Abstract
Mucosa-associated invariant T (MAIT) cells express the semi-invariant T-cell receptor TRAV1–2 and detect a range of bacteria and fungi through the MHC-like molecule MR1. However, knowledge of the function and phenotype of bacteria-reactive MR1-restricted TRAV1–2+ MAIT cells from human blood is limited. We broadly characterized the function of MR1-restricted MAIT cells in response to bacteria-infected targets and defined a phenotypic panel to identify these cells in the circulation. We demonstrated that bacteria-reactive MR1-restricted T cells shared effector functions of cytolytic effector CD8+ T cells. By analysing an extensive panel of phenotypic markers, we determined that CD26 and CD161 were most strongly associated with these T cells. Using FACS to sort phenotypically defined CD8+ subsets we demonstrated that high expression of CD26 on CD8+ TRAV1–2+ cells identified with high specificity and sensitivity, bacteria-reactive MR1-restricted T cells from human blood. CD161hi was also specific for but lacked sensitivity in identifying all bacteria-reactive MR1-restricted T cells, some of which were CD161dim. Using cell surface expression of CD8, TRAV1–2, and CD26hi in the absence of stimulation we confirm that bacteria-reactive T cells are lacking in the blood of individuals with active tuberculosis and are restored in the blood of individuals undergoing treatment for tuberculosis.
Keywords: bacteria, cell surface molecules, human, MHC, MR1 and MAIT cells, T cells
Introduction
Human mucosa-associated invariant T (MAIT) cells are a T-cell subset defined by the expression of the semi-invariant T-cell receptor-α (TCR-α) chain TRAV1–2.1,2 Molecular methods were initially used to identify the human canonical MAIT TCR-α chain (TRAV1–2/TRAJ33)1 and determine the MAIT cell requirement for the MHC-like molecule MR1.3 Subsequently, monoclonal antibodies to this TCR were generated4–6 but none so far identifies the clonotypic TRAV1–2/TRAJ33 TCR. Because, TRAV1–2 is also expressed by non-MAIT T cells, including conventional T cells1,2,7,8 and GEM (germline-encoded, mycolyl lipid-reactive) T cells,9 identification of MAIT cells by the use of α-TRAV1–2 antibody alone is insufficient.
Functionally, MAIT cells have been recently demonstrated to have the ability to detect a range of bacteria and fungi in an MR1-dependent fashion.8,10 To quantify and characterize MR1-dependent bacteria-reactive cytokine-producing T cells ex vivo, we developed an assay that uses ex vivo T-cell stimulation by HLA-Ia mismatched, pathogen-infected antigen presenting cells (APC), in the presence or absence of α-MR1 blockade.5,8 Using this assay we determined that on average 5% of CD8+ TRAV1–2+ T cells from human peripheral blood5,8 were MR1-restricted bacteria-reactive MAIT cells as assessed by production of tumour necrosis factor (TNF). Using this assay we previously found that MR1-restricted MAIT cells were absent from the blood of individuals with active tuberculosis (TB). Nonetheless, the breadth of cytokines produced by MAIT cells in response to infected APC remained to be evaluated. Although a number of studies characterized MAIT cells as TRAV1–2+ CD161hi T cells, with T helper type 1 (Th1) and Th17 functions, evaluation of MR1 restriction or microbial reactivity remained to be confirmed.4,11 Here, we found that bacteria-reactive MAIT cells failed to produce interleukin-10 (IL-10), IL-17 and Th2-associated cytokines but instead shared a Th1-like cytokine profile similar to conventional cytolytic CD8+ T-cell effectors.
The ex vivo analysis8 described above provides definitive characterization of functional bacteria-reactive MR1-restricted cells and can be used to further define the functions of MAIT cells. However, the assay has limitations because T-cell stimulation can alter the expression of phenotypic markers. Notably, down-regulation of the T-cell receptor12,13 and additional receptors after T-cell stimulation can result in incomplete evaluation of the population of interest. Specifically, CD161, a marker frequently used to identify MAIT cells, has been shown to be down-regulated on activated MAIT cells.14,15 Therefore, to ultimately define a simple phenotypic panel to identify MR1-restricted T cells with the capacity to detect and produce cytokines in response to infected cells in the absence of stimulation we screened for phenotypic markers expressed by functional MAIT cells. We found that bacteria-reactive MAIT cells preferentially expressed greater levels of cell surface markers CD26, CD150 and CD161. Using FACS-sorted subsets we demonstrated that high expression of CD26 on CD8+ TRAV1–2+ cells was highly sensitive and specific in identifying those MR1-restricted MAIT cells with the capacity to detect mycobacteria-infected cells. Using this panel in the absence of stimulation we confirm that humans with active TB lack peripheral blood MR1-restricted MAIT cells8,10 and show that these cells are restored to the blood of patients with TB who are undergoing α-TB treatment. Identification and quantification of MR1-restricted MAIT cells in the absence of stimulation will provide the basis for a better understanding of the relationship of MAIT cells in health and disease.
Methods
Human subjects
Portland, Oregon, USA
Peripheral blood mononuclear cells (PBMC) from unexposed donors, donors with latent TB (LTBI) or active TB were isolated from whole blood obtained by apheresis with informed consent under protocols approved by the Institutional Review Board at Oregon Health & Science University and as previously described.8,16
Durban, South Africa
Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee and written informed consent was obtained from all patients who were enrolled at King Edward Hospital in Durban. PBMC were obtained from HIV-negative adult subjects with culture-confirmed pulmonary TB before and after 6 months of successful treatment, defined by clinical and microbiological resolution of disease.
