Abstract
Anticoagulation and antiplatelet drugs are among the most commonly used medical drugs. In addition to the long-established heparins, hirudins, coumarins and antiplatelet drugs such as acetylsalicylic acid, numerous novel and predominantly synthetic pharmacologic agents have come onto the market in recent years. These new agents act at various sites in coagulation and have significantly broadened treatment options. Whilst immunological hypersensitivity reactions are on the whole rare, they have a considerable impact on patient management when they do occur. The present overview discusses the currently known hypersensitivity reactions to anticoagulant and antiplatelet agents, with particular attention to the newer substance classes including P2Y12 inhibitors, glycoprotein IIb/IIIb receptor antagonists, direct factor Xa inhibitors and direct thrombin inhibitors.
Keywords: thienopyridine, GP IIb/IIIa receptor antagonists, factor Xa inhibitor, direkt thrombin inhibitors, hyper sensitivity
Introduction
Antithrombotic and anticoagulant agents prevent thrombus formation by a variety of mechanisms. They can be used in a therapeutic setting for primary or secondary prevention or to treat acute thrombosis. Varying sites of action in the coagulation cascade, the fibrinolytic system or on a cellular level permit anticoagulant agents to be classified as follows:
- Antiplatelet agents prevent migration and aggregation of platelets as well as thrombus formation:
- Cyclooxygenase inhibitors (e. g. acetylsalicylic acid, ASA)
- P2Y12 inhibitors (thienopyridine-type: ticlopidine, clopidogrel, prasugrel; ticagrelor-type)
- Glycoprotein (GP) IIb/IIIa receptor antagonists (e. g. abciximab, tirofiban, eptifibatide)
- Phosphodiesterase III inhibitors (e. g. cilostazol)
- Dipyridamole
- Anticoagulant agents reduce the blood‘s ability to clot, and thus also thrombus formation:
- Vitamin K antagonists
- Coumarins
- Heparins act via factor X by activating antithrombin:
- Unfractionated heparin (high molecular weight heparin, HMWH)
- Low molecular weight heparin (LMWH)
- Synthetic pentasaccharide inhibitors of factor Xa (e. g. fondaparinux)
- Direct inhibitors of factor Xa (rivaroxaban, apixaban, edoxaban, betrixaban, darexaban, otamixaban)
- Direct thrombin inhibitors (bivalent: hirudin, lepirudin, bivalirudin; monovalent: argatroban, dabigatran)
- Antithrombin (protein obtained from blood plasma or recombinantly, for the prevention of genetic antithrombin deficiency
- Thrombolytic and fibrinolytic agents achieve thrombolysis of a pre-existing thrombus (e. g. alteplase, urokinase, tenecteplase)
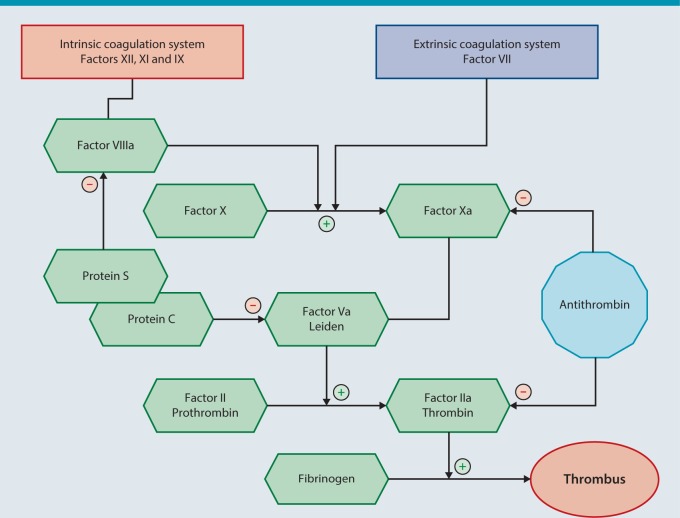
In recent years, numerous novel and predominantly synthetic pharmacologic agents that act at various sites in coagulation, thereby significantly broadening treatment options, have come onto the market (Fig. 1).
Fig. 1.
An overview of the coagulation cascade
The present article deals with hypersensitivity reactions – elicited by modern anticoagulant or antiplatelet drugs. The already well-known hypersensitivity reactions to heparins as well as the adverse drug reactions (ADR) to coumarins and ASA reported in numerous publications will not be discussed here in detail; the reader is instead referred to recently published overview articles [1, 2].
Hypersensitivity reactions to medical drugs are generally classified into four types (I–IV) according to the Coombs and Gell classification, depending on the component of the adaptive immune system predominantly involved. In addition, non-immunological reactions that primarily defy clinical differentiation from immunological reactions, i. e. intolerance or pseudo-allergic reactions, are also observed. Etiological diagnosis is oriented by the pathomechanism suspected on the basis of clinical manifestation.
Antiplatelet drugs
Cyclooxygenase inhibitors
ASA and other nonsteroidal anti-inflammatory drugs (NSAID) irreversibly inhibit cyclooxygenase 1 in platelets, leading to a reduction in thromboxane A2 (TxA2). A decrease in anti-inflammatory PGE2, as well as an increase in the sulfidoleukotrienes (cysteinyl leukotrienes) LTB4, LTC4, LTD4, is also seen. Immunological reactions to ASA mediated either cellularly or humorally have not been verified. Immediate-type hypersensitivity reactions manifest as:
Exacerbation of bronchial asthma as well as rhinosinusitis in patients with Widal‘s syndrome (Samter‘s triad), better known today as aspirin-exacerbated respiratory disease (AERD)
Exacerbation of chronic urticaria with or without concomitant angioedema in patients with this underlying disease
Anaphylactoid reactions of all degrees of severity, including cardiovascular shock
Delayed-type allergic reactions in the form of exanthemas, phototoxic reactions and, rarely, severe bullous reactions have been described in only a handful of cases [3].
