of neural structure and function are typically at their lifetime peak, but may again positively impact brain structure and function in OA as cognitive decline begins in later adulthood. Preliminary support for this hypothesis is based on a study showing enhanced white matter microstructure in high-fit children relative to low-fit children,35 as well as data from the current study linking peak VO2 and white matter microstructure in OA, but not YA. Additional studies examining the relationship between CRF and white matter across the life span are needed to further evaluate the age-dependence hypothesis.
Comparison of white matter microstructure in YA, higher fit OA, and lower fit OA
The current results support the notion that CRF contributes to successful brain aging. When OA were classified according to ACSM normative values, age-related differences in FA were eliminated in multiple white matter regions in higher fit OA, including white matter in lateral parietal lobes, as well as temporal and occipital regions. Furthermore, other white matter regions such as the genu and body of the corpus callosum, and white matter adjacent to the superior frontal gyrus and precunues, exhibited higher FA values in higher fit OA relative to lower fit OA, although FA values of the higher fit OA were lower than the YA. Nevertheless, these data provide further evidence that CRF may positively impact white matter microstructure in OA.
CRF is not a panacea for age-related neural decline, as multiple white matter regions that decline with age were not associated with peak VO2, indicating that there is regional specificity in the associations between peak VO2 and FA. Interestingly, white matter regions demonstrating an association between FA and peak VO2 were prevalent in posterior brain regions, whereas anterior brain regions tended to be more resistant to an association with peak VO2. For instance, results of our ANCOVA demonstrated equivalent FA values in YA and higher fit OA, both greater than lower fit OA, in multiple parietal and temporal regions (Fig.2A and C), yet failed to show a statistical difference in high and lower fit OA in multiple long white matter tracts connecting the frontal lobes to the parietal lobes or occipital/parietal association cortex. Interestingly, the sagittal stratum ROI, which consists of the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus, showed a pattern in which FA was equivalent in YA and higher fit OA, with both groups have greater FA relative to lower fit OA only in posterior regions (predominately in the temporal lobes) of the anatomical ROI. No such pattern was observed in the anterior portions of the ROI that extended to the frontal lobes (i.e., the anterior portion of the fronto-occipital fasciculus). Furthermore, visual inspection of Figure1B reveals relatively sparse association between FA and CRF in regions of lateral prefrontal white matter in OA, although there was evidence of an association in anterior inter-hemispheric connections such as the genu and body of the corpus callosum.
CRF likely impacts neural integrity via multiple mechanisms.11,36 Animal studies have linked wheel-running to enhanced neurogenesis, synaptogenesis, and angiogenesis (formation of new neurons, synapses, and blood vessels, respectively), as well as growth factors that support these processes (e.g., brain-derived neurotrophic factor).37,38 Exercise has also been associated with enhanced axonal regeneration39 and functional changes in myelin.40 The mechanisms underlying the regional specificity of the CRF-white matter microstructure relationship observed in the current study are unknown, and will require a better understanding of how these diverse mechanisms may impact white matter microstructure.
The multivariate impact of CRF on the brain fosters substantial enthusiasm that enhanced CRF may attenuate dementia risk41 and progression.42 Our findings that CRF may mitigate age-related white matter decline is appealing for a variety of reasons, including that aerobic activities that enhance CRF, such as walking, are inexpensive, accessible, and could potentially improve quality of life by delaying cognitive decline and prolonging independent function. A recent report has indicated that physical activity, which can enhance CRF, is positively associated with white matter microstructure in a sample of low fit OA.43 Additional research is needed to clarify the impact of specific exercise programs (e.g., strength, aerobic, or combined training) or dose of exercise (frequency, intensity, duration) on white matter microstructure, as well as to distinguish the impact of physical activity and CRF on white matter microstructure.