Mycobacteria
Mycobacterium smegmatis (strain mc2122) was used at a multiplicity of infection of three for all live infections.
Cells
A549 cells (ATCC CCL-185) were used as stimulators for direct ex vivo determination of MR1-restricted pathogen reactive MAIT cells as previously described.8
Antibodies to the following were used in this study
TRAV1–2 (OF-5A12),5 CD28, CD49d, CD8 (SK1), CD3 (OKT-3), CD4 (OKT-4), CD26 (BA5b), CD161, (HP-3G10), CD279 (EH12.2H7), CCR6 (G034E3), CCR5 (HEK/1/85a), IL-10 (JES-19F1), IL-17A (BL168), IL-2 (MQ-17H12) (BioLegend, San Diego, CA), CD150 (A12), IL-4 (8D4-8), CD107a (H4A3), granulysin (RB1), granzyme B (GB1) (BD Biosciences, San Jose, CA), TNF (IPM-2), interferon-γ (IFN-γ; 45·15), CD8β (2ST8.5H7) (Beckman Coulter, Brea, CA), IL-22 (22URT1) (eBioscience, San Diego, CA) were used.
Cytokine staining assays
For the detection of non-classical pathogen reactive CD8+ T cells including MR1-restricted pathogen reactive MAIT cells, we used an assay termed the A549 TAPI-O assay that was described previously.5,8,17 Briefly, enriched CD8+ T cells were added to M. smegmatis-infected or uninfected A549 cells at a ratio of 3 : 1 and incubated for 16 hr in the presence of TNF-α monoclonal antibody and the TNF-Processing Inhibitor 0 (TAPI-0, 10 μm) (Calbiochem, San Diego, CA).18 For the detection of CD107a, antibody was added during the culture as previously described.19 For TCR-independent stimulation, PBMC were activated with PMA (20 ng/ml, Sigma, St Louis, MO) and ionomycin (1 μm; Sigma) for 6 hr in the presence of GolgiStop (BD Pharmingen, San Diego, CA) after which cells were harvested, and stained with LIVE/DEAD® Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, CA) before being surface stained for expression of TRAV1–2, CD4, CD8, CD26, CD161, CD279, CCR6, CCR5, and CD150. For intracellular staining, cells were subsequently fixed and permeabilized with Cytofix/CytoPerm (BD Pharmingen) then stained in the presence of Perm/Wash (BD Pharmingen), with antibodies to IFN-γ, IL-2, IL-4, IL-10, IL-17A, IL-22, Granulysin, and Granzyme B. Detection of IL-10 was performed in the absence of a protein transport inhibitor as previously described.20 Acquisition was performed on a Fortessa flow cytometer with FACS Diva software (BD). Analyses were performed using Cytobank21 and FlowJo software (Tree Star, Ashland, OR).
Isolation and evaluation of FACS-sorted CD8+ T-cell subsets
CD8+ T cells were enriched from PBMC and stained with antibodies to CD26, or CD161, or CD150. Live cells lacking propidium iodide (BD Biosciences) were sorted based on defined expression levels of each cell surface marker using a BD influx cell sorter (BD Biosciences). Sorted subsets were rested overnight (37°, 5% CO2) in RPMI-1640 media (Lonza, Basel, Switzerland) supplemented with human serum (10%) and IL-2 (0·5 ng/ml; BD Biosciences) before being added to the A549 TAPI-O assay.8 Purity of sorted cell subsets was > 95% as verified by flow cytometry.
Tetramer staining
CD8+ T cells were enriched from PBMC, washed, and incubated with CFP-10 peptide/MHC tetramers (kindly provided by John Altman at the NIH Tetramer Core Facility; CFP-102–9/HLA-B*4501, CFP-1075–83/HLA-B*1502, CFP-103–11/HLA-B*0801) for 2 hr at (37°, 5% CO2) then stained with LIVE/DEAD® Fixable Dead Cell Stain Kit (Invitrogen) and subsequently stained with antibodies to TRAV1–2, CD8, CD3, CD26, CD150 and CD161.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software Inc., San Diego, CA). The non-parametric Mann–Whitney two-tailed t-test was used to assess statistically significant differences between groups and considered significant for P values < 0·05.