P2Y12 inhibitors and thienopyridines
Thienopyridines block the binding of adenosine diphosphate (ADP) to the P2Y12 ADP receptor on platelets (Fig. 2), thereby eliminating indirect activation of the GP IIb/IIIa complex and fibrinogen binding. The mechanism by which platelet aggregation is irreversibly inhibited is distinct from that of ASA. Clopidogrel and ticlopidine are both „prodrugs“ that need to be activated by cytochrome P450 (CYP) 3A, among others [4]. They are used (sometimes in combination with ASA) to prevent atherothrombotic events. Ticlopidine and clopidogrel differ in terms of their molecular structure by only one carboxyl group (COOH) side group. Although ticlopidine was the first thienopyridine to be commercially available, clopidogrel is now more commonly used due to its better side-effects profile. Indeed, ticlopidine is no longer available in Switzerland. Typical side effects of clopidogrel include gastrointestinal symptoms, headache, drowsiness and dizziness. Prasugrel, with its faster onset of action and more potent effect, is the successor to clopidogrel. It also requires initial biotransformation to an active metabolite, primarily by CYP 3A4, CYP 2B6 and to a lesser extent by CYP 2C19 and CYP 2C9. The newest member of the P2Y12 inhibitors is ticagrelor, which is not a thienopyridine. In contrast to clopidogrel and prasugrel, ticagrelor does not require metabolic activation and binds reversibly to the P2Y12 receptor.
Fig. 2.
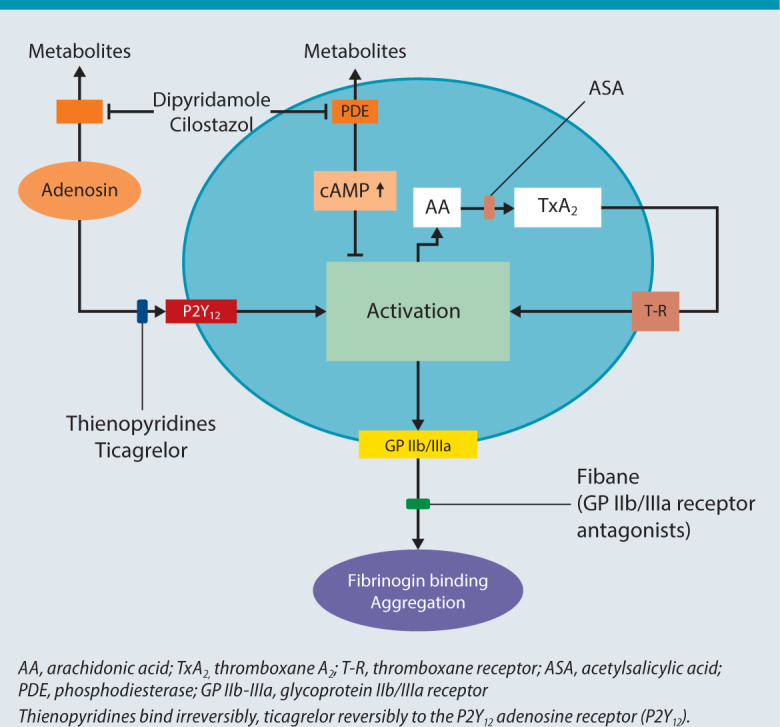
Mechanism of action of antiplatelet drugs (modified from [56])
Clopidogrel can elicit various immunological hypersensitivity reactions. Cheema et al. [5] investigated 84 patients with suspected hypersensitivity reactions to clopidogrel in whom 62 cases could be confirmed. A distinction was made between three different types of clinical manifestation involving cutaneous symptoms: the largest group (n = 49/62) exhibited truncal, itchy maculopapular exanthems. A group comprising 10 patients experienced localized well-defined yet multifocal exanthems, some demonstrating a symmetrical distribution. Angioedema with tongue and/or lip swelling and in some cases urticaria was observed in a small number of patients (3/62). Pyrexia and arthralgia were observed in two patients. Latency periods between intake and the onset of symptoms were on average 5 days in groups 1 and 2 and 1 day in group 3. In all, 42 patients underwent allergy testing. In all three patients with immediate-type reactions, intradermal testing detected sensitization to clopidogrel, partially with cross-reactivity to ticlopidine and/or prasugrel. In 34 patients with exanthem, patch testing with clopidogrel was positive, additionally showing cross-reactivity with ticlopidine in 9/34, prasugrel in 6/34 and ticlopidine plus prasugrel in 3/34. Thus it would appear that reactions are essentially either delayed-type cell-mediated allergic reactions or rarer allergic immediate-type reactions. The presence of cross-reactivity between various P2Y12 inhibitors appears to not be infrequent. None of the patients developed additional complications in the context of their allergic reaction. Interestingly, the vast majority was able to continue treatment and experienced a resolution of symptoms under short-term use of systemic corticosteroids [6, 7, 8].
