Abstract
Airway epithelial cells (AECs) express a variety of receptors, which sense danger signals from various aeroallergens/pathogens being inhaled constantly. Proteinase-activated receptor 2 (PAR-2) is one such receptor and is activated by cockroach allergens, which have intrinsic serine proteinase activity. Recently, dual oxidases (DUOX), especially DUOX-2, have been shown to be involved in airway inflammation in response to Toll-like receptor activation. However, the association between PAR-2 and DUOX-2 has not been explored in airways of allergic mice. Therefore, this study investigated the contribution of DUOX-2/reactive oxygen species (ROS) signalling in airway reactivity and inflammation after PAR-2 activation. Mice were sensitized intraperitoneally with intact cockroach allergen extract (CE) in the presence of aluminium hydroxide followed by intranasal challenge with CE. Mice were then assessed for airway reactivity, inflammation, oxidative stress (DUOX-2, ROS, inducible nitric oxide synthase, nitrite, nitrotyrosine and protein carbonyls) and apoptosis (Bax, Bcl-2, caspase-3). Challenge with CE led to up-regulation of DUOX-2 and ROS in AECs with concomitant increases in airway reactivity/inflammation and parameters of oxidative stress, and apoptosis. All of these changes were significantly inhibited by intranasal administration of ENMD-1068, a small molecule antagonist of PAR-2 in allergic mice. Administration of diphenyliodonium to allergic mice also led to improvement of allergic airway responses via inhibition of the DUOX-2/ROS pathway; however, these effects were less pronounced than PAR-2 antagonism. The current study suggests that PAR-2 activation leads to up-regulation of the DUOX-2/ROS pathway in AECs, which is involved in regulation of airway reactivity and inflammation via oxidative stress and apoptosis.
Keywords: airway epithelium, allergic asthma, cockroach extract allergens, dual oxidase-2, proteinase-activated receptor-2, reactive oxygen species
Introduction
Asthma is a common inflammatory airways disease affecting people of all nations with high prevalence in western countries. Different allergens originating from cockroach, house dust mite and ragweed, as well as other sources, play an important role in the pathogenesis of asthma.1–3 In response to these allergens, inflammatory and structural cells in the airways produce a variety of mediators such as cytokines, histamine, matrix metalloproteinases, leukotrienes and reactive oxygen species (ROS), which are associated with airway inflammation/remodelling and mucus hypersecretion.2,4,5 Reactive oxygen species such as hydrogen peroxide may be directly or indirectly involved in epithelial cell apoptosis, increased vascular permeability and inactivation of antioxidant enzymes, so contributing to asthma symptomatology.4–7
Airway epithelial cells (AECs) are constantly exposed to airborne allergens and responsible for key immune responses through a wide range of pattern recognition receptors that respond to danger signals emanating from pathogenic microbes or injured cells.2,8 Airborne allergens originating from common household pests such as cockroaches also activate AECs through proteinase-activated receptor-2 (PAR-2). Activation of PAR-2 leads to production of a variety of inflammatory mediators that are involved in the pathogenesis of asthma.9,10 This notion is supported by studies that show association of PAR-2 with airway inflammation in both people with asthma and animal models of asthma.2,11,12 PAR-2 is expressed on a variety of cells in the lung including airway epithelium.10,13 PAR-2 knockout allergic mice also develop decreased airway inflammation and airway hyper-responsiveness compared with their wild-type counterparts in response to different allergens.12,14 These data point towards a strong role of PAR-2 in the development of airway hyper-responsiveness and airway reactivity; however, signalling mechanisms following PAR-2 activation, especially in relation to oxidative stress, have not been fully elucidated in murine airway epithelium.
The NADPH oxidase homologues, dual oxidase 1 and 2 (DUOX-1 and DUOX-2) are also expressed on the airway epithelium and play an important role in host defence as well as inflammation, primarily through production of hydrogen peroxide (H2O2).15 Increasing evidence supports the role of DUOX-2 in airway reactivity/inflammation through H2O2 production at mucosal surfaces. For example, DUOX-2 is up-regulated in people with asthma, current smokers, and following viral infection.16–18 Moreover, hyperoxia-induced acute lung injury is mediated by DUOX-2-induced H2O2, and DUOX-A-deficient mice that lack maturation factor for DUOX-1/2 show decreased airway inflammation and hyper-reactivity.19,20 Toll-like receptors (TLRs) 2, 3, 4 and 5, which also sense the presence of pathogens on airway epithelium, have been recently identified to be involved in airway inflammation through DUOX-2.21,22 Histamine, which is released in response to allergic stimuli, has been shown to trigger the release of H2O2 via DUOX-1/2 on human bronchial epithelial cells and contribute to oxidative stress in the airways.23 Together, these findings suggest that DUOX-2 might be involved in the regulation of allergic airway responses. Earlier studies have shown that PAR-2 may regulate superoxide generation in different cell types, which are thought to be involved in airway inflammation.24,25 However, no study so far has looked into a possible association between PAR-2 and DUOX-2 in AECs. Given the localization of both PAR-2 and DUOX-2 on AECs and recent evidence of TLR signalling via DUOX-1/2, we hypothesized that PAR-2 activation by cockroach allergens, because of their intrinsic protease activity, might lead to activation of DUOX-2 and subsequent ROS generation during allergic airway responses.
Hence, the purpose of this study was to investigate the effect of PAR-2 activation triggered by cockroach allergens on DUOX–ROS signalling, airway reactivity and inflammation in a mouse model of allergic asthma. Our findings show for the first time that allergen sensitization and challenge with cockroach allergens extract activates DUOX-2–ROS signalling in AECs with concomitant enhancement of airway reactivity/inflammation, oxidative stress and apoptosis. All of these effects are attenuated by a small molecule antagonist of PAR-2 in the allergic airways of mice.
Materials and methods
Animals
Male BALB/c mice, 8–10 weeks of age (20–25 g), free of specific pathogens, were used in the experiments. The animals were obtained from the Experimental Animal Care Centre, College of Pharmacy, King Saud University. The animals were kept under standard laboratory conditions of 12 : 12-hr light : dark cycle and 24–26° ambient temperature. All experimental animals used in this study were under a protocol approved by the Animal Care and Research Committee of the College of Pharmacy, King Saud University.