TCR sequence analysis TRAV1–2+ CD26+ and TRAV1–2+ CD26− cells
The PBMC from D433 and D462 were stained with LIVE/DEAD® Fixable Dead Cell Stain (Invitrogen) and antibodies to CD8, TRAV1–2 and CD26. Live CD8+ TRAV1–2+ cells (200 000) were FACS sorted as CD26hi versus CD26−; DNA isolation (DNeasy; Qiagen, Hilden, Germany) and amplification and sequencing of TCRAD CDR3 regions was performed using the immunoSEQ Platform (Adaptive Biotechnologies™, Seattle, WA). The IMGT (ImMunoGeneTics, http://www.imgt.org) nomenclature was used throughout the study.22
Results
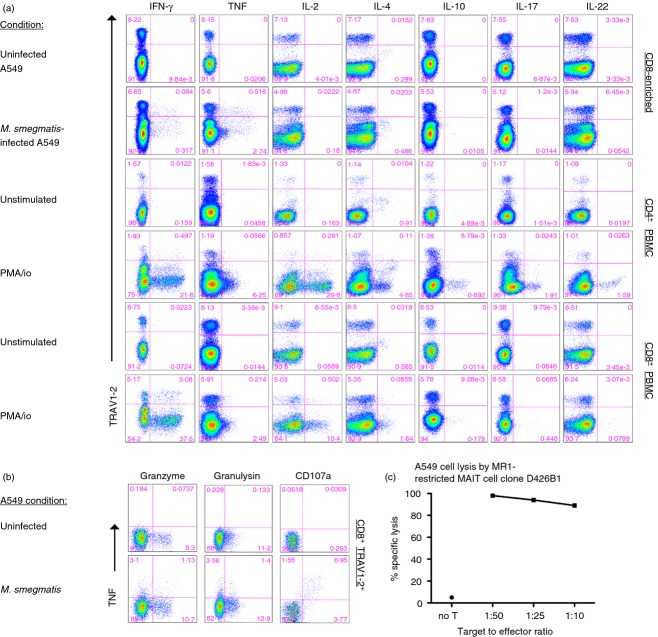
Although we previously determined that peripheral blood-derived, human MR1-restricted MAIT cells, can produce both IFN-γ and TNF in response to APC infected with a variety of bacteria and fungi,5,8,17 here we wanted to evaluate the full repertoire of cytokines produced by these CD8+ TRAV1–2+ cells. Using M. smegmatis-infected APC to stimulate MAIT cells17 we confirmed that both IFN-γ and TNF were frequently produced in response to infected APC. In contrast, CD8+ TRAV1–2+ cells did not release IL-2, IL-4, IL-10, IL-17 or IL-22, in response to infected APC, as represented in Fig.1(a) (n ≥ 3) although these cytokines were detected by CD4+ T cells after TCR-independent PMA/ionomycin stimulation. As previously shown,11,23,24 a small population of CD8+ TRAV1–2+ cells produced IL-17 but only response to TCR-independent stimulation. Hence, in response to infected APC, CD8+ TRAV1–2+ MAIT cells produced Th1 but not Th2 or Th17-associated cytokines.
Figure 1.
Mycobacteria reactive CD8+ TRAV1–2+ cells produce interferon-γ (IFN-γ), tumour necrosis factor (TNF), and are cytotoxic. (a) Representative dot plots of T-cell responses from the cytokine-staining assay are shown. CD8+ T cells enriched from peripheral blood mononuclear cells (PBMC) were incubated overnight with A549 stimulator cells left uninfected or infected with Mycobacterium smegmatis (rows 1 and 2). Alternatively, PBMC were stimulated for 6 hr with PMA/ionomycin (PMA/io). A protein transport inhibitor was added for the last 6 hr of the assay to prevent cytokine release except for the detection of interleukin-10 (IL-10) and TNF. Cells were surface stained for the expression of TRAV1–2, CD8, CD4, and then fixed and permeabilized before staining for cytokines. Cytokines are listed on the x-axis and TRAV1–2 on the y-axis. The numbers represent the frequency of events from the CD8+ gate (rows 1, 2, 5 and 6) or CD4+ gate (rows 3 and 4). Similar responses were detected from a minimum of three individuals. (b) Representative dot plots of CD8-enriched T cells from PBMC in response to uninfected or M. smegmatis infected A549 are shown. The numbers represent the frequency of events from CD8+ TRAV1–2+ gate. The y-axis represents expression of TNF and the x-axis expression of granzyme B, granulysin, or CD107a. Data are representative of a minimum of three individuals. (c) The graph depicts the per cent specific lysis of M. smegmatis-infected A549 cells by MHC-like molecule-1 (MR1) -restricted mucosa-associated invariant T (MAIT) cell clone D426B1 at different target to effector ratios (1 : 50, 1 : 25 and 1 : 10). No target cell lysis was observed in the absence of infection.
To evaluate the cytolytic potential of bacteria-reactive MAIT cells, we assessed the expression of granzyme B, granulysin and degranulation capacity using CD107a production (Fig.1b). A proportion of CD8+ TRAV1–2+ cells expressed granzyme and granulysin before stimulation. In response to infected cells on average, 28% and 26% of CD8+ TRAV1–2+ that produced TNF contained granzyme and granulysin, respectively. Furthermore, CD107a was coordinately co-expressed with TNF but not detected in response to uninfected APC. Consistent with this cytolytic potential, Fig.1(c) shows that the MR1-restricted MAIT cell clone D426B18 efficiently lysed M. smegmatis-infected epithelial cells using the FATAL assay.25 In sum, the majority of bacteria-reactive MAIT cells from human blood release Th1-like cytokines and a proportion also share cytolytic capacity.