Numerous other reports on the entities of pharmaceutical drug reactions described can be found in the literature. Drug tolerance induction/desensitization, which has proved successful in several specific cases and in small case series, can be attempted in complex cases where there is an absolute indication for a P2Y12 inhibitor [9]. A number of cases of hypersensitivity syndromes involving pancytopenia or neutropenia and/or hepatitis and generalized, partially vesiculous maculopapular exanthem and febrile state while using clopidogrel have been described [10, 11]. Isolated cases of serum sickness-like disease [12], suberythrodermic pustular psoriasis [13], acute generalized exanthematous pustulosis (AGEP) [14] and leukocytoclastic vasculitis [15, 16] are known – often with good tolerance of prasugrel, which generally appears to elicit fewer hypersensitivity reactions. Prasugrel is known to have caused immediate-type allergic reactions involving urticaria, pruritus, angioedema and dyspnea, as well as one case of hepatitis with pyrexia [17].
Yosipovitch et al. [18] reported on 16 patients with hypersensitivity reactions to ticlopidine, including several pruritic macular or maculopapular exanthems, urticaria, multiform exanthems and one fixed drug eruption. Individual cases seen under ticlopidine treatment include lichen planus-like drug eruption that was negative on epicutaneous testing yet recurred following rechallenge with ticlopidine [19], several fixed drug eruptions [20], as well as one patient with cholestatic hepatic dysfunction with exanthem and irreversible aplastic anemia with fatal outcome [21]. Strippoli et al. [22] published a case of toxic epidermal necrolysis under ticlopidine, whilst Pintor et al. [23] published a case of leukocytoclastic vasculitis. Re-exposure is contraindicated in cases of severe ADR with systemic involvement.
Glycoprotein IIb/IIIa receptor antagonists
These substances competitively and reversibly inhibit fibrinogen or von Willebrand factor binding to the GP IIb/IIIa activated receptor, thereby preventing the development of fibrinogen bridges between platelets, i. e. thrombus formation (Fig. 2). Abciximab is the fragment antigen binding (Fab) fragment of a chimeric (human/murine) monoclonal antibody to the GP IIb/IIIa receptor. Tirofiban and eptifibatide are short-acting, synthetic low-molecular-weight GP IIb/IIIa receptor antagonists.
Various types of hypersensitivity reaction to abciximab have been described. Acute severe thrombocytopenia was observed in 4 % of patients receiving abciximab for a second time. Delayed-onset thrombocytopenia (after 6–9 days on average) upon continued treatment is seen more rarely. Abciximab-specific immunoglobulin (Ig)-G or IgM antibodies that are directed against the murine fraction of the Fab fragment (human anti-murine antibodies, HAMA) and which are formed in the first few days of therapy are the most likely cause here. IgM and/or IgG human antichimeric antibodies (HACA) occur in 6 %–7 % of patients, since they bind to a C terminal human epitope of abciximab, which is located at a greater distance from the platelet membrane [24, 25]. Marked thrombocytopenia of unclear etiology is seen in 0.5 %–1 % of patients as early as after the initial dose.
Rarely, and in addition to thrombocytopenia, immediate-type allergic symptoms also occur following abciximab administration. Hawkins et al. [26] reported a case of grade-IV anaphylaxis during abciximab infusion following coronary stent implantation. Symptoms included hypotension, generalized urticaria and angioedema; an increase in mast cell tryptase was also documented. An association with abciximab could be identified by means of positive intradermal testing. However, the sensitization mechanism remained unclear, since the patient had received abciximab for the first time and had never received any other chimeric antibodies in the past. A further case of acute onset hypotension and bronchial obstruction was reported by Guzzo et al. [27], again upon first use, followed by marked thrombocytopenia the following day. Two other cases of possible anaphylaxis and thrombocytopenia detectable within hours were reported by Iakovou et al. [28]. In the cases reported by Guzzo et al. and Iakovou et al., as well as in a further case reported by Pharand et al. [29], an infusion reaction or IgG-mediated reaction need to be considered in the differential diagnosis [30]. To our knowledge, evidence of specific IgE to abciximab has not yet been found. A case of drug-induced exanthema with facial swelling, thrombopenia and eosinophilia was published by Moneret-Vautrin [31] in 2002. After 48 h, 70 % activated, predominantly CD8+ T cells were found in a biopsy specimen of the positive reaction in intradermal testing to abciximab.
The use of eptifibatide and tirofiban can also lead to (sometimes severe) thrombocytopenia. Anaphylaxis or urticaria as responses to these drugs have also been described in very rare cases. Like abciximab, tirofiban can also cause this type of reaction as early as at first exposure. The immunological mechanism of these reactions has not been conclusively elucidated.
Phosphodiesterase III Inhibitors
Cilostazol, a quinolinone derivate that acts as a phosphodiesterase III inhibitor, improves endothelial function and inhibits platelet aggregation by increasing the concentration of cyclic adenosine monophosphate (cAMP). It also inhibits the growth of vascular muscle cells. Cilostazol is approved in Germany to increase the maximum pain-free walking distance in patients with intermittent claudication. It is has been subject to special monitoring by the European Medicines Agency (EMA) since 2011 due to severe cardiovascular and hemorrhagic side effects. Skin rashes and pruritus are mentioned in the product information leaflet. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome under cilostazol and carbamazepine treatment has been published; DRESS resolved following discontinuation of both drugs and could be reproduced by challenge tests with the individual substances at a 4-week interval. Since cilostazol and carbamazepine are not structurally related, multiple drug hypersensitivity is a more likely explanation than cross-reactivity [32]. A 78-year-old female patient developed fatal toxic epidermal necrolysis (TEN) also under co-medication with carbamazepine, cilostazol and omeprazole [33]. Two further cases in the US of TEN under cilostazol have been reported to the World Health Organization (WHO)/VigiSearch. An interaction between carbamazepine and cilostazol is conceivable, since carbamazepine is a CYP-3A4 and CYP-2C19 inducer, whilst cilostazol is a CYP-3A4 and (but less significantly) CYP-2C19 substrate. Whether this link is of immunological relevance is as yet unknown.