Mice sensitization and challenge
Sensitization was performed according to the protocol described earlier with little modification.26,27 Mice were sensitized on days 1 and 6 with intraperitoneal injections of 20 μg whole-body German cockroach (Blattella germanica) extract (Greer Laboratories, Lenoir, NC), adsorbed to 4 mg alum. Non-sensitized control animals received only alum with the same volumes. Two weeks after first sensitization, the mice were challenged intransally under light anaesthesia with 50 μg cockroach extract (CE) once only on days 14 and 16. The CE at a concentration of 100 mg/ml tested positive in our earlier publication using Limulus amoebocyte assay kit, indicating that it contained > 0·05 endotoxin units/ml.12 To study the role of PAR-2 activity modulation in this model, mice were given a small molecule antagonist, ENMD-1068 (Enzo Life Sciences, Farmingdale, NY) at 5 mg/kg, intranasally before each cockroach extract challenge. Some mice were sensitized and challenged with heat-inactivated CE (HCE; inactivation carried out at 65° for 30 min) with the same protocol as described above. This procedure reduces trypsin-like proteinase activity in the CE by > 90%.12 Additionally, we used a proteinase activity inhibitor, soybean trypsin inhibitor (SBTI), and a PAR-2 agonist, trypsin, in vivo. For this purpose, one group of mice (SEN + CHAL + SBTI) was challenged with SBTI-treated CE (CE was treated with 200 μm SBTI for 30 min at 37°); the other group (SEN + CHAL + Trypsin) was given trypsin (0·2 μg, intranasally) after each CE challenge. The former neutralizes trypsin-like activity in CE and the latter is equivalent to serine protease activity contained in 50 μg CE.12
Mice were divided into following groups: Control group (CON): mice received only vehicles for sensitization and challenge; Sensitized and challenged group (SEN + CHAL): mice were sensitized and challenged with CE using the same protocol described above; Sensitized and challenged group administered ENMD-1068 (ENMD + SEN + CHAL): mice were sensitized and challenged with CE using the same protocol described above and ENMD-1068 was administered on days 14 and 16 before each allergen challenge; Sensitized and challenged group with heat-inactivated CE (SEN + CHALHCE): mice were sensitized and challenged with heat-inactivated CE using the same protocol described above; Sensitized and challenged group with heat-inactivated CE and administered ENMD-1068 (ENMD + SEN + CHALHCE): mice were sensitized and challenged with heat-inactivated CE using the same protocol described above and ENMD-1068 was administered on days 14 and 16 before each allergen challenge.
Measurement of airway reactivity in vivo
Twenty hours after final allergen challenge, airway reactivity to methacholine in conscious, unrestrained mice was assessed by a whole-body non-invasive plethysmograph (Buxco Electronics Inc., Wilmington, NC.) as described earlier.12,27 Baseline enhanced pause (Penh) was determined by exposing mice to nebulized saline. To assess the role of dual oxidase in PAR-2-mediated signalling, the SEN + CHAL group was treated with diphenyl iodonium (DPI; 1 mg/kg, intranasally) before each allergen challenge followed by a methacholine reactivity test on day 17 as described above. The mice were then exposed to increasing concentrations of aerosolized methacholine dissolved in saline (0–32 mg/ml) to obtain a dose–response and Penh values were recorded at each dose.
Bronchoalveolar lavage
The trachea was cannulated to perform bronchoalveolar lavage (BAL) 2 days after final allergen challenge; PBS was introduced into the lungs via the tracheal cannula and the total cells were counted manually in a haemocytometer chamber followed by spinning of cells onto glass slides for differential count. A differential count of at least 300 cells was made according to standard morphologic criteria on cytocentrifuged Diff-Quik stained slides. The number of cells recovered per mouse was calculated and expressed as mean ± SE per ml for each group.
Brushing of AECs from murine trachea
Airway epithelial cells from murine trachea were harvested by scraping the lumen of the trachea gently with a brush, which was made by sanding a plastic polyethylene tube. For real-time PCR, harvested AECs were immediately placed in RNAlater (Qiagen, Hilden, Germany) followed by lysing by multiple passages through an 18G needle. For ROS experiments, the tracheas were cut open longitudinally and AECs were gently scraped using a mini plastic spatula and pooled (three or four tracheas for n = 1) in oxygenated (95% O2 and 5% CO2) Krebs–Hanseleit buffer; the same samples were used for Western blot experiments. Epithelial removal was confirmed by microscopic examination of haematoxylin & eosin stained sections.
Real-time PCR
Total RNA was isolated from the murine AECs using an RNeasy micro kit according to the manufacturer's protocol (Qiagen). RNA yield and purity were measured with the Nanodrop 1000 (Thermo Scientific, Waltham, MA). This was followed by conversion of 0·5 μg of total RNA into cDNA using a High Capacity cDNA archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions as described earlier.27,28 Real-time PCR was performed on an ABI PRISM 7500 Detection System (Applied Biosystems) using Taqman Universal Mastermix (Applied Biosystems), cDNA, and FAM-labelled Taqman gene expression kit. For the real-time PCR of DUOX-1, DUOX-2, MUC5AC, Bcl-2, Bax, caspase-3 and inducible nitric oxide synthase (iNOS), the Taqman assays-on-demand gene expression kits were purchased from Applied Biosystems. 18S rRNA (Ribosomal RNA) was used as an endogenous control. The fold difference in expression of target cDNA was determined using the comparative CT method. The fold difference in gene expression of the target was calculated as described earlier.29
Western immunoblotting
Aliquots of the supernatants isolated from murine AECs (30 μg protein/well) were separated on 10% SDS–PAGE. Proteins were transferred to nitrocellulose membranes and then probed either with polyclonal goat DUOX-2, or monoclonal rabbit iNOS, or polyclonal rabbit nitrotyrosine, polyclonal rabbit Bax, or polyclonal rabbit Bcl-2, or polyclonal rabbit caspase-3 antibodies (Santa Cruz Biotechnology, Dallas, TX) at a dilution of 1 : 500 to 1 : 1000, or β-actin rabbit polyclonal antibody (Santa Cruz Biotechnology) at a dilution of 1 : 5000. This was followed by incubation with the secondary horseradish peroxidase-conjugated antibodies (anti-goat and anti-rabbit IgGs; Santa Cruz Biotechnology) for 1 hr at room temperature. For detection of bands, the membranes were treated with an enhanced chemiluminescence reagent (Amersham ECL, GE Healthcare, Chalfont St Giles, UK) for 0·5–1 min and subsequently exposed to ECL Hyperfilm. The relative expression of the protein bands was quantified by densitometric scanning of the X-ray films. Western blot values are expressed in percentage after normalization to β-actin levels.
Nitrite assay
Nitrite levels in BAL fluid were estimated by the method of Misko et al.30 The method was based upon the reaction of nitrite with 2,3-diaminonaphthalene to form the fluorescent product, 1-(H)-naphthotriazole. This assay is 50–100 times more sensitive than the well-known Griess assay. Readings of the fluorescent product were taken at 365 nm (excitation) and 450 nm (emission) on Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT). A standard curve was generated using known concentrations of sodium nitrite. Results were expressed as nmol/l.