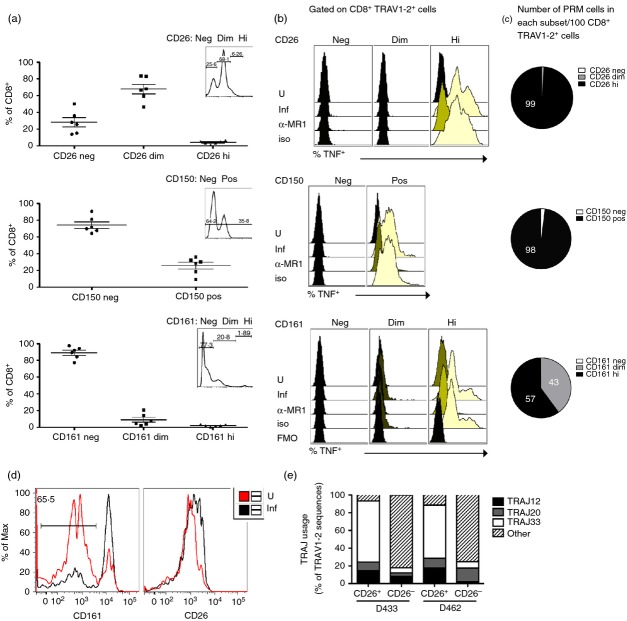
We then performed a broad analysis of phenotypic markers to identify those preferentially expressed on MR1-restricted MAIT cell clones (not shown)8 and MAIT cells evaluated directly ex vivo in response to infected APC. Specifically, we stimulated CD8+ T cells with M. smegmatis-infected APC using the A549 TAPI-O assay.17 We compared the expression of phenotypic markers on total CD8+ cells (grey histogram), CD8+ TRAV1–2+ cells (dotted line), and CD8+ TRAV1–2+ TNF+ cells (bold line) (Fig.2a). We found that in general, immunomodulatory markers, such as CD39, CD200, CD200R, Tim-3, CD160, 2B4 and FoxP3, were not detected on CD8+ TRAV1–2+ TNF+ cells (not shown). Likewise, we found that PD-1 expression did not appreciably differ between total CD8+ T cells, CD8+ TRAV1–2+ cells, and CD8+ TRAV1–2+ TNF+ cells (Fig.2a). The chemokine receptors CCR5 and CCR6 were expressed on average by only 30% and 15%, respectively, of CD8+ TRAV1–2+ TNF+ cells but were frequently expressed by total CD8+ cells and CD8+ TRAV1–2+ cells. By contrast, we found preferential expression of CD26, CD150 and CD161 by CD8+ TRAV1–2+ TNF+ cells compared with total CD8+ and CD8+ TRAV1–2+ T cells (Fig.2a,b). To determine if these markers were unique to MAIT cells we used a (CFP102-11::HLA-B44) tetramer to define a population of conventional Mycobacterium tuberculosis-specific CD8+ T cells. Here, we found that on average 50% and 75% of these cells expressed CD26hi and CD150, respectively but none had CD161hi expression (Fig.2b).
Figure 2.
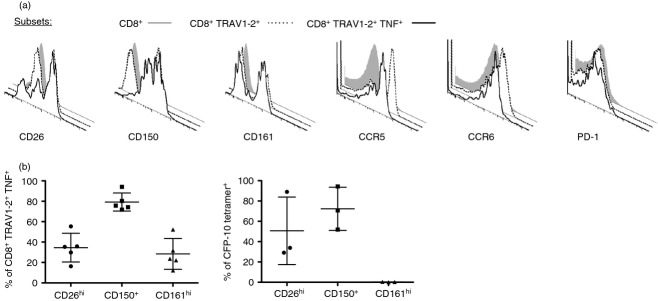
CD161, CD150 and CD26 are preferentially expressed on mycobacteria-reactive CD8+ TRAV1–2+ cells. (a,b) Representative histograms depicting CD26, CD150, CD161, CCR5, CCD6, and PD-1 expression on CD8+ (grey-filled histogram), CD8+ TRAV1–2+ (dotted line), or CD8+ TRAV1–2+ TNF+ (bold line) gated cells after incubation with M. smegmatis-infected A549 stimulator cells. (b). Left panel shows the frequency of CD8+ TRAV1–2+ TNF+ cells that express CD26hi, CD150+ and CD161hi cells after stimulation by M. smegmatis-infected A549 cells (n = 6). The right panel shows the frequency of peripheral conventional CD8+ CFP-10 tetramer+ T cells that express CD26hi, CD150+ and CD161hi from individuals (n = 3) with tuberculosis.
Although high expression of CD161 has been used to define unstimulated and MR1 tetramer-reactive MAIT cells4,15 it has been shown that CD161 receptor down-regulation occurs on MAIT cells in response to stimulation by APC loaded with fixed Escherichia coli.14 Consistent with this, we routinely found that on average only 24% of CD8+ TRAV1–2+ TNF+ cells, expressed high levels of CD161 (Fig.2a,b left panel). This finding was similar for CD26 and to a lesser extent for CD150. Therefore to directly determine if MAIT cells with the capacity to produce TNF in response to infected cells required expression of these phenotypic markers we FACS-sorted live, untreated CD8+ cell subsets (n ≥ 3) based on the negative, dim or high expression of CD26, CD150 or CD161 (Fig.3a inset; n ≥ 3). We then stimulated the sorted subsets using infected APC and analysed TNF production by the CD8+ TRAV1–2+ gated subset as represented in Fig.3(b). Regarding CD26, the top row in Fig.3(b) shows that none of the TRAV1–2+ CD26− or CD26dim cells produced TNF. In contrast the entire TRAV1–2+ CD26hi population produced TNF in an MR1-dependent manner. To further confirm the identity of this subset, we performed TCR-α chain repertoire analysis on CD8+ TRAV1–2+ CD26hi and CD8+ TRAV1–2+ CD26− cells isolated by FACS (n = 2). In the CD26hi but not the CD26− cells, > 90% of TRAV1–2 sequences were associated with MAIT-associated TRAJ usage of TRAJ12, TRAJ20 and TRAJ33 (Fig.3e).3,15,17,26 In combination, these results demonstrate that CD26hi expression on CD8+ TRAV1–2+ cells phenotypically defines MR1-restricted bacteria-reactive MAIT cells.
Figure 3.