Dipyridamole
Dipyridamole is used for the secondary prevention of ischemic insult and transient ischemic attack (TIA) and is available as a combination preparation with ASA. It inhibits phosphodiesterases in platelets, which results in reduced platelet aggregation via an increase in cAMP. The product information mentions skin rashes, urticaria, severe bronchospasm and angioedema, which, however, could also be attributable to the ASA component. Cases of severe asthma, as well as isolated cases of anaphylaxis, have been described in association with the use of adenosine (e. g. in the setting of cardiac stress testing) [34].
Salava et al. [35] reported on a female patient with generalized eczema under dipyridamole treatment and a positive patch test for type-IV sensitization. A case of dipyridamole-induced Stevens-Johnson syndrome has been reported in Taiwan [36].
Anticoagulants
Vitamin K antagonists
Hypersensitivity reactions to coumarin derivates are on the whole extremely rare. In addition to coumarin necrosis – which occurs as a result of an imbalance between vitamin K-dependent short-acting proteins C and S and the longer-acting procoagulation factors II, IX and X in the setting of a transient hypercoagulable state – maculopapular drug eruptions occur rarely, as well as urticarial, vasculitic and bullous reactions even more rarely [1]. The case of a patient with acenocoumarol-induced DRESS syndrome was published in 2013 [37]. Skin testing can be used for the diagnosis of drug eruptions and urticarial reactions. Skin testing is contraindicated in the case of coumarin necrosis; however, protein C measurement can be helpful in the diagnostic work-up.
Heparins
Heparins, negatively charged glycosaminoglycans, which bind to antithrombin III, thereby magnifying its effect, cause side effects comparatively rarely if one considers the frequency with which they are used. Delayed-type (type IV) cell-mediated reactions to LMWH occur most commonly, involving erythematous plaque formation at the site of administration, at most with secondary generalization; in these cases tolerance is usually observed with unfractionated heparin and the synthetic pentasaccharide inhibitor fondaparinux [1]. Type IV sensitization to fondaparinux with erythematous plaque formation at the injection site occurs very rarely [38]. Complex drug eruptions or severe cutaneous adverse reactions (SCAR), as well as isolated eosinophilia, are described far more rarely. Immediate-type allergic reactions largely attributable to contamination or preservatives are also very rare. Genuine IgE-mediated reactions to heparins are a rarity [39]. These need to be distinguished from a 2007 spate of anaphylaxis cases, some with fatal outcome, in response to HMWH, which were attributed to contamination with oversulfated chondroitin sulfate, leading to complement activation as well as activation of the kallikrein-kinin cascade [40].
Heparin-induced thrombocytopenia due to the formation of antibodies against heparin platelet factor-4 complexes, and the resulting hypercoagulability [41] in the setting of white-clot syndrome with predominantly cutaneous necrosis, is a feared occurrence. This phenomenon should be considered if the platelet count drops by 50 % compared with the baseline value within the first 10 (–14) days of treatment.
Direct factor Xa inhibitors
Direct factor Xa inhibitors inhibit the active site and/or substrate binding sites of the protease factor Xa, thereby producing an anticoagulatory effect. Rivaroxaban is an oral thromboprophylaxis used following hip and knee replacement surgery, as deep (recurrent) vein thrombosis and pulmonary embolism prophylaxis and treatment, as stroke prophylaxis and to prevent systemic embolism in non-valvular atrial fibrillation. Immunological side effects are on the whole very rare [42] and are given in the product information as occurring at a rate of 0.1 %–1 %. A case of rivaroxaban-induced truncal maculopapular exanthema with a partially pustulous aspect and accompanied by neutrophilia and mild eosinophilia was published in 2013. No allergy diagnostic work-up was performed. However, the time interval of only 2 and at most 5 days between the first documented use of the medication and the first sign of exanthema is extremely short for an immunological sensitization phase [43]. Only isolated hypersensitivity reactions were observed in the 7,111 patients in the rivaroxaban treatment arm of the ROCKET-AF study: toxic skin eruption (0.03 %), cutaneous vasculitis (0.01 %), erythema multiforme (0.0 %), exfoliative rash (0.01 %) and anaphylaxis or anaphylactic shock (0.01 %, respectively).
Apixaban is another oral, selective, direct and reversible inhibitor of factor Xa, also for which no reports (other than in the product information: frequency > 1/1,000, < 1/100) on possible allergic reactions can be found. Although the question of whether the use of rivaroxaban, apixaban, darexaban or edoxaban carries a higher risk for drug-induced liver injury (DILI) was posed, a meta-analysis was able to rule out this risk [46]. Edoxaban, betrixaban, darexaban, razaxaban and otamixaban, the only parenteral factor-Xa inhibitor, have been or still are in the clinical trial phase and have not yet been granted marketing approval either by the US Food and Drug Administration (FDA) or for German-speaking countries [47].