Protein carbonyls assay
The content of protein-bound carbonyls in the tracheal supernatant, an indicator of protein oxidation, was measured at 380 nm using 2,4-dinitrophenylhydrazine (DNPH) by the method of Levine et al31 as described by us previously.32 Briefly, after precipitation of proteins in tracheal supernatant by trichloroacetic acid, the pellet was dissolved in DNPH followed by further precipitation after a waiting period of 1 hr. The resulting pellet was dissolved in 6 m guanidine solution after several washes with ethanol : ethyl acetate solution. Absorbance of the sample was taken at 380 nm and carbonyl content was calculated using molar absorption coefficient of 22 000/m/cm. The final results were expressed as nmol/mg protein.
Reactive oxygen species assay
For ROS generation, the harvested AECs were incubated with 100 μm 6-carboxy-2′,7′-dichlorofluorescin diacetate (DCFH-DA) for 30 min at 37°. DCFH-DA forms a fluorescent product, DCF (dichlorofluorescein) upon oxidation with ROS. Fluorescence caused by DCF in each well was measured and recorded for 30 min at 485 nm (excitation) and 530 nm (emission) by the method of Wang and Joseph33 using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments) with temperature maintained at 37°. For in vitro experiments, the compounds trypsin (100 nm), ENMD-1068 (1 mm), polyethylene glycol (PEG)-catalase (250 U/ml) and DPI (10 μm) were incubated with harvested AECs from CE-sensitized and challenged mice for 30 min followed by measurement of DCF fluorescence as described above. The background fluorescence caused by buffer and DCF were subtracted from the total fluorescence in each well caused by the AECs in the presence of DCF. Fluorescence intensity was expressed as ROS generation (% control).
Lung histology
Lungs were removed from the thorax and fixed with formalin for histological analysis. Formalin-fixed lungs were sectioned at a thickness of 5 μm followed by staining with haematoxylin and eosin and Periodic Acid Schiff for inflammation-related morphology and mucus production, respectively. Sections were examined by bright-field microscopy.
Chemicals
Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma Chemicals (St Louis, MO).
Statistical analysis
The data were expressed as mean ± SEM and derived from two or three independent experiments. Comparisons among different groups were analysed by analysis of variance followed by Tukey's multiple comparison tests. A P value of < 0·05 was considered significant for all statistical tests. All the statistical analyses were performed using Graph Pad Prism statistical package (Graph Pad, San Diego, CA).
Results
Cockroach allergens induced activation of DUOX-2 in AECs and blockade by PAR-2 antagonism
Since TLR signalling has been linked to the activation of DUOX recently, we reasoned that PAR-2 activation by cockroach allergens may also lead to DUOX activation because it is one of the receptors that recognizes danger signals similar to TLRs. Sensitization and challenge with CE led to up-regulation of DUOX-2 at both mRNA and protein levels in AECs compared with the control group (Fig.1a,b) whereas there was no difference in DUOX-1 expression (data not shown). Sensitization and challenge with CE also led to increased ROS generation in AECs compared with the control group (Fig.1c). Pre-treatment with ENMD-1068, a PAR-2 antagonist in allergic mice, led to down-regulation of DUOX-2 and ROS generation in AECs compared with the CE-sensitized and challenged group (Fig.1a–c). Mice sensitized and challenged with heat-inactivated CE had slight increase (P > 0·05) in DUOX-2 mRNA expression and ROS generation compared with the control group (Fig.1d,e). Control mice challenged with CE also did not show any significant difference in DUOX-2 mRNA expression and ROS generation in comparison to control mice. On the other hand, mice sensitized and challenged with intact CE and then challenged with trypsin had increased DUOX-2 mRNA expression and ROS generation compared with CE sensitized and challenged mice. Furthermore, mice sensitized with intact CE and challenged with SBTI-neutralized CE had decreased DUOX-2 mRNA expression and ROS generation compared with CE-sensitized and -challenged mice (Fig.1d,e). This shows that PAR-2 activation by cockroach allergen proteinase activity leads to up-regulation of DUOX-2 and ROS generation in AECs of allergic mice.
Figure 1.
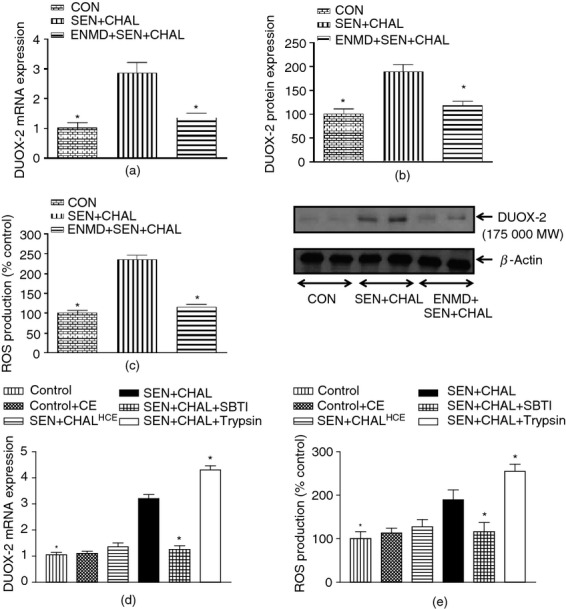
Effect of intact (a–c)/heat-inactivated cockroach extract allergen challenge and proteinase activated receptor-2 (PAR-2) activity modulators (d–e) on dual oxidase 2–reactive oxygen species (DUOX-2–ROS) signalling in airway epithelial cells (AECs) of different groups. (a) DUOX-2 mRNA expression, (b) DUOX-2 protein expression, (c) ROS generation, (d) DUOX-2 mRNA expression, and (e) ROS generation. Expression of DUOX-2 in all the groups was assessed by real-time PCR. For mRNA expression by comparative CT method using real=time PCR, first column was made as the calibrator against which the other groups were compared. ROS generation was assessed biochemically. Values are expressed as mean ± SE, n = 4 to 8/group and derived from two independent experiments. *P < 0·05, versus SEN + CHAL group.