CD26, CD150 and CD161 specifically identify functional MHC-like molecule-1 (MR1) -restricted mucosa-associated invariant T (MAIT) cells. (a) Frequency of CD8+ cells that express CD26 (neg, dim and high), CD150 (neg and pos) and CD161 (neg, dim and high) cells (n = 6) in the absence of stimulation. The insets depict the expression levels of CD26, CD150 or CD161 from CD8+ cells and represent the gating strategy used for the sorted subsets tested in (b). (b) Overlay histograms depicting the expression of tumour necrosis factor (TNF) by CD8+ TRAV1–2+ events from each sorted cell population (CD26 neg, dim, hi) (CD150 neg, pos) (CD161 neg, dim, hi) under each condition. Sorted cell populations were rested in medium overnight (37°, 5% CO2) before overnight incubation with uninfected or Mycobacterium smegmatis-infected A549 cells in the presence of α-MR1 (clone 26.5) blocking or control (msIgG2a) antibodies. The following day, the cells were stained and analysed as described in the Methods. No TNF was detected by CD8+ TRAV1–2+ cells in the CD26dim subset or by cells lacking CD26, CD150 or CD161 in response to stimulation. The fluorescence minus one (FMO) condition represents the background level of staining observed in the absence of TNF-α antibody under conditions with infected antigen-presenting cells. Similar results were obtained using peripheral blood mononuclear cells from three different individuals. (c) Average (n = 3) number of CD8+ TRAV1–2+ TNF+ cells that express CD26hi (99·2 ± 0·4), CD150hi (97·5 ± 1·8), CD161dim (43 ± 7·3), or CD161hi (56·9 ± 7·4) per 100 CD8+ TRAV1–2+ cells based on results from (a) and (b). (d) Histograms representing CD161 (left) and CD26 (right) expression on FACS-sorted CD8+ TRAV1–2+ CD26hi in response to uninfected and M. smegmatis-infected A549 cells. The frequency of cells lacking CD161hi expression after stimulation with infected cells is depicted in the left histogram. This result was representative of three biological replicates where on average 77% of stimulated cells lacked CD161hi expression. (e). TRAJ gene usage associated with mucosa-associated invariant T MAIT cells, namely TRAJ12, TRAJ20 and TRAJ33, from TRAV1–2 sequences determined from FACS-sorted CD8+ TRAV1–2+ cells expressing CD26hi or lacking CD26 from two individuals (D433 and D462). ‘Other’ represents all non-TRAJ12, TRAJ20 and TRAJ33 genes used.
In contrast to TRAV1–2+ CD26hi cells only 70% of TRAV1–2+ CD150+ cells produced TNF in an MR1-dependent fashion. Nonetheless, no bacterial reactivity was observed in the TRAV1–2+ CD150− population (Fig.3b). Similarly to TRAV1–2+ CD26hi cells, all of the TRAV1–2+ CD161hi cells produced TNF in an MR1-dependent manner (Fig.3b). Although no responses were detected from TRAV1–2+ CD161− cells, about 10% of TRAV1–2+ CD161dim cells produced TNF and were MR1 dependent. Based on the frequencies of CD8+ T cells that express dim levels of CD161 (Fig.3a) we calculated that on average over one-third of CD8+ TRAV1–2+ TNF+ cells would be CD161dim (Fig.3c). Therefore, use of CD161hi alone to quantify MAIT cells may result in the exclusion of a proportion of MR1-restricted cells with functional capacity. To confirm14 that CD161 expression on MAIT cells was altered in response to TCR-induced stimulation, CD8+ TRAV1–2+ CD26hi cells were incubated overnight with uninfected or infected A549 cells and CD161 and CD26 were determined by flow cytometry. Notably, in response to infected APC, the expression of CD161 was bi-modal. On average (n = 3), 77% of CD8+ TRAV1–2+ CD26hi cells had reduced expression of CD161 whereas CD26 was only modestly decreased (Fig.3d).
We next addressed if CD26hi and CD161hi delineated the same or distinct sets of CD8+ TRAV1–2+ T cells. In the absence of stimulation we observed that CD26hi and CD161hi were predominantly co-expressed in healthy individuals (Fig.4, top row). These results suggest that high expression of CD26 and CD161 delineates the same set of CD8+ TRAV1–2+ cells with the capacity to produce TNF in response to infected cells. We also note that we consistently detect significantly higher frequencies of bacteria-reactive MAIT cells within the CD8+ TRAV1-2+ subset by using this phenotypic panel in the absence of stimulation.
Figure 4.
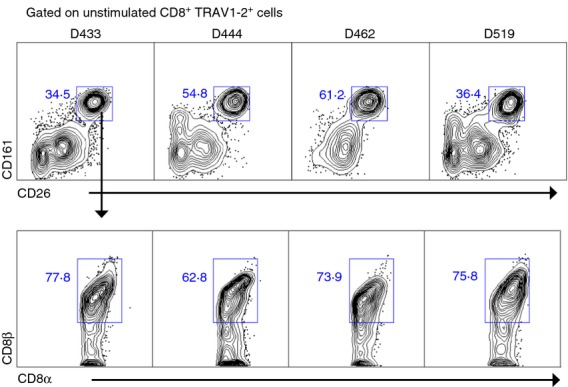
In healthy individuals high expression of CD26 or CD161 defines the same subset of CD8+ TRAV1–2+ cells that predominantly express the CD8αβ co-receptor. Expression of CD26 and CD161 on CD8α+ TRAV1–2+ cells from peripheral blood mononuclear cells of healthy individuals is shown (top row). The numbers represent the frequencies of CD8α+ TRAV1–2+ cells that co-express CD26hi and CD161hi (46 ± 13; n = 4). The bottom row represents CD8β expression (y-axis) from the CD8α+ TRAV1–2+ CD26hi CD161hi gated cells from the top row. The numbers in the plot represent the frequency of CD8α+ TRAV1–2+ CD26hi CD161hi that co-express CD8β (72·6 ± 6·7; n = 4).