Direct thrombin inhibitors
Dabigatran is an oral, specific, reversible monovalent direct thrombin inhibitor for the prevention of thrombosis, which, as a prodrug (dabigatran etexilate), first needs to be biotransformed into its active form by non-specific blood enzymes. Elimination is exclusively renal, suggesting a low potential for interaction with hepatic elimination mechanisms. The most common complications include dyspepsia and, at higher doses, in particular gastrointestinal bleeding complications [48]. According to the approval trial RE-LY, as well as the post-marketing data, hypersensitivity reactions are rare [49]. Individual cases of sometimes pronounced maculopapular exanthemas, some involving pruritus, are described [45, 50]. Although a possible association with TEN in an 86-year-old female patient was reported in one case [51], it was not possible to conclusively establish a causal link. In another case, hepatopathy developed in addition to a rash [52]. A recent meta-analysis, however, showed there to be no increased risk of hepatopathy with dabigatran [46]. Dabigatran tolerance was detected in a patient with acenocoumarol-induced DRESS syndrome [37].
Melagatran and ximelagatran were also available in Europe between 2004 and 2006 for use as prophylaxis against thromboembolism and to prevent stroke in patients with atrial fibrillation, initially with a very good benefit–risk profile. Due to an ultimately etiologically unexplained increase in liver enzymes that was not detected during pre-clinical studies and which could not be reproduced in animal models, both preparations were voluntarily withdrawn from the market by the manufacturer in 2006. However, an association between the severe course of ximelagatran-induced DILI and certain human leukocyte antigen (HLA) types could be demonstrated, pointing to a pharmacogenetic problem [53].
Argatroban, the first monovalent synthetic thrombin inhibitor to be approved, binds reversibly and competitively to the active site of thrombin. It is approved as an anticoagulant in patients with type II heparin-induced thrombocytopenia (HIT II). The accepted wisdom is that the risk of side effects increases with increasing treatment duration and that short-term use in the context of interventional cardiology procedures has relatively few side effects. According to the manufacturer‘s product information, symptoms of a possible allergic reaction to argatroban include dyspnea, cough, exanthema (possibly bullous) and general signs of vasodilation. However, a causal link to argatroban has not been possible as yet to conclusively identified. Over 95 % of patients experiencing these rare symptoms, which could possibly be attributed to a hypersensitivity reaction, were simultaneously exposed to other medications capable of eliciting allergic reactions (X-ray contrast medium, streptokinase, etc.). To our knowledge, no allergy diagnostic work-up took place.
In addition to these, two monovalent direct thrombin inhibitors, the bivalent hirudin, lepirudin, desirudin and bivalirudin are also worthy of note. Natural hirudin (obtained from the head and circumpharyngeal nerve rings of the medicinal leech Hirudo medicinalis) and genetically engineered hirudin (r-hirudin from Saccharomyces cerevisiae, e. g. lepirudin and desirudin) have not been commercially available since 2012 (lepirudin). Severe anaphylaxis has been potentially associated with the formation of specific anti-hirudin antibodies [54]. At present, only bivalirudin, a recombinant hirudin fragment, is available in German-speaking countries. Its characteristic features of a shorter half-life, enzymatic cleavage by thrombin that takes place predominantly in plasma, therefore making elimination largely independent of organ function, as well as its significantly lower risk for immediate-type hypersensitivity reactions (0.03 %) make it distinct from other hirudins. Caution is advised in patients with previous anaphylactic reaction to lepirudin, since cross-reactivity due to structural homologies cannot be ruled out [55].
Summary
The new generation of antithrombotic agents has permitted significant advances in the area of platelet aggregation inhibition and anticoagulation. To date, immunologically-mediated hypersensitivity reactions are overall relatively rare (Tab. 1); having said that, the frequently used thienopyridines can elicit numerous clinical manifestations. Moreover, relevant cross-reactivity has been demonstrated in this group. Also of importance are the rare yet severe hypersensitivity syndromes and bullous drug eruptions described for virtually all new drug groups.