Cockroach allergens induced increase in airway reactivity/inflammation and mucin secretion, and attenuation by PAR-2 antagonism
To investigate the effects of PAR-2 blockade in a model of allergic airway inflammation in response to CE sensitization and challenge, ENMD-1068 was administered before each intranasal allergen challenge. Sensitization and challenge with CE led to increased Penh in response to aerosolized methacholine dose-dependently (Fig.2a), which was attenuated significantly by ENMD-1068. Sensitization and challenge with CE also led to increased airway inflammation, as assessed by total leucocyte (Fig.2b) and eosinophil counts (Fig.2c), both of which were attenuated by ENMD-1068. Mice sensitized and challenged with heat-inactivated CE and those treated with ENMD-1068 before challenge with heat-inactivated CE had similar Penh values (P > 0·05 at all methacholine doses), which were slightly higher than in the control group (Fig.2d). On the contrary, there was an increase in total cell (P > 0·05) and eosinophil count (P < 0·05) in mice sensitized and challenged with heat-inactivated CE. Treatment with ENMD-1068 neither reduced total leucocyte count nor eosinophil count in mice sensitized and challenged with heat-inactivated CE (Fig.2e). Airway inflammation was also confirmed by histological examination, which showed blockade of inflammatory cell infiltrate into airways and perivascular regions in the ENMD-treated group (Fig.3a).
Figure 2.
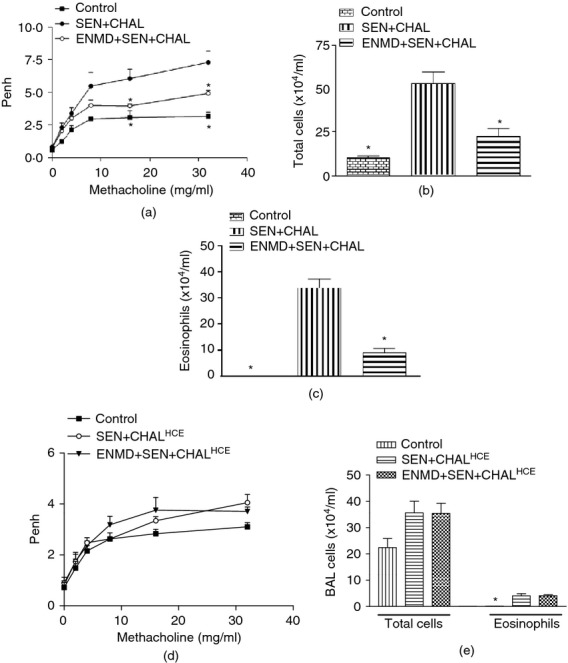
Effect of intact (a–c) and heat-inactivated (d, e) cockroach extract allergen challenge on airway reactivity and inflammation. (a) Enhanced pause (Penh), (b) total leucocyte count, (c) eosinophil count, (d) Penh, and (e) total leucocyte and eosinophil counts. Airway reactivity to methacholine was measured as Penh, 24 hr after the final allergen challenge using a Buxco system for whole body plethysmography in which mice were exposed to increasing concentrations of methacholine (0–32 mg/ml). Airway inflammation in bronchoalveolar lavage was assessed 48 hr after the final allergen challenge through total cell and eosinophil cell counts. Values are expressed as mean ± SE, n = 6 to 8/group and derived from two to three independent experiments. *P < 0·05, versus SEN + CHAL/SEN + CHALHCE group.
Figure 3.
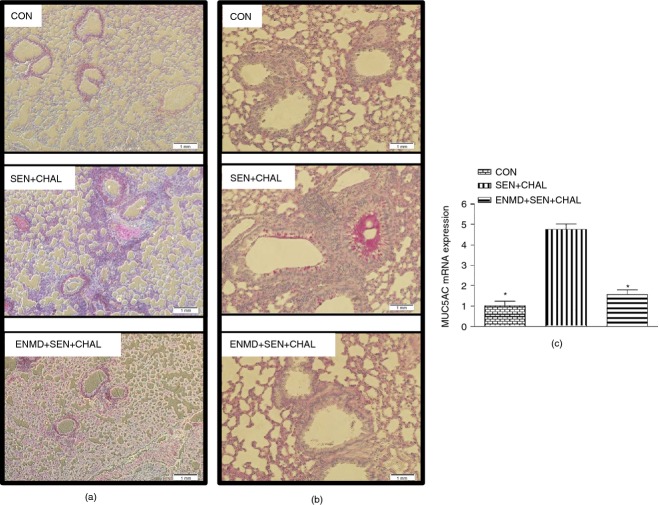
Effect of cockroach extract allergen challenge on histological parameters in allergic mice. (a) Haematoxylin & eosin (H&E) staining of lung sections, (b) periodic acid Schiff (PAS) staining of lung sections, and (c) MUC5AC expression. Expression of MUC5AC in all the groups was assessed by real-time PCR. For mRNA expression by comparative CT method using real-time PCR, first column was made as the calibrator against which the other groups were compared. Values are expressed as mean ± SE, n = 6 to 8/group and derived from two independent experiments. *P < 0·05, versus SEN + CHAL group. Each photomicrograph is a representative image from every group (n = 5 or 6/group; magnification, 100× and 200× for H&E and PAS staining, respectively).
Sensitization and challenge with CE led to increased mucin expression from AECs as shown by PAS staining (Fig.3b) and increased MUC5AC expression (Fig.3c) in AECs compared with the control group. These changes were attenuated significantly by intranasal administration of ENMD-1068 before CE allergen challenge. This shows that PAR-2 activation by CE leads to mucin secretion from AECs and antagonism of PAR-2 reverses this effect. Mice sensitized and challenged with heat-inactivated CE and those treated with ENMD-1068 before challenge with heat-inactivated CE did not have significant differences in MUC5AC expression (P > 0·05) compared with the control group mice (data not shown).
Cockroach allergens induced increase in markers of oxidative stress and apoptosis, and attenuation by PAR-2 antagonism
As PAR-2 activation led to H2O2 generation in AECs, our next hypothesis revolved around its possible detrimental effects in allergic airways. Our data show that markers of oxidative stress were increased after sensitization and challenge with CE, as shown by increased level of iNOS expression (Fig.4a), nitrite (Fig.4b), nitrotyrosine (Fig.4c) and protein carbonyls (Fig.4d). Pre-treatment with ENMD-1068 before CE challenge reversed the changes in parameters of oxidative stress induced by CE challenge in allergic mice (Fig.4a–d).
Figure 4.