We next evaluated the CD8 co-receptor usage on CD8+ TRAV1–2+ CD26hi CD161hi cells in the absence of stimulation as a number of studies have reported various forms of CD8 co-receptor usage by MAIT cells.4,15 Here, we found that on average, 73% (± 6·7) (n = 4), or the majority of circulating bacteria-reactive MAIT cells, expressed the CD8αβ co-receptor consistent with our previous findings of CD8αβ co-receptor usage by MR1-restricted bacteria-reactive MAIT cells clones 8 (Fig.4, bottom row).
We next sought to determine the utility of CD26hi, CD161hi or CD150 expression on CD8+ TRAV1–2+ cells to define MR1-restricted MAIT cells in the absence of stimulation in subjects with active TB undergoing treatment. We had previously demonstrated in a US cohort, that functional MAIT cells were undetectable in the blood of individuals with active TB, compared with uninfected individuals or those with a latent TB infection (LTBI) who demonstrated equivalent frequencies of these cells.8 Here we confirmed that CD8+ TRAV1–2+ cells that co-expressed CD26hi and CD161hi were dramatically reduced and represented < 1% of CD8+ TRAV1–2+ cells from the blood of patients with previous active TB (Fig.5a,b). This was in sharp contrast to results obtained from the blood of uninfected individuals and those with LTBI (Fig.5b), where approximately 28·4% (n = 6) and 23·4% (n = 4) of CD8+ TRAV1–2+ cells, respectively, co-expressed high levels of CD26 and CD161. In comparing CD26hi and CD161hi frequencies in infected individuals we detected a trend toward higher frequencies of MAIT cells using CD26hi compared with CD161hi. This was consistent with the possibility that some CD26hi cells expressed lower levels of CD161 because of increased MAIT cell activation.14,15 In contrast to uninfected donors (Fig.4), in M. tuberculosis-infected donors, a small but detectable CD26hi subset expressed dim levels of CD161 (Fig.5a). Hence, while both CD26hi and CD161hi markers are exquisitely specific for MAIT cells, high expression of CD26 may perform as a more sensitive and inclusive phenotypic marker for MAIT cells by capturing CD161dim cells. With regard to CD150, no significant differences were detected in the frequencies of TRAV1–2+ CD150+ cells in donors regardless of TB status (Fig.5b). This further supported the interpretation that CD150 is not a specific marker for MAIT cells.
Figure 5.
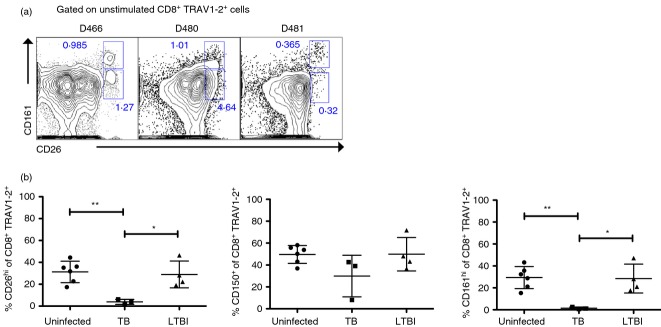
CD8+ TRAV1–2+ T cells with CD26hi expression are absent in the blood of individuals with active tuberculosis (TB). (a) Contour plots displaying CD161 and CD26 expression show reduced frequencies of CD8+ TRAV1–2+ CD26hi CD161hi cells from three individuals with active TB and the presence of a CD26hi CD161dim population. (b) Frequencies of CD26hi or CD150+ or CD161hi on CD8+ TRAV1–2+ cells from the peripheral blood mononuclear cells of uninfected (n = 6), active TB (n = 3), and latent TB infection (n = 3) donors. The unpaired two-tailed, t-test was used to determine significant differences between groups, *P < 0.05; **P < 0.01.
Finally, we wanted to evaluate the effect of successful treatment on the frequency of MAIT cells in the blood of patients with TB. To address this, we evaluated patients from Durban, South Africa undergoing anti-TB treatment. The diagnosis of TB was established based on a positive culture for M. tuberculosis obtained from either a sputum or gastric sample. Frequencies of CD8+ TRAV1–2+ CD26hi CD161hi cells were quantified before the initiation of therapy at the time of diagnosis, and following 6 months of effective therapy as defined by culture conversion at 2 months and resolution of clinical symptoms. Figure6 shows that although low frequencies of CD8+ TRAV1–2+ cells were detectable in the blood of patients with active TB before treatment, a minority of these co-expressed CD26hi and CD161hi. In sharp contrast, after 6 months of anti-TB treatment, CD8+ TRAV1–2+ cells that co-expressed CD26hi and CD161hi were dramatically increased (Fig.6). We conclude that CD8+ TRAV1–2+ MR1-restricted cells with the capacity to produce TNF in response to infected cells can be accurately quantified in the blood of humans using the phenotypic expression of CD26hi in the absence of antigenic stimulation.
Figure 6.