| Substance group according to site of action | Chemical name | Non-allergic intolerance | Immediate-type reaction | Uncomplicated rash | Hypersensitivity syndrome | Bullous skin reaction | Cytopenia | Other |
|---|---|---|---|---|---|---|---|---|
| Cyclooxygenase inhibitors | ASA and NSAID | frequent | – | rare | – | rare | – | rare |
| P2Y12 inhibitors | Thienopyridine/ticagrelor | – | rare | occasional | rare | isolated | – | rare |
| Glycoprotein IIb/IIIa receptor antagonists | Fibans/abciximab | – | rare | isolated | – | – | frequent | – |
| Phosphodiesterase inhibitors/dipyridamole | Quinolinone derivative/dipyridamole | –/– | -/rare | –/isolated | isolated/– | isolated/isolated | –/– | –/– |
| Vitamin K antagonists | Coumarins | – | isolated | rare | – | isolated | – | isolated |
| Antithrombin-III activators (heparins) | Glycosaminoglycans | – | isolated | frequent | – | rare | – | occasional |
| Direct factor Xa inhibitors | Xabans, oxazolidinones | – | iolated | rare | – | – | – | isolated |
| Direct thrombin inhibitors | bivalent polypeptide hirudin derivatives, nonpeptide thrombin inhibitors | – | – | rare | – | isolated | – | – |
No data given (–): insufficient information available
Abbreviations
- AA
Arachidonic acid
- ADP
Adenosine diphosphate
- ADR
Adverse drug reaction
- AERD
Aspirin exacerbated respiratory disease
- AGEP
Acute generalized exanthematous pustulosis
- ASA
Acetylsalicylic acid
- cAMP
Cyclic adenosine monophosphate
- COOH
Carboxyl group
- CYP
Cytochrome P450
- DILI
Drug induced liver injury
- DRESS
Drug rash with eosinophilia and systemic systems
- EMA
European Medicines Agency
- Fab
Fragment antigen binding
- FDA
US Food and Drug Administration
- GP
Glycoprotein
- HACA
Human antichimeric antibodies
- HAMA
Human anti-murine antibodies
- HIT
Heparin-induced thrombocytopenia
- HLA
Human leukocyte antigen
- HMWH
High molecular weight heparin (unfractionated heparin)
- Ig
Immunoglobulin
- LMWH
Low molecular weight heparin
- LT
Leukotriene
- NSAID
Nonsteroidal anti-inflammatory drugs
- PDE
Phosphodiesterase
- PG
Prostaglandin
- P2Y12
Adenosine receptor
- SCAR
Severe cutaneous adverse reaction
- TEN
Toxic epidermal necrolysis
- TIA
Transient ischemic attack
- T-R
Thromboxane receptor
- TxA2
Thromboxane A2
- WHO
World Health Organization
Footnotes
Cite this as Scherer Hofmeier K, Bircher AJ. Hypersensitivity reactions to modern antiplatelet and anticoagulant drugs. Allergo J Int 2015; 24:58–66 DOI: 10.1007/s40629-015-0043-7
Conflict of interest
The authors state that there are no conflicts of interest.
References
- 1.Scherer K, Tsakiris DA, Bircher AJ. Hypersensitivity reactions to anticoagulant drugs. Curr Pharm Des. 2008;14:2863–73. doi: 10.2174/138161208786369768. [DOI] [PubMed] [Google Scholar]
- 2.Bircher AJ, Harr T, Hohenstein L, Tsakiris D. Hypersensitivity reactions to anticoagulant drugs: diagnosis and management options. Allergy. 2006;61:1432–40. doi: 10.1111/j.1398-9995.2006.01227.x. [DOI] [PubMed] [Google Scholar]
- 3.Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA and GA2LEN/HANNA. Allergy. 2011;66:818–29. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 4.McCann A. Antiplatelet therapy after coronary occlusion. Australian Prescriber. 2007;30:92–96. [Google Scholar]
- 5.Cheema AN, Mohammad A, Hong T, Jakubovic HR, Parmar GS, Sharieff W, et al. Characterization of clopidogrel hypersensitivity reactions and management with oral steroids without clopidogrel discontinuation. J Am Coll Cardiol. 2011;58:1145–54. doi: 10.1016/j.jacc.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Gurbel PA, Jeong YH, Tantry US. Cutaneous clopidogrel hypersensitivity. J Am Coll Cardiol. 2011;58:1455–6. doi: 10.1016/j.jacc.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Makkar K, Wilensky RL, Burke Julien M, Herrmann HC, Spinler SA. Rash with both clopidogrel and ticlopidine in two patients following percutaneous coronary intervention with drug-eluting stents. Ann Pharmacother. 2006;40:1204–7. doi: 10.1345/aph.1G587. [DOI] [PubMed] [Google Scholar]
- 8.Campbell KL, Cohn JR, Fischman DL, Walinsky P, Mallya R, Jaffrani W, Savage MP. Management of clopidogrel hypersensitivity without drug interruption. Am J Cardiol. 2011;107:812–6. doi: 10.1016/j.amjcard.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Lokhandwala J, Best PJM, Henry Y, Berger PB. Allergic reactions to clopidogrel and cross-reactivity to other agents. Curr Allergy Asthma Rep. 2011;11:52–7. doi: 10.1007/s11882-010-0152-9. [DOI] [PubMed] [Google Scholar]
- 10.Comert A, Akgun S, Civelek A, Kavala M, Sarigül S, Yildirim T, Arsan S. Clopidogrel-induced hypersensitivity syndrome associated with febrile pancytopenia. Int J Dermatol. 2005;44:882–4. doi: 10.1111/j.1365-4632.2005.02366b.x. [DOI] [PubMed] [Google Scholar]
- 11.Doogue MP, Begg EJ, Bridgman P. Clopidogrel hypersensitivity syndrome with rash, fever and neutropenia. Mayo Clin Proc. 2005;80:1368–70. doi: 10.4065/80.10.1368. [DOI] [PubMed] [Google Scholar]