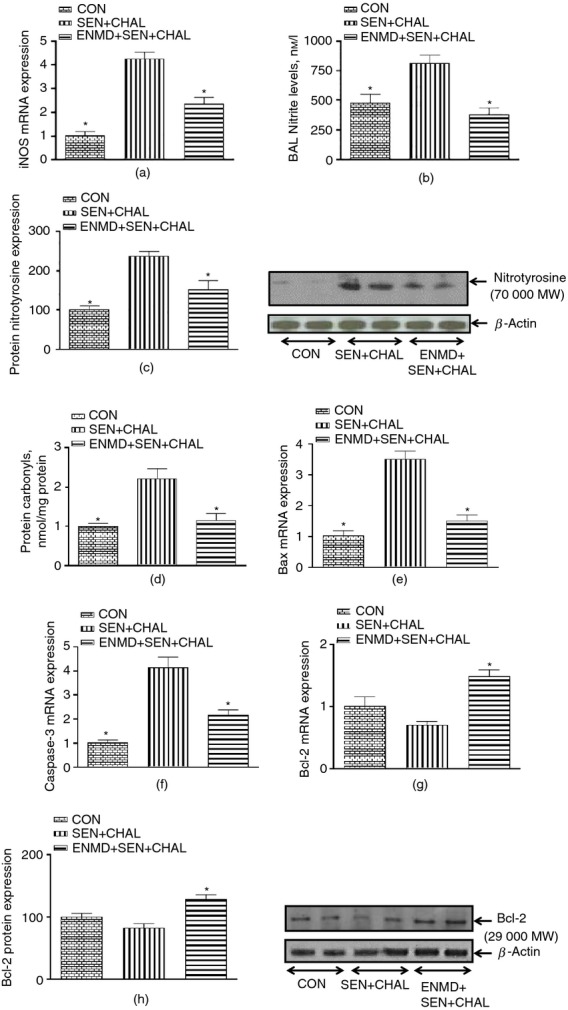
Effect of cockroach extract allergen challenge on parameters of oxidative stress and apoptotic markers in airway epithelial cells (AECs)/trachea of allergic mice. (a) Inducible nitric oxide synthase (iNOS) expression, (b) nitrite levels, (c) 3-NT, (d) protein carbonyls, (e) Bax, (f) caspase-3, and (g, h) Bcl-2. Expression of iNOS and pro-/anti-apoptotic genes in all the groups was assessed by real-time PCR. For mRNA expression by comparative CT method using real-time PCR, the first column was made as the calibrator against which the other groups were compared. 3-NT and Bcl-2 levels were assessed by Western blot whereas nitrite and protein carbonyls were assessed biochemically. Values are expressed as mean ± SE, n = 4 to 8/group and derived from two independent experiments. *P < 0·05, versus SEN + CHAL group.
The next question was whether oxidative stress was associated with increased apoptosis in AECs. Sensitization and challenge with CE led to increased apoptotic markers in epithelial cells as evidenced by increased Bax (Fig.4e) and caspase-3 expression (Fig.4f), and decreased Bcl-2 expression (both mRNA and protein; Fig.4g,h). Pre-treatment of allergic mice with ENMD-1068 reversed the changes in apoptotic markers induced by CE sensitization and challenge (Fig.4e–h). Hence, all of these data suggest that PAR-2 activation induced by CE in allergic mice not only leads to increased oxidative stress in the airways but also apoptosis and these effects may be initiated by DUOX-2 mediated signalling.
Inhibition of DUOX-2 in vitro/in vivo in allergic mice attenuates oxidative stress, apoptotic markers and airway reactivity/inflammation
Until now, we have only shown that PAR-2 is responsible for DUOX-2 up-regulation in the allergic airways, increased oxidative stress and airway reactivity/inflammation. If DUOX-2 was indeed responsible for all of these changes after PAR-2 activation, then its inhibition by DPI should attenuate the changes induced by cockroach allergens. Treatment of CE-sensitized and -challenged mice with DPI both in vivo (Fig.5a) and in vitro (Fig.5b) resulted in significant reduction of ROS generation from AECs. PEG-catalase treatment in vitro showed around 85% reduction in ROS generation from AECs, suggesting that H2O2 is the predominant oxidizing species in AECs of CE-sensitized and -challenged mice (Fig.5b). Moreover, PAR-2 activation by trypsin in vitro further resulted in enhancement of ROS generation from AECs of CE-sensitized and -challenged mice (Fig.5b). Next, effects of DUOX-2 inhibition were determined on oxidative stress and apoptotic parameters after in vivo treatment of CE-sensitized and -challenged mice with DPI. DUOX-2 inhibition by DPI led to a decrease in oxidative stress markers, i.e. nitrotyrosine and protein carbonyl levels (Fig.6a,b), as well as apoptotic markers, i.e. Bax, caspase-3 and Bcl-2 expression (Fig.6c–e). Finally, it was confirmed whether DUOX-2-mediated oxidative stress and apoptosis in airways contributed functionally to parameters related to allergic airway responses (airway reactivity, inflammation and mucin gene expression). Indeed, pre-treatment of allergic mice with DPI led to decrease not only in airway reactivity but also inflammation (total leucocyte count) compared with CE-sensitized and -challenged mice (Fig.7a,b). MUC5AC expression was also attenuated by DPI pre-treatment in allergic mice (Fig.7c). Surprisingly, DUOX-2 inhibition by DPI in allergic mice showed only 15% reduction in eosinophil count (not significant; data not shown) and similar iNOS/nitrite levels compared with CE-sensitized and -challenged mice (data not shown); this suggests that PAR-2-mediated DUOX-2 signalling is not responsible for iNOS up-regulation and eosinophilic infiltration. However, overall our data show that DUOX-2-generated ROS after cockroach allergen challenge contributes significantly in the induction of oxidative stress and apoptosis in the airways with parallel enhancement of allergic airway responses.
Figure 5.
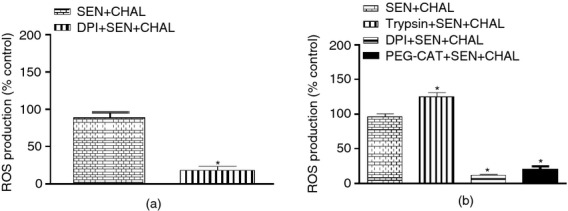
Effect of the dual oxidase (DUOX) inhibitor, diphenyl iodonium (DPI); proteinase activated receptor-2 (PAR-2) agonist, trypsin; and scavenger of hydrogen peroxide, polyethylene glycol (PEG-CAT) on reactive oxygen species (ROS) generation from airway epithelial cells (AECs) of allergic mice. (a) ROS generation after in vivo DPI treatment, and (b) ROS generation after in vitro treatment of PEG-CAT, DPI and trypsin. ROS generation was assessed biochemically. Values are expressed as mean ± SE, n = 3 or 4/group and derived from two independent experiments. *P < 0·05, versus SEN + CHAL group.
Figure 6.