Limitations
The current study was cross-sectional, and other cohort factors such as genetics, diet, or blood pressure could have influenced the results. The current study reports associations between CRF and white matter microstructure, and does not necessarily represent a causal relationship between CRF and white matter microstructure. Although YA, like OA, represented the full spectrum of ACSM percentile scores and had a similar ranges in raw peak VO2 values (YA range: 30.8; OA range: 34.0), it is possible that the lack of association observed within YA may be attributable to a limited sample size. The sample had limited ethnic and racial diversity.
Acknowledgments
This work was supported by the Department of Veterans Affairs, Rehabilitation Research & Development Service (Career Development Award e7822w awarded to S. M. H.), the Department of Veterans Affairs, Clinical Science Research & Development Service (M. V.), and the National Institute on Aging (K24AG035007 awarded to R. A. S.). Assistance with participant recruitment was provided by the Massachusetts Alzheimer’s Disease Research Center (P50-AG005134) and the Boston University Alzheimer’s Disease Center (P30-AG13846). The authors thank Kelly Allsup, MA, Pantel Vokonas, MD, Huiting Liu, Margaret Cadden, and Rebecca Lysiak for assistance with data collection. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States Government. The authors have no conflicts of interest to report.
Conflict of Interest
Dr. Hayes and Dr. Verfaellie reports grants from Department of Veterans Affairs, during the conduct of the study.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. White matter regions showing reduced FA in older adults relative to younger adults.
Table S2. White matter regions showing peak VO2 by age group interaction.
Table S3. Results of hierarchical regression models with nuisance variables entered in Step 1.
References
- Walhovd KB, Westlye LT, Amlien I, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, et al. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, et al. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, et al. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 2015;25:983–990. doi: 10.1093/cercor/bht289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci. 2013;5:31. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell CR, Gunstad J, Waldstein SR, et al. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014;69:455–462. doi: 10.1093/gerona/glt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML, Forman DE. The effects of aerobic exercise on cognitive and neural decline in aging and cardiovascular disease. Curr Geriatr Rep. 2014;3:282–290. doi: 10.1007/s13670-014-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer CS, Searcy JL, Bridges MT, et al. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PLoS One. 2011;6:e26812. doi: 10.1371/journal.pone.0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br J Sports Med. 2011;45:1208–1215. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- Marks BL, Madden DJ, Bucur B, et al. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, et al. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Erickson KI, Simonsick EM, et al. Physical activity predicts microstructural integrity in memory-related networks in very old adults. J Gerontol A Biol Sci Med Sci. 2014;69:1284–1290. doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Simonsick EM, Erickson KI, et al. Cardiorespiratory fitness and brain diffusion tensor imaging in adults over 80 years of age. Brain Res. 2014;1588:63–72. doi: 10.1016/j.brainres.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Bastin ME, Munoz Maniega S, et al. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology. 2012;79:1802–1808. doi: 10.1212/WNL.0b013e3182703fd2. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Forman DE, Verfaellie M. Cardiorespiratory fitness is associated with cognitive performance in older but not younger adults. J Gerontol B Psychol Sci Soc Sci. 2014 doi: 10.1093/geronb/gbu167. Dec 20. pii: gbu167. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Shepel J, Williams VJ, et al. Associations between T(1) white matter lesion volume and regional white matter microstructure in aging. Hum Brain Mapp. 2014;35:1085–1100. doi: 10.1002/hbm.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Holtrop JL, et al. Aerobic fitness is associated with greater white matter integrity in children. Front Hum Neurosci. 2014;8:584. doi: 10.3389/fnhum.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. J Appl Physiol. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, et al. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Ying Z, de Vellis J, Gomez-Pinilla F. Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia. 2007;55:966–975. doi: 10.1002/glia.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Honea RA, Billinger SA, et al. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging. 2012;33:1624–1632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Chaddock-Heyman L, Voss MW, et al. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One. 2014;9:e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. White matter regions showing reduced FA in older adults relative to younger adults.
Table S2. White matter regions showing peak VO2 by age group interaction.
Table S3. Results of hierarchical regression models with nuisance variables entered in Step 1.