- 12.Phillips EJ, Knowles SR, Shear NH. Serum sickness-like reaction associated with clopidogrel. Br J Clin Pharmacol. 2003;56:583. doi: 10.1046/j.0306-5251.2003.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meissner M, Bier C, Wolter M, Kaufmann R, Gille J. Suberythrodermic pustular psoriasis induced by clopidogrel. Br J Dermatol. 2006;155:630. doi: 10.1111/j.1365-2133.2006.07380.x. [DOI] [PubMed] [Google Scholar]
- 14.Ellerbroek JC, Cleveland MG. Clopidogrel-associated acute generalized exanthematous pustulosis. Cutis. 2011;87:181–5. [PubMed] [Google Scholar]
- 15.Shetty RK, Madken M, Naha K, Vivek G. BMJ Case reports. 2013. Leucocytoclastic vasculitis as a late complication of clopidogrel therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erpolat S, Nazli Y, Colak N, Yenidunya S. Leucocytoclastic vasculitis associated with clopidogrel. Cut Ocular Toxicology. 2012;31:171–3. doi: 10.3109/15569527.2011.627578. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Ruiz M, Carbonell-Porras A, Garcia-Reyne A, Lopez-Medrano F. Management of a hypersensitivitity reaction to thienopyridines: prasugrel-induced fever and hepatitis resolved after switching to clopidogrel. Rev Esp Cardiol. 2012;65:767–76. doi: 10.1016/j.recesp.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Yosipovitch G, Rechavia E, Reinmesser M, David M, Tikva P. Adverse cutaneous reactions to ticlopidine in patients with coronary stents. J Am Acad Dermatol. 1999;41:473–6. doi: 10.1016/S0190-9622(99)70124-6. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa M, Nishijima S. Lichen planus-type drug eruption resulting from ticlopidine. Int J Dermatol. 2005;44:436–7. doi: 10.1111/j.1365-4632.2005.02266.x. [DOI] [PubMed] [Google Scholar]
- 20.Schmutz JL, Barbaud A, Trechot PF. Premier cas de toxidermie pigmentée fixe à la cétirizine (Zyrtec, Virlix) et à la ticlopidine (Ticlid) avec épidermotest positif. Ann Dermatol Venereol. 2002;129:458. [PubMed] [Google Scholar]
- 21.Ceylan G, Kirimli, Akarsu M, Ündar B, Güneri S. Early ticlopidine-induced hepatic dysfunction, dermatitis and irreversible aplastic anaemia after coronary artery stenting. Am J Hematol. 1998;59:260. doi: 10.1002/(SICI)1096-8652(199811)59:3<260::AID-AJH16>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Strippoli D, Russo G, Simonetti V, Motolese A. Lyell syndrome due to ticlopidine. G Ital Dermatol Venereol. 2011;146:497–500. [PubMed] [Google Scholar]
- 23.Pintor E, Sanmartin M, Azcona L, Hernandez R, Fernandey-Cruz A, Macaya C. Vasculitis leucocitoclastica en relacion con ticlopidina. Rev Esp Cariol. 2001;54:114–6. doi: 10.1016/S0300-8932(01)76272-0. [DOI] [PubMed] [Google Scholar]
- 24.Curtis BR, Divgi A, Garritty M, Aster RH. Delayed thrombocytopenia after treatment with abciximab: a distinct clinical entity associated with the immune response to the drug. J Thromb Haemost. 2004;2:985–92. doi: 10.1111/j.1538-7836.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 25.Tcheng JE, Kereiakes DJ, Lincoff AM, George BS, Kleiman NS, Sane DC, et al. Abciximab readministration: results of the ReoPro Readministration Registry. Circulation. 2001;104:870–5. doi: 10.1161/hc3301.094533. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins C, Gatenby P, McGill D. Severe hypotension complicating primary angioplasty: allergy to abciximab. Allergy. 2003;58:688–9. doi: 10.1034/j.1398-9995.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 27.Guzzo JA, Nichols TC. Possible anaphylactic reaction to abciximab. Cathet Cardiovasc Intervent. 1999;48:71–3. doi: 10.1002/(SICI)1522-726X(199909)48:1<71::AID-CCD14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Iakovou Y, Mangina A, Milissari E, Cokkinos DV. Acute profound thrombocytopenia associated with anaphylactic reaction after abciximab therapy during percutaneous coronary angioplasty. Cardiology. 2001;95:215–6. doi: 10.1159/000047375. [DOI] [PubMed] [Google Scholar]
- 29.Pharand C, Palisaitis DA, Hamel D. Potential anaphylactic shock with abciximab readministration. Pharmacotherapy. 2002;22:380–3. doi: 10.1592/phco.22.5.380.33196. [DOI] [PubMed] [Google Scholar]
- 30.Scherer K, Spoerl D, Bircher AJ. Adverse drug reactions to biologics. J Dtsch Dermatol Ges. 2010;8(6):411–26. doi: 10.1111/j.1610-0387.2010.07339.x. [DOI] [PubMed] [Google Scholar]
- 31.Moneret-Vautrin DA, Morisset M, Vignaud JM, Kanny G. T-cell mediated allergy to abciximab. Allergy. 2002;57:269–70. doi: 10.1034/j.1398-9995.2002.1n3435.x. [DOI] [PubMed] [Google Scholar]