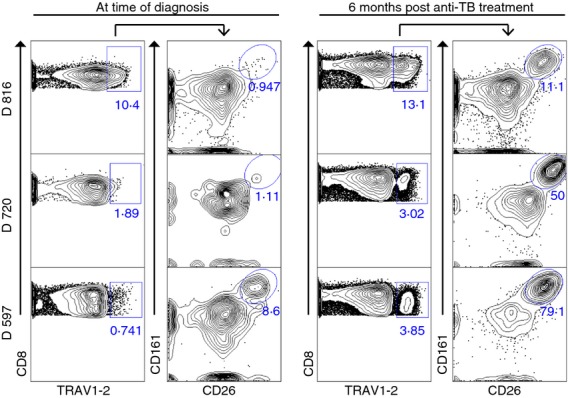
CD8+ TRAV1–2+ CD26hi CD161hi cells are restored in blood of patients with (TB) following TB treatment. Contour plots displaying the frequencies of CD8+ TRAV1–2+ cells from individual patients (n = 3) with active TB at the time of diagnosis (left column) and 6 months after TB treatment (right column). Increased frequencies of CD8+ TRAV1–2+ gated cells co-expressing CD161hi CD26hi cells after treatment.
Discussion
In this study, our goal was to broadly characterize the function of human peripheral blood MAIT cells and identify a simple surface phenotypic panel to identify and quantify MR1-restricted MAIT cells with functional capacity under conditions of health and disease. Using infected APC as stimulators we determined that MAIT cells predominantly display the functional attributes of conventional cytolytic effector CD8+ T cells. Specifically, ex vivo MAIT cells produced TNF and IFN-γ and a proportion contained granules associated with cytolytic T cells and the capacity to lyse infected cells as previously reported27 In response to infected APC, MAIT cells did not produce cytokines associated with Th2 responses, IL-10 or IL-17. Although we did not detect any MR1-restricted bacteria-reactive TRAV1–2+ IL-17 producing cells,14 in our study we detected a small subset of CD8+ TRAV1–2+ cells that produced IL-17 (< 1%) but only in response to TCR-independent stimulation.11 In aggregate, we find that in response to infected APC, MAIT cells produce Th1-like cytokines and share functional characteristics with cytolytic CD8+ effector T cells.
Phenotypically, MAIT cells have been associated with high expression of CD161.4,10,11,14,28 Specifically, TRAV1–2+ CD161hi cells express TCRs associated with the canonical MAIT semi-invariant TCR TRAV1-2/TRAJ33 4 and are reactive to MR1 ligands,6 as shown by MR1 tetramer staining.15 Here we confirmed that the CD8+ TRAV1–2+ CD161hi subset was entirely comprised of bacteria-reactive MR1-restricted T cells. Nonetheless, a substantial number of these cells were present in the CD161dim compartment. As previously shown,14 we confirmed that stimulation of MAIT cells with infected cells resulted in CD161 down-regulation. Notably, CD161 down-regulation has been associated with chronic activation of MAIT cells in HIV-infected individuals14 and a decrease in CD8+ CD161hi cells in HIV-infected and HIV/TB-co-infected individuals.29,30 Nonetheless, Fernandez et al.31 noted a decrease of MR1 tetramer-positive cells in patients with HIV. However these were all assessed as CD161hi cells. Therefore, circulating TRAV1–2+ CD161dim cells may represent those that have undergone recent activation. While high expression of CD161 is specific for MAIT cells, its use alone may underestimate the absolute MAIT cell population.
In this study we determined that high expression of CD26 by TRAV1-2+ CD8+ T cells could identify an MR1-restricted T cell with the capacity to produce TNF in response to infected APC. This was supported by genotypic TCR analysis showing that > 95% of all TCR sequences from sorted CD8+ TRAV1–2+ CD26hi cells expressed MAIT-associated TCR.3,15,17,26 As no CD8+ TRAV1–2+ cells in the CD26dim or CD26− subsets produced TNF in response to infected cells we conclude that the combination of CD8, TRAV1–2 and CD26hi can be used to define with high specificity and sensitivity MAIT cells from human blood. CD26 or dipeptidyl peptidase-4 expression on T cells has been associated with activated and/or memory T cells.32,33 Indeed, a proportion of HLA-Ia restricted M. tuberculosis-specific CD8+ T cells were CD26hi, demonstrating that the expression of this molecule is not specific to MAIT cells. At this point, the role of CD26 in T-cell immunity remains controversial and its function on MAIT cells is unknown.34
We also evaluated CD150 for its potential to define a MAIT cell. We found that while CD150 was as sensitive as CD161 and CD26 in the detection of CD8+ TRAV1–2+ TNF+ cells, its poor specificity limits its utility and would result in an inaccurate over-estimation of MAIT cells. This was best illustrated by the inability of CD150 to reflect the decreased MAIT cell frequency in those with active TB.
In this study, we have focused on the definition of MR1-restricted MAIT cells from the peripheral blood using APC infected with M. smegmatis. Although it is possible that functionally and phenotypically distinct MAIT cell subsets would be elicited by APC infected with different microorganisms, at this point we have no evidence to support this. Indeed we found that in response to Salmonella typhimurium and Candida albicans, ex vivo MAIT cells produced TNF17 consistent with the current findings. With regard to phenotype, we find the majority of human MAIT cells express the CD8 co-receptor. Although TRAV1–2/TRAJ33+ cells were originally defined in the CD4− CD8− double-negative T-cell subset,1,3,4 these cells are present at very low frequencies as shown by MR1 tetramer reactivity.15 Potentially the low frequencies of this subset have hindered further evaluation, leaving open the possibility that double-negative MAIT cells represent a unique subset in humans.