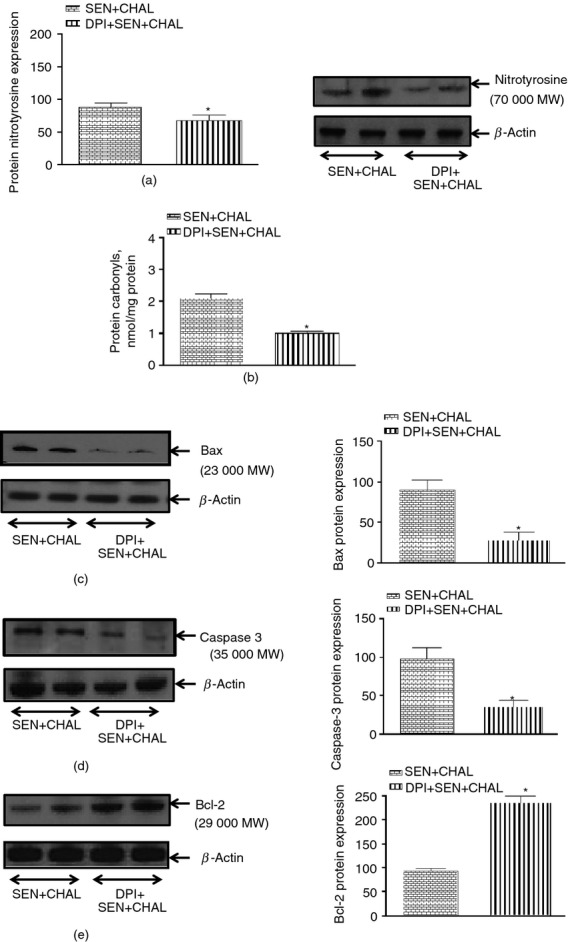
Effect of dual oxidase (DUOX) inhibitor, diphenyl iodonium (DPI) on cockroach extract allergen-induced changes in markers of oxidative stress and apoptosis in airway epithelial cells (AECs) of allergic mice. (a) 3-NT, (b) protein carbonyls, (c) Bax, (d) caspase-3 and (e) Bcl-2. Expression of pro-/anti-apoptotic markers was assessed by Western blot whereas protein carbonyls were measured biochemically. Values are expressed as mean ± SE, n = 5 or 6/group and derived from two independent experiments. *P < 0·05, versus SEN + CHAL group.
Figure 7.
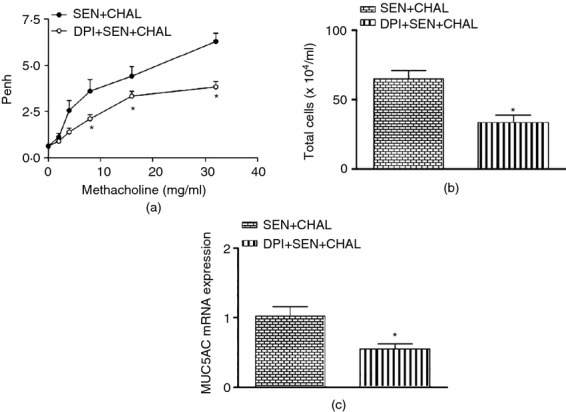
Effect of dual oxidase (DUOX) inhibitor, diphenyl iodonium (DPI) on cockroach extract allergen-induced changes characteristic of allergic asthma. (a) Enhanced pause (Penh), (b) airway inflammation, and (c) MUC5AC expression. Airway reactivity to methacholine was measured as Penh, 24 hr after the final allergen challenge using a Buxco system for whole body plethysmography in which mice were exposed to increasing concentrations of methacholine (0–32 mg/ml). Airway inflammation in bronchalveolar lavage was assessed 48 hr after the final allergen challenge through total cell and eosinophil cell counts. Expression of MUC5AC in all the groups was assessed by real-time PCR. For mRNA expression by comparative CT method using real-time PCR, the first column was made as the calibrator against which the other groups were compared. Values are expressed as mean ± SE, n = 5 or 6/group and derived from two independent experiments. *P < 0·05, versus SEN + CHAL group.
Discussion
This is the first study to show that PAR-2 activation by cockroach allergens leads to activation of DUOX-2 and subsequent ROS generation in murine AECs. Furthermore, PAR-2 blockade by a small molecule antagonist, ENMD-1068, before allergen challenge prevented ROS generation and subsequent airway hyper-responsiveness and airway inflammation. This suggests that cockroach allergen proteinases cleave PAR-2 and lead to activation of DUOX-2–ROS signalling in the airway epithelium of allergic mice.
The aeroallergens originating from house dust mite and cockroach have been shown to cause release of several mediators via activation of PAR-2 on AECs and other inflammatory cells. These mediators are thought to be responsible for various aspects of airway hyper-responsiveness and airway inflammation.3,10,12,13 This has been confirmed by proteinase inhibitors, which attenuate allergen-induced airway hyper-responsiveness and inflammation in various animal models of asthma.34,35 Furthermore, allergic mice that lack PAR-2 show decreased airway inflammation and hyper-reactivity compared with wild-type mice in response to different allergens.12,14 Among various inflammatory mediators, PAR-2 activation has been shown to cause release of superoxide radicals from various inflammatory cells.24,25 However, no study has investigated the effect of PAR-2 activation on DUOX signalling during allergic responses in murine airway epithelial cells.
The respiratory epithelium is the most important first line of defence against inhaled microorganisms and allergens by evoking immune responses through a variety of receptors that recognize and respond to pathogen-associated molecular patterns. Recently, H2O2 is reported to be released in response to different pathogenic stimuli primarily from NADPH oxidase homologues, DUOX-1 or DUOX-2.2,15,21 Controlled H2O2 production by these two enzymes in AECs takes care of the invading pathogens and forms an important physical barrier. However, during airway inflammation, DUOX up-regulation may be detrimental because of excessive production of H2O2, which may be used by several pathways to further amplify oxidative potential of H2O2 and cause cellular damage. This is supported by findings that show increased H2O2 in BAL fluid/exhaled breath condensates and products of oxidative damage in the airways of people with asthma.36–38
It was thought for a long time that increased H2O2 production is the result of dismutation of superoxide radicals, which is increased in people with asthma.32,36,37 However, recent findings have modified this concept and shown an important role of DUOX-2 especially in the context of airway inflammation. This is supported by recent findings in people with asthma as well as animal studies.17,19 Our study also showed up-regulation of DUOX-2 and subsequent ROS generation from murine AECs after CE allergen challenge. We have shown earlier that CE allergens contain serine proteinase activity and activate PAR-2,12 so increase in ROS production in our study was the result of DUOX-2 up-regulation after PAR-2 cleavage because there was no induction of DUOX-1 after CE allergen challenge.