- 32.Kang SY, Kim JY, Kim MY, Lee SY, Kim MH, Kim TW, et al. Drug-induced hypersensitivity syndrome/drug reactions with eosinophilia and systemic symptoms syndrome induced by cliostazol and carbamazepine. J Dermatol. 2012;39:723–4. doi: 10.1111/j.1346-8138.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 33.Concepción Martín I, Fernández de Palencia Espinosa MA, Garrido Corro B, De La Rubia Nieto A. [Toxic epidermal necrolysis with fatal ending due to the concurrent use of carbamazepine, cilostazol and omeprazol: a case report] Farm Hosp. 2011;35:217–8. doi: 10.1016/j.farma.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Weinmann P, Moretti JL, Leynadier F. Anaphylaxis-like reaction induced by dipyridamole during myocardial scintigraphy. Am J Med. 1994;97:488. doi: 10.1016/0002-9343(94)90331-X. [DOI] [PubMed] [Google Scholar]
- 35.Salava A, Alanko K, Hyry H. Dipyridamole-induced ecxematous drug eruption with positive patch test reaction. Contact Dermatitis. 2012;67:101–18. doi: 10.1111/j.1600-0536.2012.02043.x. [DOI] [PubMed] [Google Scholar]
- 36.Jao T, Tsai TH, Jeng JS. Aggrenox (Asasantin retard)-induced Stevens-Johnson syndrome. Br J Clin Pharmacol. 2008;67:264–5. doi: 10.1111/j.1365-2125.2008.03340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinero-Saavedra M, Prados Castano M, Ortega Camarero M, Leguisamo Milla S. DRESS syndrome induced by acenocoumarol with tolerance to warfarin and dabigatran: a case report. Blood Coagul Fibrinolysis. 2013;24:576–8. doi: 10.1097/MBC.0b013e32835facc8. [DOI] [PubMed] [Google Scholar]
- 38.Hohenstein E, Tsakiris D, Bircher AJ. Delayed-type hypersensitivity to the ultra-low-molecular-weight heparin fondaparinux. Contact Dermatitis. 2004;51(3):149–51. doi: 10.1111/j.0105-1873.2004.0426c.x. [DOI] [PubMed] [Google Scholar]
- 39.Harr T, Scherer K, Tsakiris DA, Bircher AJ. Immediate type hypersensitivity to low molecular weight heparins and tolerance of unfractioned heparin and fondaparinux. Allergy. 2006;61:787–8. doi: 10.1111/j.1398-9995.2006.01063.x. [DOI] [PubMed] [Google Scholar]
- 40.Kishimoto TH, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–67. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovecchio F. Heparin-induced thrombocytopenia. Clinical Toxicology. 2014;52:579–83. doi: 10.3109/15563650.2014.917181. [DOI] [PubMed] [Google Scholar]
- 42.Godoy Monzon D, Iserson KV, Cid A, Vazquez JA. Oral thromboprophylaxis in pelvic trauma: a standardized protocol. J Emergency Med. 2012;43:612–7. doi: 10.1016/j.jemermed.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Yates J, Choudhry M, Keys G. A case report describing a suspected rivaroxaban hypersensitivity reaction in a surgical patient. J Clin Pharm Ther. 2013;38:159–61. doi: 10.1111/jcpt.12013. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Food and Drug Administration, ed. FDA draft briefing document for the Cardiovascular and Renal Drugs Advisory Committee (CRDAC). 2011. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandrenalDrugsAdvisoryCommittee/ucm270796.pdf
- 45.To K, Reynolds C, Spinler SA. Rash associated with dabigatran etexilate. Pharmacotherapy. 2013;33:e23–7. doi: 10.1002/phar.1203. [DOI] [PubMed] [Google Scholar]
- 46.Caldeira D, Barra M, Santos AT, de Abreu D, Pinto FJ, Ferreira JJ, Costa J. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart. 2014;100:550–6. doi: 10.1136/heartjnl-2013-305288. [DOI] [PubMed] [Google Scholar]
- 47.Davies EM, Packard KA, Knezevich JT, Campbell JA. New and emerging anticoagulant therapy for atrial fibrillation and acute coronary syndrome. Pharmacotherapy. 2011;31:975–1016. doi: 10.1592/phco.31.10.975. [DOI] [PubMed] [Google Scholar]
- 48.Bilen O, Teruya J. Complications of anticoagulation. Dis Mon. 2012;58:440–7. doi: 10.1016/j.disamonth.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Thorne K, Jayathissa S, Dee S, Briggs N, Taylor J, Reid S, et al. Adherence and outcomes of patients prescribed dabigatran (Pradaxa) in routine clinical practice. Intern Med J. 2014;44:261–5. doi: 10.1111/imj.12370. [DOI] [PubMed] [Google Scholar]
- 50.Whitehead H, Boyd JM, Blais DM, Hummel J. Drug-induced exanthem following dabigatran. Ann Pharmacother. 2011;45:e53. doi: 10.1345/aph.1Q317. [DOI] [PubMed] [Google Scholar]
- 51.Tsoupris A, Tzimas T, Gkabrelas K, Akritidis N. Iron complex, dabigatran and toxic epidermal necrolysis syndrome: a case-report. J Clin Pharm Ther. 2013;38:177–8. doi: 10.1111/jcpt.12032. [DOI] [PubMed] [Google Scholar]
- 52.U.S. Food and Drug Administration, ed. 2010. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularRenal/DrugsAdvisoryCommittee/UCM247244.pdf
- 53.Keisu M, Andersson TB. Drug-induced liver injury in humans: the case of ximelagatran. Handb Exp Pharmacol. 2010;196:407–18. doi: 10.1007/978-3-642-00663-0_13. [DOI] [PubMed] [Google Scholar]
- 54.Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003;108:2062–5. doi: 10.1161/01.CIR.0000096056.37269.14. [DOI] [PubMed] [Google Scholar]
- 55.Warkentin TE, Koster A. Bivalirudin: a review. Expert Opin Pharmacother. 2005;6:1349–71. doi: 10.1517/14656566.6.8.1349. [DOI] [PubMed] [Google Scholar]
- 56.Schrör K. Antiaggregatorische Therapie. In: Pötsch B, Madlener K, editors. Hämostaseologie. 2. Aufl. Springer: Heidelberg - Berlin; 2010. pp. 795–800. [Google Scholar]