Although we demonstrate that CD26hi and CD161hi are specific markers to define MR1-restricted MAIT cells from human blood, it is not known if these markers will identify MAIT cells from other tissue sites. At present, MAIT cells defined in a number of ways have been identified in the blood,1,3–5,8,10 the liver 11 and mucosal sites such as the lung,8,10 small intestine3 and rectum.14 CD161hi expression has routinely been used to define MAIT cells at these different tissue sites.10,11,14,28 In our experience, we do not reliably detect CD161 on bacteria-reactive MR1-restricted MAIT cells from human lungs, where MAIT cells display an activated profile (not shown).8 Additional studies will be required to characterize the function and phenotype of MAIT cells from different tissue sites.
Here we have validated a simple phenotypic flow cytometric panel for the identification and quantification of MR1-restricted MAIT cells with functional capacity from human blood. In sum, our results demonstrate that human MR1-restricted T cells share the general effector profile of CD8+ cytolytic T cells and that CD26hi expression on CD8+ TRAV1–2+ cells can identify MR1-restricted T cells in the absence of antigenic stimulation. We successfully used this phenotypic panel to demonstrate that MR1-restricted MAIT cells were virtually absent in the blood of patients with TB and returned to the circulation in patients that had undergone treatment. We believe the ability to enumerate these cells ex vivo will be useful in defining the role of the cells in the setting of human disease.
Acknowledgments
We thank Dr Ted H. Hansen for his gift of the α-MR1 antibody and John Altman for CFP-10 tetramers. We thank Erin Merrifield for her expert assistance with human subject protocols. We thank Yunus Moosa and Faieza Sahid for their collection of samples and clinical data on the patients from Durban.
Funding was provided by National Institutes of Health (NIH) grant AI095776, under the NIAID funded MIST Consortium (mucosal.org) and T32AI007387, and in part by the Burroughs Wellcome Fund/ASTMH Postdoctoral Fellowship in Tropical Infectious Diseases and Merit Review Awards # I01 BX001231 and I01 BX000533 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development and resources and the use of facilities at the VA Portland Health Care System. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Disclosures
The authors have no conflicts of interest to declare.
Author contributions
DML, MCG and PKS conceived and designed the experiments. PKS, EBW, RJN and SSP performed the experiments. MCG, DML, PKS, EBW, RJN and SSP analysed the data. DML, MCG, WRB, TN and VOK contributed reagents, materials or analysis tools. MCG, DML and DAL wrote the paper.
References
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8-αβ T cells demonstrates preferential use of several V beta genes and an invariant TCR-α chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilloy F, Treiner E, Park SH, et al. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted αβ T cell subpopulation in mammals. J Exp Med. 1999;189:1907–21. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–9. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Martin E, Treiner E, Duban L, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MC, Eid T, Smyk-Pearson S, et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6:35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–23. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- Tynan FE, Reid HH, Kjer-Nielsen L, et al. A T cell receptor flattens a bulged antigenic peptide presented by a major histocompatibility complex class I molecule. Nat Immunol. 2007;8:268–76. doi: 10.1038/ni1432. [DOI] [PubMed] [Google Scholar]
- Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, Kasmar A, de Jong A, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14:706–13. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourhis L, Martin E, Peguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–8. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–9. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- Cantrell DA, Davies AA, Crumpton MJ. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985;82:8158–62. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–51. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Leeansyah E, Ganesh A, Quigley MF, et al. Activation, exhaustion and persistent decline of the anti-microbial MR1-restricted MAIT cell population in chronic HIV-1 infection. Blood. 2012;121:1124–35. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–20. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165:925–30. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- Gold MC, McLaren JE, Reistetter JA, et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J Exp Med. 2014;211:1601–10. doi: 10.1084/jem.20140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney D, Quigley MF, Asher TE, et al. Isolation of viable antigen-specific CD8+ T cells based on membrane-bound tumor necrosis factor (TNF)-α expression. J Immunol Methods. 2011;369:33–41. doi: 10.1016/j.jim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Muris AH, Damoiseaux J, Smolders J, Cohen Tervaert JW, Hupperts R, Thewissen M. Intracellular IL-10 detection in T cells by flow cytometry: the use of protein transport inhibitors revisited. J Immunol Methods. 2012;381:59–65. doi: 10.1016/j.jim.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Kotecha N, Krutzik PO, Irish JM. Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom. 2010;10:10 7. doi: 10.1002/0471142956.cy1017s53. Chapter: Unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP, Pommie C, Ruiz M, et al. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27:55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Billerbeck E, Kang YH, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A. 2010;107:3006–11. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle CJ, Delrow J, Joslyn RC, et al. Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161hi CD8α semi-invariant T cells. Blood. 2011;118:2752–62. doi: 10.1182/blood-2011-02-334698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- Lepore M, Kalinicenko A, Colone A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Dusseaux M, Bohineust A, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XZ, Jo J, Tan AT, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190:3142–52. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- Cosgrove C, Ussher JE, Rauch A, et al. Early and non-reversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2012;121:951–61. doi: 10.1182/blood-2012-06-436436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EB, Akilimali NA, Govender P, et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS ONE. 2013;8:e83474. doi: 10.1371/journal.pone.0083474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CS, Amarasena T, Kelleher AD, Rossjohn J, McCluskey J, Godfrey DI, Kent SJ. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol. 2014;93:177–88. doi: 10.1038/icb.2014.91. [DOI] [PubMed] [Google Scholar]
- Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J Immunol. 1987;138:1346–50. [PubMed] [Google Scholar]
- Fox DA, Hussey RE, Fitzgerald KA, et al. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984;133:1250–6. [PubMed] [Google Scholar]
- Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29:295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]