The PAR-2 activation after CE allergen challenge was responsible for enhanced airway reactivity and inflammation via the DUOX-2–ROS pathway. This observation was based on the attenuation of a CE-induced increase in airway reactivity and inflammation by ENMD-1068 and DPI. Inhalation of H2O2 has been reported to produce airway reactivity and inflammation even in naive mice.39 Therefore it is not surprising that recent studies are beginning to show an important role of H2O2 generated by DUOX-2 in airway inflammation as well as hyper-reactivity.17,19 Enhanced ROS production has been reported in previous studies in allergic airways and shown to correlate with increased airway constriction, infiltration of inflammatory cells, vascular hyperpermeability and exaggerated mucus production.4,5,32,37,38
Proteinase-activated receptor 2-mediated regulation of DUOX-2 activity is not surprising because other receptors that recognize and respond to danger signals at mucosal surfaces also lead to production of ROS via DUOX. For example, TLR-2, -3, -4 and -5, which also sense the presence of pathogens on airway epithelium, have been recently shown to be associated with inflammation through DUOX-2. Ryu et al21 showed recently that β-glucans–TLR-2 and lipopolysaccharide–TLR-4 signalling pathways were essential for triggering allergic rhinitis and allergic asthma, respectively, through DUOX-2-generated ROS. DUOX-A-deficient mice that lack maturation factors for DUOX-1 and -2 show decreased airway hyper-reactivity and inflammation.19 Yu et al18showed that TLR-3 activation by poly(I:C) led to cleavage of soluble tumour necrosis factor receptor type 1 partly via DUOX-2-generated ROS from human bronchial epithelial cells. Moreover, people with asthma have been shown to have increased expression of DUOX-2 compared with those without asthma.17 Histamine, which is released in response to allergic stimuli, has been shown to trigger the release of H2O2 via DUOX-1/2 on human bronchial epithelial cells and contribute to oxidative stress in the airways.23 PAR-2 adds to this expanding repertoire of receptors that can respond to allergic stimuli and modify the environment of AECs through production of ROS. However, we cannot rule out the possibility that some of the observed effects on DUOX-2, airway reactivity and inflammation may be PAR-2 independent because of the presence of low lipopolysaccharide activity in the CE preparation, and this is a limitation of our study.
Hydrogen peroxide produced in AECs may react with nitrite present in the airways to produce nitrating species.7,17,36 H2O2 may diffuse into the nearby tissue where eosinophil peroxidase and/or myeloperoxidase may use both H2O2 and nitrite to cause oxidative modification of proteins and loss/alteration of function.5,40 Indeed, our study shows increased iNOS expression in AECs along with increased nitrite levels in BAL fluid. Concomitantly, nitrotyrosine and protein carbonyl levels were increased in CE-sensitized and -challenged mice, suggesting that H2O2 generated by DUOX-2 after PAR-2 activation may be responsible for these observations. However, DPI administration did not lead to decreased iNOS expression and nitrite levels but led to decreased nitrotyrosine/protein carbonyl levels, suggesting that DUOX-2 inhibition may be responsible for decreased oxidative stress predominantly via a decrease in H2O2-mediated reactions. A recent study has shown that H2O2 generated by DUOX-2 is responsible for oxidative modification of proteins in AECs.17
Increased oxidative stress/ROS generation may lead to apoptosis of AECs, as has been reported in earlier reports.4,40–42 However, in this study we investigated ROS-mediated apoptosis in the context of PAR-2 activation. It is well known that apoptotic signals converge on the mitochondria via activation of pro-apoptotic members of the Bcl-2 family, such as Bax, whereas Bcl-2 serves as an anti-apoptotic protein. Upon activation of Bax, possibly by ROS, there is subsequent activation of effector caspases such as caspase-3 which ultimately leads to apoptosis.4,43 These apoptotic changes were observed in AECs after CE allergen challenge as indicated by increased Bax and caspase 3 expression with a concomitant reduction in Bcl-2 expression. CE-induced changes on apoptotic markers were reversed by ENMD-1068 as well as DPI. This possibly suggests the involvement of DUOX-2 in PAR-2-mediated increase in markers of apoptosis on AECs after CE allergen challenge. Kim et al20 have shown recently that DUOX-2 is involved in apoptosis of AECs in response to hyperoxia. Our study also points towards the importance of DUOX-2 signalling with respect to apoptosis in AECs.
Increased apoptosis may lead to several pathophysiological changes in the airways. For example, it can lead to epithelial shedding and increase permeability to otherwise non-permeable substances.4,41 A compromised epithelial barrier is thought to be responsible for enhanced entry of antigens to dendritic cells, leading to their maturation and an increase in allergic responses and further contributing to airway hyper-responsiveness and remodelling.2,5,8 Our study shows that PAR-2 antagonism can rescue the epithelium from these damaging effects of DUOX-2-generated ROS and help in the maintenance of epithelial integrity. Increased ROS may also inactivate the antioxidant enzymes as has been reported in the earlier studies. Antioxidant enzymes oxidatively modified by ROS consequently lose their protective function thereby contributing to epithelial apoptosis/inflammation in response to allergens.4,5,32,40,43 Similar mechanisms may be responsible for the increased apoptosis observed in our study. This is supported by observations in our study showing increased nitrotyrosine, as well as protein carbonyls, formation in the airways, both of which are a consequence of ROS-mediated modification of proteins and serve as important markers of oxidative insult on proteins.
DUOX-1/2 has also been implicated in augmenting mucin expression in the airway, a characteristic feature of allergic asthma that may lead to airway remodelling during chronic inflammation. Recently, it has been shown that TLR signalling leads to MUC5AC up-regulation via DUOX-1/2. For example, flagellin, a TLR-5 agonist induced DUOX-2-generated H2O2 and MUC5AC expression in nasal epithelium and these effects were absent in DUOX-2 knockout mice.22 Another report showed DUOX-1-mediated H2O2 was responsible for increased MUC5AC expression in response to TLR-4 activation.44 Furthermore, NOX4, a non-phagocytic NADPH oxidase, was also reported to be involved in MUC5AC expression in human nasal epithelial cells via H2O2 production.45 Our study confirms these earlier observations and shows that DUOX-2 is involved in exaggerated mucin secretion in response to PAR-2 activation and may contribute to airway remodelling during chronic airway inflammation. Our study further shows that DUOX-1/2 may be differentially regulated in response to different danger signals being encountered at airway mucosal surfaces.
In conclusion, our study shows a significant contribution of DUOX-2–ROS pathway in PAR-2-mediated effects on airway reactivity, inflammation, oxidative stress and apoptosis in airways of allergic mice. Our study also shows that antagonism of PAR-2 activation by ENMD-1068 leads to attenuation of allergic airway responses caused by CE allergen challenge. Our findings suggest that PAR-2 antagonism may be used as a therapeutic strategy to counteract airway hyper-reactivity and inflammation.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research and College of Pharmacy Research Center, King Saud University for their funding and support.
Glossary
- AEC
airway epithelial cell
- BAL
bronchoalveolar lavage
- CE
cockroach extract
- DUOX
dual oxidase
- H2O2
hydrogen peroxide
- iNOS
inducible nitric oxide synthase
- i.n
intranasal
- NO
nitric oxide
- PAR-2
proteinase-activated receptor 2
- ROS
reactive oxygen species
Disclosures
The authors declare no conflict of interest.
References
- Janssen-Heininger YM, Poynter ME, Aesif SW, et al. Nuclear factor κB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc. 2009;6:249–55. doi: 10.1513/pats.200806-054RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zeng X, He S. Evaluation on potential contributions of protease activated receptors related mediators in allergic inflammation. Mediators Inflamm. 2014:829068. doi: 10.1155/2014/829068. . doi: 10.1155/2014/829068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Siddiqui N, Alharbi NO, Alharbi MM. Airway and systemic oxidant-antioxidant dysregulation in asthma: a possible scenario of oxidants spill over from lung into blood. Pulm Pharmacol Ther. 2014;29:31–40. doi: 10.1016/j.pupt.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Masood A, Siddiqui N. Oxidant–antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis. 2008;2:215–35. doi: 10.1177/1753465808094971. [DOI] [PubMed] [Google Scholar]
- Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of NO synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–37. [PubMed] [Google Scholar]
- Grainge CL, Davies DE. Epithelial injury and repair in airways diseases. Chest. 2013;144:1906–12. doi: 10.1378/chest.12-1944. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, Befus AD, Moqbel R. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol. 2000;106:537–45. doi: 10.1067/mai.2000.109058. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol. 2001;107:679–85. doi: 10.1067/mai.2001.114245. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Amadesi S, Dabbagh K, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–21. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- Arizmendi NG, Abel M, Mihara K, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011;186:3164–72. doi: 10.4049/jimmunol.0903812. [DOI] [PubMed] [Google Scholar]
- Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–30. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–10. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal. 2009;11:2453–65. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Betsuyaku T, Suzuki M, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid Redox Signal. 2008;10:705–14. doi: 10.1089/ars.2007.1941. [DOI] [PubMed] [Google Scholar]
- Voraphani N, Gladwin MT, Contreras AU, et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. 2014;7:1175–85. doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Lam J, Rada B, Leto TL, Levine SJ. Double-stranded RNA induces shedding of the 34-kDa soluble TNFR1 from human airway epithelial cells via TLR3-TRIF-RIP1-dependent signaling: roles for dual oxidase 2- and caspase-dependent pathways. J Immunol. 2011;186:1180–8. doi: 10.4049/jimmunol.1001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R. Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic Biol Med. 2013;65:38–46. doi: 10.1016/j.freeradbiomed.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Ryu JC, Kwon Y, Lee S, Bae YS, Yoon JH, Ryu JH. Dual oxidase 2 in lung epithelia is essential for hyperoxia-induced acute lung injury in mice. Antioxid Redox Signal. 2014;21:1803–18. doi: 10.1089/ars.2013.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Yoo JY, Kim MJ, et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013;131:549–61. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- Joo JH, Ryu JH, Kim CH, et al. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxid Redox Signal. 2012;16:57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- Rada B, Boudreau HE, Park JJ, Leto TL. Histamine stimulates hydrogen peroxide production by bronchial epithelial cells via histamine H1 receptor and dual oxidase. Am J Respir Cell Mol Biol. 2014;50:125–34. doi: 10.1165/rcmb.2013-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–22. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- Lim SY, Tennant GM, Kennedy S, Wainwright CL, Kane KA. Activation of mouse protease-activated receptor-2 induces lymphocyte adhesion and generation of reactive oxygen species. Br J Pharmacol. 2006;149:591–9. doi: 10.1038/sj.bjp.0706905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park MS, Jung KH, Song J, Kim YA, Cho HJ, Min BI, Bae H. Treatment with pyranopyran-1, 8-dione attenuates airway responses in cockroach allergen sensitized asthma in mice. PLoS One. 2014;9:e87558. doi: 10.1371/journal.pone.0087558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Fan M, Ansari HR, et al. Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1335–44. doi: 10.1152/ajplung.00416.2006. [DOI] [PubMed] [Google Scholar]
- Rievaj J, Davidson C, Nadeem A, Hollenberg M, Duszyk M, Vliagoftis H. Allergic sensitization enhances anion current responsiveness of murine trachea to PAR-2 activation. Pflugers Arch. 2012;463:497–509. doi: 10.1007/s00424-011-1064-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–6. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garlard D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–72. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Chhabra SK, Masood A, et al. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–8. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–6. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Clark JM, Abraham WM, Fishman CE, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. Am J Respir Crit Care Med. 1995;152:2076–83. doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- Maryanoff BE, de Garavilla L, Greco MN, Haertlein BJ, Wells GI, Andrade-Gordon P, Abraham WM. Dual inhibition of cathepsin G and chymase is effective in animal models of pulmonary inflammation. Am J Respir Crit Care Med. 2010;181:247–53. doi: 10.1164/rccm.200904-0627OC. [DOI] [PubMed] [Google Scholar]
- Horváth I, Donnelly LE, Kiss A, Kharitonov SA, Lim S, Chung KF. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med. 1998;158:1042–6. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- Antczak A, Nowak D, Shariati B, Król M, Piasecka G, Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir J. 1997;10:1235–41. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–6. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim SR, Park SJ, et al. Hydrogen peroxide induces vascular permeability via regulation of vascular endothelial growth factor. Am J Respir Cell Mol Biol. 2006;35:190–7. doi: 10.1165/rcmb.2005-0482OC. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Janocha AJ, Aronica MA, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–97. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–44. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Bucchieri F, Puddicombe SM, Lordan JL, et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Bio. 2002;27:179–85. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- Li W, Yan F, Zhou H, et al. P. aeruginosa lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly through Duox1 in vitro and in vivo. PLoS One. 2013;8:e63945. doi: 10.1371/journal.pone.0063945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Park YD, Moon UY, Kim JH, Jeon JH, Lee JG, Bae YS, Yoon JH. The role of Nox4 in oxidative stress-induced MUC5AC overexpression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:598–609. doi: 10.1165/rcmb.2007-0262OC. [DOI] [PubMed] [Google Scholar]